Abstract
Over the past two decades, most guidelines have advocated early dialysis initiation on the basis of studies showing improved survival in patients starting dialysis early. These recommendations led to an increase in the proportion of patients initiating dialysis with an estimated glomerular filtration rate (eGFR) >10 ml/min/1.73 m2, from 20% in 1996 to 52% in 2008. During this period, patients starting dialysis with an eGFR ≥15 ml/min/1.73 m2 increased from 4% to 17%. However, recent studies have failed to substantiate a benefit of early dialysis initiation and some data have suggested worse outcomes in patients starting dialysis with a higher eGFR. Several reasons for this seemingly paradoxical observation have been suggested, including the fact that patients requiring early dialysis are likely to have more severe symptoms and comorbidities, leading to confounding by indication, as well as biological mechanisms that causally relate early dialysis therapy to adverse outcomes. Dialysis reinitiation in patients with a failing renal allograft encounters similar problems. However, unique factors associated with a failed allograft means that the optimal timing of dialysis initiation in failed transplant patients might differ from that in transplant-naive patients. In this Review, we will discuss studies of dialysis initiation and compare risks and benefits of early versus late dialysis therapy.
Introduction
In 1997, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative recommended that dialysis should tart at a urea clearance (Kt/Vurea) of <2.0 per week.1 This value corresponds to a glomerular filtration rate (GFR) of approximately 10.5 ml/min/1.73 m2. The updated guidelines from 2006 suggest that dialysis should start before chronic kidney disease (CKD) stage 5 (GFR <15 ml/min/1.73 m2) is achieved if patients have symptoms associated with existing comorbidities and insufficient renal function.2 A European Best Practice guideline published in 2002 recommended that dialysis be started when the GFR is between 8 ml/min/1.73 m2 and 10 ml/min/1.73 m2 if patients have symptoms such as signs or uremia and uncontrolled blood pressure.3 This guideline was revised in 2011, however, and states “it should be taken into account that the majority of patients will be symptomatic and need to start dialysis with GFR in the range 6–9 ml/min/1.73 m2.”4 Given the said guidelines, dialysis therapy was initiated between 10 ml/min/1.73 m2 and 15 ml/min/1.73 m2 in most patients with CKD in the USA.5 Data from the US Renal Data System (USRDS) show that between 1996 and 2008, the proportion of patients initiating hemodialysis with an estimated GFR (eGFR) of >10 ml/min/1.73 m2 increased from 20% to 52%, and those with a starting eGFR of ≥15 ml/min/1.73 m2 increased from 4% to 17%.6, 7 Interestingly, observational studies from the past decade and a randomized, controlled trial published in 2010 failed to support the early initiation of dialysis in patients with CKD.6, 8–18
In contrast to transplant-naive patients with CKD, only limited data are available in failed kidney transplant patients who need dialysis reinitiation. Failed kidney transplant patients seem to have worse clinical outcomes than their transplant-naive counterparts,19, 20 including worse survival on hemodialysis 19 or peritoneal dialysis therapy.21–26 Indeed, the incidence and prevalence of dialysis-dependent end-stage renal disease (ESRD) as a result of post-transplantation complications have increased dramatically, from 0.3 per million and 3.3 per million in 1996–1998 to 1.4 per million and 4.8 per million in 2006–2008, respectively.5 During 2006–2008, the annual cost of hemodialysis and transplantation per patient was US$77,506 and $26,668, respectively, suggesting that each extra year with a functioning ransplant instead of on dialysis would save $50,838.5 Theoretically, over $500 million could have been saved during this time period if each year 10,000 transplanted patients had remained on transplantation for one extra year before initiating dialysis. Similar findings are seen in transplant-naive patients. During 2006–2008, the annual cost per patient on hemodialysis and per high-risk patient with CKD (patients with CKD and heart failure) was $77,506 and $35,000, respectively, which suggests that each extra year that dialysis is delayed would save approximately $40,000.5 Therefore, although there are important clinical and economic implications from the findings of dialysis initiation studies in transplant-naive patients with CKD, revisiting this issue in failed kidney transplant patients could have even more overarching consequences, especially as the ideal timing of dialysis initiation in failed kidney transplant patients and the financial implications are unknown. Given the exposure to immunosuppressive drugs and other unique factors associated with a failing allograft, the timing of dialysis initiation could have different implications in failed transplant patients and in their transplant naive counterparts. In this Review, we will examine data pertaining to the association between the eGFR at initiation of dialysis and outcomes in both transplant-naive patients with CKD and failed kidney transplant patients.
Early versus late dialysis initiation
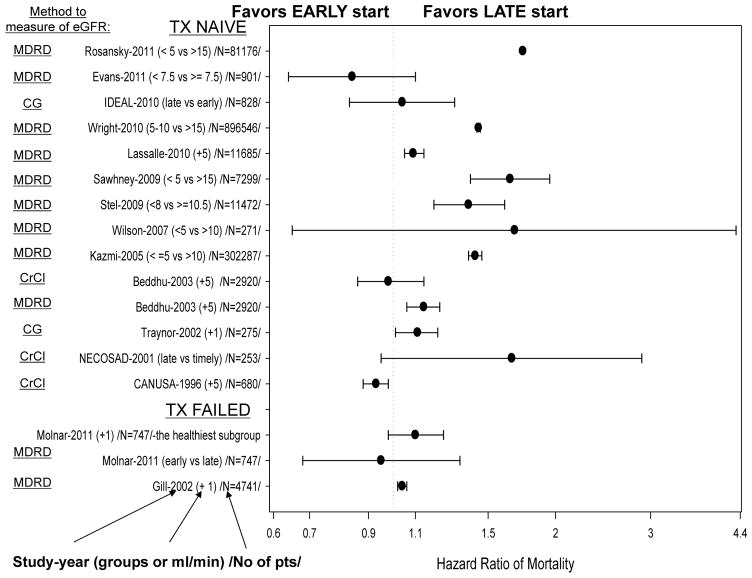
Since the mid-1970s, some observational studies have supported the early initiation of dialysis in patients with CKD. These studies were small and some did not adjust for age or comorbidities, which differed significantly between the early and late dialysis initiation groups.27–33 Despite the rather weak evidence, early initiation was almost universally recommended as the better choice. A number of studies published in the past decade, however, including a randomized controlled trial, have casted doubt on the benefits of early initiation of dialysis in patients with CKD.6, 9, 10, 13, 16–18, 34 Figure 1 summarizes studies that have compared early versus late dialysis initiation in transplant-naive patients with CKD and in failed kidney transplant patients.
Figure 1.
Landmark studies of early versus late initiation of dialysis treatment in transplant-naive patients with chronic kidney disease and failed kidney transplant patients.
Data in support of early dialysis initiation
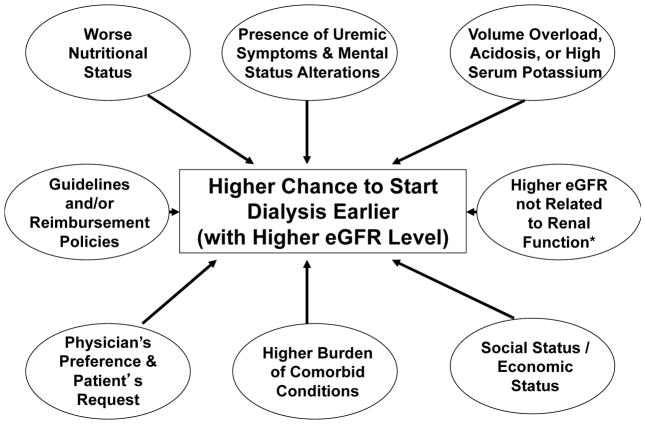
Several factors are associated with early initiation of dialysis (Figure 2). Practice guidelines outlined by the National Kidney Foundation Disease Outcomes Quality Initiative recommended that renal replacement therapy should be considered when the GFR declined below 10.5 ml/min/1.73 m2, and should be implemented if there was unintentional weight loss, a decrease in weight-normalized protein intake of <0.8 g/kg per day, or if there were clinical signs or symptoms of uremia.1 These recommendations were based on the assumption that early initiation of dialysis might improve the nutritional status of patients on dialysis, thus leading to improved survival.27–29, 32 These guidelines led to a notable trend towards earlier initiation of hemodialysis (starting eGFR ≥10 ml/min/1.73 m2) from 1996 to 2006 in the USA.35
Figure 2.
Potential factors associated with early versus late initiation of dialysis treatment.
To the best of our knowledge, the few studies that support early dialysis initiation are mostly from 15–20 years ago.27–33 A study from 1976 found that earlier dialysis initiation had superior results,27 with follow-ups in 1978 and 1985.28, 29 However, in the latter Italian studies there were no data on age, comorbidity, quality of life, or the censoring rules for the survival analysis. Ratcliffe et al.30 compared the survival of patients in early (mean 4-year follow-up by nephrologists before initiation of dialysis) versus late referral groups in the UK. Of the patients referred late and starting dialysis with residual renal function (RRF) <6 ml/min/1.73 m2, 70% of patients required prolonged hospitalization, during which mortality was 13%. By contrast, in the early referral group who had RRF >6 ml/min/1.73 m2 at dialysis start, only 9% of patients required prolonged hospitalization and mortality was only 4%.30 Similar results have been found in other studies.31,32 Perhaps the most convincing data in support of early dialysis initiation came from the Canada-USA (CANUSA) study of patients on peritoneal dialysis.33, 36 For patients with an initial GFR >3.8 ml/min, the 12-month and 24-month survival was 94.7% and 82.1%, respectively, compared with 90.8% and 73.6% for those with an initial GFR <3.8 ml/min (P = 0.015).33, 37 Although patients with an initial renal Kt/V >0.71 had better survival than those with an initial Kt/V <0.71, the difference was not statistically significant (P = 0.10).33, 37 Controlling for age, diabetes mellitus, cardiovascular disease, country, and serum albumin concentration, the death risk of 0.95 for a 0.5 ml/min greater GFR at initiation of dialysis was notable.33, 37 It is important to note that mortality can also be high in patients with a low eGFR who are not on dialysis. A study from 2011 showed that the mortality among non-dialyzed patients increased significantly at an eGFR <7.5 ml/min (HR 4.65, 95% CI 2.28–9.49) compared with an eGFR 7.5–10 ml/min.18
The studies discussed above were all observational and therefore have important limitations. The apparent survival gain of early dialysis initiation in these studies is likely owing to lead-time bias (rather than actual improvement in the course of the disease).8 Another major source of bias in these studies is confounding by indication, whereby the severity of a patient’s symptoms might determine the timing of dialysis initiation. Although a randomized controlled trial would avoid this issue the magnitude of confounding by indication could be somewhat mitigated by using novel statistical techniques such as the propensity score10 or instrumental variables in observational studies. To our knowledge only one study used these methods, and the results did not support early initiation of dialysis, while several predictors of early initiation were identified.10 Another important source of bias and error in these studies is the use of eGFR instead of true creatinine clearance. An early study showed that patients with low creatinine levels were malnourished and that predialysis patients with CKD with protein–energy wasting, including sarcopenia or low meat intake (as a result of diminished appetite or recommended low-protein diet), had lower serum creatinine levels and perhaps lower creatinine clearance rates but paradoxically higher eGFR calculated using the Modification of Diet in Renal Disease (MDRD) formula at initiation of dialysis.38 Serum creatinine has been suggested to be a good indicator of muscle mass under steady-state conditions in patients on maintenance hemodialysis.39–41 Indeed, Beddhu et al. reported that higher estimated initiation GFR (MDRD), but not higher creatinine clearance, was associated with increased risk of death.10 Whether the observed associations are due to confounding by indication and/or other sources of bias, or whether they are true and causal associations with biological plausibility is therefore not clear (Figure 3).
Figure 3.
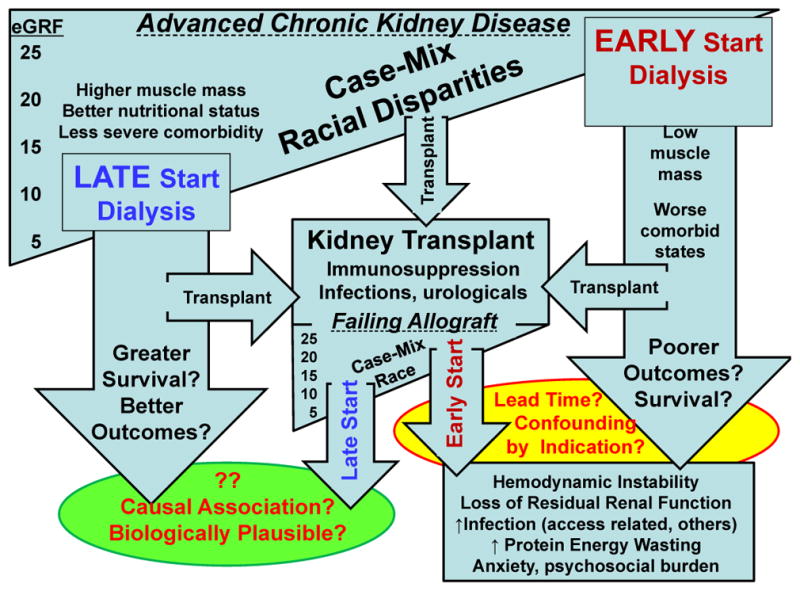
Outcome differentials in early versus late dialysis initiation in transplant-naive and failed kidney transplant patients.
Data against early dialysis initiation
Several studies have questioned the beneficial effects of early dialysis initiation.42 In one observational study, a small beneficial effect of early dialysis initiation of 2.5 months in the first 3 years was found after the start of dialysis, but this gain was apparently an overestimation caused by the lead time.8 In another study, higher eGFR at dialysis initiation as associated with an increased risk of death, although in a subgroup of patients with measured creatinine clearance this calculation of renal function as not significantly associated with decreased or increased mortality.10 Traynor et al. found that higher creatinine clearance at initiation of dialysis was associated with elevated mortality risk.9 Similarly, a large observational study from the USA found a dose-dependent increase in mortality with earlier dialysis initiation.16 After correcting for other factors, patients who initiated dialysis with a higher eGFR experienced a 44% increased mortality risk compared with patients with an eGFR of 5–10 ml/min/1.73 m2 whereas those who initiated dialysis at the lowest eGFR (<5 ml/min/1.73 m2) had a 12% lower death risk.16 Similar results were reported in a large observational study from Europe.13
An important limitation of the studies mentioned above is that the early and late initiation groups of patients had significantly different case-mix and risk factor profiles (Figure 3). Patients who start dialysis early are more likely to be men, to have diabetes mellitus, more severe heart failure or coronary heart disease, lower serum albumin levels and more comorbidities, and are less likely to have glomerulonephritis or polycystic kidney disease; thus, these patients are ‘sicker’.6, 10, 15, 18 Although comorbidity is an important confounder, the increased risk of death is not fully explained by comorbidities.11, 12, 15 Wilson et al. reported that pre-existing morbid conditions are more important determinants of 2-year dialysis survival than the time of dialysis initiation.12 This finding was confirmed in a study that included more than 10,000 patients, in which higher eGFR was associated with an increased mortality risk.15 This association was weakened but remained statistically significant after adjusting for age and comorbidity.15 Hence, the above-mentioned observational studies are prone to biases related to lead time, patient selection, referral time and the confounding by indication (Figure 3).
Data from randomized controlled trials that examined the optimal timing for the initiation of dialysis were lacking until 2010, when the results of the Initiating Dialysis Early and Late (IDEAL) study were published.17, 34 Patients were eligible for the IDEAL study if they had progressive CKD (including a failing kidney transplant) and an eGFR between 10 ml/min/1.73 m2 and 15 ml/min/1.73 m2. The eGFR was estimated using the Cockcroft–Gault equation and corrected for body-surface area.43 Patients were randomly assigned to either commence dialysis with an eGFR of 10.0–14.0 ml/min/1.73 m2 (early-start group) or to continue to receive routine medical care and commence dialysis with an eGFR of 5.0–7.0 ml/min/1.73 m2 (late-start group).34 During a median follow-up of 3.6 years, 37.6% (152 of 404 patients) of early starters and 36.6% (155 of 424 patients) of late starters died (hazard ratio [HR] for early initiation 1.04, 95% CI 0.83–1.30; P = 0.75).17 The authors concluded that, with careful clinical management, dialysis can be safely delayed for some patients until the eGFR falls below 7 ml/min/1.73 m2 or until traditional clinical indicators are present.17 Although randomized, controlled trials provide the best type of evidence, questions always remain about the generalizability of their results.44 In the IDEAL study, the mean predialysis eGFR differed only slightly between the two groups (2.2 ml/min by Cockcroft–Gault and 1.8 ml/min by MDRD equation).45 This difference is smaller than what was prespecified by the study protocol. Moreover, there was a striking difference in the rate of uremia, which developed in only 7% of patients in the early-start group compared with 73% in the late-start group. Worsening uremia might affect health-related quality of life. Indeed, the quality of life was significantly better in the first year of dialysis in patients with early compared with late start of dialysis in one study.46 Additionally, the IDEAL study is not representative of incident dialysis patients in the USA and Europe.4, 7 Data from the USRDS suggest that approximately 50% of incident dialysis patients are aged >65 years whereas the mean age of patients in the IDEAL study was 60 years. In addition, approximately 40% of patients in the IDEAL study had cardiovascular disease, whereas USRDS data suggest that this complication occurs in approximately 60% of patients with stage 4 or 5 CKD.7 The results of the IDEAL study should therefore by interpreted with caution.47
Dialysis in failed transplant patients
In the USA, kidney allograft loss is an important cause of dialysis initiation. The incidence of ESRD owing to post-transplant complications was four-times higher in 2006–2008 than 10 years earlier (Figure 4).5 A similar trend in Europe and Australia might exist, but neither the ERA-EDTA registry nor ANZDATA collect data relating to the number of patients who return to dialysis therapy following a failed kidney transplant. An analysis of the USRDS database found that 1-year, 2-year and 3-year all-cause mortality was 16%, 25% and 33%, respectively, in failed kidney transplant patients on dialysis.47 Cardiac and infectious diseases were the main causes of death.47 The use of peritoneal dialysis compared with hemodialysis was associated with similar early and overall mortality among patients returning to dialysis after a failed transplant.48 Interestingly, these patients had worse survival than renal allograft recipients whose dialysis therapy was delayed49 Indeed, current evidence indicates that, despite similar comorbidity profiles, the survival of patients on dialysis after a failed transplant is worse than incident waitlisted transplant-naive patients on dialysis.19
Figure 4.
Return to dialysis therapy in failed renal transplant recipients from 1991–2009 from the United States Renal Data System.
There are some potential explanations for this survival difference (Table 1). First, failed transplant patients on dialysis have an increased risk of infection caused by the effects of exposure to chronic immunosuppression. Septicemia rates in these patients are particularly high during the first few months after starting dialysis, which might be related in part to the use of temporary hemodialysis catheters during which time residual immunosuppression might continue for months to years following dialysis reinitiation50 Second, compared with incident transplant-naive patients on dialysis, failed transplant patients might receive suboptimal predialysis care which could contribute to morbidity after dialysis reinitiation.47, 51, 52 Failed transplant patients usually have increased exposure to uremia owing to longer dialysis vintage and therefore are more predisposed to the effects of anemia, erythropoietin resistance, hypoalbuminemia, mineral and bone disorders, and other conditions associated with reduced survival.20, 52–54 Third, the presence of a failed allograft remnant might be an ongoing source of chronic inflammation, which is a known risk factor for mortality both in patients on dialysis and in kidney transplant recipients.55, 56 Early initiation of dialysis with an allograft that is still viable is likely to be associated with such adverse immunological and inflammatory reactions, although this remains to be proven. Finally, in incident patients on dialysis, preserved urine output is a strong predictor of patient survival.57 However, little is known about the impact of transplant RRF on survival in failed transplant patients on dialysis. A decline in RRF is known to occur faster in failed transplant patients than in transplant-naive patients.26 Based on striking differences between transplant-naive and failed kidney transplant patients, including exposure to immunosuppressive medications, studies in transplant-naive patients with CKD might not be generalizable to patients with a failed renal allograft.
Table 1.
Mechanisms associated with poor outcomes in failed kidney transplant patients
| Mechanism | Potential remedies |
|---|---|
| Chronic immunosuppression and steroid exposure and increased infection rates prior to dialysis therapy | Consider reduced immunosuppressive medication dose with eGFR <25 ml/min |
| Increased septicemia risk with temporary hemodialysis catheters while residual immunosuppression continues | Avoid temporary or permanent catheters during the first months after re-starting dialysis |
| Suboptimal predialysis care given higher priority to immunosuppression | Refer to non-transplant nephrologists with expertise in optimal dialysis preparation |
| Increased exposure to uremia owing to longer dialysis vintage | Assure more aggressive treatment of uremic conditions |
| Increased predisposition to effects of anemia, PEW, MBD, and other conditions associated with reduced survival | Better treatment of anemia, malnutrition, osteodystrophy and other comorbidities [Au: okay?] |
| Presence of failed allograft remnants as a source of chronic inflammation | Consider transplant nephrectomy |
| Early initiation of dialysis with a viable allograft more likely to be associated with immunological and inflammatory reactions | Avoid early dialysis reinitiation as long as transplant kidney is viable |
| Unknown impact of RRF from transplant vs native kidneys on survival | Examine source of RRF |
| Other transplant-specific morbid states, including acute rejection, urological complications | Assure optimal care during transplant era |
| Conditions associated with dialysis therapy outcomes | Address challenges related to dialysis therapy |
Abbreviations: eGFR, estimated glomerular filtration rate; MBD, mineral and bone disorder; PEW, protein–energy wasting; RRF, residual renal function.
Dialysis reinitiation in transplant patients
To the best of our knowledge, only two studies have assessed the association between eGFR at reinitiation of dialysis and mortality in failed kidney transplant patients.47, 58 Gill et al. examined 4,741 failed kidney transplant patients for more than 1 year after reinitiation of dialysis in the USA.48 During the follow-up period, 1,016 patients (21%) died, mostly owing to cardiac (36%) and infectious (17%) causes. The eGFR was significantly higher at dialysis reinitiation in patients who died than in survivors (9.7 ± 4.8 ml/min/1.73 m2 versus 8.0 ± 3.7 ml/min/1.73 m2). Each ml/min/1.73 m2 increase in eGFR at dialysis reinitiation was associated with a 4% increased risk of death during dialysis (HR 1.04, 95% CI 1.02–1.06).47 Although this study is pioneering, it has several limitations that are similar to those seen in studies of transplant-naive patients, including confounding by indication, that is, the sickest patients tend to require dialysis initiation at higher RRF levels.47 This confounding by indication could have been addressed at least partially with the use of propensity scores as was done in one study of transplant-naive patients with CKD10 and a recent study in failed transplant recipient,58 or by using an instrumental variable. In the recent cohort study of 747 failed kidney transplant patients who had returned to dialysis therapy,58 a propensity score for the likelihood of early (eGFR≥10.5 but <15 ml/min/1.73m2) vs. late (eGFR<10.5 ml/min/1.73m2) dialysis therapy reinitiation was fitted by logistic regression. Even though patients with early initiation of dialysis appeared sicker (more diabetic and heart disease patients with lower BMI), there was no survival advantage of earlier dialysis therapy in any group.58 Higher eGFR upon re-initiation of dialysis treatment exhibited a trend towards higher mortality risk, and this trend was even more prominent among the healthiest subgroups of patients identified by the propensity score including women and younger individuals (see Figure 1). To date, neither additional observational studies nor any randomized controlled trials have answered the important question as to whether early or late reinitiation of dialysis is better in failed kidney transplant patients.
Factors that modify dialysis outcomes
Some potential factors might modify the association between dialysis reinitiation and outcomes in failed kidney transplant recipients. First, the cause and time course of first transplant failure might modify this association. Patients with a primary nonfunctioning graft or hyperacute rejection may required immediate allograft nephrectomy have no renal function and belong to late reinitiation group. Second, differences found in observational studies among patients who start early versus late dialysis might relate to predialysis care by a renal care professional, as well as other markers of optimal dialysis initiation such as vascular access type and location of dialysis initiation59 Third, late referral to a nephrologist also has an impact on survival. In a meta-analysis from 2007, late referral was associated with a significantly increased risk of all-cause mortality (risk ratio 1.99, 95% CI 1.66–2.39) and 1-year mortality (risk ratio 2.08, 95% CI 1.31–3.31) compared with early referral.60 Fourth, the dialysis dose at initiation of dialysis might have an impact on the association between dialysis reinitiation and outcomes. Fifth, the type of dialysis modality at initiation also has an effect on mortality although it is not known whether starting hemodialysis early would have a different effect from starting peritoneal dialysis early. Finally, the timing of reduction of immunosuppression and graft removal might have an effect on this association. In patients with late graft failure (graft survival >1 year) removal of the graft may improves survival and it enables the physician to stop immunosuppression shortly after the reinitiation of dialysis.61
Biological contributors to outcomes
A number of biological factors might causally contribute to increased risk of mortality upon early initiation of dialysis (Table 2). In patients who start dialysis relatively early the harmful aspects of some of these factors might be substantially mitigated or perceived to be less when compared with patients who prolong the dialysis-free period, but have lower residual renal function and uremia. Thrice-weekly hemodialysis treatment could lead to subtle but cumulative mechanical and oxidative stress on the cardiovascular system and could give rise to hemodynamic instability. Although biocompatible membranes seem to be associated with better survival than cellulose membranes, bioincompatibility still remains a problem that might contribute to elevated inflammation or infection and oxidative stress.62 Moreover, the infection rate is extremely high after initiation of dialysis. In a US Medicare cohort of patients who newly started dialysis between 1996 and 2001, the 1-year incidence of infection-related hospitalizations was 32% for those who received hemodialysis and 24% for those who received peritoneal dialysis; the 3-year incidence of infection-related hospitalizations exceeded 50% in both groups.63 However, in the HEMO study, most infection-related hospitalizations were not attributed to vascular access.64 Additionally, the frequency of infection-related hospitalizations relating to access was disproportionately higher among patients with catheters than among those with grafts or fistulas.64
Table 2.
Potential biological factors contributing to poor outcomes upon early dialysis initiation
| Potentially harmful factor | Dialysis modality | Pathophysiological consequences |
|---|---|---|
| Mechanical stress of dialysis therapy | Hemodialysis more than peritoneal dialysis | Vascular and endothelial damage |
| Membrane bioincompatibility | Hemodialysis | Inflammation, complement activation |
| Oxidative stress | Hemodialysis and peritoneal dialysis | Inflammation, oxidative stress |
| Infection including bacteremia related to vascular access | Hemodialysis | Acceleration of atherosclerosis, inflammation |
| Infections and peritonitis related to peritoneal access | Peritoneal dialysis | Acceleration of atherosclerosis, inflammation |
| Non-access related infections including pneumonia | Hemodialysis and peritoneal dialysis | Acceleration of atherosclerosis, inflammation |
| Repetitive low blood pressure episodes upon excessive ultrafiltration | Hemodialysis and peritoneal dialysis | Repeated bouts of acute tubular necrosis, loss of residual renal function, ischemic events |
| Ischemic limbs related to vascular access | Hemodialysis | Steal syndrome, necrotic fingers |
| Damage to peritoneum related to peritoneal dialysis | Peritoneal dialysis | Fibrosing peritonitis |
| Increased exposure to toxic medications such as ESA | Hemodialysis and peritoneal dialysis | Thromboembolic events, increased platelet activation |
| Low serum creatinine levels from low muscle mass (sarcopenia) | Hemodialysis and peritoneal dialysis | Increased PEW rates and risk of death |
| Use of anticoagulation therapy (heparin) | Hemodialysis | Risk of bleeding |
| Heightened anxiety upon each dialysis treatment | Hemodialysis and peritoneal dialysis | Reduced quality of life, depression, increased risk of death |
| Social burden and psychological stigma of dialysis dependency | Hemodialysis and peritoneal dialysis | Malfunctioning social status, impaired work and social prospects, loss of independency, depression |
| Frequent commuting to dialysis clinic | Hemodialysis more than peritoneal dialysis | Increased risk of motor-vehicle and other accidents |
| Immunological reactions from the remnant renal allograft (upon early dialysis reinitiation) | Hemodialysis and peritoneal dialysis | Bouts of acute rejection and inflammation, allograft rupture and bleeding |
| Reduced albumin levels | Peritoneal dialysis more than hemodialysis | Decreased pool of antioxidative albumin, PEW |
| Loss of vital nutrients and molecules such as carnitine and vitamin C | Hemodialysis more than peritoneal dialysis | PEW, anemia |
| Frequent blood loss and iron depletion | Hemodialysis more than peritoneal dialysis | Iron deficiency, resistant anemia requiring increased ESA doses |
Abbreviations: ESA, erythropoietin-stimulating agent; PEW, protein–energy wasting.
Repeated bouts of acute tubular necrosis as a result of low blood pressure during each hemodialysis session65 could lead to faster loss of residual kidney function and frequent ischemic events with hypotensive episodes. Exposure to toxic medications might also have a role. Patient anxiety that accompanies each hemodialysis treatment, along with fatigue and lightheadedness following sessions, might aggravate harm.66
Another factor that could explain the association between high eGFR and mortality is sarcopenia. eGFR is an inaccurate measure of true GFR in patients with stage 5 CKD as it more accurately reflects muscle mass, [not detectable by BMI.67 Therefore, a high eGFR in patients with stage 5 CKD largely reflects sarcopenia (leading to low creatinine levels), not higher renal function.68–70 Sarcopenia is in turn a predictor of poor outcomes in hemodialysis.71 Early-start dialysis based on creatinine clearance as a measure of kidney function (a better measure in stage 5 CKD) might or might not have an adverse effect on mortality risk.10 Moreover, most equations used to estimate GFR tend to overestimate GFR in kidney transplant recipients. A study examining 12 different methods found that Walser and MDRD equations gave the best performance to estimate GFR in kidney transplant recipients.72
Prolonging the predialysis period
For decades, there have been minority camps within the nephrology community advocating late-start dialysis initiation. A nonrandomized study suggested increased survival in the early dialysis period if patients were supplemented with a low-protein diet.73 The strategy of maintaining a low protein diet (0.6–0.7 g/kg per day) while providing amino acids and proteins of high biological value can be a method to protect kidney function. In many countries outside the USA, ketoanalogues are used routinely, as are indoxyl sulfate modulators and absorbents. Increased circulating indoxyl sulfate levels are associated with inflammation, oxidative stress, endothelial dysfunction, vascular calcification and mortality in patients with CKD.74 Thus, the removal of indoxyl sulfate by AST-120 (an oral indoxyl sulfate absorbent) could ameliorate the progression of not only CKD, but also of cardiovascular disease and osteodystrophy related to CKD.75, 76 More recently, bardoxolone methyl, an oral antioxidant inflammation modulator, was associated with improvement in the eGFR in patients with advanced CKD and type 2 diabetes mellitus at 24 weeks.77 The improvement persisted at 52 weeks, indicating that bardoxolone methyl could have promise for the treatment of patients with CKD.77 Even though the results of this study are promising, it is important to note that only eGFR (MDRD) was measured, which is based on serum creatinine level and hence muscle mass.41 The most common adverse event noted in this study was muscle spasm, which was 2–3-fold higher in the bardoxolone methyl group. Moreover, in the treatment group, patients reported 3–4-times higher rates of loss of appetite and weight loss of several kilograms.77 In addition to the aforementioned interventions, salt and fluid restriction with or without diuretics might contribute to prolongation of the dialysis-independent period and late initiation of dialysis. Finally, alkali therapy, such as bicarbonate slows the rate of progression of renal failure to ESRD and improves nutritional status among patients with CKD.78–80
Conclusions
Since the emergence of recent observational studies and the IDEAL trial, the European Best Practice Guidelines have changed their recommendations of dialysis initiation. The presence of symptoms, diabetes mellitus and rapid deterioration of RRF has now become more important than eGFR in making the decision regarding dialysis initiation. These guidelines suggest that the majority of patients will be symptomatic and need to start dialysis with an eGFR in the 6–9 ml/min/1.73 m2 range.4, 67 Similar recommendations for late dialysis start have been suggested in an increasing number of publications.42, 44
We feel that starting dialysis early solely based on an eGFR is not justified and could in fact be harmful in some cases of transplant-naive and failed transplant patients. Although other studies indicate that starting dialysis with an eGFR <7 ml/min/1.73 m2 is not necessarily associated with an increased mortality risk, these results may reflect survivor bias. The concept of depending on this single metric for such a crucial decision is likely to be flawed and alternative and reliable measures are required. In failed kidney transplant patients there is an even larger paucity of evidence for or against early reinitiation of dialysis treatment. The available limited data suggest, however, that failed kidney transplant patients differ considerably from transplant-naive patients with CKD, and hence the association between initiation of dialysis and outcomes may differ substantially. Observational and controlled studies are needed to examine this important area of daily clinical practice in the nephrology and transplantation arenas.
Key points.
Late initiation of dialysis is not associated with worse mortality in transplant-naive patients with chronic kidney disease (CKD)
Current guidelines suggest that the majority of transplant-naive patients will be symptomatic and need to start dialysis with estimated glomerular filtration rate (eGFR) in the 6–9 ml/min/1.73 m2 range
In failing kidney transplant patients, there is a paucity of evidence for or against early reinitiation of dialysis treatment
There are several biological factors, such as hemodynamic instability, loss of residual renal function and higher infection rate, which can contribute to the increased mortality risk upon dialysis initiation as compared to extended dialysis-free period in both transplant-naïve and failing transplant patients.
There are additional factors, such as different predialysis care by transplant nephrologists, immunosuppressive regimen modifications and residual function of renal allograft, which may modify the association between dialysis reinitiation and outcomes in failed kidney transplant recipients
Starting dialysis early solely based on eGFR is not justified and could in fact be harmful in some cases, and alternative and more reliable measures of the clinical conditions are required
Review criteria.
Material for this Review was obtained by searching the PubMed database using the following terms (alone and in various combinations): “dialysis”, “peritoneal dialysis”, “hemodialysis”, “initiation”, “start”, “transplantation”, “kidney transplantation”, “graft loss”, “graft failure”, “glomerular filtration rate”, “creatinine clearance”, “outcome” and “mortality”. Data for this Review were obtained by searching USRDS, ERA-EDTA and ANZDATA databases. Selected materials were full-length, English-language papers, with a focus on studies of dialysis initiation and on papers published since 1980. The reference lists of identified papers were searched for further relevant material.
Acknowledgments
M. Z. Molnar received grants from the National Development Agency (KTIA-OTKA-EU 7KP-HUMAN-MB08-A-81231) from the Research and Technological Innovation Fund, and was also supported by the Hungarian Kidney Foundation. K. Kalantar-Zadeh was supported by research grants from the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health (K24 DK091419 and R01 DK078106) and a philanthropic grant from H. Simmons.
Biographies
Miklos Z. Molnar, MD, PhD, is a Research Associate at Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, CA, USA and Associate Professor of Medicine at the Semmelweis University in Budapest, Hungary. He obtained an MD from the Semmelweis University Medical School in Budapest, Hungary. His main research interests are epidemiology and outcomes, anemia, inflammation, nutrition and sleep disorders in kidney transplant recipients.
Akinlolu O. Ojo, MD, MPH, MBA, PhD, is the Director of Clinical Research, Division of Nephrology and Comprehensive Kidney Program, University of Michigan, Ann Arbor, MI, USA. He obtained an MD from the University of Lagos in Lagos, Nigeria. His research interests include outcomes registry analysis and clinical trials in kidney transplantation. His research focuses on the hypertensive and diabetic renal disease, health disparities in end-stage renal disease, including kidney transplantation and cardiovascular disease in kidney transplant recipients.
Suphamai Bunnapradist, MD, MS, is the Director of Research for the UCLA Kidney Transplant Program and Professor of Medicine at David Geffen School of Medicine at UCLA, Los Angeles, CA, USA. He obtained an MD with honors from Chulalongkorn University in Bangkok, Thailand. His research interests include outcomes registry analysis and clinical trials in kidney transplantation and his research focuses on the evaluation of immunosuppressants in transplantation, hepatitis in renal transplantation and the optimal utilization of organs.
Csaba P. Kovesdy, MD, FASN, is Chief of Nephrology at Salem Veterans Affairs Medical Center in Salem, VA and Clinical Associate Professor of Medicine at the University Of Virginia School Of Medicine in Charlottesville, VA, USA. He obtained an MD from the University of Pecs Medical School in Pecs, Hungary. His main research interests are epidemiology and outcomes, anemia, bone and mineral disorders and nutrition in nondialysis-dependent chronic kidney disease.
Kamyar Kalantar-Zadeh, MD, MPH, PhD, is Associate Professor of Medicine, Pediatrics and Epidemiology at UCLA David Geffen School of Medicine and UCLA School of Public Health, and Director of the Harold Simmons Center for Chronic Disease Research and Epidemiology at Los Angeles Biomedical Research Institute at Harbor-UCLA, CA, USA. He is a medical graduate of the Universities of Bonn and Erlangen-Nuremberg in Germany and a board-certified practicing physician in internal medicine, nephrology and pediatrics. His research interests include longevity in chronic diseases, cachexia, malnutrition, inflammation, minerals and bone, anemia and patient-centered outcomes.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
M. Z. Molnar and K. Kalantar-Zadeh researched data to include in the manuscript. M. Z. Molnar, C. P. Kovesdy and K. Kalantar-Zadeh contributed to discussion of content for the article. All authors contributed equally to the writing of the article and to the reviewing and editing of the manuscript before submission.
References
- 1.National Kidney Foundation. DOQI Clinical Practice Guidelines for hemodialysis and peritoneal dialysis adequacy. Am J Kidney Dis. 1997;30:S67–136. doi: 10.1016/s0272-6386(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48:S1–322. [Google Scholar]
- 3.The European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) European best practice guidelines for haemodialysis. When to start dialysis. Nephrol Dial Transplant. 2002;17:S10–11. [Google Scholar]
- 4.Tattersall J, Dekker F, Heimburger O, Jager KJ, Lameire N, Lindley E, Van Biesen W, Vanholder R, Zoccali C. When to start dialysis: updated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) study. Nephrol Dial Transplant. 2011;26:2082–2086. doi: 10.1093/ndt/gfr168. [DOI] [PubMed] [Google Scholar]
- 5.US Renal Data System. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. USRDS 2010 Annual Data Report. [Google Scholar]
- 6.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171:396–403. doi: 10.1001/archinternmed.2010.415. [DOI] [PubMed] [Google Scholar]
- 7.US renal Data System. Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. USRDS 2009 Annual Data Report. [Google Scholar]
- 8.Korevaar JC, Jansen MA, Dekker FW, Jager KJ, Boeschoten EW, Krediet RT, Bossuyt PM. When to initiate dialysis: effect of proposed US guidelines on survival. Lancet. 2001;358:1046–1050. doi: 10.1016/S0140-6736(01)06180-3. [DOI] [PubMed] [Google Scholar]
- 9.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13:2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 10.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, Cheung AK. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14:2305–2312. doi: 10.1097/01.asn.0000080184.67406.11. [DOI] [PubMed] [Google Scholar]
- 11.Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, Kausz AT. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46:887–896. doi: 10.1053/j.ajkd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Wilson B, Harwood L, Locking-Cusolito H, Chen SJ, Heidenheim P, Craik D, Clark WF. Optimal timing of initiation of chronic hemodialysis? Hemodial Int. 2007;11:263–269. doi: 10.1111/j.1542-4758.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- 13.Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, Finne P, Ioannidis GA, Salomone M, Traynor JP, Zurriaga O, Verrina E, Jager KJ. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24:3175–3182. doi: 10.1093/ndt/gfp264. [DOI] [PubMed] [Google Scholar]
- 14.Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A. Survival and dialysis initiation: comparing British Columbia and Scotland registries. Nephrol Dial Transplant. 2009;24:3186–3192. doi: 10.1093/ndt/gfp189. [DOI] [PubMed] [Google Scholar]
- 15.Lassalle M, Labeeuw M, Frimat L, Villar E, Joyeux V, Couchoud C, Stengel B. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77:700–707. doi: 10.1038/ki.2010.14. [DOI] [PubMed] [Google Scholar]
- 16.Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, Ragasa R, Goldfarb-Rumyantzev AS. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010;5:1828–1835. doi: 10.2215/CJN.06230909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 18.Evans M, Tettamanti G, Nyren O, Bellocco R, Fored CM, Elinder CG. No survival benefit from early-start dialysis in a population-based, inception cohort study of Swedish patients with chronic kidney disease. J Intern Med. 2011;269:289–298. doi: 10.1111/j.1365-2796.2010.02280.x. [DOI] [PubMed] [Google Scholar]
- 19.Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49:294–300. doi: 10.1053/j.ajkd.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Caldes Ruisanchez S, Marcen Letosa R, Amezquita Orjuela Y, Fernandez Lucas M, Rivera Gorrin M, Galeano Alvarez C, Fernandez Rodriguez A, Teruel Briones JL, Quereda Rodriguez-Navarro C. Dialysis after kidney transplant failure: do patients start in a worse condition than the general population with chronic kidney disease? Nefrologia. 2011;31:51–57. doi: 10.3265/Nefrologia.pre2010.Nov.10610. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge H, Bammens B, Lemahieu W, Maes BD, Vanrenterghem Y. Comparison of peritoneal dialysis and haemodialysis after renal transplant failure. Nephrol Dial Transplant. 2006;21:1669–1674. doi: 10.1093/ndt/gfl010. [DOI] [PubMed] [Google Scholar]
- 22.Badve SV, Hawley CM, McDonald SP, Mudge DW, Rosman JB, Brown FG, Johnson DW. Effect of previously failed kidney transplantation on peritoneal dialysis outcomes in the Australian and New Zealand patient populations. Nephrol Dial Transplant. 2006;21:776–783. doi: 10.1093/ndt/gfi248. [DOI] [PubMed] [Google Scholar]
- 23.Mujais S, Story K. Patient and technique survival on peritoneal dialysis in patients with failed renal allograft: a case-control study. Kidney Int Suppl. 2006:S133–137. doi: 10.1038/sj.ki.5001930. [DOI] [PubMed] [Google Scholar]
- 24.Duman S, Asci G, Toz H, Ozkahya M, Ertilav M, Sezis M, Ok E. Patients with failed renal transplant may be suitable for peritoneal dialysis. Int Urol Nephrol. 2004;36:249–252. doi: 10.1023/b:urol.0000034678.84908.9b. [DOI] [PubMed] [Google Scholar]
- 25.Sasal J, Naimark D, Klassen J, Shea J, Bargman JM. Late renal transplant failure: an adverse prognostic factor at initiation of peritoneal dialysis. Perit Dial Int. 2001;21:405–410. [PubMed] [Google Scholar]
- 26.Davies SJ. Peritoneal dialysis in the patient with a failing renal allograft. Perit Dial Int. 2001;21(Suppl 3):S280–284. [PubMed] [Google Scholar]
- 27.Bonomini V, Albertazzi A, Vangelista A, Bortolotti GC, Stefoni S, Scolari MP. Residual renal function and effective rehabilitation in chronic dialysis. Nephron. 1976;16:89–102. doi: 10.1159/000180589. [DOI] [PubMed] [Google Scholar]
- 28.Bonomini V, Feletti C, Scolari MP, Stefoni S. Benefits of early initiation of dialysis. Kidney Int Supplement. 1985;17:S57–59. [PubMed] [Google Scholar]
- 29.Bonomini V, Vangelista A, Stefoni S. Early dialysis in renal substitutive programs. Kidney Int Supplement. 1978:S112–116. [PubMed] [Google Scholar]
- 30.Ratcliffe PJ, Phillips RE, Oliver DO. Late referral for maintenance dialysis. Br Med J (Clin Res Ed) 1984;288:441–443. doi: 10.1136/bmj.288.6415.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jungers P, Zingraff J, Albouze G, Chauveau P, Page B, Hannedouche T, Man NK. Late referral to maintenance dialysis: detrimental consequences. Nephrol Dial Transplant. 1993;8:1089–1093. [PubMed] [Google Scholar]
- 32.Tattersall J, Greenwood R, Farrington K. Urea kinetics and when to commence dialysis. Am J Nephrol. 1995;15:283–289. doi: 10.1159/000168850. [DOI] [PubMed] [Google Scholar]
- 33.Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1996;7:198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 34.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Dempster J, Fraenkel MB, Harris A, Harris DC, Johnson DW, Kesselhut J, Luxton G, Pilmore A, Pollock CA, Tiller DJ. The Initiating Dialysis Early and Late (IDEAL) study: study rationale and design. Perit Dial Int. 2004;24:176–181. [PubMed] [Google Scholar]
- 35.Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009;76:257–261. doi: 10.1038/ki.2009.161. [DOI] [PubMed] [Google Scholar]
- 36.McCusker FX, Teehan BP, Thorpe KE, Keshaviah PR, Churchill DN. How much peritoneal dialysis is required for the maintenance of a good nutritional state? Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Kidney Int Supplement. 1996;56:S56–61. [PubMed] [Google Scholar]
- 37.Churchill DN. An evidence-based approach to earlier initiation of dialysis. Am J Kidney Dis. 1997;30:899–906. doi: 10.1016/s0272-6386(97)90102-5. [DOI] [PubMed] [Google Scholar]
- 38.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Pappas LM, Cheung AK. Creatinine production, nutrition, and glomerular filtration rate estimation. J Am Soc Nephrol. 2003;14:1000–1005. [Google Scholar]
- 39.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R, Anker SD. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, Krishnan M, Nissenson AR, Danovitch GM, Kalantar-Zadeh K. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant. 2011;11:725–736. doi: 10.1111/j.1600-6143.2011.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noori N, Kovesdy CP, Bross R, Lee M, Oreopoulos A, Benner D, Mehrotra R, Kopple JD, Kalantar-Zadeh K. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am J Kidney Dis. 2011;57:130–139. doi: 10.1053/j.ajkd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosansky S, Glassock RJ, Clark WF. Early start of dialysis: a critical review. Clin J Am Soc Nephrol. 2011;6:1222–1228. doi: 10.2215/CJN.09301010. [DOI] [PubMed] [Google Scholar]
- 43.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 44.Lindholm B, Davies S. End-stage renal disease in 2010: Timing of dialysis initiation and choice of dialysis modality. Nat Rev Nephrol. 2011;7:66–68. doi: 10.1038/nrneph.2010.178. [DOI] [PubMed] [Google Scholar]
- 45.Faust J, Schreiner O. Early versus late initiation of dialysis. N Engl J Med. 2010;363:2368–2369. doi: 10.1056/NEJMc1010323. author reply 2369–2370. [DOI] [PubMed] [Google Scholar]
- 46.Korevaar JC, Jansen MA, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT. Evaluation of DOQI guidelines: early start of dialysis treatment is not associated with better health-related quality of life. Am J Kidney Dis. 2002;39:108–115. doi: 10.1053/ajkd.2002.29896. [DOI] [PubMed] [Google Scholar]
- 47.Gill JS, Abichandani R, Kausz AT, Pereira BJ. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int. 2002;62:1875–1883. doi: 10.1046/j.1523-1755.2002.00640.x. [DOI] [PubMed] [Google Scholar]
- 48.Perl J, Hasan O, Bargman JM, Jiang D, Na Y, Gill JS, Jassal SV. Impact of dialysis modality on survival after kidney transplant failure. Clin J Am Soc Nephrol. 2011;6:582–590. doi: 10.2215/CJN.06640810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, Callard SE, Dickinson DM, Schmouder RL, Leichtman AB. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation. 1998;66:1651–1659. doi: 10.1097/00007890-199812270-00014. [DOI] [PubMed] [Google Scholar]
- 50.Johnston O, Zalunardo N, Rose C, Gill JS. Prevention of sepsis during the transition to dialysis may improve the survival of transplant failure patients. J Am Soc Nephrol. 2007;18:1331–1337. doi: 10.1681/ASN.2006091017. [DOI] [PubMed] [Google Scholar]
- 51.Gill JS, Abichandani R, Khan S, Kausz AT, Pereira BJ. Opportunities to improve the care of patients with kidney transplant failure. Kidney Int. 2002;61:2193–2200. doi: 10.1046/j.1523-1755.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- 52.Almond MK, Tailor D, Marsh FP, Raftery MJ, Cunningham J. Increased erythropoietin requirements in patients with failed renal transplants returning to a dialysis programme. Nephrol Dial Transplant. 1994;9:270–273. [PubMed] [Google Scholar]
- 53.Lopez-Gomez JM, Perez-Flores I, Jofre R, Carretero D, Rodriguez-Benitez P, Villaverde M, Perez-Garcia R, Nassar GM, Niembro E, Ayus JC. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol. 2004;15:2494–2501. doi: 10.1097/01.ASN.0000137879.97445.6E. [DOI] [PubMed] [Google Scholar]
- 54.Wanner C, Zimmermann J, Schwedler S, Metzger T. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Supplement. 2002:99–102. doi: 10.1046/j.1523-1755.61.s80.18.x. [DOI] [PubMed] [Google Scholar]
- 55.Molnar MZ, Czira ME, Rudas A, Ujszaszi A, Lindner A, Fornadi K, Kiss I, Remport A, Novak M, Kennedy SH, Rosivall L, Kovesdy CP, Mucsi I. Association of the malnutrition-inflammation score with clinical outcomes in kidney transplant recipients. Am J Kidney Dis. 2011;58:101–108. doi: 10.1053/j.ajkd.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 56.Rambod M, Bross R, Zitterkoph J, Benner D, Pithia J, Colman S, Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2009;53:298–309. doi: 10.1053/j.ajkd.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068–1081. doi: 10.1053/j.ajkd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 58.Molnar MZ, Streja E, Kovesdy CP, Hoshino J, Hatamizadeh P, Glassock R, Ojo A, Kalantar-Zadeh K. Estimated Glomerular Filtration Rate at Re-Initiation of Dialysis and Mortality in Failed Kidney Transplant Recipients. Nephrol Dial Transplant. 2012 doi: 10.1093/ndt/gfs004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendelssohn DC, Malmberg C, Hamandi B. An integrated review of “unplanned” dialysis initiation: reframing the terminology to “suboptimal” initiation. BMC Nephrol. 2009;10:22. doi: 10.1186/1471-2369-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan MR, Dall AT, Fletcher KE, Lu N, Trivedi H. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med. 2007;120:1063–1070. doi: 10.1016/j.amjmed.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 61.Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS. Nephrectomy after transplant failure: current practice and outcomes. Am J Transplant. 2007;7:1961–1967. doi: 10.1111/j.1600-6143.2007.01884.x. [DOI] [PubMed] [Google Scholar]
- 62.Bloembergen WE, Hakim RM, Stannard DC, Held PJ, Wolfe RA, Agodoa LY, Port FK. Relationship of dialysis membrane and cause-specific mortality. Am J Kidney Dis. 1999;33:1–10. doi: 10.1016/s0272-6386(99)70251-9. [DOI] [PubMed] [Google Scholar]
- 63.Chavers BM, Solid CA, Gilbertson DT, Collins AJ. Infection-related hospitalization rates in pediatric versus adult patients with end-stage renal disease in the United States. J Am Soc Nephrol. 2007;18:952–959. doi: 10.1681/ASN.2006040406. [DOI] [PubMed] [Google Scholar]
- 64.Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol. 2003;14:1863–1870. doi: 10.1097/01.asn.0000074237.78764.d1. [DOI] [PubMed] [Google Scholar]
- 65.Orofino L, Marcen R, Quereda C, Villafruela JJ, Sabater J, Matesanz R, Pascual J, Ortuno J. Epidemiology of symptomatic hypotension in hemodialysis: is cool dialysate beneficial for all patients? Am J Nephrol. 1990;10:177–180. doi: 10.1159/000168077. [DOI] [PubMed] [Google Scholar]
- 66.Cukor D, Coplan J, Brown C, Friedman S, Cromwell-Smith A, Peterson RA, Kimmel PL. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:484–490. doi: 10.2215/CJN.00040107. [DOI] [PubMed] [Google Scholar]
- 67.Liberek T, Warzocha A, Galgowska J, Taszner K, Clark WF, Rutkowski B. When to initiate dialysis--is early start always better? Nephrol Dial Transplant. 2011;26:2087–2091. doi: 10.1093/ndt/gfr181. [DOI] [PubMed] [Google Scholar]
- 68.Grootendorst DC, Michels WM, Richardson JD, Jager KJ, Boeschoten EW, Dekker FW, Krediet RT. The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant. 2011;26:1932–1937. doi: 10.1093/ndt/gfq667. [DOI] [PubMed] [Google Scholar]
- 69.Noori N, Kovesdy CP, Dukkipati R, Kim Y, Duong U, Bross R, Oreopoulos A, Luna A, Benner D, Kopple JD, Kalantar-Zadeh K. Survival predictability of lean and fat mass in men and women undergoing maintenance hemodialysis. The American journal of clinical nutrition. 2010;92:1060–1070. doi: 10.3945/ajcn.2010.29188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalantar-Zadeh K, Cano NJ, Budde K, Chazot C, Kovesdy CP, Mak RH, Mehrotra R, Raj DS, Sehgal AR, Stenvinkel P, Ikizler TA. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol. 2011;7:369–384. doi: 10.1038/nrneph.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, Luna A, Gomez M, Luna C, Bross R, Nissenson AR, Kalantar-Zadeh K. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaspari F, Ferrari S, Stucchi N, Centemeri E, Carrara F, Pellegrino M, Gherardi G, Gotti E, Segoloni G, Salvadori M, Rigotti P, Valente U, Donati D, Sandrini S, Sparacino V, Remuzzi G, Perico N Investigators MSSS. Performance of different prediction equations for estimating renal function in kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:1826–1835. doi: 10.1111/j.1600-6143.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- 73.Coresh J, Walser M, Hill S. Survival on dialysis among chronic renal failure patients treated with a supplemented low-protein diet before dialysis. J Am Soc Nephrol. 1995;6:1379–1385. doi: 10.1681/ASN.V651379. [DOI] [PubMed] [Google Scholar]
- 74.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int Suppl. 1997;62:S23–28. [PubMed] [Google Scholar]
- 76.Niwa T. Uremic toxicity of indoxyl sulfate. Nagoya J Med Sci. 2010;72:1–11. [PMC free article] [PubMed] [Google Scholar]
- 77.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. Bardoxolone Methyl and Kidney Function in CKD with Type 2 Diabetes. N Engl J Med. 2011 doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 78.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 2008;73:192–199. doi: 10.1038/sj.ki.5002647. [DOI] [PubMed] [Google Scholar]
- 80.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78:303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]