Abstract
The neuronal isoform of cytoplasmic polyadenylation element-binding protein (CPEB) is a regulator of local protein synthesis at synapses and is critical in maintaining learning-related synaptic plasticity in Aplysia. Previous studies indicate that the function of Aplysia CPEB can be modulated by conversion to a stable prion-like state, thus contributing to the stabilization of long-term memory on a molecular level. Here, we used biophysical methods to demonstrate that Aplysia CPEB, like other prions, undergoes a conformational switch from soluble α-helix-rich oligomer to β-sheet-rich fiber in vitro. Solid-state NMR analyses of the fibers indicated a relatively rigid N-terminal prion domain. The fiber form of Aplysia CPEB showed enhanced binding to target mRNAs as compared to the soluble form. Consequently, we propose a model for the Aplysia CPEB fibers that may have relevance for functional prions in general.
Although significant knowledge of cellular and molecular mechanisms underlying the acquisition and early storage of implicit and explicit long-term memory has been gained, the mechanisms by which memories are maintained for long periods of time are still not fully understood1,2. Because proteins normally have relatively short half-lives, of hours or days, the question remains: How can the change in molecular composition of a synapse be maintained for long periods of time, as is required for long-term memory? We previously found one answer to this conundrum in a work describing a prion-like regulator of local protein synthesis at the synapse in the marine snail Aplysia californica: the cytoplasmic polyadenylation element–binding protein Aplysia CPEB3,4. This provided physiological evidence that the prion-like properties of Aplysia CPEB might explain the self-sustained, continuous molecular turnover at the synapse5.
Generally, CPEB proteins bind a six-nucleotide sequence, the cytoplasmic polyadenylation element (CPE) at the 3′ untranslated regions (3′ UTRs) of mRNAs, and recruit the polyadenylation machinery required for translational activation6,7. The neuronal isoform of Aplysia CPEB differs from other isoforms of CPEB in having an N-terminal domain that is rich in glutamine and asparagine8, similar to the QN-rich domain of certain yeast prions (Sup35p, Ure2p and Rnq1p)9–11. When prions convert from soluble form to self-perpetuating infectious form, they undergo a conformational change from a globular or an unstructured form to an aggregated β-sheet– enriched amyloid structure12–14. Previous studies have shown that Aplysia CPEB exists in at least two different physical conformations, one of which is an aggregated self-perpetuating form3,5 (Fig. 1). Moreover, in vitro-formed fibers of isolated prion domains of Aplysia CPEB are able to induce conformational changes and can self-perpetuate in yeast cells15. These findings are consistent with the idea that the N-terminal QN-rich extension confers a new ability upon Aplysia CPEB to function as a biologically useful prion in the nervous system. Recent evidence indicates that the aggregated form of Aplysia CPEB is in the active conformation and is essential for the maintenance of long-term synaptic plasticity in Aplysia5. The homologs of CPEB in Drosophila (Orb2)16 and in mice (mCPEB3)17 have similarly been found to be essential for long-term memory. The potential generality of this hypothesis is underscored by the identification of isoforms of CPEB with QN-rich terminal regions in humans18.
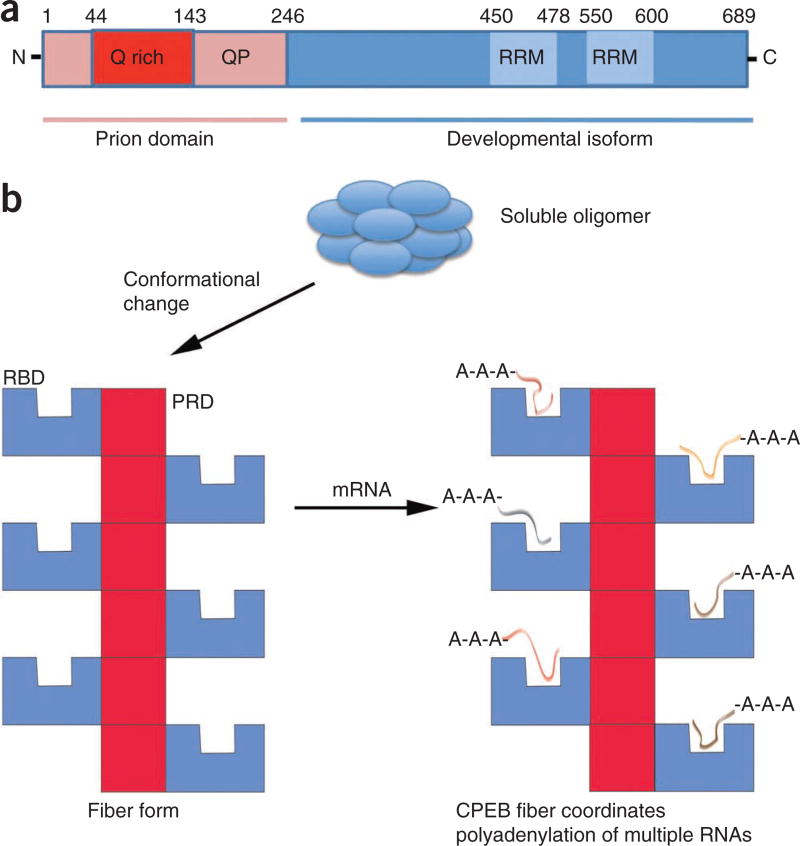
Figure 1. Schematic representation of the neuronal isoform of Aplysia CPEB.
(a) Domain organization in the neuronal isoform of Aplysia CPEB. Pink, prion domain (PRD); blue, RNA-binding domain (RBD), which alone also represents the developmental isoform of Aplysia CPEB. The neuronal isoform (expressed only in neurons) and developmental isoform (expressed in other tissues also) of Aplysia CPEB differ only in the PRD. Two RNA-recognition motifs (RRM), a glutamine-rich domain (Q rich) and a glutamine- and proline-rich domain (QP) are shown. (b) Model showing the assembly of Aplysia CPEB fibers that can efficiently regulate the coordinated polyadenylation of multiple RNAs in a localized manner. Red, PRD; blue, RBD. The PRDs are stacked together to form the rigid fiber axis, and the RBDs are solvent exposed and oriented outside the fiber axis.
To date, to our knowledge, there has been no direct biophysical evidence for the existence of Aplysia CPEB in two forms similar to the different conformational states of other known prions. Here we demonstrate that Aplysia CPEB can exist in at least two different conformational forms: an α-helix-rich form and a β-sheet-rich amyloid form. Our solid-state NMR results indicate that the prion-like N-terminal domain of Aplysia CPEB is not entirely composed of β-sheets but also has helical and random-coil signatures. We propose a model for the aggregated form of Aplysia CPEB in which the N-terminal domains form the fiber axis, whereas the RNA-binding domains project out of the fiber axis.
RESULTS
The soluble form of Aplysia CPEB exists as oligomers
To characterize the different conformational forms of Aplysia CPEB in vivo, we used total soluble-protein extract from the central nervous system (CNS) of Aplysia under native conditions. The Aplysia CNS tissues were briefly ground at 4 °C and centrifuged (Online Methods). The supernatant was collected and fractionated by gel-filtration chromatography. All fractions obtained after gel filtration were analyzed by western blotting using an antibody raised against the Aplysia CPEB sequence (Online Methods). These analyses indicate that CPEB extracted from Aplysia CNS tissues exists as a high-molecular-weight oligomer or mixture of oligomers of ten or more units (>80% of the mass of detected protein has an apparent molecular weight of >670 kDa) (Fig. 2 and Supplementary Fig. 1a,b). No monomer was detected by this method. However, consistent with the observations reported previously5, trace amounts of oligomers were found in the molecular-weight range expected for a trimer or tetramer. Control experiments done with Aplysia α-tubulin (which exists as a monomer as well as a polydispersed polymer in vivo; Fig. 2c) and with kinesin heavy chain (which is a dimer in Aplysia) showed the expected molecular-weight distribution pattern in our experimental condition. The apparent molecular weight of each fraction was obtained from a calibration curve (Supplementary Fig. 2b).
Figure 2. The soluble form of Aplysia CPEB exists as oligomers in Aplysia neurons and in E. coli expressing Aplysia CPEB.
(a) Gel-filtration analysis of native total soluble-protein extract from Aplysia CNS (red), native extract of recombinant Aplysia CPEB purified from E. coli (blue) and affinity-purified Aplysia CPEB in PBS–2 M urea buffer (green). (b) Western blot analysis using Aplysia CPEB antibody of the fractions obtained from gel filtration of Aplysia neuronal extracts (red in a). Fractions 21–23 are near the void volume of the column (corresponding to molecular weights >670 kDa). Fractions 31 and 32 correspond to molecular weights in the range of 210–280 kDa, indicative of trace amounts of trimer or tetramer. (c) Control experiment for the western blot analysis of the different fractions, using anti–α-tubulin antibody. As expected, soluble α-tubulin is present in the fractions corresponding to monomer through very high-molecular-weight oligomers.
Recombinant Aplysia CPEB was expressed in Escherichia coli cells, and the native lysate of these cells was analyzed similarly by chromatography followed by western blotting (Supplementary Fig. 1c). The data also showed a very high apparent molecular weight (>670 kDa) for Aplysia CPEB in the soluble form, with a trace amount of smaller oligomers. Moreover, the affinity-purified, soluble Aplysia CPEB in phosphate-buffered saline (PBS) containing 2 M urea was analyzed by similar methods, which revealed that large soluble oligomers (larger than decamers) were the dominant forms present in the soluble state. Because the protein in all these three cases eluted near the void volume of the column (670 kDa), determination of the exact apparent molecular weight was not possible, and only a lower molecular-weight cutoff could be determined. Nevertheless, in all three cases more than 80% of the soluble protein was in the form of a high-molecular-weight oligomer, the majority of which had a size of a decamer or larger oligomer.
The similarity in the elution profiles for the native extract from Aplysia, the native extract from freshly lysed E. coli cells expressing Aplysia CPEB (Supplementary Fig. 1c) and the affinity-purified protein suggests that the oligomeric form of Aplysia CPEB is likely to be similar to the oligomeric form in the in vivo material in Aplysia as compared to recombinant material, lending relevance to our biophysical studies in vitro.
The soluble form of purified Aplysia CPEB in PBS with 2 M urea was further characterized by size-exclusion chromatography followed by light scattering. We found from light-scattering experiments that the oligomeric state of soluble Aplysia CPEB in PBS with 2 M urea buffer has 12–20 units and a radius of gyration (Rg) similar to the hydrodynamic radius (Rh), which is around 25 nm. Because the average Rg value is approximately equal to the average Rh value, these oligomers might have a hollow spherical shape under this condition (Supplementary Fig. 2a,b). When the concentration of urea in the PBS buffer was increased to 4 M, these oligomers started to disaggregate into trimer- to monomer-sized particles (Supplementary Fig. 2c) with an extended conformation evident from combined size-exclusion chromatography (SEC) and light-scattering analyses. Although the protein in PBS with 4 M urea buffer eluted at a higher apparent mass than the protein in PBS with 2 M urea, the corresponding light-scattering signal showed a very low molar mass (around monomer to trimer). This might represent some sort of unfolding of the protein, owing to the increase in concentration of urea in the buffer causing an extended conformation for the protein.
Does Aplysia CPEB exist in two different conformations?
A relatively simple experiment confirmed that Aplysia CPEB indeed forms two distinct conformations with different aggregation and functional capacity. Purified recombinant Aplysia CPEB in urea-containing phosphate buffer gradually formed an insoluble aggregate (in 1–2 weeks for a protein solution with a concentration of approximately 1 mg ml−1 or higher in the presence of 2 M urea) and did so more rapidly when the protein concentration was higher or the urea concentration lower. We then tested the ability of the soluble and fiber forms of Aplysia CPEB to bind RNA. To probe the RNA binding function, soluble or insoluble Aplysia CPEB was incubated with the 3′ UTRs of mRNAs coding for N-actin (neuronal actin), which included the CPE elements and were 32P enriched. The samples were then filtered through a nitrocellulose membrane, which preferentially binds protein but not RNA19. 32P-radiolabeled CPE-mutated N-actin RNA was used as one of the controls in this experiment4. To eliminate any error due to inherent nonspecific binding of RNA to the insoluble protein, two insoluble Aplysia CPEB protein samples were used: the full-length Aplysia CPEB mutated at the RNA-binding domain, and the isolated N-terminal domain of Aplysia CPEB (prion domain, PRD). We found that, compared with the soluble form, the insoluble full-length Aplysia CPEB binds RNA with a significantly greater affinity (P < 0.05; Student’s t test; Fig. 3), which suggests the existence of two functionally different forms of Aplysia CPEB in vitro. This is notable in light of the in vivo data on Aplysia CPEB reported previously, which showed that positive function in long-term memory-related synaptic facilitation is associated with the aggregated form of this protein5.
Figure 3. RNA-binding assay.
RNA-binding efficiency of full-length Aplysia CPEB (FL CPEB; red), isolated prion domain (PRD; cyan) and full-length Aplysia CPEB mutated at the RNA-binding domain (FL CPEB mRBD; brown) bound to a 32P-labeled N-actin RNA 3′ UTR probe. As shown, full-length Aplysia CPEB in its soluble form (pink) displayed minimal RNA binding, and negative-control N-actin RNA mutated at the CPE element allowed roughly the same binding as that of mutated protein to wild-type N-actin RNA. The conformational change of Aplysia CPEB resulted in a significant increase in binding affinity toward its target mRNAs, compared to that of the soluble form (P < 0.05; Student’s t test; n = 6). Error bars, s.e.m.
Secondary structure of the two forms of Aplysia CPEB
The secondary structure of the soluble form of Aplysia CPEB was determined by far-UV (180–350 nm) CD. The CD spectrum of the Aplysia CPEB protein in PBS with 2 M urea, pH 7.4, showed an α-helix-rich secondary structure (Fig. 4a). Deconvolution of the CD spectrum by using algorithms in DICHROWEB (Contin, Selcon and K2D)20,21 showed that the soluble form has 10% or less β-sheet content, 40–50% α-helix content and 30–40% random coil.
Figure 4. Secondary-structure analysis of two conformational forms of full-length Aplysia CPEB.
(a) CD spectrum of purified recombinant, soluble full-length Aplysia CPEB (blue) in PBS–2 M urea buffer, indicating a helix-rich secondary structure. For comparison, the CD spectrum of an α-helix–rich protein, BSA in PBS with 2 M urea (red), is shown. (b) FTIR analysis of fibers formed from full-length recombinant Aplysia CPEB. The spectrum of Aplysia CPEB (red) is compared with those of helix-rich protein BSA (blue) and β-sheet–rich protein Aβ42 amyloid (green). The amide I band of full-length Aplysia CPEB at 1,622–1,627 cm−1 indicates a β-sheet–enriched secondary structure comparable with the spectrum of Aβ42. α-helix–rich proteins are predicted to have an amide I band peak maximum at 1,656 cm−1, as in the case of BSA. As shown, this peak (1,656 cm−1) is absent in the spectra of Aplysia CPEB and Aβ42. (c) Confocal projection image showing thioflavin-T staining of the fiber form of full-length Aplysia CPEB, indicative of an amyloid-like structure. The soluble form showed only background fluorescence.
The secondary structure of the fiber form of Aplysia CPEB was analyzed by FTIR spectroscopy, focusing on the amide I region (1,600– 1,700 cm−1). The amide I band, in which β-sheets appear between 1,637 and 1,630 cm−1, has been described to shift below 1,630 cm−1 for β-sheets in amyloid-like structures22. FTIR analysis of the fiber form of Aplysia CPEB showed an amide I band at 1,622–1,627 cm−1, which suggests that this form of the protein is predominantly in a β-sheet-rich conformation (Fig. 4b). Manual deconvolution of the spectrum suggests that the aggregated form of Aplysia CPEB consists of about 40–50% β-sheets. Synthetic amyloid (Aβ42) and BSA (a helix-rich protein) were also analyzed as controls. To further test whether the fiber form of Aplysia CPEB has an amyloid-like structure, we used thioflavin-T, a dye that can specifically stain amyloids. Unlike the soluble form, the fiber form of Aplysia CPEB showed a bright fluorescence when stained with thioflavin T23 (Fig. 4c).
X-ray powder diffraction of Aplysia CPEB fibers
We further characterized the fibers of purified full-length recombinant Aplysia CPEB (expressed in E. coli) by X-ray powder diffraction. The fibers formed in PBS were washed three times with water and collected by centrifugation. These fibers were loaded to a loop (as the sample holder) and placed on the path of the X-ray for 1 h. The diffraction pattern of Aplysia CPEB showed two well-defined rings at 10.7 Å and 4.7–4.8 Å (Fig. 5a) for the intersheet and interstrand distances, respectively, consistent with an amyloid structure. Yeast prions such as Rnq1p, Sup35p and Ure2p showed similar diffraction patterns for their aggregated fiber form24,25, which supported the idea that Aplysia CPEB adopts a β-sheet-rich fiber structure. However, in unusual cases, helical fibers may show prominent diffraction at similar spacing26. Taken together with all the other biophysical analyses and dye-staining experiments on Aplysia CPEB fibers, our X-ray diffraction data support a β-sheet-rich (amyloid) secondary structure for these fibers. In contrast, polyethylene glycol-precipitated soluble Aplysia CPEB showed no fluorescence with thioflavin T and did not give a diffraction pattern corresponding to an amyloid structure (data not shown). We repeated the experiment by placing the wet fibers in a sealed capillary to avoid any potential drying of the fibers due to the use of a loop as a sample holder. The radial projection of the diffraction pattern obtained from the wet fibers (Fig. 5b) showed a sharp peak at 4.7 Å and slightly broader peak around 10.7–11 Å, indicative of amyloid structures.
Figure 5. X-ray powder diffraction analyses of Aplysia CPEB fibers.
(a) X-ray powder diffraction pattern of nonoriented full-length Aplysia CPEB fibers. The graph is generated from the X-ray diffraction pattern by using ImageJ44. (b) Radial projection of the X-ray diffraction pattern of wet Aplysia CPEB fibers in a sealed glass capillary. The resultant diffraction pattern subtracted from the background diffraction of the capillary and water is shown. (c) Kinetics of fiber formation of Aplysia CPEB, showing no lag phase for the fiber formation of full-length Aplysia CPEB upon addition of soluble full-length Aplysia CPEB (1 mg ml−1) in PBS containing 2 M urea to a 40× excess of PBS containing thioflavin T.
Rapid kinetics of fiber formation
The kinetics of fiber formation of the full-length Aplysia CPEB were recorded by using real-time fluorescence measurements in the presence of the amyloid-staining dye thioflavin T. To determine whether the conformational change of Aplysia CPEB into fibers has a lag phase, the soluble protein in PBS with 2 M urea was directly added to a 40-fold-volume excess of PBS containing thioflavin T, and the fluorescence intensity was measured over time. The data were collected at intervals of 0.1 s. An instantaneous increase in emission intensity following the addition of the protein solution to the PBS-dye solution indicated an apparent lack of a lag phase on the seconds timescale, thus offering evidence for the lack of stable intermediates in the process under the conditions we studied (Fig. 5c). This result is analogous to that for other functional amyloids (for example, Pmel17, condensed peptide hormones or ALF)27–30.
Furthermore, we found that Aplysia CPEB fibers, once formed, can be redissolved in 6–8 M urea or 4–6 M guanidine hydrochloride solution. This indicates that the fiber formation of Aplysia CPEB is a reversible process in vitro.
Limited proteolysis of the fiber form of Aplysia CPEB
To identify the fragment involved in the fiber core formation, we carried out limited proteolysis with trypsin and proteinase K followed by MS. We found three fragments missing in the fiber form of Aplysia CPEB and present in the soluble form after trypsin digestion and mass spectrometry. Sequencing of these fragments revealed that they are associated with the N terminus of Aplysia CPEB (Supplementary Fig. 4 and 5). The SDS-PAGE analysis of the trypsin-digested full-length Aplysia CPEB showed a 20- to 25-kDa protease-resistant fragment (Supplementary Fig. 4d). We could not find any protease-resistant fragment in an analogous experiment carried out using the soluble form of full-length Aplysia CPEB. These observations suggest that when Aplysia CPEB converts from the soluble form to the fiber form, the conformational change makes the N terminus of the protein resistant to enzymatic digestion. These data are consistent with the model (Fig. 1b) that we derived from the solid-state NMR data indicating that the N terminus of the Aplysia CPEB fiber is rigid and that it forms the fiber axis. The sequence of the trypsin-resistant fragment of Aplysia CPEB fibers has four intact trypsin-digestion sites (Supplementary Fig. 4f).
Proteinase-K digestion of the PRD and full-length Aplysia CPEB fibers showed identical protease-resistant fragments around 7 kDa (Supplementary Fig. 4e). This is consistent with the idea that this fragment might have derived from the N terminus (PRD) of CPEB. To further confirm the position of this fragment, western blot analysis was carried out using an antibody raised against the C terminus of the Aplysia CPEB protein. The absence of staining suggests that these peptide fragments belong to the N terminus of the protein. Amino acid analysis showed a glutamine-rich sequence for this fragment, but the exact sequence of the fragment could not be determined from amino acid analysis and MS (data not shown). The details of these experiments are given in Online Methods.
Solid-state NMR analysis of Aplysia CPEB
To probe the structure of the fiber form of Aplysia CPEB, we used solid-state NMR spectroscopy, including cross-polarization31, refocused INEPT (insensitive nuclei enhanced by polarization transfer)32 and direct excitation. The fibers of full-length Aplysia CPEB formed in PBS were collected by centrifugation and washed with deionized water to reduce salt-induced sample heating. After the washing, the protein was centrifuged at 14,000 r.p.m. for 30 min to form a compact pellet. This pellet was loaded into the NMR rotor by centrifugation. The cross-polarization experiment selectively detects the static parts of the sample33, whereas refocused INEPT detects the dynamic portions of the protein. The direct-excitation experiment highlights both the dynamic and the static part of the sample. Solid-state NMR data on full-length Aplysia CPEB fibers showed the presence of both static and dynamic domains (Fig. 6a). Other prion proteins like HET-s have also been found to have such dynamical heterogeneity in their amyloid forms34–36. Similarly, the N terminus of Pmel17 fibers has a polymorphism that was revealed by solid-state NMR, which indicates that there may not need to be a unique structure for an amyloid to be functional37.
Figure 6. 1D solid-state NMR spectra of the fiber form of full-length Aplysia CPEB and the isolated prion domain, indicating dynamic RNA-binding domains and static prion domains.
(a) 13C 1D spectra of recombinant full-length Aplysia CPEB in hydrated insoluble (fiber) form recorded at a 1H frequency of 600 MHz and 13 kHz MAS. The initial 13C magnetization was generated by using either a simple 90 °C 13C pulse (direct excitation, DE), Hartman-Hahn cross-polarization (CP) or a refocused INEPT. (b) Corresponding 13C 1D spectra of the isolated prion domain of Aplysia CPEB (PRD) recorded at 750 MHz 1H frequencies and 12 kHz MAS. (c) Temperature dependence of 13C cross-polarization 1Ds of full-length Aplysia CPEB fiber, recorded at 750 MHz 1H frequency, 12 kHz MAS using a set temperature of 220 K and 260 K. The dynamic parts of the fibril, predominantly located in the RNA-binding domain, freeze out at low temperatures and become visible using solid-state NMR pulse sequences (CP).
The PRD also showed similar NMR spectroscopic signatures (indicating the presence of both dynamic and static regions in the protein). However, the refocused INEPT spectrum of the PRD has much less signal associated with the dynamic region of the protein compared to full-length Aplysia CPEB (Fig. 6b), which indicates relatively high rigidity at the N terminus of the protein. The 1D spectra showed that Aplysia CPEB fibers are dynamically heterogeneous. We investigated the temperature dependence of Hartman-Hahn cross-polarization 13C 1D spectra of full-length Aplysia CPEB fibers by recording the spectra at 220 K and 260 K. Because the dynamic parts of the fibrils freeze out (or become rigid) at low temperature, we found an increase in signal intensity on the cross-polarization spectra recorded at a lower temperature (Fig. 6c). This confirms the presence of dynamic parts in the structure of Aplysia CPEB fibers.
Aplysia CPEB fibers were characterized by 2D solid-state NMR spectroscopy. The 2D 13C-13C dipolar assisted rotational resonance (DARR) spectra of the PRD (~200 amino acids at the N terminus; Fig. 7a) was recorded. Previously tabulated average positions are indicated for the Cα–Cβ and Cα–CO cross-peaks of α-helical, β-sheet and random-coil conformations for serine, alanine, threonine, leucine and glutamine in the 2D DARR (Fig. 7a)38. The assignments of serine, alanine, threonine and leucine were based on their particular chemical shifts, and glutamine could be assigned by the detection of a complete spin system and the fact that this amino acid comprises ~40% of the PRD sequence, thus resulting in the strongest cross-peaks of the entire spectrum (Supplementary Fig. 3b).
Figure 7. 2D solid-state NMR spectra on the N-terminal prion-like domain of Aplysia CPEB.
(a) 13C–13C DARR spectrum of the PRD recorded at a 1H frequency of 750 MHz, 12 kHz MAS and mixing time of 50 ms. The average Cα–Cβ cross-peak positions corresponding to an α-helical (red), extended β-sheet (orange) and random-coil (blue) conformation for the amino acids threonine, serine, glutamine and alanine, as well as the corresponding Cα–CO cross-peaks for threonine and glutamine, are marked with ellipsoids. The full 13C connectivity of glutamine in α-helical versus β-sheet conformations are also shown with red and orange bars, respectively. (b) Overlay of 13C–13C DARR spectrum of full-length Aplysia CPEB (recorded at a 1H frequency of 900 MHz, 20 kHz MAS and a mixing time of 50 ms) with the DARR spectrum of the isolated prion domain shown in a. (c) 1H–13C INEPT-HETCOR spectra of full-length Aplysia CPEB fiber. Tentative amino acid assignments are indicated.
The PRD fibers are clearly not composed entirely of β-sheets but also of helices and random-coil elements, on the basis of chemical shifts for serine, alanine and leucine, which are present in the PRD. The presence of two complete and separated spin systems for glutamines, one in a β-sheet conformation, the other in an α-helical conformation, indicates that the PRD has a mixed secondary structure (Supplementary Fig. 3b). This observation is in accordance with the results obtained from the FTIR spectroscopy of Aplysia CPEB fibers, which showed about 50–60% β-sheet and the rest 40–50% random-coil and α-helix structures. From the FTIR spectra of the protein it was difficult, however, to distinguish between random-coil and helical structures.
The 2D DARR spectra of full-length Aplysia CPEB fibers gave qualitatively the same result as the 2D DARR spectrum of the PRD (Fig. 7 and Supplementary Fig. 3), which suggests that most of the signals in the spectra of the full-length Aplysia CPEB spectrum arise from the static PRD, and the RNA-binding domain might be relatively dynamic and, therefore, missing in our spectra. This interpretation is supported by the fact that we observed a marked increase in signal by freezing the full-length Aplysia CPEB fibers (Fig. 6c). We hypothesize that in the structure of the full-length Aplysia CPEB, the PRD is very rigid, and the C-terminal RNA-binding domain is quite dynamic (Fig. 1b).
The flexible part of Aplysia CPEB was further analyzed by using an INEPT-based heteronuclear correlation (HETCOR)39 spectrum in a similar way as was reported for HET-s (residues 218-289) and huPrP (residues 23-144)34,35,40. The HETCOR spectrum of Aplysia CPEB (Fig. 7c) showed that most of the amino acids in the dynamic part of the protein are either in α-helical or in random-coil conformation (Supplementary Table 1).
In summary, on the basis of NMR observations, we propose a model for the coordinated polyadenylation of CPE-containing RNAs by the amyloid form of Aplysia CPEB (illustrated in Fig. 1b). The RNA-binding assays (Fig. 3) and limited proteolysis experiments (Supplementary Fig. 4) provide additional support for this model.
DISCUSSION
Previous work suggested that Aplysia CPEB in vivo has prion-like properties and that the aggregated form was the functional state of the protein3–5. These findings motivated us to ask whether Aplysia CPEB exists in vitro in multiple conformations with distinct secondary structures. To this end, we have expressed and purified recombinant Aplysia CPEB and found the protein to be highly insoluble. Recombinant Aplysia CPEB is soluble in phosphate buffer in the presence of 2 M urea, but at lower concentrations of urea the protein promptly forms insoluble aggregates. Biophysical analyses of these two forms confirmed that Aplysia CPEB has an α-helix–rich structure in its soluble form and a β-sheet–rich structure in the insoluble aggregated form, with a smaller percentage of helical and random-coil signatures.
The fiber form of Aplysia CPEB showed the biophysical signature of an amyloid in FTIR, thioflavin-T staining and X-ray diffraction analyses. Solid-state NMR spectra showed that the PRD of Aplysia CPEB is not solely composed of β-sheets but also has helical and random-coil stretches. The alanine, leucine and also some of the glutamine shifts in Figure 7a and Supplementary Figure 3b, in particular, indicate a partial helical content in the PRD fiber of Aplysia CPEB. Owing to the lack of site-specific assignments, it is difficult to know whether the structure of Aplysia CPEB is polymorphic, comparable to many pathological amyloids, or has a unique structure characteristic of functional prions, similar to the HET-s prion. Prior solid-state NMR studies have shown that the nonprion domains of a number of prion proteins such as HET-s, Ure2p and Rnq1 can be large, sometimes even globular, domains of mixed secondary structure. However, an extended β-sheet conformation was shown to be the dominant secondary structure of the prion domains of all of these proteins24,36,41. Our NMR data for the prion domain of Aplysia CPEB revealed the possibility of a new, stable and insoluble β-sheet–rich prion domain with mixed secondary structure containing loops and α-helices. The fact that we detect mixed secondary structure is somewhat of a departure and may result from the use of a larger domain with over 200 amino acids. This is of particular note because another bioinformatics and mutagenesis study from our laboratory predicts that the glutamine-rich regions of Aplysia CPEB could have a propensity to form α-helical coiled-coil structures42. Whether such coiled coils are the basis of the aggregated state or merely a prelude to β-sheet formation is unclear. Because positive functional amyloid is induced just before the onset of the persistent synaptic facilitation underlying long-term memory, it would clearly be a very dramatic process in the cell that may require regulation of the formation and dismantling of the amyloid. One advantage of having an extensive nonsheet structure, as for example a coiled-coil structure, is that the nonsheet structure could have a regulatory role in these aggregation processes. Moreover, as β-sheet structures are rigid and not accessible to regulation, the nonsheet coiled-coil structure could provide a mechanism for the regulated switching from a soluble to a β-sheet structure and back again. Thus the structure model has the advantage of β-sheet rigidity necessary for a translation-machine model and the flexibility of the coiled coil needed for regulation.
Solid-state NMR experiments on full-length Aplysia CPEB indicate that the protein has both very rigid and very dynamic parts: the rigidity of the fibers is apparently associated with the PRD of the protein. In contrast, the C terminus or the RNA-binding domain of the protein is very flexible and could not be observed with solid-state NMR. Dynamical heterogeneity has been reported for other prions as well. This observation leads us to propose a model for the gain of function during aggregation of Aplysia CPEB, in which the prion domains of the protein are stacked together to form the fiber axis (Fig. 1). The RNA-binding domains, by contrast, are dynamic and exposed on the surface of the fiber, free to bind RNA. A similar model was proposed to explain the activity of amyloid-like fibrils of RNase A43. A locally concentrated arrangement of RNA-binding sites could be advantageous for translation at the synapse. It could facilitate localized polyadenylation of multiple RNAs, and it might, therefore, coordinate polyadenylation of a population of inter-related RNAs during synthesis of new proteins that are immediately used at the synapse for the maintenance and stabilization of long-term memory.
METHODS
Methods and any associated references are available in the online version of the paper.
ONLINE METHODS
Plasmids
All plasmid constructs used in this study have been described previously3–5.
The full-length Aplysia CPEB and isolated PRD, containing the first 213 amino acids at the N terminus, were expressed and purified as recombinant proteins. They were cloned in frame into the histidine-tag vector pRSETA (Novagen). The His6-CPEB fusion protein was expressed in E. coli and purified by Ni-affinity chromatography under denaturing conditions (Qiagen).
The neuronal actin 3′ UTR and mutated CPE-actin plasmids were also described elsewhere4. The N-actin 3′ UTR was obtained by PCR using the 5′ primer 5′-GGGAATTCGTCTGGAGCCACCAACAC-3′ and 3′ primer 5′-CG GATCCATTTATTAACATTGTATAAAAAATACAGTTGAAC-3′. To mutate the CPE element, the 3′ primer was changed to 5′-CGGATCCATTTATTAACATTG TATGGGAAATACAGT-TGAAC-3′. The N-actin 3′ UTRs were cloned into the pCR2.1 vector (Invitrogen). Plasmids were linearized by using BamH1 and used for in vitro transcription to prepare [32P]UTP labeling of RNA (Ambion).
The sequence of full-length Aplysia CPEB and PRD were expressed in E. coli. The bold-faced letters show the beginning and end of native Aplysia CPEB sequence.
CPEB:
MRGSHHHHHHGMASMTGGQQMGRDLYDDDDKDRWGSMQAMAVAS QSPQTVDQAISVKTDYEDNQQEHIPSNFEIFRRINALLDNSLEANNVSCSQS QSQQQQQQTQQQQQQQQQQQQQQHLQQVQQQRLLKQQQQQAQRQ QIQQQLLQQQQQKQQLQQQQQQEQLQQQQLQLQQQLQQQLQHIQKEP SSHTYTPGPSPELQSVLNYANVPLSKSAAFNCNNSSSYSVGPTPVQSPVTPSP AASAVTVNSPSYGNFQLFGENAFDSTTPFQSDGTSQSHSRSLANDSDPMVV MSPGRDSIIPLSPTEKILYQNFLLSKQAQGENTALPPSPPHEIMPLSPLEKKLY SNLLSKHTQGMRAINSTSPLQTPLTPPRSPQEVLYASMPAAQVGESSSVIDM MSRMDLSGRNQQADYSGTLAFLDAHNVLRRRTPSSSRSRSVMERSAPSSYF ANLDPYAIDRAARLHRNAAAVSEASCTWSGHLPPRNHKNPVYSPKVFLG GVPWDITESGLQAAFSKYGHLKIEWPGKDGYVHLLFDVEKSVRSLLQACT HDFSNGDYFYKISSRRMRSKEVQVIPWVLADSNHVFRPSQRLESNKTVFV GALHGMITAEALGRIMSDLFGNVVYAGIDTDKHKYPIGSGRVTFSSRKSYM KAVQAAFVEIKTPKFTKKLQVDPYLGDAICSLCNSHQGNYFCRDLLCFK YLCRSCWYWQHAPDSMRQHRPLTRNTKSSLSLYPYDVPDYA
PRD:
MEGDIHMRGSHHHHHHGMASMTGGQQMGRDLYDDDDKDRWGS MQAMAVASQSPQTVDQAISVKTDYEDNQQEHIPSNFEIFRRINALLDNSL EANNVSCSQSQSQQQQQQTQQQQQQQQQQQQQQHLQQVQQQRLL KQQQQQAQRQQIQQQLLQQQQQKQQLQQQQQQEQLQQQQLQLQQ QLQQQLQHIQKEPSSHTYTPGPSPELQSVLNYANVPLSKSAIIIIDPGSLIRL LTKPERKLSWLLPPLSN
Another form of Aplysia CPEB doubly mutated at the RNA binding domain, C664A H672A (CPEB-mRBD), was also obtained from K. Si.
An Aplysia anti-CPEB antibody was generated against 17 amino acids (LCNSHQGNYFCRDLLC) at the C terminus of the full-length Aplysia CPEB protein (Covance)1. Anti-Aplysia kinesin heavy chain1 (ApKHC1) antibody was raised in rabbit (Covance) as described previously45 and mouse anti–α-tubulin antibodies were purchased from Sigma.
Protein preparation
Full-length Aplysia CPEB or the isolated PRD was expressed in bacteria and purified as previously described3–5 with some modifications. After a two-step purification using metal-affinity chromatography followed by ion-exchange chromatography, protein with a purity >95% (on the basis of SDS PAGE and silver staining) was obtained.
Aplysia CNS extract preparation
Neuronal extracts were prepared from the Aplysia CNS by using ice-cold buffer containing 50 mM Tris-HCL, pH 7.5, 1 mM EDTA, 300 mM NaCl and protease inhibitors (Roche) and phosphatase inhibitors (Sigma)45. The CNS tissues were briefly ground in this buffer at 4 °C, kept on a rotator for 20 min and centrifuged at 10,000 r.p.m. for 20 min, and the supernatant was used for the chromatography analysis.
Gel-filtration or size-exclusion chromatography
All SEC experiments were done at 4 °C. The native Aplysia CNS extract was injected into a Superdex 200 column (120 ml, GE), and the elution was collected as 2-ml fractions. All fractions (60 fractions for one run) were concentrated in Amicon Ultra units, m.w. cutoff 10 kDa, Millipore). Volumes of 20 µl of the concentrated fractions were loaded onto a denaturing polyacrylamide gel (SDS-PAGE) followed by western blotting using anti–Aplysia CPEB antibody. Extract from E. coli cells that express recombinant Aplysia CPEB was also analyzed in a similar way. Similar analyses were performed for Aplysia α-tubulin and Aplysia kinesin on the same blotted membrane.
Purified full-length Aplysia CPEB in PBS with 2 M or 4 M urea was analyzed by SEC followed by online static laser light scattering. The soluble protein was filtered (650 µm) and injected onto the SEC column (TSKG6000PW, Tosoh Corp.) equipped with a UV detector and an LS detector (90°). SEC served as a fractionation step based on the size of the particles. The LS detector gave the weight average molecular weight. The radius of gyration (Rg) and hydrodynamic radius (Rh) distribution of the particles were derived from the LS signal.
RNA-binding assay
For the filter-binding assays, nitrocellulose filters (Millipore, HAWP025S0) were preincubated in the RNA binding buffer at room temperature for 30 min and kept in the same buffer until ready to bind RNA. The binding buffer is 50 mM HEPES buffer, pH 7.8, containing RNAse inhibitors SUPERaseIn (1 unit per µl; Ambion), yeast tRNA (1 µg/ml), BSA (1 µg/ml), salmon sperm DNA (1 µg/ml; Ambion) and phosphatase inhibitors (1:100 v/v; Sigma). Soluble oligomers and freshly prepared aggregates (2 µg protein, determined by measuring absorbance at 280 nm) were incubated with in vitro–transcribed 32P-labeled wild-type and mutant N-actin RNAs (6,000 µCi) at 25 °C for 30 min. Binding reactions were conducted in siliconized RNAse-free 1.5-ml tubes (Ambion). Each treatment had four replicates. The protein-RNA mixture was then transferred onto high-protein-binding nitrocellulose filters (HAWP025S0, Millipore) and placed on a Millipore vacuum filtration manifold apparatus. A brief vacuum was applied, and filters were washed four times with 3 ml binding buffer. The filters were then collected and air dried, and radioactivity was measured by scintillation counter and analyzed by using SigmaPlot.
Circular dichroism and Fourier-transform infrared spectroscopy
CD spectra were recorded with a J-720 CD spectrometer (Jasco) scanning at 20 nm/min, with a bandwidth of 1 nm and data spacing of 0.5 nm, using a 0.1-cm cuvette. Five individual scans were averaged and subtracted from the background.
Infrared spectra of the fibers were recorded by using a PerkinElmer FTIR system purged with nitrogen, employing the diffused reflectance infrared Fourier transform (DRIFT) spectroscopy method. All spectra recorded had a minimum of 512 scans. The background was subtracted from the sample spectrum to obtain the final data.
Thioflavin-T staining and fluorescence measurement
Purified Aplysia CPEB protein expressed in E. coli, in PBS containing 2 M urea was used for this study. The fibers were produced by adding 0.5–1 mg/ml solution of the protein to a 40× volume of PBS containing around 10–25 µM concentration of thioflavin T (Sigma).
The fibers were visualized by using a confocal microscope to measure fluorescence at an excitation maximum of 445 nm and an emission maximum of 485 nm.
Real-time measurements of thioflavin-T fluorescence were collected by using a spectrofluorometer (Fluoromax 3). PBS buffer with dye excited at 425 nm (which is preferable to 445 nm, as it avoids the overlap of Raman scattering with the fluorescence emission), and the fluorescence emission was monitored at 480 nm as a function of time. Upon the addition of protein solution to the PBS-dye solution, a sudden increase in fluorescence intensity was observed, showing a spontaneous formation of amyloid fibers.
X-ray powder diffraction
Aplysia CPEB fibers were loaded on a loop and were placed in a 1.54-Å-wavelength X-ray beam generated by a rotating anode source operated at 50 kV and 100 mA, coupled with a multilayer focusing optics. The samples were rotated 180° perpendicular to the X-ray beam. Diffraction data were collected for 120 min by using a Raxis IV two-dimensional-imaging plate detector placed 15 cm from the sample. Background diffraction was subtracted from the sample diffraction data to obtain the final diffraction spectra.
Diffraction of wet fibers in a glass capillary was done by loading the fibers in the capillary (0.7 mm) with a drop of water. The diffraction pattern was recorded for 20 min. The background diffraction due to the capillary and water was subtracted to get the resulting diffraction pattern of the fibers.
Limited proteolysis and mass-spectroscopic analysis
Proteolysis of soluble and insoluble Aplysia CPEB was carried out by using two different proteases: proteinase K and trypsin. For the proteinase-K treatments, proteinase K (final concentration 1 µg/µl) was added to soluble and insoluble Aplysia CPEB and incubated at 37 °C for 30 min. Protease digests were analyzed by SDS-PAGE and MS. Trypsin digestions were carried out overnight. The pellet and soluble fractions were analyzed by SDS PAGE and MS fingerprinting (Columbia University Protein Core Facility).
Digestion and LC-MS or MS analysis of soluble and insoluble Aplysia CPEB
Insoluble Aplysia CPEB fibrils were suspended in ultrapure water and centrifuged. The supernatant was removed and the wash repeated. The solid material was suspended in 25 mM Tris-HCl, pH 7.5, reduced with DTT and alkylated with iodoacetamide. Trypsin (Roche) was added to the tube, which was incubated at 32 °C for 2 h. This was followed by addition of endoproteinase Asp-N and continued incubation overnight at 32 °C. The soluble form of Aplysia CPEB was reduced, alkylated and digested in the same way. After digestion, both preparations were centrifuged to remove any remaining solid material and desalted by using a pipette tip packed with C18 reverse-phase support.
LC-MS and MS analyses were done on a Waters Ultima Q-T hybrid quadrupole time-of-flight mass spectrometer with a nanoelectrospray source. Capillary voltage was set at 1.8 kV and cone voltage at 32 V; collision energy was set according to mass and charge of the ion, from 18 eV to 50 eV. Chromatography was performed on an LC Packings HPLC with a C18 PepMap column, using a linear acetonitrile gradient with flow rate of 200 nl/min. Raw data files were processed by using the MassLynx ProteinLynx software, and .pkl files were submitted for searching at www.matrixscience.com with the Mascot algorithm.
Solid-state NMR
All samples used for solid-state NMR analysis were labeled uniformly with 13C and 15N, as described in a reported procedure46,47. A brief summary of the labeling procedure is as follows: the bacterial culture grown overnight were transferred to fresh LB medium (2 l) containing ampicillin (100 µg/ml) and chloramphenicol (34 µg/ml) at 37 °C and were shaken for 2 h. When an OD600 between 0.6 and 0.8 was reached, the cells were pelleted and washed with 2 l ice-cold M9 buffer and redissolved in 1 l of M9 minimal medium containing 0.5 g of 15NH4Cl and 4 g of 13C-labeled glucose (Cambridge Isotope Lab). After 1 h of equilibration at 25 °C, protein expression was induced with 1 mM IPTG, and the cells were harvested after 12 h. The purification procedure was the same as for the unlabeled protein. After the fibers were formed in PBS (pH 7.4) at 4 °C, they were washed with deionized water to remove the salt, which can lead to sample heating and reduce the quality of the NMR spectra. Afterward, the wet fibers were centrifuged into an MAS rotor. The PRD fibers had about 2 mg of protein. The full-length samples measured at 600 MHz and 900 MHz contained around 1 mg of the protein.
CP spectra were done with a 13C field of 50 kHz, adjusting the 1H RF-field strength to the ω1H = ω13C+ωMAS condition. Unless noted otherwise, we kept the sample at temperatures between 0 and 10 °C. The 13C 1D CP, direct excitation, refocused INEPT and 2D DARR spectra of PRD fibers, as well as the low-temperature cross-polarization 13C 1Ds spectra of full-length Aplysia CPEB, were recorded on a Bruker Advance 750 spectrometer using a 4 mm triple-resonance HXY probe operating at 12 kHz MAS. For the 1D spectra, 1,024 acquisitions were added. The 2D DARR spectra were recorded with a mixing time of 50 ms and a spectral width of 50 kHz in both dimensions. The direct dimension was sampled with 1,024 complex points, and 32 acquisitions were added for every 512 points in the indirect dimension. The 13C 1D CP, direct excitation, refocused INEPT and 2D INEPT-HETCOR spectra of full-length Aplysia CPEB were recorded on a 600 MHz InfinityPlus spectrometer using a 3.2 mm T3 probe. The 1D spectra were recorded at 20 kHz MAS, adding 1,024 acquisitions. The 2D INEPT-HETCOR was recorded at 1l kHz by using spectral widths of 50 kHz and 20 kHz for the direct 13C and indirect 1H dimension, respectively. For the direct dimension, 1,024 complex points were recorded, and 384 acquisitions were added for every 128 points in the indirect dimension. The 2D DARR spectrum of full-length Aplysia CPEB was recorded on a Bruker Advance II 900 spectrometer using a 3.2 mm E-free probe operating at 20 kHz MAS. The mixing time was 50 ms, and the spectral width was 57 kHz in both dimensions. For the direct dimension, 1,024 complex points were recorded, and 32 acquisitions were added for every 512 points in the indirect dimension.
Data were processed by using Topspin 1.3 (Bruker), and multidimensional spectra were analyzed by using CARA 1.8 (www.cara.nmr.ch).
Acknowledgments
We are extremely grateful to K. Si (Stowers Institute for Medical Research, Kansas City, Missouri, USA) for providing the plasmids used in this study and to S. Jokusch (Columbia University) for helping us with FTIR spectra and real-time fluorescence measurement. We would like to acknowledge and thank J. Lidestri, (Columbia University) and H. Park (Scripps Research Institute) for assisting with X-ray diffraction analyses of the fibers. We are grateful to K. Nakanishi and N. Berova (Columbia University) for their guidance in CD analysis and to M.A. Gawinowicz (Columbia University) for MS analysis of the protein fragments. This work was supported by the Howard Hughes Medical Institute, US National Institutes of Health (grant MH075026; E.R.K.) and US National Science Foundation (grant MCB0316248; A.E.M.).
Footnotes
Note: Supplementary information is available in the online version of the paper.
Author Contributions
B.L.R., S.V.P. and A.B.S. designed the project after discussing with E.R.K., A.E.M. and W.A.H. All the experiments were done by B.L.R. except NMR and RNA-binding experiments. NMR experiments were done by A.B.S. and RNA-binding experiments by S.V.P. B.L.R., A.B.S., E.R.K. and A.E.M. wrote the paper.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron Medline. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Bailey CH, Kandel ER. Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia. Prog. Brain Res. 2008;169:179–198. doi: 10.1016/S0079-6123(07)00010-6. [DOI] [PubMed] [Google Scholar]
- 3.Si K, Lindquist S, Kandel ER. A neuronal isoform of the Aplysia CPEB has prion-like properties. Cell. 2003;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- 4.Si K, et al. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in Aplysia. Cell. 2003;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- 5.Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell Medline. 2010;140:421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 6.McGrew LL, Dworkin-Rastl E, Dworkin MB, Richter JD. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989;3:803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- 7.Gebauer F, Richter JD. Mouse cytoplasmic polyadenylylation element binding protein: an evolutionarily conserved protein that interacts with the cytoplasmic polyadenylylation elements of c-mos mRNA. Proc. Natl. Acad. Sci. USA. 1996;93:14602–14607. doi: 10.1073/pnas.93.25.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Schwartz JH. The cytoplasmic polyadenylation element binding protein and polyadenylation of messenger RNA in Aplysia neurons. Brain Res. 2003;959:68–76. doi: 10.1016/s0006-8993(02)03729-0. [DOI] [PubMed] [Google Scholar]
- 9.DePace AH, Santoso A, Hillner P, Weissman JS. A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell. 1998;93:1241–1252. doi: 10.1016/s0092-8674(00)81467-1. [DOI] [PubMed] [Google Scholar]
- 10.Glover JR, et al. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell. 1997;89:811–819. doi: 10.1016/s0092-8674(00)80264-0. [DOI] [PubMed] [Google Scholar]
- 11.Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 12.Sondheimer N, Lindquist S. Rnq1: an epigenetic modifier of protein function in yeast. Mol. Cell. 2000;5:163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- 13.Prusiner SB. Prions. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison PM, Bamborough P, Daggett V, Prusiner SB, Cohen FE. The prion folding problem. Curr. Opin. Struct. Biol. 1997;7:53–59. doi: 10.1016/s0959-440x(97)80007-3. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich SU, Lindquist S. Protein-only mechanism induces self-perpetuating changes in the activity of neuronal Aplysia cytoplasmic polyadenylation element binding protein (CPEB) Proc. Natl. Acad. Sci. USA. 2011;108:2999. doi: 10.1073/pnas.1019368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumdar A, et al. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell. 2012;148:515–529. doi: 10.1016/j.cell.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Pavlopoulos E, et al. Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell. 2011;147:1369–1383. doi: 10.1016/j.cell.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagase T, et al. Prediction of the coding sequences of unidentified human genes. XV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1999;6:337–345. doi: 10.1093/dnares/6.5.337. [DOI] [PubMed] [Google Scholar]
- 19.Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat. Struct. Mol. Biol. 2004;11:1215–1222. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- 20.Lobley A, Whitmore L, Wallace BA. DICHROWEB: an interactive website for the analysis of protein secondary structure from circular dichroism spectra. Bioinformatics. 2002;18:211–212. doi: 10.1093/bioinformatics/18.1.211. [DOI] [PubMed] [Google Scholar]
- 21.Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32:W668–W673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zandomeneghi G, Krebs MR, McCammon MG, Fandrich M. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 2004;13:3314–3321. doi: 10.1110/ps.041024904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeVine H., III Thioflavine T interaction with synthetic Alzheimer′s disease β-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickner RB, Dyda F, Tycko R. Amyloid of Rnq1p, the basis of the [PIN+] prion, has a parallel in-register β-sheet structure. Proc. Natl. Acad. Sci. USA. 2008;105:2403–2408. doi: 10.1073/pnas.0712032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shewmaker F, et al. The functional curli amyloid is not based on in-register parallel β-sheet structure. J. Biol. Chem. 2009;284:25065–25076. doi: 10.1074/jbc.M109.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jabet C, Sprague ER, VanDemark AP, Wolberger C. Characterization of the N-terminal domain of the yeast transcriptional repressor Tup1. Proposal for an association model of the repressor complex Tup1 × Ssn6. J. Biol. Chem. 2000;275:9011–9018. doi: 10.1074/jbc.275.12.9011. [DOI] [PubMed] [Google Scholar]
- 27.Dueholm MS, et al. Functional amyloid in Pseudomonas. Mol. Microbiol. Medline. 2010;77:1009–1020. doi: 10.1111/j.1365-2958.2010.07269.x. [DOI] [PubMed] [Google Scholar]
- 28.Fowler DM, et al. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maji SK, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnhart MM, Chapman MR. Curli biogenesis and function. Annu. Rev. Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pines A, Gibby MG, Waugh JS. Proton-enhanced NMR of dilute spins in solids. J. Chem. Phys. 1973;59:569–590. [Google Scholar]
- 32.Burum DP, Ernst RR. Net polarization transfer via a J-ordered state for signal enhancement of low-sensitivity nuclei. J. Magn. Reson. 1980;39:163–168. [Google Scholar]
- 33.Levitt MH. Spin Dynamics: Basics of Nuclear Magnetic Resonance. Wiley; 2001. [Google Scholar]
- 34.Siemer AB, et al. Observation of highly flexible residues in amyloid fibrils of the HET-s prion. J. Am. Chem. Soc. 2006;128:13224–13228. doi: 10.1021/ja063639x. [DOI] [PubMed] [Google Scholar]
- 35.Helmus JJ, Surewicz K, Nadaud PS, Surewicz WK, Jaroniec CP. Molecular conformation and dynamics of the Y145Stop variant of human prion protein in amyloid fibrils. Proc. Natl. Acad. Sci. USA. 2008;105:6284–6289. doi: 10.1073/pnas.0711716105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loquet A, et al. Prion fibrils of Ure2p assembled under physiological conditions contain highly ordered, natively folded modules. J. Mol. Biol. 2009;394:108–118. doi: 10.1016/j.jmb.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Hu KN, McGlinchey RP, Wickner RB, Tycko R. Segmental polymorphism in a functional amyloid. Biophys. J. 2011;101:2242–2250. doi: 10.1016/j.bpj.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spera S, Ikura M, Bax A. Measurement of the exchange rates of rapidly exchanging amide protons: application to the study of calmodulin and its complex with a myosin light chain kinase fragment. J. Biomol. NMR. 1991;1:155–165. doi: 10.1007/BF01877227. [DOI] [PubMed] [Google Scholar]
- 39.Bax A, Freeman R. Investigation of complex networks of spin-spin coupling by two-dimensional NMR. J. Magn. Reson. 1981;44:542–561. [Google Scholar]
- 40.Helmus JJ, Surewicz K, Surewicz WK, Jaroniec CP. Conformational flexibility of Y145Stop human prion protein amyloid fibrils probed by solid-state nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 2010;132:2393–2403. doi: 10.1021/ja909827v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritter C, et al. Correlation of structural elements and infectivity of the HET-s prion. Nature. 2005;435:844–848. doi: 10.1038/nature03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiumara F, Fioriti L, Kandel ER, Hendrickson WA. Essential role of coiled coils for aggregation and activity of Q/N-rich prions and polyQ proteins. Cell Medline. 2010;143:1121–1135. doi: 10.1016/j.cell.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambashivan S, Liu Y, Sawaya MR, Gingery M, Eisenberg D. Amyloid-like fibrils of ribonuclease A with three-dimensional domain-swapped and native-like structure. Nature. 2005;437:266–269. doi: 10.1038/nature03916. [DOI] [PubMed] [Google Scholar]
- 44.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puthanveettil SV, et al. A new component in synaptic plasticity: upregulation of kinesin in the neurons of the gill-withdrawal reflex. Cell. 2008;135:960–973. doi: 10.1016/j.cell.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marley J, Lu M, Bracken C. A method for efficient isotopic labeling of recombinant proteins. J. Biomol. NMR. 2001;20:71–75. doi: 10.1023/a:1011254402785. [DOI] [PubMed] [Google Scholar]
- 47.Siemer AB, McDermott AE. Solid-state NMR on a type III antifreeze protein in the presence of ice. J. Am. Chem. Soc. 2008;130:17394–17399. doi: 10.1021/ja8047893. [DOI] [PubMed] [Google Scholar]