Abstract
Purpose of review
The erythroid progenitors BFU-E (burst-forming unit-erythroid) and CFU-E (colony-forming unit-erythroid) have a critical role in erythropoiesis. They represent a heterogeneous and poorly characterized population of cells with modifiable self-renewal, proliferation and differentiation capabilities. This review focuses on the current state of erythroid progenitor biology with regard to immunophenotypic identification and regulatory programs; in addition, we will discuss the therapeutic implications of using these erythroid progenitors as pharmacologic targets.
Recent findings
Erythroid progenitors are classically defined by the appearance of morphologically defined colonies in semisolid cultures. However, these prior systems preclude a more thorough understanding of the composite nature of progenitor populations. Recent studies employing novel flow cytometric and cell-based assays have helped to redefine hematopoiesis, and suggest that erythroid progenitors may arise from different levels of the hematopoietic tree. Moreover, the identification of cell surface marker patterns in human BFU-E and CFU-E enhance our ability to perform downstream functional and molecular analyses at the population and single cell level. Advances in these techniques have already revealed novel subpopulations with increased self-renewing capacity, roles for erythroid progenitors in globin gene expression, and insights into pharmacologic mechanisms of glucocorticoids and pomalidomide.
Summary
Immunophenotypic and molecular characterization resolves the diversity of erythroid progenitors and may ultimately lead to the ability to target these progenitors to ameliorate diseases of dyserythropoiesis.
Keywords: Human erythroid progenitors, cell differentiation, erythropoiesis, pomalidomide, glucocorticoids
Introduction
Although identified in the 1970s, few studies have focused exclusively on detailed cellular and molecular characterization of the erythroid progenitors, BFU-Es and CFU-Es, which are central to the generation of red blood cells during erythropoiesis. The development of new methodologies has enabled the isolation and molecular characterization of erythroid progenitors. This review focuses on recent advances made in erythropoiesis, including immunophenotyping, regulation of self-renewal and differentiation, and the pharmacological targeting of erythroid progenitors for the treatment of anemia.
Hematopoiesis revisited: implications for erythroid differentiation
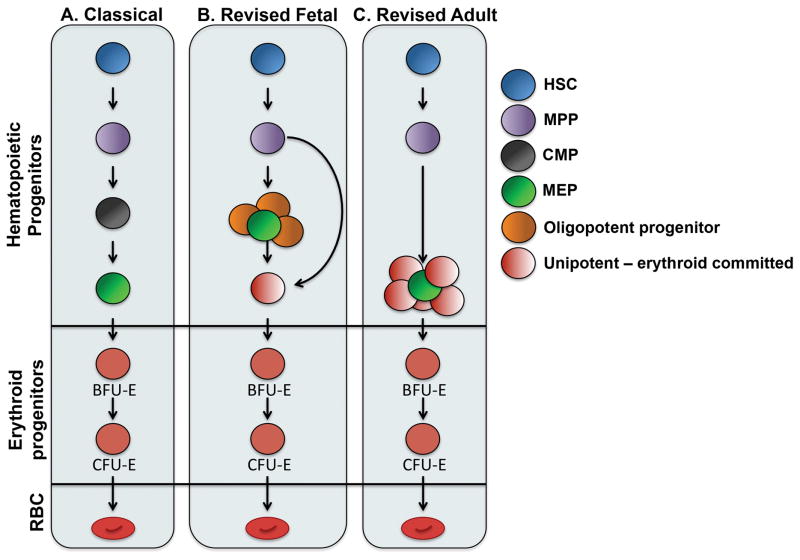
The conventional hematopoietic hierarchy depicts multipotent long-term and short-term hematopoietic repopulating stem cells at the apex, which give rise to progressively more differentiated hematopoietic progenitors and terminal blood cell lineages. The first branch point after the multipotent progenitor (MPP) divides lymphoid and myelo-erythroid lineages at the common lymphoid progenitor (CLP) and common myeloid progenitor (CMP) (1). Two additional hematopoietic progenitors emerge from CMPs including the oligopotent granulocyte macrophage progenitor (GMP) and bipotent megakaryocyte-erythroid progenitor (MEP) (2). Further commitment of MEPs toward the erythroid lineage is marked by the formation of BFU-E, the first erythroid restricted progenitor (3). BFU-Es ultimately differentiate into the erythropoietin(EPO)-sensitive CFU-E, which then progress through a series of erythroblast stages, reticulocyte maturation and ultimately terminal red cell formation (4). Thus, in this classical model of hematopoiesis, erythropoiesis relies on the stepwise differentiation of increasingly erythroid restricted hematopoietic progenitors (Figure 1A).
Figure 1. Erythroid lineage potential arises from different progenitor subsets according to revised hematopoietic hierarchy.
(A) Classical view of hematopoiesis suggests that erythroid progenitors are derived from discrete multipotent progenitors populations that undergo a series of differentiation steps whereby these progenitor cells become increasingly erythroid restricted. (B–C) New insights reveal that hematopoiesis is specific of the developmental stage and progenitor populations are heterogeneous. A simplified view of these findings with an emphasis on erythroid differentiation is depicted. During fetal hematopoiesis (B), erythroid potential arises from multipotent, oligopotent and unipotent progenitors. Conversely, the adult erythroid lineage (C) is derived from multipotent stem and predominantly unipotent progenitors. Finally, and common to both, erythroid progenitors can arise from a bypass directly from the multipotent progenitor.
Studies over the last decades in mice and more recently humans, have revised this model of hematopoiesis through the identification of alternative differentiation pathways and subpopulations within these previously defined progenitor stages. Hematopoietic progenitors may branch earlier than previously thought, as megakaryo-erythroid and myelo-lymphoid potential have been shown to diverge at a lymphoid-primed multipotent progenitor (LMPP); this route bypasses MPP and CMP (5, 6). Alternatively, myelo-erythroid output may predominantly rely on myeloid-restricted progenitors with long-term repopulating activity. These cells undertake a “myeloid bypass” whereby oligo- and bi-potent progenitors with self-renewing capacity at the single cell level give rise to erythroid, megakaryocyte and granulocyte-macrophage lineages (7). There is also growing evidence that the bipotent MEP contains multiple populations with varying lineage biases (8*, 9*).
Furthermore, hematopoiesis appears to be developmental stage specific. Through the use of novel single cell lineage potential assays and single cell transcriptome analyses, Notta et al. recently demonstrated that the fetal hematopoietic tree consists of multi-, oligo- and uni-potent progenitors, whereas adult hematopoiesis relies mostly on unipotent progenitors (Figure 1B–C). In addition, a higher amount of erythroid-megakaryocyte progenitors were shown to branch directly from the multipotent and oligopotent fractions during fetal hematopoiesis suggesting that the regulation of erythroid biased programs differ in fetal and adult hematopoietic progenitors (10**). It remains to be determined whether the fetal and adult erythroid progenitors derived from varying levels of the hematopoietic tree also differ at transcriptional and functional levels (i.e. higher self-renewal, differential globin gene expression). Future investigations should focus on these progenitors to identify whether all erythroid progenitors possess “memory” from their hematopoietic predecessors and whether this carries physiologic significance.
Isolation and characterization of megakaryocyte-erythroid and erythroid progenitors
Initial studies first identified the erythroid restricted progenitors, BFU-E and CFU-E, as well as early BFU-E with megakaryocyte potential by the morphology of hematopoietic colonies formed in semisolid media (11*). Later studies confirmed the existence of a discrete bipotent MEP using colony forming assays and a limited panel of cell surface markers by flow cytometry (12). Although crucial for our basic understanding of erythroid progenitor biology, these early studies were unable to discern the considerable variability among progenitor populations that advances in flow cytometry, genomic approaches (e.g. global and single cell RNA-seq) and novel in vitro culture systems have more recently highlighted.
An extensive discussion of the MEP is beyond the scope of this review; however, it should be noted that in contrast to murine hematopoiesis (13), a clear consensus for the cell surface markers that define human MEPs is lacking. As summarized in Table 1, MEPs have been characterized using a combination of cell surface markers including IL-3R, FLT3, MPL (assessed using BAH1 clone and other antibodies), CD36, CD41, CD71 and CD105 (8*, 9*, 10**, 14**). Using a combination of approaches including functional, single cell transcriptomics and lineage potential assays, these studies reveal that the identification of MEP and their subpopulations appears highly dependent on gating strategy; this likely reflects their extensive heterogeneity.
Table 1.
Current gating strategies used to identify MEP, BFU-E and CFU-E by flow cytometry.
| Progenitor population | Species | Cell surface markers | Citation |
|---|---|---|---|
| Megakaryocyte-erythroid progenitor (MEP) | |||
| Mouse | Lin− flk2− IL7Rα− c-kit+ FcgRII/III− CD34− CD105+/− CD150+/− | (13) | |
| Human | CD34+ CD38+ CD7+ CD10− FLT3− CD45RA− MPL+/− (BAH1 clone) CD71+/− | (10) | |
| Human | Lin− CD34+ CD38+ IL-3R− CD45RA− CD71+/− CD105+/− | (8) | |
| Human | Lin− CD34+ CD38mid CD45RA− FLT3− MPL+ CD36− CD41− | (14) | |
| Human | Lin− CD34+ CD38+ IL-3R− CD45RA− CD71 CD41 | (9) | |
| Burst forming unit-erythroid (BFU-E) | |||
| Mouse | c-kit+ CD45+ Ter119− CD71low | (15) | |
| Mouse (stress) | CD34− CD133− c-kit+ Sca-1+ | (19) | |
| Human | IL-3R− GPA− CD34+ CD36− | (17) | |
| Human | CD34+ CD123− CD71low TβRIIIlow/hi | (18) | |
| Human (stress) | CD34− CD133− c-kit+ (19) | ||
| Colony forming unit-erythroid (CFU-E) | |||
| Mouse | c-kit+ CD45− Ter119− CD71hi | (15) | |
| Mouse | Lin− c-kit+ Sca-1− IL-7Rα− IL3Rα− CD41− CD71+ | (16) | |
| Human | IL-3R− GPA− CD34− CD36+ | (17) | |
To date, few gating strategies exist for erythroid progenitors, and therefore considerably less is known about their heterogeneity (Table 1). Murine studies identify fetal liver BFU-Es and CFU-Es as c-Kit+CD45+Ter119−CD71low and c-Kit+CD45−Ter119−CD71hi, respectively (15), while murine bone marrow CFU-Es are characterized as Lin−c-Kit+Sca-1−IL-7Rα−IL3Rα−CD41−CD71+ cells (16). The immunophenotypic identity of human erythroid progenitors was less clear, until Li et al. first demonstrated that BFU-E reside in the IL-3R−GPA−CD34+CD36− fraction, and IL-3R−GPA−CD34−CD36+ denotes CFU-E. These populations give rise to characteristically pure colonies in methylcellulose and discrete gene expression signatures by RNAseq (17**). More recently, in the murine system, Gao et al. used single cell RNAseq to demonstrate that the transient expression of Type-III Transforming Growth Factor-β Receptor (TβRIII) distinguished early (large cluster forming) BFU-E and late (small cluster forming) BFU-E in fetal livers (18*). Whether these TβRIII− cells represent a novel more immature BFU-E or a functional subpopulation of BFU-E requires further investigation.
Despite these pivotal studies, the utility of these markers may only apply to steady-state erythropoiesis. Indeed, stress erythroid progenitors express the cell surface markers CD34−CD133−c-Kit+Sca-1+ and CD34−CD133−c-Kit+ in mice and human-derived cells, respectively (19*). Thus, erythroid progenitors represent a heterogeneous population of cells, and moving forward, it will be critical to understand the regulatory mechanisms that underlie this diversity.
Regulation of erythroid progenitor self-renewal, proliferation and differentiation
Erythroid progenitors uniquely act as a critical interface between the hematopoietic stem cell and the more mature stages, dictating programs that eventually control the function of terminal erythroblasts. Indeed, terminal erythroblasts are largely pre-programmed, and as cells enter terminal erythroid differentiation they possess little or no capabilities for proliferation and self-renewal (20**). This programming confines erythroblasts to one division per stage with a single proerythroblast giving rise to 16 or 32 enucleate reticulocytes. Therefore, an active area of investigation lies in the identification and understanding of how these progenitors integrate soluble, cell-contact and cell-autonomous signals in order to control red cell mass and differentiation programs.
Cytokines and growth factors
Soluble factors that govern erythroid progenitor physiology come from diverse families of signaling molecules, and often work synergistically to amplify progenitor numbers. For example, stem cell factor (SCF), the ligand for c-kit receptor, cooperates with erythropoietin (EPO), TGF-α, insulin-like growth factor 1 (IGF-1) and IL-3 in regulating erythroid progenitor biology. Studies using erythroid cell lines and primary CFU-Es demonstrate that the synergy of SCF and EPO act at the receptor and downstream effector levels (21–24). Moreover, SCF and TGF-α increases growth and self-renewal of avian erythroid progenitors (25). Additional factors that synergize with SCF include IL-3 and IGF-1. IL-3 and SCF increase BFU-Es in liquid cultures (26), and SCF and IGF-1 enhance peripheral blood erythroid progenitors ex vivo (27).
IL-3 also functions independently of SCF at the BFU-E stage. IL-3 stimulates BFU-E proliferation (28), and may regulate self-renewal as evidenced by the emergence of sub-colonies in methylcellulose (29). Further, IL-3 with activin A induces BFU-E mitogenesis and increased colony numbers (30).
TGF-β exerts a pro-differentiation role during early erythropoiesis. The expression of CD105 (endoglin) on human erythroid progenitors accelerates their differentiation in the presence of TGF-β (8*), and TβRIII expression appears restricted to BFU-E subpopulations with diminished self-renewing capacity in vitro (18*).
Despite the cooperative nature of these signaling molecules, murine studies implicate EPO and SCF as the sole endogenous factors required for mammalian erythroid progenitors. SCF/c-kit mutant and EPO-R deficient mice both exhibit severe anemia with varying erythroid progenitor defects, whereas erythropoiesis remains unaffected in the absence of IL-3R (31–33). Activating mutations in the c-kit receptor prevent erythroblast maturation, and expand progenitors (34*). Further, EPO acts directly on CFU-Es to promote survival as opposed to proliferation (35). In the absence of EPO, CFU-Es undergo apoptosis through the FAS-FAS ligand pathway, and fail to progress into terminal erythroid differentiation (36, 37).
Extracellular signals may also promote the expansion and differentiation of specialized erythroid progenitors during times of erythropoietic stress. Studies in mice and more recently in humans have shown that in addition to EPO and SCF, hypoxia, bone morphogenic protein 4 (BMP4), growth differentiation factor-15 (GDF-15) and hedgehog contribute to the expansion of BFU-Es during stress erythropoiesis. These progenitors also express higher levels of genes associated with self-renewal such as Pu.1 and GATA2 (19).
A largely unanswered question is how and in what context cytokines shape the transcriptional landscape and composition of erythroid progenitor subpopulations as well as the functional consequences of soluble factor signaling.
Cell intrinsic
Considerably less is known regarding how intrinsic factors such as transcriptional, post-transcriptional and translational regulators control erythroid progenitor function. However, it is well appreciated that discrete gene subsets must be turned on and off in progenitors and terminal erythroblasts to facilitate the proper balance of self-renewal and differentiation. The reciprocal expression of the GATA family of transcription factors, GATA2 and GATA1, during erythropoiesis exemplifies this necessity (38). These studies indicate that the expression of self-renewal genes by sustained GATA2 expression could theoretically lead to increased levels of erythroid progenitors (39). Although GATA2 functions in multiple hematopoietic lineages, its overexpression leads to megakaryocyte differentiation (40, 41) suggesting that the careful dosage of transcription factors is needed for self-renewing pro-erythroid transcriptional programs. A similar dose-dependent mechanism may underlie PU.1-mediated BFU-E self-renewal. PU.1 is highly expressed in multi- and oligo-potent progenitors as well as in more committed myeloid and lymphoid cells, and is proposed to mediate BFU-E self-renewal at low levels (42). Fetal liver cells null for PU.1 show perturbations of erythroid progenitor numbers including attenuated proliferative capacity, accelerated differentiation and failure to respond to SCF and EPO (43). The exact molecular mechanisms remain elusive as PU.1 binding partners and gene targets have yet to be defined in primary erythroid progenitor populations.
In addition to transcriptional controls, RNA stability and accessibility to translational machinery could also determine erythroid progenitor self-renewal. The RNA binding protein ZFP36L2, which is strongly induced following glucocorticoid receptor agonism in murine progenitors, promotes BFU-E self-renewal by negatively regulating gene transcripts associated with terminal erythroid differentiation and delaying the BFU-E to CFU-E transition (44). An additional, largely unstudied RNA binding protein in erythroid progenitors is LIN28B. LIN28B regulates the expression of the let7 family of microRNAs and HMGA2, which plays a critical role in fetal hematopoiesis (45**, 46*). Furthermore, fetal CFU-Es exhibit increased responsiveness to EPO, and therefore, the LIN28B-let7-HMGA2 axis may possess an unappreciated function during fetal erythropoiesis (47). Since ectopic expression of LIN28B has already been shown to induce HbF in adult erythroid progenitors (48), investigation into whether this also affects erythroid progenitor self-renewal should be explored.
The erythroblastic island
These critical niches for erythropoiesis are composed of a central macrophage surrounded by differentiating erythroid cells at all stages of maturation beginning with the CFU-E. Through still poorly elucidated mechanisms, it is hypothesized that BFU-E migrate towards these islands, and following differentiation to CFU-E, adhere to macrophages through a number of different molecules (49, 50**, 51*). Whether central macrophages promote CFU-E self-renewal or merely increase proliferation of CFU-E/Pro-EB requires further investigation. However, in vitro and murine studies clearly demonstrate an essential function for erythroblastic islands during steady-state and stress erythropoiesis (52, 53).
Erythroid progenitors as a target for treating disordered erythropoiesis
As previously mentioned, erythroid progenitors are crucial for determining the overall red cell mass. It is therefore not surprising that erythroid defects such as the inherited bone marrow failure syndromes (e.g. Diamond Blackfan anemia [DBA]) demonstrate a paucity of erythroid progenitors, specifically CFU-Es (54*, 55). Although two major theories, (i) increased p53 levels or (ii) translational defect of GATA1, have been proposed as central to the pathophysiology of DBA, it is generally accepted that the apoptosis of the EPO-responsive CFU-E causes the red cell failure associated with the disease (56–58). Thus, understanding why these progenitor cells are exquisitely sensitive to abnormal translational and how to pharmacologically target these progenitors remains a fundamental goal.
There is a limited number of pharmacologic agents that directly target erythroid progenitors. Since activin and BMP signaling stimulates BFU-E self-renewal, and TGF-β accelerates erythroid differentiation, therapeutic interventions aimed at the TGF-β superfamily may ameliorate certain cases of anemia. Indeed, inhibition of the TβRI kinase by Galunisertib augments the number of immature early-BFU-E (18*). The ligand trap activin receptor type IIA and IIB fusion proteins, RAP-011 and RAP-536 respectively, stimulate red cell production in normal and ineffective erythropoiesis; these molecules preclude activation of SMAD2/3 by growth differentiation factor-11 (GDF-11) (59, 60). Whether these compounds act at the level of the BFU-E and/or CFU-E in addition to their action on terminally differentiating erythroblasts is unclear.
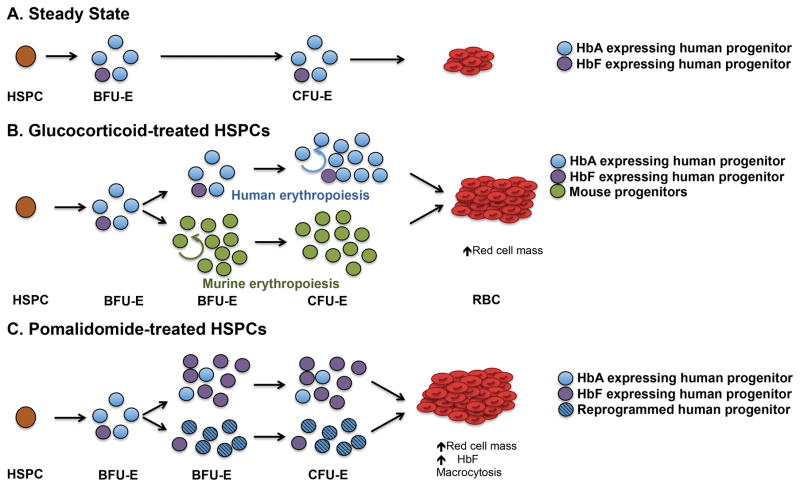
Glucocorticoids (e.g. prednisone and dexamethasone) are the current standard of care for DBA and other hematologic conditions including warm autoimmune hemolytic anemia. They are hypothesized to act at the level of the erythroid progenitors (61). However, the mechanism of action of glucorticoids in erythropoiesis remains unclear. Conflicting findings in murine and human systems have generated controversy regarding the exact progenitor stage at which glucocorticoids act (44, 62*, 63*). Furthermore, in vitro studies utilizing dexamethasone as an expansion agent prevent a stage-specific assessment of its function on erythroid progenitors (64). Together, glucocorticoids may exhibit species specificity with the targeted population being BFU-E and CFU-E in mice and humans, respectively. Indeed, data from others (61) and our unpublished data suggest that in humans, dexamethasone acts at the CFU-E stage (Figure 2A,B). Further experimentation using purified human erythroid progenitors is currently being explored to understand the molecular and stage-specific actions of glucocorticoids.
Figure 2. Pomalidomide and glucocorticoids are hypothesized to act on different erythroid progenitor populations.
(A) During steady-state erythropoiesis in the adult, the BFU-E population contains a small minority of HbF-producing cells (purple), and cells possess limited self-renewal. BFU-Es differentiate into CFU-Es followed by erythroid precursors and erythrocytes containing mainly adult hemoglobin (HbA). (B) Glucocorticoids appear to affect erythroid progenitors in a species-specific manner. In mice, glucocorticoids increase BFU-E self-renewal (green), whereas increased CFU-Es may result from glucocorticoid treatment in humans. The proportion of HbF-producing cells (purple) remains unchanged by glucocorticoid treatment. (C) Pomalidomide exerts its effect at BFU-E or on the BFU-E to CFU-E transition to induce HbF and increase BFU-E numbers through two potential mechanisms: (i) stimulate the expansion of pre-programmed HbF producing BFU-E (purple) or (ii) reprogram adult progenitors to prevent γ-globin repression (striped).
There is increasing evidence that the immunomodulatory drugs (IMiDs), pomalidomide and lenalidomide, target erythroid progenitors at the level of the BFU-E or during the transition of the BFU-E to CFU-E stage. Lenalidomide increases CFU-Es suggesting that these drugs could be used to stimulate red blood cell production (65). Alternatively, IMiDs also induce fetal hemoglobin (HbF) production in adult-derived CD34+ HSPCs, and pomalidomide appears to exert this effect at the level of the BFU-E (66**). A provocative implication from these studies is that globin gene regulatory programs reside in erythroid progenitors. Indeed, Papayonnopoulou and collaborators provide early evidence of this phenomenon, and imply that a minority of “pre-programmed” adult erythroid progenitors give rise to erythroblasts expressing HbF (67, 68). Therefore, the most effective pharmacologic agents might exploit the regulatory mechanisms in erythroid progenitors to either enrich this small population of “HbF programmed cells” or override γ-globin silencing in the majority of BFU-Es engaged with adult globin expression regulatory factors (Figure 2C).
Conclusion
The rapidly growing technological advances such as genomic, proteomic and cell biological characterization of single cells and advances in flow cytometry approaches are likely to add to our understanding of the role, regulation and differentiation potential of erythroid progenitors, and their contribution to red cell production. Ultimately, these findings will lead to the discovery of new therapies for the treatment of disorders of erythropoiesis.
Keypoints.
Recent advances reveal that erythroid progenitors are not a uniform population, but rather, composed of different subpopulations with variable levels of self-renewing capabilities.
The discovery of cell surface marker panels that resolve BFU-Es and CFU-Es populations enables the study of erythroid progenitor dynamics in vivo and in vitro.
An active area of investigation is how erythroid progenitors integrate soluble, cell intrinsic and microenvironment signals to regulate self-renewal, proliferation and differentiation.
BFU-Es and CFU-Es may represent appealing population of cells for pharmacologic stimulation of fetal hemoglobin and red blood cell mass.
Due to the molecular and cellular heterogeneity of erythroid progenitors, future studies should use single cell approaches whenever feasible.
Acknowledgments
This work was partly supported by NIH Grants DK26263 (to NM), and by the Pediatric Cancer Foundation (to LB). LB is the recipient of an Allied World St. Baldrick’s Foundation Scholar Award. BMD is a past recipient of an American Society of Hematology Physician-Scientist Career Development Award.
Footnotes
Conflict of Interest Disclosure:
None.
References
- 1.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10(2):120–36. doi: 10.1016/j.stem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2(6):640–53. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dore LC, Crispino JD. Transcription factor networks in erythroid cell and megakaryocyte development. Blood. 2011;118(2):231–9. doi: 10.1182/blood-2011-04-285981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koury MJ. Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev. 2014;28(2):49–66. doi: 10.1016/j.blre.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121(2):295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Mansson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26(4):407–19. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154(5):1112–26. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- *8.Mori Y, Chen JY, Pluvinage JV, Seita J, Weissman IL. Prospective isolation of human erythroid lineage-committed progenitors. Proc Natl Acad Sci U S A. 2015;112(31):9638–43. doi: 10.1073/pnas.1512076112. This study resolves three subpopulations in the megakaryocyte-erythroid progenitor population using CD105 and CD71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Psaila B, Barkas N, Iskander D, Roy A, Anderson S, Ashley N, et al. Single-cell profiling of human megakaryocyte-erythroid progenitors identifies distinct megakaryocyte and erythroid differentiation pathways. Genome Biol. 2016;17:83. doi: 10.1186/s13059-016-0939-7. This study uses a combination of single cell transcriptomics and flow cytometry to identify subpopulations within the classically defined MEP fraction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **10.Notta F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351(6269):aab2116. doi: 10.1126/science.aab2116. This study provides evidence that fetal and adult hematopoietic progenitor differentiation differs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Koury MJ. Tracking erythroid progenitor cells in times of need and times of plenty. Exp Hematol. 2016;44(8):653–63. doi: 10.1016/j.exphem.2015.10.007. A comprehensive review on the state of erythropoiesis during steady-state or stress conditions. [DOI] [PubMed] [Google Scholar]
- 12.Vannucchi AM, Paoletti F, Linari S, Cellai C, Caporale R, Ferrini PR, et al. Identification and characterization of a bipotent (erythroid and megakaryocytic) cell precursor from the spleen of phenylhydrazine-treated mice. Blood. 2000;95(8):2559–68. [PubMed] [Google Scholar]
- 13.Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1(4):428–42. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- **14.Sanada C, Xavier-Ferrucio J, Lu YC, Min E, Zhang PX, Zou S, et al. Adult human megakaryocyte-erythroid progenitors are in the CD34+CD38mid fraction. Blood. 2016;128(7):923–33. doi: 10.1182/blood-2016-01-693705. This study develops a new in vitro megakaryocyte-erythroid potential assay and gating strategy to enrich human MEPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flygare J, Rayon Estrada V, Shin C, Gupta S, Lodish HF. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood. 2011;117(12):3435–44. doi: 10.1182/blood-2010-07-295550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terszowski G, Waskow C, Conradt P, Lenze D, Koenigsmann J, Carstanjen D, et al. Prospective isolation and global gene expression analysis of the erythrocyte colony-forming unit (CFU-E) Blood. 2005;105(5):1937–45. doi: 10.1182/blood-2004-09-3459. [DOI] [PubMed] [Google Scholar]
- **17.Li J, Hale J, Bhagia P, Xue F, Chen L, Jaffray J, et al. Isolation and transcriptome analyses of human erythroid progenitors: BFU-E and CFU-E. Blood. 2014;124(24):3636–45. doi: 10.1182/blood-2014-07-588806. First cell surface marker scheme used to detect human BFU-E and CFU-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Gao X, Lee HY, da Rocha EL, Zhang C, Lu YF, Li D, et al. TGF-beta inhibitors stimulate red blood cell production by enhancing self-renewal of BFU-E erythroid progenitors. Blood. 2016;128(23):2637–41. doi: 10.1182/blood-2016-05-718320. This work demonstrates that the use of single cell transcriptome-wide approaches can resolve novel erythroid progenitor subpopulations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Xiang J, Wu DC, Chen Y, Paulson RF. In vitro culture of stress erythroid progenitors identifies distinct progenitor populations and analogous human progenitors. Blood. 2015;125(11):1803–12. doi: 10.1182/blood-2014-07-591453. The authors provide cell surface markers and ex vivo culture system for mouse and human stress progenitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *20.Stadhouders R, Cico A, Stephen T, Thongjuea S, Kolovos P, Baymaz HI, et al. Control of developmentally primed erythroid genes by combinatorial co-repressor actions. Nat Commun. 2015;6:8893. doi: 10.1038/ncomms9893. The authors demonstrate that chromatin occupancy of different regulatory complexes allow for lineage-specific genes expression following erythroid differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munugalavadla V, Kapur R. Role of c-Kit and erythropoietin receptor in erythropoiesis. Crit Rev Oncol Hematol. 2005;54(1):63–75. doi: 10.1016/j.critrevonc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Klingmuller U, Acurio A, Hsiao JG, Lodish HF. Functional interaction of erythropoietin and stem cell factor receptors is essential for erythroid colony formation. Proc Natl Acad Sci U S A. 1997;94(5):1806–10. doi: 10.1073/pnas.94.5.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui X, Krantz SB, You M, Zhao Z. Synergistic activation of MAP kinase (ERK1/2) by erythropoietin and stem cell factor is essential for expanded erythropoiesis. Blood. 1998;92(4):1142–9. [PubMed] [Google Scholar]
- 24.Tan BL, Hong L, Munugalavadla V, Kapur R. Functional and biochemical consequences of abrogating the activation of multiple diverse early signaling pathways in Kit. Role for Src kinase pathway in Kit-induced cooperation with erythropoietin receptor. J Biol Chem. 2003;278(13):11686–95. doi: 10.1074/jbc.M207068200. [DOI] [PubMed] [Google Scholar]
- 25.Steinlein P, Wessely O, Meyer S, Deiner EM, Hayman MJ, Beug H. Primary, self-renewing erythroid progenitors develop through activation of both tyrosine kinase and steroid hormone receptors. Curr Biol. 1995;5(2):191–204. doi: 10.1016/s0960-9822(95)00040-6. [DOI] [PubMed] [Google Scholar]
- 26.Papayannopoulou T, Brice M, Blau CA. Kit ligand in synergy with interleukin-3 amplifies the erythropoietin-independent, globin-synthesizing progeny of normal human burst-forming units-erythroid in suspension cultures: physiologic implications. Blood. 1993;81(2):299–310. [PubMed] [Google Scholar]
- 27.Panzenbock B, Bartunek P, Mapara MY, Zenke M. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood. 1998;92(10):3658–68. [PubMed] [Google Scholar]
- 28.Serke S, Huhn D. Effects of various recombinant human hemopoietic growth factors (rhEpo, rhG-CSF, rhGM-CSF, rhIl-3) on the growth of peripheral blood progenitor cells (BFU-E, CFU-GM) Blut. 1990;61(1):25–9. doi: 10.1007/BF01739430. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JL, Marley SB, Blackett NM, Szydlo R, Goldman JM, Gordon MY. Interleukin 3 (IL-3), but not stem cell factor (SCF) increases self-renewal by human erythroid burst-forming units (BFU-E) in vitro. Cytokine. 1998;10(1):49–54. doi: 10.1006/cyto.1997.0256. [DOI] [PubMed] [Google Scholar]
- 30.Mizuguchi T, Kosaka M, Saito S. Activin A suppresses proliferation of interleukin-3-responsive granulocyte-macrophage colony-forming progenitors and stimulates proliferation and differentiation of interleukin-3-responsive erythroid burst-forming progenitors in the peripheral blood. Blood. 1993;81(11):2891–7. [PubMed] [Google Scholar]
- 31.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90(4):1345–64. [PubMed] [Google Scholar]
- 32.Nishinakamura R, Nakayama N, Hirabayashi Y, Inoue T, Aud D, McNeil T, et al. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity. 1995;2(3):211–22. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83(1):59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- *34.Haas N, Riedt T, Labbaf Z, Bassler K, Gergis D, Frohlich H, et al. Kit transduced signals counteract erythroid maturation by MAPK-dependent modulation of erythropoietin signaling and apoptosis induction in mouse fetal liver. Cell Death Differ. 2015;22(5):790–800. doi: 10.1038/cdd.2014.172. This study demonstrates that the crosstalk between SCF and EPO signaling pathways at the progenitor stages mediates murine erythroid differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248(4953):378–81. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- 36.Kelley LL, Koury MJ, Bondurant MC, Koury ST, Sawyer ST, Wickrema A. Survival or death of individual proerythroblasts results from differing erythropoietin sensitivities: a mechanism for controlled rates of erythrocyte production. Blood. 1993;82(8):2340–52. [PubMed] [Google Scholar]
- 37.De Maria R, Testa U, Luchetti L, Zeuner A, Stassi G, Pelosi E, et al. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood. 1999;93(3):796–803. [PubMed] [Google Scholar]
- 38.Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J Biol Chem. 2010;285(41):31087–93. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briegel K, Lim KC, Plank C, Beug H, Engel JD, Zenke M. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 1993;7(6):1097–109. doi: 10.1101/gad.7.6.1097. [DOI] [PubMed] [Google Scholar]
- 40.Huang Z, Dore LC, Li Z, Orkin SH, Feng G, Lin S, et al. GATA-2 reinforces megakaryocyte development in the absence of GATA-1. Mol Cell Biol. 2009;29(18):5168–80. doi: 10.1128/MCB.00482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikonomi P, Rivera CE, Riordan M, Washington G, Schechter AN, Noguchi CT. Overexpression of GATA-2 inhibits erythroid and promotes megakaryocyte differentiation. Exp Hematol. 2000;28(12):1423–31. doi: 10.1016/s0301-472x(00)00553-1. [DOI] [PubMed] [Google Scholar]
- 42.Back J, Dierich A, Bronn C, Kastner P, Chan S. PU.1 determines the self-renewal capacity of erythroid progenitor cells. Blood. 2004;103(10):3615–23. doi: 10.1182/blood-2003-11-4089. [DOI] [PubMed] [Google Scholar]
- 43.Fisher RC, Slayton WB, Chien C, Guthrie SM, Bray C, Scott EW. PU.1 supports proliferation of immature erythroid progenitors. Leuk Res. 2004;28(1):83–9. doi: 10.1016/s0145-2126(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Prak L, Rayon-Estrada V, Thiru P, Flygare J, Lim B, et al. ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature. 2013;499(7456):92–6. doi: 10.1038/nature12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Copley MR, Babovic S, Benz C, Knapp DJ, Beer PA, Kent DG, et al. The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat Cell Biol. 2013;15(8):916–25. doi: 10.1038/ncb2783. This is the first study to provide evidence that Lin28b is a cell autonomous factor involved in fetal hematopoiesis. [DOI] [PubMed] [Google Scholar]
- *46.Rowe RG, Wang LD, Coma S, Han A, Mathieu R, Pearson DS, et al. Developmental regulation of myeloerythroid progenitor function by the Lin28b-let-7-Hmga2 axis. J Exp Med. 2016;213(8):1497–512. doi: 10.1084/jem.20151912. The authors show that the Lin28b/Let7/Hmga2 axis is responsible for a fetal differentiation program in murine myeloerythroid progenitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rich IN, Kubanek B. The ontogeny of erythropoiesis in the mouse detected by the erythroid colony-forming technique. I. Hepatic and maternal erythropoiesis. J Embryol Exp Morphol. 1979;50:57–74. [PubMed] [Google Scholar]
- 48.Lee YT, de Vasconcellos JF, Yuan J, Byrnes C, Noh SJ, Meier ER, et al. LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo. Blood. 2013;122(6):1034–41. doi: 10.1182/blood-2012-12-472308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–8. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **50.Giger KM, Kalfa TA. Phylogenetic and Ontogenetic View of Erythroblastic Islands. Biomed Res Int. 2015;2015:873628. doi: 10.1155/2015/873628. An outstanding review on erythroblastic islands with an evolutionnary perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Hom J, Dulmovits BM, Mohandas N, Blanc L. The erythroblastic island as an emerging paradigm in the anemia of inflammation. Immunol Res. 2015;63(1–3):75–89. doi: 10.1007/s12026-015-8697-2. Our recent review on the putative crosstalk between macrophages and erythroblasts during anemia of inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19(4):429–36. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhodes MM, Kopsombut P, Bondurant MC, Price JO, Koury MJ. Adherence to macrophages in erythroblastic islands enhances erythroblast proliferation and increases erythrocyte production by a different mechanism than erythropoietin. Blood. 2008;111(3):1700–8. doi: 10.1182/blood-2007-06-098178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *54.Means RT., Jr Pure red cell aplasia. Blood. 2016;128(21):2504–9. doi: 10.1182/blood-2016-05-717140. A recent concise review on this bone marrow failure syndrome. [DOI] [PubMed] [Google Scholar]
- 55.Narla A, Vlachos A, Nathan DG. Diamond Blackfan anemia treatment: past, present, and future. Semin Hematol. 2011;48(2):117–23. doi: 10.1053/j.seminhematol.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellis SR, Lipton JM. Diamond Blackfan anemia: a disorder of red blood cell development. Curr Top Dev Biol. 2008;82:217–41. doi: 10.1016/S0070-2153(07)00008-7. [DOI] [PubMed] [Google Scholar]
- 57.Sankaran VG, Ghazvinian R, Do R, Thiru P, Vergilio JA, Beggs AH, et al. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J Clin Invest. 2012;122(7):2439–43. doi: 10.1172/JCI63597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ludwig LS, Gazda HT, Eng JC, Eichhorn SW, Thiru P, Ghazvinian R, et al. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat Med. 2014;20(7):748–53. doi: 10.1038/nm.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dussiot M, Maciel TT, Fricot A, Chartier C, Negre O, Veiga J, et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in beta-thalassemia. Nat Med. 2014;20(4):398–407. doi: 10.1038/nm.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suragani RN, Cadena SM, Cawley SM, Sako D, Mitchell D, Li R, et al. Transforming growth factor-beta superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med. 2014;20(4):408–14. doi: 10.1038/nm.3512. [DOI] [PubMed] [Google Scholar]
- 61.Chan HS, Saunders EF, Freedman MH. Diamond-Blackfan syndrome. II. In vitro corticosteroid effect on erythropoiesis. Pediatr Res. 1982;16(6):477–8. doi: 10.1203/00006450-198206000-00015. [DOI] [PubMed] [Google Scholar]
- *62.Vignjevic S, Budec M, Markovic D, Dikic D, Mitrovic O, Diklic M, et al. Glucocorticoid receptor mediates the expansion of splenic late erythroid progenitors during chronic psychological stress. J Physiol Pharmacol. 2015;66(1):91–100. These recent findings highlight a putative role for the glucocorticoid receptor at the CFU-E stage during murine erythropoiesis. [PubMed] [Google Scholar]
- *63.Lee HY, Gao X, Barrasa MI, Li H, Elmes RR, Peters LL, et al. PPAR-alpha and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature. 2015;522(7557):474–7. doi: 10.1038/nature14326. A novel role for PPAR-α in the regulation of erythroid progenitors self-renewal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fibach E, Manor D, Oppenheim A, Rachmilewitz EA. Proliferation and maturation of human erythroid progenitors in liquid culture. Blood. 1989;73(1):100–3. [PubMed] [Google Scholar]
- 65.Narla A, Dutt S, McAuley JR, Al-Shahrour F, Hurst S, McConkey M, et al. Dexamethasone and lenalidomide have distinct functional effects on erythropoiesis. Blood. 2011;118(8):2296–304. doi: 10.1182/blood-2010-11-318543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **66.Dulmovits BM, Appiah-Kubi AO, Papoin J, Hale J, He M, Al-Abed Y, et al. Pomalidomide reverses gamma-globin silencing through the transcriptional reprogramming of adult hematopoietic progenitors. Blood. 2016;127(11):1481–92. doi: 10.1182/blood-2015-09-667923. Our work demonstrating that fetal hemoglobin induction by pomalidomide requires reprogramming of erythroid progenitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papayannopoulou TH, Brice M, Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci U S A. 1976;73(6):2033–7. doi: 10.1073/pnas.73.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stamatoyannopoulos G, Nakamoto B, Kurachi S, Papayannopoulou T. Direct evidence for interaction between human erythroid progenitor cells and a hemoglobin switching activity present in fetal sheep serum. Proc Natl Acad Sci U S A. 1983;80(18):5650–4. doi: 10.1073/pnas.80.18.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]