Abstract
Attachment, affect, and sex shape responsivity to psychosocial stress. Concurrent social contexts influence cortisol secretion, a stress hormone and biological marker of hypothalamic–pituitary–adrenal axis activity. Patterns of attachment, emotion status, and sex were hypothesized to relate to bifurcated, that is, accentuated and attenuated, cortisol reactivity. The theoretical framework for this study posits that multiple individual differences mediate a cortisol stress response. The effects of two psychosocial stress interventions, a modified Trier Social Stress Test for Teens and the Frustration Social Stressor for Adolescents were developed and investigated with early adolescents. Both of these protocols induced a significant stress reaction and evoked predicted bifurcation in cortisol responses; an increase or decrease from baseline to reactivity. In Study I, 120 predominantly middle-class, Euro-Canadian early adolescents with a mean age of 13.43 years were studied. The girls' attenuated cortisol reactivity to the public performance stressor related significantly to their self-reported lower maternal-attachment and higher trait-anger. In Study II, a community sample of 146 predominantly Euro-Canadian middle-class youth, with an average age of 14.5 years participated. Their self-reports of higher trait-anger and trait-anxiety, and lower parental attachment by both sexes related differentially to accentuated and attenuated cortisol reactivity to the frustration stressor. Thus, attachment, affect, sex, and the stressor contextual factors were associated with the adrenal-cortical responses of these adolescents through complex interactions. Further studies of individual differences in physiological responses to stress are called for in order to clarify the identities of concurrent protective and risk factors in the psychosocial stress and physiological stress responses of early adolescents.
Keywords: Cortisol, Adolescence, Stress, Attachment, Affect, Sex
Introduction
Although developmental psychologists no longer view adolescence as a time of inevitable sturm und drang, it is undoubtedly the time between childhood and maturity that brings many emotional, social, cognitive, and physical challenges, as well as opportunities (Gunnar et al. 2009; Shih et al. 2006; Stewart et al. 2013). Normative stressors do not necessarily eventuate in major psychosocial or physiological disruption. However, youth with adjustment problems resulting from gene expression or negative early experiences and their sequellae may exhibit atypical stress responses and may, in consequence, be vulnerable to psychosocial difficulties during adolescence. Boyce and colleagues (Bauer et al. 2002; Essex et al. 2011a) suggested that by examining concurrent psychosocial and physiological responses of individuals in the face of a stressor, researchers can achieve a more adequate understanding of stress reactivity. The present studies examine the relationships between early adolescents' physiological responses to experimentally generated psychosocial stressors and self-reported affect, attachment perception, and sex. As part of this examination, two laboratory stress protocols were developed and evaluated for their capacity to induce measurable psychological and physiological stress responses in adolescents. The framework guiding this research posits the importance of a bio-psycho-social analysis, assuming that dynamic biological processes and responses are mutually influenced by individual experiences and the psychosocial context (Jeliki et al. 2007; Susman and Rogol 2004).
Cortisol Reactivity to Stress
Physiological stress reactivity is typically monitored by examining the activation of the hypothalamic–pituitary–adrenal (HPA) system (Hellhammer et al. 2009; Kirschbaum and Hellhammer 2007) via salivary cortisol. The nature of a stimulus and an individual's perception of it can initiate a cortisol stress response (Kudielka et al. 2009). If a stimulus is perceived as low in controllability or predictability and/or high in social-evaluative threat, stress may be experienced (Dickerson and Kemeny 2004), thereby activating the HPA-axis (Kirschbaum and Hellhammer 2007; Levine 2005), with the stress hormone cortisol being one marker of psychosocial stress. Novel and challenging stimuli are likely to increase HPA-axis activity, setting off a constellation of emotional, cognitive, and behavioral responses as well as metabolic changes (Gunnar and Quevedo 2007; Schore 2012).
Examination of salivary cortisol is a non-invasive method for evaluating physiological stress responsivity. Relative salivary-cortisol change from basal levels index metabolic changes characteristic of normative stress reactivity (Essex et al. 2011b; Kirschbaum 2010; Wessa et al. 2006). Individual differences exist, however, with moderate levels of glucocorticoids being associated with good physical and behavioural health and anomalously high or low levels associated with less optimal functioning (Gunnar and Quevedo 2007). Gunnar and Quevedo (2007) encouraged exploring individual differences in the effects of stress on development.
Some previous research findings have reported heightened or accentuated responding to stressors (Quirin et al. 2008; van de Wiel et al. 2004), while others have reported blunted or attenuated reactivity under stress (Beaton et al. 2006; Cicchetti et al. 2010; Ruttle et al. 2011; Stewart et al. 2013). Attenuated reactivity has been reported in successful cognitive behavioral stress management interventions (e.g., Hammerfald et al. 2006). It is, therefore, hypothesized that within samples of participants in stress reactivity studies there can be either accentuated or attenuated responding, a bifurcation that deserves a more detailed exploration (Thompson et al. 2015). Further, Del Guidice et al. (2011) proposed an adaptive calibration model of stress responses that posits that the coordination and regulation of stress responding during development results in individual differences in patterns of responsivity. The findings to date, however, have not been consistent in reliably identifying relevant individual-difference factors influencing responses to stress (Bagner et al. 2010). Based on Del Guidice, Ellis and Shirtcliff's model, a constellation of psychosocial and physiological variables, including attachment ideation, affect, and sex were identified in the current study as likely candidates to be associated with normative, accentuated, and/or attenuated physiological reactivity.
Attachment
Early-life stress has been shown to have lifespan consequences for physiological stress reactivity (Loman and Gunnar 2010; Pesonen and Räikkönen 2011). Early social attachment experiences and resultant emotion-regulation (Ainsworth et al. 1978; Schore and McIntosh 2011; Weinfield et al. 1999) affect both healthy development and maladaptation (Luthar 2003; Nachmias et al. 2008; Natsuaki et al. 2009). Positive early relationships have been shown to protect against vulnerability, whereas negative attachments can have either internalizing or externalizing consequences (Howe 2011; Schore 2012).
There have been few studies of the relationship between concurrent adolescent attachment ideation and their stress reactivity. It is known that early negative attachment experiences may alter the secretion of cortisol such that levels will be either elevated or suppressed later in development (Susman 2006). For example, if children experience social separation or parental loss, cortisol is elevated with insecurely attached 12- to 24-month-olds, showing higher baseline adrenocortical activity than securely attached infants in the Strange Situation (Bugental et al. 2003; Martorell 2002). Further, adults who had experienced early parental-loss exhibited greater cortisol accentuation than non-loss adults (Luecken 1998). However, the effects of early experience on life-course attachment relationships require longitudinal study (Howe 2011) and are currently under both investigation and considerable debate (e.g., Fivush and Waters 2015). This raises a research question as to how attachment relations are associated with adolescent stress responses and, more specifically, whether profiles of early adolescents will demonstrate an expected bifurcation in stress reactivity, with certain current attachment ideations being associated with profiles of accentuated responsivity and others with attenuated responsivity. This exploration would address a notable gap in the literature.
Anger
Attachment disruptions can result in emotional distortions. Bowlby (1973) associated anger experiences and expressions with attachment distress, making anger a potential variable of interest. Experiences and expressions of anger have been operationalized in terms of their state and trait manifestations with state anger indexing concurrent feelings ranging from irritation to rage, whereas trait anger reflects a relatively persisting disposition to such feelings (Deffenbacher 1992; Spielberger 1999). The negative cognitive attributions and physiological changes associated with anger may not necessarily precede an aggressive act (Kassinove and Sukhodolsky 1995). However, the biological correlates of anger are not often considered independently from aggression (Denson et al. 2009), the already-well-established correlate of stress responding. In consequence, although Rudolph et al. (2010) are a notable exception, there has until recently been a dearth of studies specifically linking anger experiences/expression, independent of aggression, and cortisol reactivity. Anger expression predicted early morning elevations in salivary cortisol in adults (Steptoe et al. 2000) and high anger scores predicted higher cortisol levels in children (Martorell 2002). However, children with high cortisol were from families that were low on anger expression in Granger et al. (1998) and child anger was not necessarily related to family anger. The extensive literature on anger experiences and expressions has not included systematic investigations of their relationships with concurrent stress until recently (e.g., Johnson et al. 2014) or their associations with adolescent concurrent attachment reports (such as Konishi and Hymel 2014). This poses a second research question: whether reports of trait anger associate with cortisol responses, and whether individual differences in anger are related to cortisol accentuation and/or attenuation.
Sex
Similarities and differences have been shown in physiological stress responses of adult males and females (Kudielka et al. 2007a) and of some boys and girls (Susman and Pajer 2004). These differences are thought to emanate from differential biological mechanisms as well as gendered expectations for the physical and psychosocial expression of stress, with females going beyond “fight or flight” reactions and exhibiting “tend or befriend” responses as well (Taylor et al. 2000). Little is known about how differential gender-socialization experiences with attachment and emotional traits associate interactively with stress responses. Gunnar et al. (2009) reported sex-differential responding at ages 11 and 13 years. Furthermore, in response to a corticotropin releasing hormone (CRH) pharmacological challenge test, adolescent boys showed a greater cortisol increase than did girls (Dorn et al. 1995). Psychological self-assessments in stressful situations, and especially internalized sensitivity to stress in certain girls, could mediate sex differences in cortisol secretion (Natsuaki et al. 2009). Some sex-difference inconsistencies might emanate from differences in affective responses to previous psychosocial trauma (Bagner et al. 2010; Cicchetti et al. 2010; Klein and Corwin 2002; Perry 2001). van den Bos et al. (2014) did not confirm Ordaz and Luna's (2012) expectation of sex differences in cortisol responses to a psychosocial stressor. So a research question remains as to the place of sex differences in stress responding and arises specifically in examining a bifurcation of cortisol reactivity. That is, will one sex or the other tend to exhibit more accentuated or attenuated cortisol responding in the face of an experimental stressor?
Two studies are reported in the present paper. Each study hypothesized that a bifurcated pattern of cortisol response would be evident within community samples of early adolescents (i.e., either increasing or decreasing from baseline to reactivity levels) and that this bifurcation would be associated with self-reported parental attachment relationships, affect status, and sex. Although not a causal examination, exploring these relationships could identify indices of relative resilience or vulnerability in response to the adolescent development of the experience and expression of psychosocial stress responses. The contributions of such potentially critical psychosocial processes and their interactions were examined within a multi-factorial investigation of adolescent cortisol stress responses using two modified stress protocols.
Protocols for experimentally eliciting stress responses have primarily been developed for adults. Most notably, the Trier Social Stress Test (TSST) is a gold standard for the investigation of stress reactivity and reliably induces cortisol change scores two to four times basal levels (Kirschbaum and Hellhammer 2007). Procedural adaptations for the investigation of reactivity in children and adolescents have primarily been achievement-related (e.g., test taking, competitive cognitive or psychomotor tasks) and/or anxiety-provoking tasks (e.g., public speaking, improvisation, invasive medical procedures) (Kirschbaum, 2010). In the current research, adaptations of the Trier Social Stress Test for children (TSST-C: Buske-Kirschbaum et al. 1997) were used to investigate individual differences in adolescent stress reactivity. The development and evaluation of these two laboratory protocols for inducing psychological and physiological stress responses in adolescents was another primary objective of the current study.
Analysis
Prior to each study, a power analysis was conducted to determine the number of participants needed to achieve a power level of .90, using an alpha of .05, assuming a medium effect size (Cohen and Cohen 1983). For both studies, the data were checked for accuracy of input, missing values, distribution of variables, assumptions of multivariate analysis, as well as univariate and multivariate outliers. Examination of missing data showed that mean substitution could be used for subsequent analysis; the assumptions of normality, linearity, and homogeneity of variance were satisfied; and multicollinearity was determined not to be problematic. Preliminary anlyses were conducted with and without univariate and multivariate outliers. No differences were found in the main or interaction effects so outliers were included.
Due to the exploratory nature of the studies, alpha was set at .05 to enhance the chance of identifying effects while still providing reasonable protection against Type I errors (Tabachnick and Fidell 2001). All multivariate F's were based on Pillai's V Trace, which is relatively more robust and less sensitive to violations of homogeneity of variance than other multivariate tests of significance (Cohen and Cohen 1983). Significant effects were followed up with post hoc analyses using the regression plot approach described by Aiken and West (1991). Finally, results were re-examined using an alternative model analysis; Area Under the Curve with respect to increase (AUCi; Pruessner et al. 2003), which emphasizes changes over time. SPSS (PC 20) was used to perform the analyses.
Study I
Introduction
The purpose of the first study was to establish the TSST-T as an appropriate means of inducing cortisol-responsivity (a stress response) in an adolescent sample; determine whether change in cortisol levels from baseline to reactivity would be bifurcated within the sample; and, explain cortisol-responsivity patterns in the context of social, behavioral, and emotional variables. To that end, this study investigated the relationship between cortisol reactivity and the psychosocial processes of attachment relations, anger, and gender.
Method
Participants
Four hundred and eighty-five, eighth- and ninth-grade boys and girls, with parental consent and participant assent, were recruited through local schools and pre-assessed using the trait-anger scale of the State-Trait Anger Expression Inventory-2 (STAXI-2: Spielberger 1999). This was done to ensure that a community sample of teenagers were recruited with the most extreme trait anger responses to maximize the potential for detecting affect and stress reactivity relationships. One hundred twenty students: 60 (30 girls, 30 boys) performing in the highest 20th percentile on trait anger and 60 (30 girls, 30 boys) in the lowest 20th percentile were ultimately selected for this research on anger, attachment, sex, and stress. All 120 of these selected recruits agreed to continue participating in the study. Participants had a mean age of 13.43 years with either a female puberty mean score of 3.05 or a male puberty mean score of 2.78 (out of a maximum 5) on the Pubertal Development Scale (PDS: Petersen et al. 1988). This well-standardized pubertal status index was used as a potential moderator of sex in the event that main effect differences in cortisol responses were established. Authors of the PDS reported internal consistency scores ranged from .68 to .77 and concurrent validity with physician ratings ranged from .61 to .67 (Petersen et al. 1988). Participants were predominantly Euro-Canadian from two-parent (72 % married), college/university educated (56 % of mothers and 50 % of fathers), and employed (94 % of mothers and 97 % of fathers) families. They were randomly assigned to a medium-stress treatment or a low-stress control condition, as described below, counterbalanced for anger status and sex.
Procedure
The Trier Social Stress Test for Children (TSST-C; Kudielka et al. 2007b), a protocol that reliably induces moderate stress (Buske-Kirschbaum et al. 1997), was slightly modified for teenagers (TSST-T) in the present study. Like the TSST-C, the TSST-T moderate-stress condition participants were instructed to generate an oral narrative from a story stem before confederate judges and then engage in a serial mental-subtraction task. Unlike the TSST-C, judges were still-faced instead of friendly, participants were told that judges would evaluate their performance, and transportation was provided from the participants' school to the laboratory. The need for comparable low-stress control protocols has been identified in the literature. To that end, social-evaluative components were eliminated and controllability was increased to produce a parallel, but low-stress comparison/control condition for this and the second study. Sessions were conducted between 15:30 and 18:30 when diurnal cortisol is relatively low, varies minimally, and leaves room for reactivity measurement.
Participants were driven by car for 15 min from their school to the laboratory by a youth-experienced mature adult whose intent was to establish a positive, non-threatening context for them. The 90-min laboratory protocol can be divided into 3 phases: anticipation, test/treatment, and recovery.
Anticipation Phase (20 min)
During the anticipation phase, participants spent 10 min rapport-building in the Lab with a graduate research-assistant at the conclusion of which, a second assistant collected the first saliva sample (Time – 10). Participants were questioned as to whether they had eaten, drunk stimulants, or exercised within the last hour and whether they smoked or were using prescription medications (birth control pills or treatments for asthma, for instance). Having been instructed and screened in advance, none reported any of the above counter-indicated behaviors. The participant and first assistant continued a low-key interaction for a second 10 min, at which time a second, basal cortisol sample (Time 0) was taken.
Test Phase (20 min)
Participants then immediately received information about the nature of the moderate- or low-stress condition, to whichever they had randomly been assigned. This marked the beginning of the test or treatment phase. Moderate-stress participants were shown the experimental room, wherein a tape recorder, video camera, microphone, and large clock were in prominent view, and then given 5 min to prepare a “good” narrative from the standard story-stem. The Tim +10 min saliva sample followed this preparation time, and immediately preceded the actual TSST-T stress intervention. Each of the 60 adolescents assigned to this condition was then returned to the experimental room, where two young-adult female confederate judges (university student research assistants) were seated at a table. Participants were instructed to stand behind a podium to complete the story stem in exactly 5 min. They were told their performance would be graded. They next performed the serial-subtraction-task for 3 min (beginning by subtracting 13 from 2037) and were instructed by a judge to start over after any error. The confederate judges were still-faced throughout both tasks. Time +20 saliva was sampled immediately following the subtraction task.
Recovery Phase (50 min)
During the response and recovery phases following the stress procedure, participants completed pencil-and-paper questionnaires in a post-experimental room for 50 min (the total session took approximately 80–90 min). The fifth and six cortisol samples were collected at protocol Times +45 and +60. The procedure is outlined in the first column of Table 1 (the second and third columns show the low-stress condition and the procedure used in Study II).
Table 1. Stress condition time line for TSST-T, low-stress, and FSS-A protocols.
| TSST-T | Low stress | FSS-A | |
|---|---|---|---|
| 3:30 p.m. | Rapport building/demographic information (pre-experimental room) | Rapport building/demographic information (pre-experimental room) | Rapport building/demographic information (pre-experimental room) |
| 3:39 p.m. | Relaxed conversation | Relaxed conversation/conversation topic form | Relaxed conversation/debate topic form |
| Time-10 | [Saliva 1] | [Saliva 1] | [Saliva 1] |
| 3:49 p.m. | Relaxed conversation | Relaxed conversation | Relaxed conversation |
| Time 0 | [Saliva 2] | [Saliva 2] | [Saliva 2] |
| 3:59 p.m. | Introduction to tasks (preparation room) | Introduction to tasks (preparation room) | Introduction to tasks (preparation room) |
| Time +10 | [Saliva 3] | [Saliva 3] | [Saliva 3] |
| 4:02 p.m. | Story-stem task (5 min) Subtraction task (3 min) (experimental room) |
Story/conversation (5 min) Subtraction task (3 min) (experimental room) |
Debate (5 min) Subtraction task (3 min) (experimental room) |
| 4:11 p.m. | |||
| Time +20 | [Saliva 4] | [Saliva 4] | [Saliva 4] |
| 4:14 p.m. | PSS, demographics (post-experimental room) | PSS, demographics/STAXI, STAI (post-experimental room) | PSS, STAXI, STAI (post-experimental room) |
| 4:35 p.m. | Debriefing/randomized questionnaires | Debriefing/randomized questionnaires | Debriefing/randomized questionnaires |
| Time +45 | [Saliva 5] | [Saliva 5] | [Saliva 5] |
| 4:50 p.m. | Study I TSST-T | Study I low stress | |
| Time +60 | [Saliva 6] | [Saliva 6] | |
| 4:55 p.m. | Study II TSST-T | Study II low stress | Study II FSS-A |
| Time +60 | [Saliva 6] | [Saliva 6] | [Saliva 6] |
Example for Participant 1
The first 20 min and last 50 min were identical for both moderate- and low-stress conditions. During the control-treatment phase in the low-stress condition, the 60 control-participants tape recorded their own story and then serially subtracted the same sums using a calculator, without judges present. Pizza and juice was provided at the end of the protocol for all participants.
Measures
Attachment, anger, and saliva cortisol were assessed in addition to the demographic and pubertal information that was collected.
Attachment The Inventory of Parent and Peer Attachment (IPPA; Armsden and Greenberg 1987) assesses adolescents' perceptions of their relationship security with their mother/mother figure, father/father figure, and peers. Each index (mother, father, peer) has 25 items, answered on a 5-point ordinal scale with higher scores representing higher reported attachment. This study focused on parental (both mother and father) attachment. Internal consistencies reported by the authors of the IPPA ranged from .72 to .93. Concurrent validity correlations with the Family Environment Scale were in the .52–.78 range (Armsden and Greenberg 1987).
Anger Participants completed the State-Trait Anger Expression Inventory-2 (STAXI-2; Spielberger 1999). This 57-item measure of anger proneness (Deffenbacher 1992) has an Anger Expression Index and six subscales: State Anger, Trait Anger, Anger Expression-Out, Anger Expression-In, Anger Control-Out, and Anger Control-In. Subscales are computed by summing item scores, rated on a 4-point scale, with higher scores indicating greater anger. According to Spielberger (1999), internal consistencies ranged from .65 to .85, and concurrent validity correlations with the Buss-Durkee Hostility Inventory were in the .66–.71 range. This study focused on the 10-item trait-anger subscale, a relatively stable measure of anger-proneness as reported by Deffenbacher (1992).
Saliva Collection and Analysis
Participants chewed on a short straw to produce saliva and signaled when they estimated they had enough saliva to half-fill a 2-ml polypropylene vial for each of the six saliva samples collected. They passively drooled through a second straw into the vial. Once vials were sufficiently filled, they were capped and stored in a freezer until shipped on dry ice to the Pennsylvania State University Behavioral Endocrinology Laboratory and assayed in duplicate. The averaged intra- and inter-coefficients of variation were 10.8 and 9.2 % respectively; which are acceptable ratings within the field of endocrinology (Susman 2001).
Hypotheses
It was expected that medium-stress TSST-T adolescents would have a significant cortisol stress response (relative change from basal to reactivity levels), greater than low-stress participants. These findings would confirm the adapted TSST-T as an effective procedure for reliably inducing a stress response. Adolescents in the TSST-T condition with accentuated cortisol response were expected to report more positive parental attachment and lower trait anger, whereas attenuated responsivity were expected to be associated with more negative attachment relations and higher trait anger, especially for girls (Obradovic 2012).
Results
Table 2 provides means and standard deviations for the dependent and independent variables.
Table 2. Descriptive statistics for variables in Study I.
| M | SD | Min. | Max. | |
|---|---|---|---|---|
| TSST-T (N = 60) | ||||
| Cortisol Time 1 | .165 | .085 | .045 | .408 |
| Cortisol Time 2 | .156 | .091 | .041 | .392 |
| Cortisol Time 3 | .145 | .084 | .037 | .376 |
| Cortisol Time 4 | .157 | .105 | .035 | .548 |
| Cortisol Time 5 | .230 | .222 | .033 | 1.280 |
| Cortisol Time 6 | .169 | .134 | .027 | .655 |
| Low-stress (N = 60) | ||||
| Cortisol Time 1 | .167 | .093 | .035 | .567 |
| Cortisol Time 2 | .156 | .079 | .033 | .518 |
| Cortisol Time 3 | .142 | .075 | .026 | .460 |
| Cortisol Time 4 | .133 | .065 | .023 | .406 |
| Cortisol Time 5 | .112 | .061 | .022 | .334 |
| Cortisol Time 6 | .096 | .049 | .021 | .197 |
| Cortisol change (μg/dl) | .074 | .197 | −.14 | .93 |
| Mother attachment | 97.80 | 20.77 | 41.00 | 125.00 |
| Father attachment | 89.73 | 18.64 | 34.00 | 123.00 |
| Trait anger | 21.11 | 7.33 | 11.00 | 40.00 |
N = 120
A 2 (sex) × 2 (stress condition) MANOVA with change score (Time 0 vs. Time 45) as a dependent variable revealed a significant stress condition effect [F(1,116) = 19.89, p = .001, ]. The TSST-T protocol was effective in eliciting a significant cortisol stress response. Post hoc comparisons using independent t-tests adjusted for multiple comparisons revealed that the TSST-T participant's baseline (Time 0) and maximum change cortisol scores (Time 45) were significantly different from each other (t = −2.92, p = .005). In addition, cortisol change scores in the TSST-T condition were different from those in the low stress condition (t = 4.46, p = .001). Finally, change scores from baseline to maximum change for low-stress participants were not significantly different from each other, providing evidence for the protocol as a comparable control. (TSST-T and low-stress condition curves are drawn in grey in Fig. 1.)
Fig. 1. Study I mean cortisol responses of accentuators, attenuators, TSST-T and low-stress Condition over time.
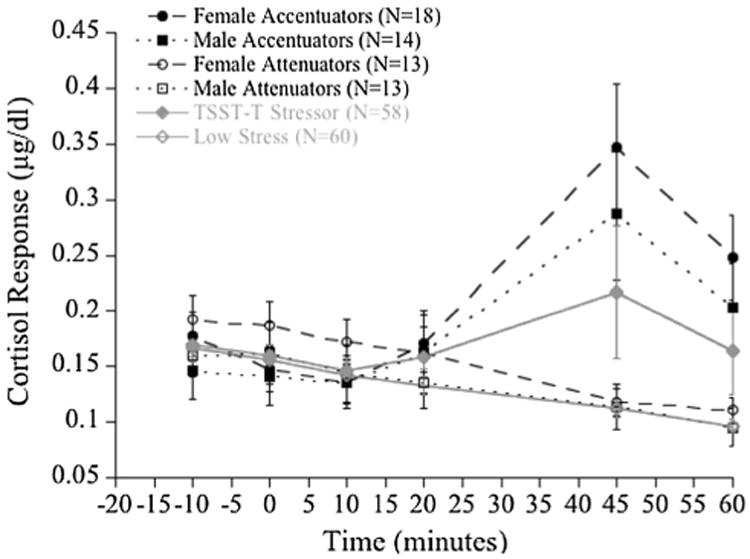
Inspection of the TSST-T cortisol change scores revealed the expected bifurcated pattern, with 32 participants (18 girls and 14 boys) increasing from baseline to reactivity levels, while 26 decreased (13 girls and 13 boys; see Fig. 1 for the bifurcated curves in black). A group variable was created, identifying participants as either accentuators or attenuators, and a 2 × 2 MANOVA of cortisol response (accentuators/attenuators) and sex (girls/boys) as independent measures and parental attachment and trait-anger as the dependent measures was performed. The influence of puberty status was examined and found to be nonsignificant.
A significant cortisol response by sex interaction was established [F(5,51) = 3.01, p = .019, ]. The canonical correlation for this effect was .48, accounting for 22 % of the variance. Post hoc univariate F tests showed that girls in the cortisol-response-attenuated group had lower maternal-attachment scores than girls in the cortisol-response-accentuated group. The main effect of sex was also significant [F(5,51) = 3.36; p = .011, ]. The canonical correlation, .50, accounted for 24 % of the variance. An examination of post hoc univariate F tests revealed that the effect is accounted for by both maternal-(p = .003) and paternal-attachment (p = .034), whereby girls reported both lower maternal- and paternal-attachment than boys.
This bifurcated pattern was reexamined using Area Under the Curve with respect to increase (AUCi; Pruessner et al. 2003). One significant difference was established. Using the group variable of accentuation/attenuation as the independent variable and parental attachment and trait anger as dependent measures, a significant father attachment by trait anger interaction emerged [F(1,11) = 4.76; p = .034, ]. Attenuators had lower father-attachment and higher trait-anger than accentuators. As recommended by Susman (1997), girls and boys were examined separately. Girls also evidenced a significant father-attachment by trait-anger interaction [F(1,11) = 4.53; p = .047, ], with attenuators having lower father-attachment and higher trait-anger than accentuators. No significant differences were found with boys.
Discussion
As predicted, the bifurcated cortisol response pattern was related to parental attachment and trait anger, when exposed to a stress-inducing event from which the youth may have perceived they could not readily flee—but only for girls. An attenuated response was generated from girls who reported parental attachment insecurity and higher trait anger. When considering change scores, maternal attachment was most relevant; whereas AUCi revealed trait anger within the context of lower father attachment most pertinent. An accumulation of such stress-generating experiences may result in a suppression of adolescent responses to an unpredictable, unavoidable stressor due to a feeling of powerlessness. Attenuated responding could assist girls in dissociating from the potentially unwanted challenge (Obradovic 2012). The sex main effect suggests boys and girls to be differentially responsive to physiological, social, emotional and behavioral context interactions such that boys might interpret or respond differently from girls in their perceptions of their maternal and paternal relationships (Burghy et al. 2012).
Study II
Introduction
The bifurcated cortisol pattern established in Study I, as well as the relationship between cortisol reactivity, attachment, anger, and sex, raised several questions for further study. Was this bifurcated pattern replicable with a non-selected community sample? Were the sex-related individual differences associated with the bifurcation replicable (Del Giudice 2011; Löckenhoff et al. 2008)? Could the gendered individual differences be dependent on the type of stressor? Would a frustration-provoking stressor better elucidate the relationship between anger and cortisol responses, especially for boys (Allen et al. (2002). What other affective variables might relate to bifurcation? For instance, what part might anxiety play in this stress reactivity (see Peckins et al. 2012)?
Dorn et al. (1993) reported an inverse relationship between anxiety and pregnant adolescents' cortisol reactivity. Those teens with mental-health referrals and greater anxiety had lower cortisol stress responses. Peckins et al. (2012) reported violence exposure to be associated with both cortisol reactivity and generalized anxiety in a non-clinical adolescent sample with greater exposure to violence and higher anxiety related to lower cortisol-reactivity. As with the construct of anger (Spielberger 1999), differentiated operationally experiences of anxiety as state (concurrent worry or fear) and trait (enduring feelings of such discomfort) emotions (Spielberger 1988). While Takahashi et al. (2006) reported a positive relationship between trait anxiety and basal cortisol of male college students, their reactivity to psychosocial stress was unrelated to trait anxiety, suggesting that HPA response saturation caused high chronic/basal-cortisol but attenuated acute stress-reactivity. Shirotsuki et al. (2009) also reported attenuated responses to the Trier Stress Test of socially anxious males. Beaton et al. (2006) interpreted similar blunted reactivity of both genders as adaptive, allowing socially-anxious individuals to more effectively address threatening situations, as Saxbe et al. (2012) suggested of family members in aggressive homes. Deschênes et al. (2012) also reported evidence of an association between anger and generalized anxiety. While anxiety related to salivary cortisol stress responses (Leininger and Skeel 2012), questions arise as to its role in stress reactivity bifurcation.
Method
Participants
Participants were recruited through classroom and noon hour information sessions at local schools. Parental and participant informed consents were obtained. One hundred forty-six adolescents (73 girls, 73 boys), average age of 14 years 6 months (range 13–16 years), were randomly assigned to the FSS-A (37 girls, 37 boys), TSST-T (18 girls, 18 boys), or Low Stress (18 girls, 18 boys) condition, counterbalancing for gender. Unlike in Study I, participants were not pre-selected for trait-anger scores. The mean female puberty-score was 2.70 and the male puberty-score was 2.82 out of a maximum of 5 (PDS: Petersen et al. 1988). Participants were predominantly Euro-Canadian (60 %) from two-parent (57 % married) college/university educated (84 % of mothers; 77 % of fathers), and employed (71 % of mothers; 87 % of fathers) families.
Procedure
Differential responding to varying psychosocial stressors has been associated with interactions between sex and emotional status (Klimes-Dougan et al. 2001; McBurnett et al. 2000; Steiner et al. 2002). Anger- or frustration-provoking social stressors have resulted in greater male stress responsivity, especially for youth who have greater anger problems or been diagnosed with disruptive behaviour disorders (Brain and Susman 1997; van Goozen et al. 2004). Gendered stress responses could account for the differential behavioral patterns found in Study I. The modified TSST-T used in Study I is an example of a social performance anxiety-provoking task. Anxiety-provoking stressors may be more salient for girls and frustration-provoking stressors, more salient for boys. Further, a frustration stressor might better elucidate relationships between stress responses and affect. Therefore, a new but comparable standardized psychosocial stress task called the Frustration Social Stressor for Adolescents (FSS-A) was developed for Study II, affording a more nuanced test of the relationship between cortisol response, parental attachment, affect (both anger and anxiety), and sex.
The FSS-A, designed to induce moderate frustration in a laboratory setting, followed the same format as the TSST-T in Study I. It was piloted with 19, 13- to 15-year-old adolescents, eliciting anticipated physiological (cortisol, heart rate) stress responses and self-reported stress increases during the challenge. Cortisol change from baseline to peak level was significant (t = −2.165, p = .045). Heart rate reactivity was also significant (t = −2.214, p = .040). The majority of participants (63.2 %) reported that they experienced stress during the challenging tasks, with 31.6 % reported being “somewhat stressed”, and an additional 31.6 % reported feeling “quite a bit” or being “extremely” stressed. Based on these preliminary findings, the FSS-A was administered to a larger, non-selected, community sample of 74 adolescents in Study II. These participants' responses were compared with 36 low-frustration-stress and 36 TSST-T control groups, for a total of 146 participants. The protocol was administered in quiet rooms in participants' schools with only a minimal settling period offered prior to participation. The FSS-A, also a three-phase 90-min protocol, is outlined in the second column of Table 1 and involves the following:
Anticipation Phase (20 min)
Rapport was established and demographic information, as well as information about recent eating, exercise, smoking, birth control and other medications were obtained from participants. Participants then indicated their position on a list of value-laden issues developmentally relevant to adolescents (i.e., independence, trust, loyalty to friends, family relationships, peer pressure) that were derived from the board game (http://scruplesgame.com, accessed on July 22, 2016). Participants rated the degree of frustration they would feel debating with someone who disagreed with their position. The creation and application of an evaluated peer debate on a value-laden topic was based on evidence that conflict-related stressors involving parent–child and peer debates are effective in provoking frustration in adolescents (Allen et al. 2002; Klimes-Dougan et al. 2001). Saliva samples were taken at 10 and 20 min (Time −10 and Time 0). In Study II, saliva samples were collected using a “salivette”, a cylindrical cotton swab that fits into a centrifugation tube. This is an improved, less invasive method of saliva collection developed after conducting Study I.
Test Phase (20 min)
The test phase began when participants were informed that they would debate the issue they reported as most frustrating with a same-sex (confederate) peer; it would be video and audio recorded; an opposite-sex judge would rate their argument; and they would be assessed for good performance. Participants were shown the experimental classroom, including judge's table, tape recorder, video camera, podium, microphone, and large clock, and given 5 min to prepare for the debate. A 5-min debate was held with a youthful same-sex research assistant who argued the opposing view. The same mental-subtraction task as in Study I followed. The confederate research-assistant remained still-faced throughout the debate, “evaluating” performance. Saliva samples were taken 30 min into the protocol (immediately following debate preparation) and at 40 min (after completion of the serial-subtraction task) (Time +10 and Time +20).
Recovery Phase (50 min)
Participants filled out three questionnaires, were debriefed, and then completed the remaining questionnaires. Saliva samples were taken at 65 and 85 min into the protocol (Time +45 and Time +65). This was a slight deviation from the TSST-T saliva sampling time in Study I to accommodate debriefing. Pizza and soda pop/juice were then provided.
The low-stress control procedure included the above three phases but involved a friendly discussion on an enjoyable topic chosen by participants and the serial subtraction task using a calculator. The TSST-T protocol was the same as in Study I with a slight deviation in saliva sampling at Time +65 instead of Time +60.
Measures
In addition to the demographic information, pubertal status, six salivary cortisol samples, the Inventory of Parent and Peer Attachment, and the State-Trait Anger Expression Inventory-2 (Trait Anger Subscale) described in Study I, participants also responded to the state-anger subscale on the State-Trait Anger Expression Inventory-2 and were administered the State-Trait Anxiety Inventory (Spielberger 1988).
Attachment and Anger
In this study, internal consistency for each of the parental attachment scales (mother and father) on the IPPA was .95. For the STAXI-2, the reliability coefficient for the trait-anger subscale was .81 and .91 for the state-anger subscale. The 15-item state-anger subscale on the STAXI-2 was used to rate emotional responses to the stress protocol and to help distinguish bifurcated cortisol patterns.
Anxiety
The State-Trait Anxiety Inventory (STAI; Spielberger 1988), a 40-item self-report questionnaire, measures trait anxiety (how respondents generally feel) and state anxiety (how respondents feel right now). Participants rate each item on a 4-point scale and subscales are computed by summing item scores, with higher scores indicating greater anxiety. Internal consistency ranged from .86 to .95 and concurrent validity correlations with the IPAT Anxiety Scale and Taylor Manifest Anxiety Scale were in the .73–.85 range according to Spielberger (1988). For this study, the reliability coefficient for the trait-anxiety subscale was .88 and .91 for the state-anxiety subscale.
Saliva Collection and Analysis
Participants chewed for 60 s on a cotton salivette that was transferred into the centrifuge tube. Samples were stored before analysis a freezer with a standard temperature of −20 °C. Cortisol analyses were conducted at the Department of Cellular & Physiological Sciences Laboratory at the University of British Columbia using Salimetrics (HS-Cortisol) High Sensitivity Salivary Cortisol Enzyme Immunoassay Kits. Assay sensitivity is 0.007 μg/dl. It has an inter-assay coefficient of 3.41 %, an intra-assay coefficient of 2.92 %, and a required sample volume of 25 μl.
Hypotheses
The FSS-A protocol was designed to be an effective frustration-provoking psychosocial stressor. A difference between cortisol baseline and reactivity levels was predicted, indicating its ability to induce a measurable stress response. FSS-A adolescents were expected to have greater cortisol change scores than low-stress participants and the FSS-A adolescents were expected to report higher state-anger than state-anxiety, providing some validation for the FSS-A as a frustration inducing protocol. A bifurcated cortisol pattern was expected such that those with attenuated cortisol reactivity would demonstrate higher trait-anger and trait-anxiety, lower state-anger and state-anxiety, and lower parental-attachment.
Results
Table 3 provides means and standard deviations for the dependent and independent variables.
Table 3. Descriptive statistics for variables in Study II.
| M | SD | Min. | Max. | |
|---|---|---|---|---|
| FSS-A (N = 74) | ||||
| Cortisol Time 1 | .200 | .146 | .051 | .812 |
| Cortisol Time 2 | .189 | .139 | .046 | .660 |
| Cortisol Time 3 | .177 | .139 | .043 | .732 |
| Cortisol Time 4 | .176 | .125 | .036 | .571 |
| Cortisol Time 5 | .183 | .139 | .044 | .748 |
| Cortisol Time 6 | .127 | .091 | .036 | .518 |
| Low-stress (N = 36) | ||||
| Cortisol Time 1 | .181 | .123 | .032 | .723 |
| Cortisol Time 2 | .169 | .116 | .029 | .590 |
| Cortisol Time 3 | .146 | .092 | .028 | .464 |
| Cortisol Time 4 | .134 | .101 | .023 | .526 |
| Cortisol Time 5 | .106 | .063 | .025 | .333 |
| Cortisol Time 6 | .083 | .039 | .022 | .193 |
| TSST-C (N = 36) | ||||
| Cortisol Time 1 | .168 | .112 | .036 | .577 |
| Cortisol Time 2 | .175 | .146 | .032 | .693 |
| Cortisol Time 3 | .153 | .129 | .032 | .577 |
| Cortisol Time 4 | .165 | .133 | .026 | .685 |
| Cortisol Time 5 | .205 | .172 | .027 | .692 |
| Cortisol Time 6 | .136 | .104 | .022 | .450 |
| Cortisol change (μg/dl) | .03 | .13 | −.30 | .64 |
| Mother attachment | 93.63 | 20.14 | 28.00 | 125.00 |
| Father attachment | 88.20 | 19.47 | 42.00 | 123.00 |
| Trait anger | 20.00 | 5.22 | 10.00 | 38.00 |
| State anger | 49.68 | 7.96 | 44.00 | 80.00 |
| Trait anxiety | 43.98 | 9.10 | 23.00 | 63.00 |
| State anxiety | 47.78 | 10.31 | 30.00 | 76.00 |
N = 146
A significant multivariate main effect for stress condition [2 (sex) × 3 (stress condition) MANOVA] showed the FSS-A protocol to be an effective elicitor of cortisol stress responding [F(2,140) = 4.91, p = .009, ]. Post hoc comparisons using independent t tests adjusted for multiple comparisons showed significant differences between cortisol change scores (Time 0 to Time 45) for adolescents in the FSS-A condition and the low-stress control condition (t = 2.64, p = .009) but not between the FSS-A and TSST-T, confirming the comparability of the FSS-A to the TSST-T (Fig. 2 shows the efficacy of the FSS-A protocol for inducing a stress response in comparison to the TSST-T and low-stress condition). Paired sample t-tests revealed a significant difference in cortisol from baseline to peak reactivity for FSS-A participants (t = −2.38, p = .02). Furthermore, change scores from baseline to maximum change for low-stress participants were not significantly different from each other, again providing evidence for the protocol as an effective control.
Fig. 2. Study II FSS-A, TSST-T, and Low Stress mean cortisol responses over time.
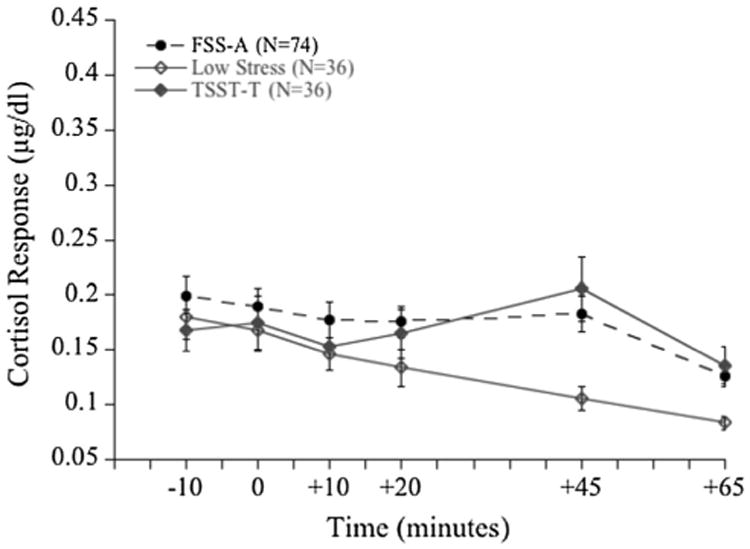
The FSS-A was expected to be a frustration-provoking stressor (with higher state anger than state anxiety) and a between-subjects one-way MANOVA revealed significant multivariate effects for stress condition for both state anger [F(4,286) = 7.81, p = .001, ] and state anxiety [F(4,286) = 9.27, p = .0001, ]. Post-hoc comparisons indicated that the FSS-A induced both significantly greater state anger and state anxiety than the low-stress condition (t = 4.32, p = < .001 and t = 4.41, p =<.0001, respectively). As expected, a paired samples t test showed FSS-A participants reporting higher state-anger than -anxiety (t = 2.01, p = .048).
Inspection of FSS-A cortisol change scores again revealed the bifurcated pattern as in Study I, with 51 % of participants (19 girls and 19 boys) increasing from baseline to peak response (accentuated), while the other 49 % (18 girls and 18 boys) decreased or attenuated (see Fig. 3). Interestingly, there was a subgroup among the attenuators whose baseline scores were notably higher than those of other participants. The individual-differences explanation of the bifurcated cortisol reactivity pattern was more complicated in Study II than in Study 1.
Fig. 3. Study II mean cortisol responses of accentuators, attenuators, FSS-A, TSST-T and low-stress condition over time.

A 2 × 2 MANOVA for cortisol response (accentuators/attenuators) and sex (girls/boys) as independent measures and parental attachment, trait anger, trait anxiety, state anger, and state anxiety as dependent measures was performed. Neither effects for cortisol response nor a cortisol response by sex interaction were established. The influence of puberty status and use of oral contraception was examined and found to be non-significant.
Using AUCi (increase: Pruessner et al. 2003) as an alternative method of analysis, the bifurcated pattern was analyzed with accentuators and attenuators as independent variables and parental attachment, trait anger, trait anxiety, state anger, and state anxiety as dependent measures. Trait-anxiety scores were significantly higher for accentuators than attenuators [F(1,23) = 5.85; p = .019, ]. Interactions were also established for response by mother attachment [F(1,23) = 4.19; p = .046, ] and mother attachment by trait anger [F(1,23) = 3.93; p = .05, ]. Attenuators were more responsive, reporting higher mother-attachment, and lower trait-anger. Female accentuators reported significantly higher trait-anxiety [F(1,23) = 5.80; p = .032, ] and a significant cortisol response differential in a father-attachment by state-anxiety interaction [F(1,23) = 5.47; p = .036, ] with attenuators, reporting higher father-attachment, and lower state-anxiety. Male attenuators reported significantly higher trait anger [F(1,23) = 6.71; p = .024, ].
Discussion
The FSS-A effectively induced frustration and elicited a stress response in adolescents, providing evidence that a psychosocial challenge involving a peer-related debate can be an effective stressor as Allen et al. (2002) suggested. In addition, the majority of participants (76 %) reported that they had experienced subjective stress during the challenge tasks and that it was the tasks themselves and not the cortisol measurement that was stressful (as reported on the Subjective Stress Scale, an adapted 18-item questionnaire designed to assess participants' subjective stress levels at different times during the laboratory experiment, the level of stress induced by different components of the experiment, their sense of control, as well as the positive and negative affect they experienced; Lakey and Heller 1988). Further, the FSS-A was comparably effective for both girls and boys. This may allow researchers to use this interpersonal experimental stress procedure to elucidate individual differences in adolescent stress responses that have previously been complicated by sex differences.
The anticipated bifurcated physiological response pattern was replicated in Study II using a different stress protocol, indicating the need to examine and explain stress responses within the context of both accentuated and attenuated responding (Del Guidice et al. 2011; Susman 2006). Overall, participants in Study II reported positive attachment relationships and low levels of trait- anger and -anxiety, which is not surprising, given that it was an unselected community sample. Even so, relationships between attachment, anger, anxiety, and physiological stress responses emerged. Parental attachment moderated the relationship between anger, anxiety, and the bifurcated cortisol reactivity. However, those adolescents reporting more positive mother- and father-attachment and lower trait-anger and -anxiety exhibited attenuated responding when faced with a frustration stressor. Less positive parental-attachment and higher trait-anxiety were associated with cortisol stress accentuation. In terms of sex-differentiated cortisol responses, for girls, higher state-anxiety and higher trait-anxiety along with higher trait-anger within the context of lower parental-attachment were associated with accentuation; whereas for boys, higher trait-anger related to attenuation. Overall, these findings were contrary to the expected higher trait-anger and trait-anxiety, and lower parental attachment association with attenuated cortisol reactivity. Unlike Study 1, where lower mother- and father-attachment related to cortisol attenuation, positive parental attachment, especially to fathers, provided a stronger model for attenuated responsivity to frustration in Study II. This result highlights the importance of taking type of stressor and individual differences in participant populations into account when interpreting adolescent stress responses.
The unexpectedly higher parental attachment associated with attenuation in this study of moderate stress is also contrary to what has become an accepted pattern in the clinical stress literature: that physiological disengagement reflects adaptation to risk factors such as dysfunctional attachment, chronic anger, lack of perceived self-efficacy, and coping resources (e.g., Hart et al. 1995). However, a large number of the participants in this normative sample demonstrated physiological attenuation in response to a moderate stressor that closely simulate naturally occurring interpersonal social conflicts and school curriculum tasks encountered by most students on a regular basis. This raises the question of whether or not physiological response attenuation might be a positive biological response adaptation in such situations, especially in light of the fact that attenuators in this study reported higher parental attachment than accentuators. There also might be different “types” of attenuators or contexts for attenuation. As noted earlier, some participants had average baseline cortisol levels and then attenuated while others started with above average baseline cortisol levels. Sample sizes constrained post hoc exploration of these two attenuating groups.
General Discussion
The cortisol response literature offers many examples of stress response accentuation, which is an increase from baseline to reactivity score (Bugental et al. 2003; Martorell 2002) and many reports of participant attenuation, a decrease from baseline to reactivity (e.g., Hart et al. 1995). Identifying individual differences contributing to this bifurcation is a complicated research agenda that will ultimately reduce a significant gap in the literature. To that end, two studies were conducted that hypothesized that a bifurcated pattern of cortisol response would be evident within community samples of early adolescents and that this bifurcation would be associated with self-reported parental attachment relationships, affect status, and sex. The framework guiding this research posited the importance of a bio-psycho-social analysis, assuming that dynamic biological processes and responses are mutually influenced by individual experiences and the psychosocial context (Jeliki et al. 2007; Susman and Rogol 2004). This research is also unique in that the two early-adolescent community samples experienced different stress protocols, with both clearly bifurcating, and relationships found between individual difference characteristics and cortisol response patterns. Although the variables afford promise in investigating attenuation and accentuation, none was singularly associated with participants' reactivity propensity.
The development and application of two ecologically valid adolescent-appropriate psychosocial experimental stress procedures makes an important contribution to stress research. In the first study, the adapted standardized TSST-T protocol was effective in eliciting a significant cortisol stress response, confirming it as a successful stressor. In the second study, the FSS-A provides a newly standardized frustration-related interpersonal stress procedure where one had not previously been developed. Again, the overall normative response change patterns showed the FSS-A to be effective in producing a stress response. Furthermore, state anger was associated with cortisol responsivity, showing the FSS-A to be a successful frustration stimulus. The comparative effectiveness of the FSS-A for both boys and girls allows researchers to use the stress procedure better to elucidate propensities to respond to frustration with anger that may have been previously complicated by gender differences (Dorn et al. 1995). Results and conclusions drawn from research using these two ecologically sound procedures increases generalizability and can therefore provide greater insight into adolescent stress responses in real life situations that are not always readily observable for research purposes. This will provide opportunities to examine cortisol stress response patterns and their associated individual difference variables.
Another contribution of this work is in highlighting the importance of inspecting within-group variations in the direction of cortisol change under stress. Thompson et al. (2015) have similarly differentiated their infant participants as “increasers”, “no-changers” and “decreasers” to characterize their participants' different patterns of cortisol responding. The cortisol responses of the community samples of early adolescents in both of the present studies bifurcated at approximately fifty percent (i.e., half of the youths accentuating and the other half attenuating). The findings raise the question of whether physiological disengagement might be an adaptive physiological response in some stressful situations for normative adolescents as opposed to a vulnerable response to risk factors as found in clinical samples (Cicchetti et al. 2010; Perry 2001). If psychosocial stressors such as those simulated by the TSST-T and the FSS-A are perceived as somewhat unavoidable in everyday adolescent life, Study II suggests that it might be the extent to which adolescents attenuate or accentuate rather than the direction of a physiological response that might be a clearer marker of adaptation risk. Testing this hypothesis in future research is therefore recommended.
The adaptive calibration model of Del Guidice et al. (2011) can accommodate the two stress-reactivity pathways found in both Study I and Study II. Experience of unpredictable environments may precipitate vigilance in individuals that could predispose a heightened sensitivity that females express by attenuation and males, by accentuation. Del Guidice et al. suggest that chronic stressors can precipitate low emotionality, more expressed by impulsivity in males and detachment and low levels of parental attachment in females. This model predicts, then, both hyper- and hypo-responsivity, and sets a platform for research that could differentiate maladaptive from adaptive functioning, as suggested by Obradovic (2012).
Examining within-group variations in the direction of cortisol change under stress also potentiates theorizing and encourages examining the roles of individual differences in stress reactivity as they interact with other variables. Self-reported attachment related in this study to reactivity in differentiated ways depending upon affective states, sex, and the nature of the stressor. An initial hypothesis was that the ample literature on aggression and stress reactivity (Steptoe et al. 2000) potentially obscured an underlying relationship between stress responses and anger. This assumption was in part based on the theoretical perspective that attachment perceptions would set a protective response pattern when an organism is under subsequent attachment threat that a clear candidate modulator would be anger, according to Bowlby (1973). But this was too simple a story. While selecting participants on the extremes of the scored anger continuum in Study I maximized the likelihood of detecting the influence of trait anger on reactivity, this alone was not a determining factor. However, along with attachment reports, trait anger was partially effective in discriminating girls that reported low attachment to mothers and fathers as showing attenuated cortisol reactivity. But there was no comparable differentiation of boys' individual differences, even though they also bifurcated in their responses to stress. Girls reported overall higher levels of parental attachment but this factor did not elucidate the sex differences between girls and boys in the attenuated responses revealed by inspecting the response bifurcation.
In the second study, anger (both trait and state), anxiety (both trait and state), and attachment reports were examined. Parental attachment and in particular father-attachment was a significant associate of anger and anxiety. Mother- and father-attachment and trait- and state-anxiety discriminated accentuating from attenuating girls, while trait anger differentiated responses within the boys' group. It is amply evident that it will be complex interactions as opposed to single variables that contribute to bifurcated-group cortisol stress-responses.
Limitations
The contributions made by this research should be considered in the context of several limitations. First, larger sample sizes would allow for more detailed examination of stress response directional patterns, not just accentuation or attenuation from baseline. It is entirely possible that there are more than two patterns. Indeed, a preliminary, post hoc cluster analysis in Study II produced five cortisol change-score patterns. Second, participants in this research were school-based and recruited through a volunteer self-selection process requiring parent/guardian consent. Youth and families with more developmental challenges likely were not included and, therefore, results would not generalize to all adolescents. Furthermore, the normative population sample in these studies makes it difficult to compare results to those found in similar studies involving clinically-referred adolescents. Also, such demographic variables as ethnic and cultural backgrounds and experiences, and general physical health and depression, while not the focus of this study are worth exploration. Finally, the exploration of individual differences relied on self-report measures that have potential limitations related to respondents' understanding of the questions, social desirability and impression management, ability to recall information, and degree of knowledge regarding the information being questioned.
Future Directions
Future directions for these inquiries include further testing and validating the stress procedures for adolescents, recruiting larger samples, and targeting marginalized youth and clinical samples using the same stress procedures to gain a greater understanding of stress responsivity patterns of all adolescents. Further improvements to the protocols could be made, such as extending the duration of the rapport building phase (Balodis et al. 2010) and using relaxation techniques during that period (e.g., relaxing music, watching relaxing scenes on a computer) as suggested by Gordis et al. 2006). Examination of other variables including attachment style, self-efficacy, competency, and past trauma (Diong et al. 2005; Howard and Medway 2004) could have potential in explaining individual differences in stress responses. In terms of determining sex differences in cortisol responding, moderating variables such as race, SES, background stress, menstrual cycle phase, and physical health should be considered. In addition, coping strategies that associate with stress reactivity could provide insight into cortisol stress response bifurcation patterns (Klimes-Dougan et al. 2001). Investigating other physiological markers (e.g., heart rate, skin conductance) and hormones related to stress (e.g., alpha-amylase, testosterone, and dehydroepiandrosterone) warrants mention. These additional variables could provide clearer relationships between individual differences in cortisol responses. From a clinical perspective, the exploration of physiological reactivity following attachment-focused and other psychotherapeutic implementations such as the cognitive-behavioral stress-management approach reported by van de Wiel et al. (2004) and Hammerfald et al. (2006) could be beneficial to the understanding of clinical and other intervention investigations.
Conclusions
Inevitable experiences of moderate, every-day stress are present in adolescence as in all other life phases. Through an understanding of both adaptive and disruptive developmental processes, an investigation of youth stress responses and the factors that influence those responses can provide a more comprehensive and complex adolescent development theory (Howe 2011; Schore 2012). Within the context of a theoretically based biopsychosocial conceptual model, the current studies explored individual differences in early adolescent attachment, affect and sex and bifurcated stress responses. In Study I, girls' attenuated cortisol reactivity to the public performance stressor (the TSST-T) related significantly to their self-reported lower maternal-attachment and higher trait-anger. In Study II, self-reports of higher trait-anger and trait-anxiety, and lower parental attachment by both sexes related differentially to accentuated and attenuated cortisol reactivity to the frustration stressor (the FSS-A). Thus, attachment, affect, sex, and contextual factors were associated with both the accentuated and attenuated adrenal-cortical stress responses of these early adolescents through complex interactions, providing support for their relevance in the examination of adolescent stress responding (Thompson et al. 2015). The current studies also established two new experimental protocols, the TSST-T and FSS-A, as reliable and valid interpersonal experimental procedures for exploring stress reactivity. This makes a significant contribution to future adolescent stress research in that standardized protocols provide an opportunity to explore different individual difference variables, populations, family configurations, and therapeutic interventions (Kudielka et al. 2009). They also make it possible to monitor and index response change patterns over the life course (Howe 2011; Kirschbaum 2010). Youth-friendly practitioners appreciate the challenges their clients face and this appreciation can be enriched by research findings that help identify the multi-factorial nature of the biopsychosocial variables that enhance or constrain youth thriving in psychosocial contexts.
Acknowledgments
We thank the adolescent participants in this study and appreciate the cooperation of their teachers and parents who assisted in supporting the data collection in Fredericton NB and Vancouver BC Canada. We also thank the numerous research assistants who made the research possible. We also offer thanks to E. Leslie Cameron for drawing the figures.
Funding: Funding was generously provided by MindCareNew Brunswick for Study I and the Healthy Early Learning Partnership (HELP) at UBC for Study II.
Biographies
Catherine Ann Cameron is Honorary Professor of Psychology at the University of British Columbia and Emerita Professor at the University of New Brunswick. Her developmental research includes cross-cultural studies of deception, cultural research on thriving and mediated early communications.
Stacey McKay is in private practice at McKay Psychological Services in Fredericton NB Canada. She is also an Instructor and Clinical Associate at the University of New Brunswick.
Elizabeth J. Susman is the Jean Phillips Shibley Professor of Biobehavioral Health in the Department of Biobehavioral Health, at The Pennsylvania State University. Her research program integrates behavioral endocrinology and developmental psychology.
Katherine Wynne-Edwards is Professor of Comparative Endocrinology in Comparative Biology and Experimental Medicine in the Faculty of Veterinary Medicine at the University of Calgary. Her research includes hormonal changes in men becoming fathers and the impact of lifestyle on individual hormone dynamics.
Joan M. Wright is in private practice at Joan Wright & Associates in Fredericton NB Canada.
Joanne Weinberg is Professor Emerita and Distinguished University Scholar in the Department of Cellular & Physiological Sciences in the Faculty of Medicine at the University of British Columbia. Her research focuses on the investigation of how early life experiences alter brain and biological development.
Footnotes
Authors' Contributions C.A.C. conceived of the two studies in collaboration with J.M.W. for Study I and S.M. for Study II. She also wrote first drafts of the manuscript and coordinated the development of its final form. J.M.W. designed and conducted Study I. S.M. designed and conducted Study II. She also performed the final statistical analyses of both Studies I and II and co-wrote the manuscript. E.J.S. collaborated in the design of both Studies I and II and the analyses of Study I. K.W.-E. and J.W. provided guidance in the conduct and analysis of Study II. All authors were consulted and they approved the final version of this paper.
Conflicts of interest The authors report no conflict of interests.
Ethical Approval Institutional Ethics Review Boards at the University of New Brunswick and the Fredericton NB School Board and the University of British Columbia and the Vancouver BC School Board approved the conduct of Studies I and II respectively. The ethical standards of the Canadian Psychological Association and the guidelines of the Society for Research in Child Development were rigorously adhered to.
Informed Consent Written informed consent was obtained from the parents/guardians of all participants and informed assent was obtained from all participants.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Hillsdale, NJ: Laurence Erlbaum; 1978. [Google Scholar]
- Allen JP, Hauser ST, O'Connor TG, Bell KL. Prediction of peer-rated adult hostility from autonomy struggles in adolescent-family interactions. Development and Psychopathology. 2002;14:123–137. doi: 10.1017/s0954579402001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armsden GC, Greenberg MT. The inventory of parent and peer attachment: Relationships to wellbeing in adolescence. Journal of Youth and Adolescence. 1987;16:427–454. doi: 10.1007/BF02202939. [DOI] [PubMed] [Google Scholar]
- Bagner DM, Sheinkopf SJ, Vohr BR, Lester BM. A preliminary study of cortisol reactivity and behavior problems in young children born premature. Developmental Psychobiology. 2010;52:574–582. doi: 10.1002/dev.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Wynne-Edwards KE, Olmstead MC. The other side of the curve: Examining the relationship between pre-stressor physiological responses and stress reactivity. Psychoneuroendocrinology. 2010;35:1363–1373. doi: 10.1016/j.psyneuen.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce T. Associations between physiological reactivity and children's behavior: Advantages of a multi-system approach. Journal of Developmental and Behavioral Pediatrics. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beaton EA, Schmidt LA, Ashbaugh AR, Santesso DL, Antony MM, McCabe RE, et al. Low salivary cortisol levels among socially anxious young adults: Preliminary evidence from a selected and non-selected sample. Personality and Individual Differences. 2006;41:1217–1228. doi: 10.1016/j.paid.2006.02.020. [DOI] [Google Scholar]
- Bowlby J. Attachment and loss: Vol 2 Separation: Anxiety and anger. New York: Basic Books; 1973. [Google Scholar]
- Brain PF, Susman EJ. Hormonal aspects of aggression and violence. In: Stoff DM, Breiling J, Maser JD, editors. Handbook of antisocial behavior. New York, NY: Wiley; 1997. pp. 314–323. [Google Scholar]
- Bugental DB, Martorell GA, Barraza V. The hormonal costs of subtle forms of infant maltreatment. Hormones and Behavior. 2003;43:237–244. doi: 10.1016/S0018-506X(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81(1):252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd. New Jersey, NY: Lawrence Erlbaum; 1983. [Google Scholar]
- Deffenbacher JL. Trait-anger: Theory, findings and implications. In: Butcher JN, Spielberger CD, editors. Advances in personality assessment. Vol. 9. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. pp. 177–201. [Google Scholar]
- Del Giudice M. Sex differences in romantic attachment: A meta-analysis. Personality and Social Psychology Bulletin. 2011;37(2):193–214. doi: 10.1177/0146167210392789. [DOI] [PubMed] [Google Scholar]
- Del Guidice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1017/(4)S0954579412000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune responses: A meta-analysis of acute laboratory social stressors and emotion inductions. Psychological Bulletin. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- Deschênes SS, Dugas MJ, Fracalanza K, Koerner N. The role of anger in generalized anxiety disorder. Cognitive Behaviour Therapy. 2012;41(3):261–271. doi: 10.1080/16506073.2012.666564. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diong SM, Bishop GD, Enkelmann HC, Tong EMW, Why YP, Ang JCH, et al. Anger, stress, coping, social support and health: Modeling the relationships. Psychology and Health. 2005;20(4):467–495. doi: 10.1080/0887044040512331333960. [DOI] [Google Scholar]
- Dorn LD, Burgess ES, Susman EJ, von Eye A, DeBellis MD, Gold PW, et al. Response to oCRH in depressed and non-depressed adolescents: Does gender make a difference? Journal of the American Academy of Child and Adolescent Psychiatry. 1995;35(6):764–773. doi: 10.1097/00004583-199606000-00016. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Peterson AC. Cortisol reactivity and anxiety and depression in pregnant adolescents: A longitudinal perspective. Psychoneuroendocrinology. 1993;18(3):219–239. doi: 10.1016/0306-4530(93)90006-7. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce TW, Hertzman C, Lam LL, Armstrong JM, Neumann SMA, et al. Epigenetic vestiges of early developmental adversity: Childhood exposure and methylation in adolescence. Child Development. 2011a;84(1):58–75. doi: 10.1111/j.1467-8624.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, et al. Influence of early life stress on later hypothalamic–pituitary–adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2011b;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivush R, Waters TEA. Patterns of attachments across the lifespan. In: Scott RA, Kosselyn SM, editors. Emerging trends in the behavioral sciences: An interdisciplinary, searchable, and linkable resource. Wiley Online Library; 2015. [DOI] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Tricket PK. Paper presented at Biennial Meeting of the Society for Research on Adolescence. San Francisco, CA: 2006. Salivary alpha-amylase and cortisol response to social stress among maltreated and comparison youth. [Google Scholar]
- Granger D, Serbin L, Schwartzman A, Lehoux P, Cooperman J, Ikeda S. Children's salivary cortisol, internalising behaviour problems, and family environment: Results from the Concordia Longitudinal Risk Project. International Journal of Behavioral Development. 1998;22:707–728. doi: 10.1080/016502598384135. [DOI] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus–pituitary– adrenal activity over transition to adolescence: Normative changes and associations with puberty. Developmental Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol in maltreated children: Evidence of relations between neuroendocrine activity and social competence. Development and Psychopathology. 1995;7:11–26. [Google Scholar]
- Hammerfald K, Eberle C, Grau M, Kinsperger A, Zimmerman A, Ehlert U, et al. Persistent effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects: A randomized controlled trial. Psychoneuroendocrinology. 2006;31:333–339. doi: 10.1016/j.psyneuen.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Howard MS, Medway FJ. Adolescents' attachment and coping with stress. Psychology in the Schools. 2004;41(3):391–403. doi: 10.1002/pits.10167. [DOI] [Google Scholar]
- Howe D. Attachment across the lifecourse: A brief introduction. Basingstoke UK: Palgrave Macmillan; 2011. [Google Scholar]
- Jeliki H, Bobek DL, Phelps E, Lerner RM, Lerner JV. Using positive youth development to predict contribution and risk behaviors in early adolescence: Findings from the first two waves of the 4-H Study of Positive Youth Development. International Journal of Behavioral Development. 2007;31:263–273. doi: 10.1177/0165025407076439. [DOI] [Google Scholar]
- Johnson MD, Galambos NL, Krahn HJ. Depression and anger across 25 years: Changing vulnerabilities in the VSA model. Journal of Family Psychology. 2014;28(2):225–235. doi: 10.1037/a0036087. [DOI] [PubMed] [Google Scholar]
- Kassinove H, Sukhodolsky DG. Anger disorders: Basic science and practice issues. In: Kassinove H, editor. Anger disorders: Definition, diagnosis, and treatment. Washington, DC: Taylor & Francis; 1995. pp. 1–26. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C. Trier Social Stress Test. In: Stolerman IP, editor. Encyclopedia of psychopharmacology. Berlin: Springer; 2010. pp. 1–3. [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol. In: Fink G, editor. Encyclopedia of stress. 2nd. Vol. 3. Oxford: Academic Press; 2007. pp. 405–409. [Google Scholar]
- Klein LC, Corwin EJ. Seeing the unexpected: How sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Current Psychiatry Reports. 2002;4(6):441–448. doi: 10.1007/s11920-002-0072-z. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13(3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Konishi C, Hymel S. An attachment perspective on anger among adolescents. Merrill-Palmer Quarterly. 2014;60(1):53–79. doi: 10.1353/mpq.2014.0000. [DOI] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Sex differences in human stress response. In: Fink G, editor. Encyclopedia of stress. 2nd. Vol. 3. Oxford: Academic Press; 2007. pp. 469–473. [Google Scholar]
- Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently: Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Wüst S, Kirschbaum C, Hellhammer DH. Trier Social Stress Test. In: Fink G, editor. Encyclopedia of stress. 2nd. Vol. 3. Oxford: Academic Press; 2007. pp. 776–781. [Google Scholar]
- Lakey B, Heller K. Social support from a friend, perceived support, and social problem solving. American Journal of Community Psychology. 1988;16(6):811–824. doi: 10.1007/BF00930894. [DOI] [PubMed] [Google Scholar]
- Leininger S, Skeel R. Cortisol and self-report measures of anxiety as predictors of neuropsychological performance. Archives of Clinical Neuropsychology. 2012;27:318–328. doi: 10.1093/arclin/acs035. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Löckenhoff CE, Costa PT, Jr, Lane RD. Age differences in descriptions of experiences in oneself and others. Journal of Gerontology: Psychological Sciences. 2008;63B(2):92–99. doi: 10.1093/geronb/63.2.p92. Online ISSN 1758-5368, Print ISSN 1079-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience and Biobehavioral Reviews. 2010;34:867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosomatic Medicine. 1998;60:765–772. doi: 10.1097/00006842-199811000-00021. [DOI] [PubMed] [Google Scholar]
- Luthar SS, editor. Resilience and vulnerability: Adaptation in the context of childhood adversities. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Martorell GA. Maternal and child adrenocortical responses as a function of attachment classification, temperament, and maternal care giving attributions. Dissertation Abstracts International. 2002;62(7-B):3401. [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57(1):38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- Nachmias N, Gunnar M, Mangelsdorf S, Parriz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 2008;67(2):508–522. doi: 10.1111/j.1467-8624.1996.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: Sex differences in the role of cortisol reactivity to interpersonal stress. Journal of Clinical Child & Adolescent Psychology. 2009;38(4):513–524. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic J. How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development? Development and Psychopathology. 2012;24:371–387. doi: 10.1017/S0954579412000053. [DOI] [PubMed] [Google Scholar]
- Ordaz S, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37:1135–1157. doi: 10.1016/j.psyneuen.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckins MK, Dockray S, Eckenrode JL, Heaton J, Susman EJ. The longitudinal impact of exposure to violence on cortisol reactivity in adolescence. Journal of Adolescent Health. 2012;51(4):366–372. doi: 10.1016/j.jadohealth.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry BD. The neurodevelopmental impact of violence in childhood. In: Schetky D, Benedek E, editors. Textbook of child and adolescent forensic psychiatry. Washington, DC: American Psychiatric Press Inc; 2001. pp. 221–238. [Google Scholar]
- Pesonen AK, Rämikkönen K. Mental health of children evacuated during World War II. In: Fitzgerald HE, Puura K, Tomlinson M, Paul C, editors. International perspectives on children and mental health, Volume 1: Development and context. Santa Barbara, CA: Praeger Publishers; 2011. pp. 197–216. [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of puberty status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Quirin M, Pruessner JC, Kuhl J. HPA system regulation and attachment anxiety: Individual differences in reactivity and awakening cortisol. Psychoneuroendocrinology. 2008;33:581–590. doi: 10.1016/j.psyneuen.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Peer victimization and aggression: Moderation by individual differences in salivary cortisol and alpha-amylase. Journal of Abnormal Child Psychology. 2010;35:843–856. doi: 10.1007/s10802-010-9412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Fisher DBD, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59:123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Margolin G, Shapiro LA, Baucom BR. Does dampened physiological reactivity protect youth in aggressive family environments? Child Development. 2012;83(3):821–830. doi: 10.1111/j.1467-8624.2012.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schore AN. The science of the art of psychotherapy. New York NY: Norton; 2012. [Google Scholar]
- Schore AN, McIntosh J. Family law and the neuroscience of attachment, Part 1. Family Court Review. 2011;49:501–512. [Google Scholar]
- Shih JH, Eberhart NK, Haammen CL, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child & Adolescent Psychology. 2006;35(1):103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- Shirotsuki K, Izawa S, Sugaya N, Yamada KC, Ogawa N, Ouchi Y, et al. Salivary cortisol and DHEA reactivity to psychosocial stress in socially anxious males. International Journal of Psychophysiology. 2009;72:198–203. doi: 10.1016/j.ijpsycho.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. STAI: State-Trait Anxiety Inventory (self-evaluation questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1988. [Google Scholar]
- Spielberger CD. STAXI-2: State-Trait Anger Expression Inventory—2 (professional manual) Lutz, FL: Psychological Assessment Resources; 1999. [Google Scholar]
- Steiner H, Ryst E, Berkowitz J, Gschwendt MA, Koopman C. Boys' and girls' responses to stress: Affect and heart rate during a speech task. Journal of Adolescent Health. 2002;30:14–21. doi: 10.1016/s1054-139x(01)00387-1. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Cropley M, Griffith J, Kirschbaum C. Job strain and anger expression predict early morning elevations in salivary cortisol. Psychosomatic Medicine. 2000;62:286–292. doi: 10.1097/00006842-200003000-00022. [DOI] [PubMed] [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. Journal of Abnormal Child Psychology. 2013;41(7):1015–1026. doi: 10.1007/s10802-013-9740-1. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Modeling developmental complexity in adolescence: Capturing the future of biology and behavior in context. Journal of Research in Adolescence. 1997;7(3):283–306. doi: 10.1111/1532-7795.ep11339787. [DOI] [Google Scholar]
- Susman EJ. Mind-body interaction and development: Biology, behavior, and context. European Psychologist. 2001;6(3):163–171. doi: 10.1027/1016-9040.6.3.163. [DOI] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Pajer K. Biology-behavior integration and antisocial behavior in girls. In: Putallaz M, Bierman K, editors. Aggression, antisocial behavior and violence among girls: A developmental perspective. New York: Guilford Publications; 2004. [Google Scholar]
- Susman EJ, Rogol A. Puberty and psychological development. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology. New York: Wiley; 2004. pp. 15–44. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4th. Needham Heights, MA: Allyn and Bacon; 2001. [Google Scholar]
- Takahashi T, Ikeda K, Ishikawa M, Kitamura N, Tsukasaki T, Nakama D, et al. Anxiety, reactivity, and social stress-induced cortisol elevations in humans. Neuroendocrinology Letters. 2006;26(4):351–354. [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107(3):411–429. doi: 10.1037/0033-295X.107.3.411. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Morgan G, Jurado KA. A longitudinal study of infant cortisol response during learning events. Monographs of the Society for Research in Child Development. 2015;80(4):319. doi: 10.1111/mono.12218. [DOI] [PubMed] [Google Scholar]
- van den Bos E, de Rooij M, Miers AC, Bokhorst CL, Westenberg PM. Adolescents' increasing stress response to social evaluation: Pubertal effects on cortisol and alpha-amylase during public speaking. Child Development. 2014;85(1):220–236. doi: 10.1111/cdev.12118. [DOI] [PubMed] [Google Scholar]
- van de Wiel NM, van Goozen SH, Matthys W, Snoek H, van Engeland H. Cortisol and treatment effect in children with disruptive behavior disorders: A preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(8):1011–1018. doi: 10.1097/01.chi.0000126976.56955.43. [DOI] [PubMed] [Google Scholar]
- van de Wiel NM, Matthys SH, Snoek H, van Engeland H. Cortisol and treatment effect in children with disruptive behavior disorders: A preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(8):1011–1018. doi: 10.1097/01.chi.0000126976.56955.43. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Snoek H, Matthys W. Evidence of fearlessness in behaviourally disordered children: A study on startle reflex modulation. Journal of Child Psychology and Psychiatry. 2004;45(4):884–892. doi: 10.1111/j.1469-7610.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- Weinfield NS, Sroufe LA, Egeland B, Carlson EA. The nature of individual differences in infant-caregiver attachment. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research and clinical practice. New York, NY: Guilford; 1999. pp. 68–88. [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]


