Abstract
Inhalation of oxidant gases has been implicated in adverse outcomes in pregnancy, but animal models to address mechanisms and studies to identify potential pregnancy-specific therapies are lacking. Herein we show that inhalation of bromine at 600 parts per million for 30 minutes by pregnant mice on the 15th day of embryonic development results in significantly lower survival after 96 hours than an identical level of exposure in non-pregnant mice. On the 19th embryonic day, bromine-exposed pregnant mice have increased systemic blood pressure, abnormal placental development, severe fetal growth restriction, systemic inflammation, increased levels of circulating anti-angiogenic short fms-like tyrosine kinase one, and evidence of pulmonary and cardiac injury. Treatment with tadalafil, an inhibitor of type 5 phosphodiesterase, by oral gavage one hour post-exposure and then once daily thereafter, attenuated systemic blood pressures, decreased inflammation, ameliorated pulmonary and cardiac injury, and improved maternal survival (from 36% to 80%) and fetal growth. These pathological changes resemble those seen in preeclampsia. Non-pregnant mice did not exhibit any of these pathological changes, and were not affected by tadalafil. These findings suggest that pregnant women exposed to bromine may require particular attention and monitoring for signs of preeclampsia-like symptoms.
Keywords: Hemodynamics, Vascular Biology, Pregnancy, Heart Failure, Preeclampsia, Lung Edema
INTRODUCTION
Recent epidemiological evidence indicates that inhalation of noxious gases such as NO2, N2O3, SO2, and O3 during pregnancy is associated with fetal growth restriction (FGR) and/or the development of preeclampsia-type symptoms 1, 2. Additional observational studies have further corroborated this association between inhaled toxicants and preeclampsia-like symptoms. For instance, maternal cigarette smoking impairs fetal growth via excess placenta-derived secreted frizzled-related protein 1 (FRP1) 3. Additionally, in a state-wide birth cohort, maternal exposure during pregnancy to fine particulate matter (PM2.5) and ozone was positively associated with small-for-gestational age and low birth weight births 4. Finally, formaldehyde inhalation has been strongly linked to fetal growth restriction and adverse outcomes in pregnancy 5.
Exposure to halogens (e.g. chlorine (Cl2) or bromine (Br2)) causes significant risk to human populations resulting in both pulmonary and systemic injury followed by death from respiratory and cardiac failure; reviewed in 6. Over 56 million tons of halogens are produced each year worldwide for use in the manufacture of flame-retardants, medicinal compounds, gasoline additives, dyes, and water disinfectants. Accidental exposures during transit of halogens has occurred in Geneva Switzerland (1988, Br2), Graniteville, SC (2005, Cl2) and Chelyabinsk Russia (2011, Br2), resulting in significant short and long term morbidity and mortality 7.
In addition to major industrial accidents and/or intentional release of halogens, a common source of halogen inhalation toxicity are swimming pool sanitation accidents 8. Furthermore, pre-existing viral infections increase the severity of lung injury secondary to halogen exposure 9. Accordingly, there is an extensive body of literature available regarding halogen inhalation lung injury both in humans and in animal models 10. Yet, there is a paucity of systematic studies addressing mechanisms of lung and systemic injury caused by halogen gas injury in pregnancy. We observed that exposure of pregnant mice to concentrations of Br2, likely to be encountered in the vicinity of industrial accidents, resulted in increased mortality as compared to non-pregnant mice; furthermore, surviving pregnant mice developed preeclampsia-like symptoms and their fetuses failed to thrive.
Preeclampsia is a life-threatening complication of pregnancy characterized by maternal hypertension and is considered severe when accompanied by pulmonary or systemic pathologies 11. Preeclampsia is commonly associated with fetal growth restriction (FGR). Circulating factors, in part released by an injured placenta such as soluble fms-like tyrosine kinase-1 (sFLT-1), soluble endoglin (sENG) and inflammatory cytokines, have been mechanistically linked to features of preeclampsia such as increased blood pressure, decreased trophoblast maturation and proliferation and retarded fetal growth 12, 13. sFLT-1 and sENG, in particular, are thought to promote endothelial dysfunction, by inhibiting vascular endothelial growth factor (VEGF) 14. Since inhibitors of type 5 phosphodiesterase (PDE5i) are efficient in treating endothelial dysfunction, they have been used successfully in animal models of preeclampsia and are in clinical trials for the treatment of preeclampsia 15, 16.
We hypothesized that pregnancy sensitizes mice to Br2 inhalation injury through a potential amplifying effect on endothelial dysfunction by placenta-derived inflammatory and anti-angiogenic mediators. We further hypothesized that treatment with the PDE5i, tadalafil, can counteract pregnancy-specific Br2 toxicity via a beneficial effect on blood pressure and perfusion. In a series of experiments we found that exposure of pregnant mice at gestational day 14.5 (E14.5) to Br2 in concentrations likely to be encountered in industrial accidents 17 (600 parts per million (ppm) for 30 min) caused a significantly higher rate of mortality compared to non-pregnant female and male mice. We show that this is associated with severe FGR, cardiac and pulmonary injuries, and development of preeclampsia-like features. Furthermore, maternal mortality and morbidity, as well as fetal growth restriction, were alleviated by maternal post-exposure administration of tadalafil.
METHODS
(Please see Online Supplement for detailed description of methods, procedures and reagents used).
Animals
Pathogen-free, age-matched male (20–25 g), non-pregnant female (20–25 g), and pregnant female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in the University of Alabama at Birmingham Animal Facility under standard conditions with food and water access ad libitum. All procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC #20343).
Exposure of mice to Br2
Mice were exposed to Br2 for 30 or 45 min at 600 ppm Br2 as described previously 10 at E14.5. After exposure, mice were returned to room air. Control mice were exposed to air for 30 or 45 min under identical conditions.
Tadalafil administration
Stock solutions of tadalafil (Santa Cruz Biotechnology, Santa Cruz, CA, SC-208412), were administered to pregnant and non-pregnant mice via oral gavage at 2 mg/kg body weight at one h post-exposure and every 24 h thereafter. Control mice received equal volumes of sterile saline by gavage.
Statistics
Survival between treatment groups under different experimental conditions and at different time points was evaluated using the Pearson chi-squared test, and Fisher’s exact test. The overall survival rates were estimated according to the Kaplan-Meier method and compared using the log-rank test. To eliminate unequal variances, data was log transformed. No outliers were identified via ROUT. One-way (for pregnant-only variables such as placental analyses) or two-way (for analyses that included pregnant and non-pregnant data) ANOVA (for three or more groups) was used to compare continuous variables. Analysis was conducted using Graphpad Prism ver. 7 software (La Jolla, CA). A p-value < 0.05 was considered statistically significant in the two-tailed statistical tests that were used. All data is represented as mean ± standard error of the mean (SEM).
RESULTS
Br2 exposure causes higher mortality and loss of body weight in pregnant mice compared to non-pregnant mice
We exposed male and non-pregnant female mice and pregnant mice to Br2 (Figure 1A) at E14.5 to an exposure level that causes minimal lethality in non-pregnant mice (600ppm for 30 min). All mice exposed to Br2 lost weight similarly by E16.5 (Figure 1B). However, non-pregnant mice began to gain weight after E16.5. In contrast, pregnant mice continued to lose weight until either death or euthanasia at E18.5.
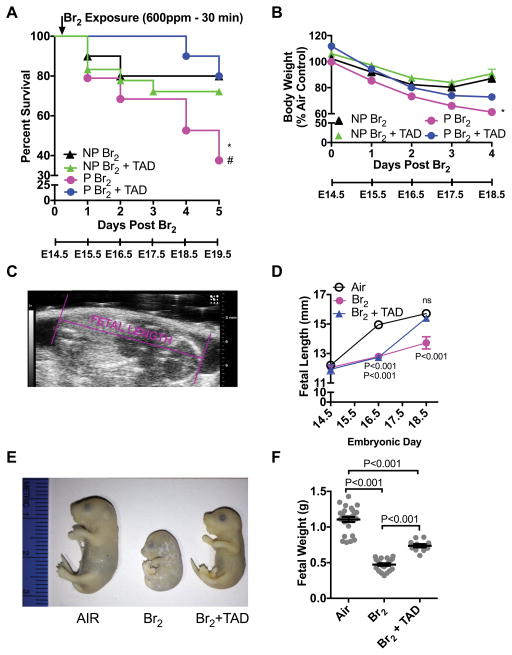
Figure 1. Pregnant mice exhibit increased body weight loss and mortality and fetal growth restriction.
Non-pregnant (NP) and pregnant (P) (E14.5) mice were exposed to air or to Br2 at 600 ppm for 30 min and returned to room air; they received tadalafil (TAD; 2 mg/kg BW in 0.1 ml of sterile saline) or vehicle via oral gavage at 1 h post-exposure and every 24 h thereafter. Body weights and survival times were recorded daily. A) Kaplan-Meyer curves of pregnant and non-pregnant mice with tadalafil or vehicle, post Br2 exposure. Non-pregnant mice exposed to Br2 and returned to room air lived longer than similarly exposed pregnant mice (* = P<0.05). Tadalafil improved survival times of pregnant mice post Br2 (# = P<0.05) but not of non-pregnant mice; n=10–19; Log-Rank Test. B) Body weights normalized to weights of air-exposed mice. Pregnant mice exposed to Br2 and returned to room air exhibit more severe weight loss compared to similarly exposed non-pregnant mice (* = P<0.05). Tadalafil administration mitigated weight loss in pregnant mice at four days post-exposure, but has no effect in non-pregnant mice; n=6–8; ANOVA. C) Representative ultrasound of a fetus showing how fetal length was measured. D) Summary data of fetal length measurements at E14.5, E16.5, & E18.5. Exposure to Br2 resulted in decreased fetal lengths which were restored to their air control values at E18.5 in the tadalafil group; n= 6–10 pups (2 pups per litter) for each condition; ANOVA; p values as compared to the corresponding air controls for the indicated gestational age. E) Representative photograph of paraformaldehyde-fixed fetuses at E18.5 for the indicated conditions. Fetuses of Br2-exposed pregnant mice exhibit severe fetal growth restriction, and tadalafil improves fetal growth. F) Fetal weights were recorded after extraction of fetuses at E18.5. Fetal weights of fetuses from Br2-exposed pregnant mice weighed considerably less and were partially rescued by tadalafil (TAD); n=pups (11–25) (2 pups per litter); ANOVA. All data are means±S.E.M.
Male and non-pregnant female mice exposed to Br2 at 600 ppm for 30 min had a mean survival of 80% within five days post-exposure. However, pregnant females exposed to Br2 had significantly lower mean survival (36%) than non-pregnant mice. Treatment of Br2-exposed mice with tadalafil post-exposure increased survival to 80%. Additionally, tadalafil had no effect on mortality in non-pregnant mice exposed to similar or even greater doses of Br2 (Figure S1: please see http://hyper.ajajournals.org). These data suggest a pregnancy-specific mechanism of injury. Subsequent studies were performed to identify potential mechanisms involved.
Br2 inhalation injury causes severe fetal growth restriction, and treatment with tadalafil partially rescues fetal growth
Pregnant mice were exposed to Br2 at 600 ppm for 30 min or air at E14.5 and then returned to room air with or without tadalafil at 2 mg/kg by gavage daily, starting one h post-Br2 exposure. Fetal length, measured at E14.5, E16.5, and E18.5 in utero with echosonography (Figure 1C), was decreased at both E16.5 and E18.5 as compared with air controls (Figure 1D). Tadalafil treatment returned fetal lengths to air control levels by E18.5 (Figures 1D and 1E). Also, fetuses of pregnant mice exposed to 600ppm Br2 for 30 min weighed less as compared to fetuses of air breathing mice (Figure 1E); administration of tadalafil increased fetal weight. Fetuses of Br2-exposed mice showed no signs of viability (breathing, movement, or skin turning pink) after delivery via cesarean section on E18.5, as opposed to the fetuses of air-exposed mice which all demonstrated these signs. Even though fetuses of mice exposed to Br2 exhibited no signs of viability when delivered at E18.5 by cesarean section, prior ultrasonography at E18.5 detected heartbeats in all but one fetus. With such a marked decrease in fetal growth following Br2 exposure, we next investigated whether this was due to injury to the placenta.
Br2 inhalation injury hinders placenta development and induces inflammation and production of the anti-angiogenic molecule sFLT-1
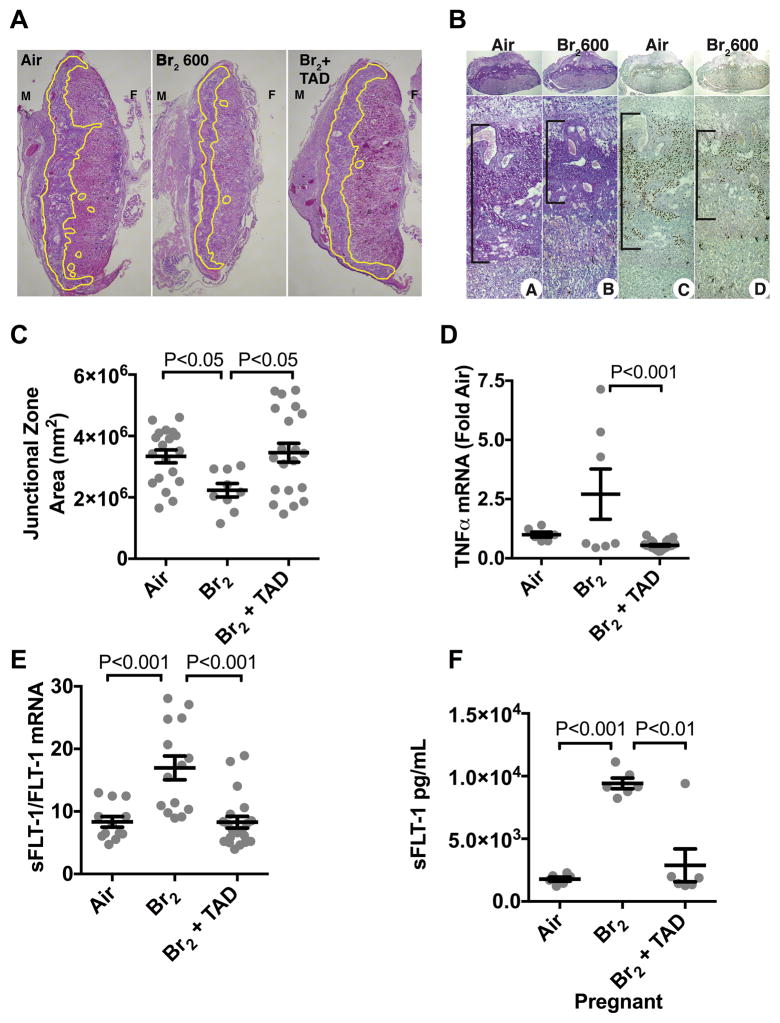
Fetal growth is dependent on normally developing placenta, and abnormal placenta development or placenta injury can negatively affect maternal physiology by sustaining an inflammatory state and producing anti-angiogenic molecules. As shown in Figures 2A–C, there were significant reductions of the placental junctional zones in mice exposed to Br2 (despite similar placental weights) and a return to baseline values in tadalafil treated mice. Visualization of glycogen-containing cells by PAS staining and detection of CDX2 by immunohistochemistry corroborated the findings of altered placenta development by detecting a reduced area occupied by glycogen-containing and CDX2-positive cells.
Figure 2. Exposure of pregnant mice to Br2 damages their placentas.
Pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air and received tadalafil (TAD) or vehicle. A–B) Representative H&E stained (A) placenta sections at E18.5 with the junctional zone demarcated with yellow highlighting (A) as well as (B) PAS staining (left) and CDX2 staining (right) of Br2-exposed pregnant mice revealed a reduced junctional zone (B: black bars) at E18.5; tadalafil administration restored junctional zones to normal size. C) Junctional zone areas at E18.5 for the indicated groups; n=9–20; ANOVA. D–E) TNFα mRNA (n=6–23) was reduced in Br2-exposed pregnant mice treated with tadalafil. sFLT-1/FLT-1 mRNA (=12–18) was increased in placentas of Br2-exposed pregnant mice at E18.5 (ANOVA), and was reduced to air control values by tadalafil. F) sFLT-1 in plasma at E18.5 increased in pregnant mice exposed to Br2 and was reduced with tadalafil; ANOVA. Only one placenta per pregnant mother was used. All data are means±S.E.M.
Placental TNFα mRNA was significantly reduced in Br2-exposed mice that received tadalafil (Figure 2D). This indicated a less inflamed state in placentas of Br2-exposed mice with PDE5 inhibition. sFLT-1:FLT-1 mRNA ratio increased in placentas of mothers exposed to Br2 (Figure 2E). Tadalafil therapy post-Br2 decreased the sFLT-1:FLT-1 mRNA ratio to air control levels. These changes in sFLT-1 mRNA levels were reflected by a five-fold increase of circulating sFLT-1 levels in maternal plasma of Br2-exposed mice, which was normalized by tadalafil therapy (Figure 2F).
Pregnant mice exhibit signs of severe lung injury 96 h post-Br2 exposure, which are absent in non-pregnant mice
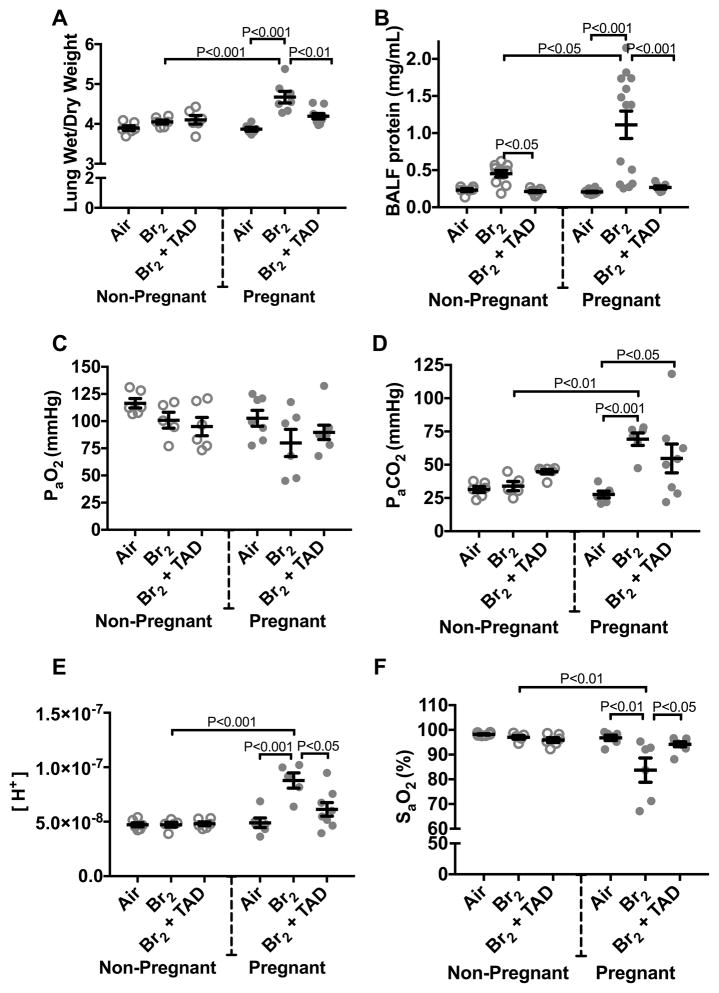
Br2 inhalation in non-pregnant animals is known to cause a temporary increase in alveolar epithelial permeability. This leads to increased protein content in the bronchoalveolar lavage (BAL) fluid, which begins to resolve after three days 10. Our data show that non-pregnant mice exposed to 600 ppm for 30 min have lung wet/dry weight ratios and BAL fluid protein values similar to those of the corresponding air controls three days post-exposure. Pregnant mice, however, had increased lung wet/dry weight ratios and BAL fluid protein at E18.5. Treatment with tadalafil significantly reduced wet/dry lung weight ratios and BAL fluid protein (Figures 3A and 3B).
Figure 3. Pregnant mice exhibit pulmonary injury 96 h post-exposure.
Non-pregnant and pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air, then administered tadalafil (TAD) or vehicle. A) Lung wet/dry weight ratios at E18.5 are increased in pregnant mice exposed to Br2 and are reduced by tadalafil. B) Protein concentrations in the bronchoalveolar lavage fluid (BALF) of pregnant mice were increased at E18.5 and returned to the air control values following administration of tadalafil (TAD). C–F) PaO2 was unchanged, PaCO2 increased, [H+] increased and SaO2 was decreased in pregnant mice exposed to Br2 at E18.5. Administration of Tadalafil returned [H+] and SaO2 to their air control values but did not improve PaCO2; All data n=6–14 mice; ANOVA; means±S.E.M.
PaO2 was unaffected by Br2 inhalation (Figure 3C), however, there was a significant increase of PaCO2 in pregnant mice (Figure 3D) accompanied by a severe drop in pH (to 7.05 Figure 3E). These findings are consistent with alveolar hypoventilation. Similar findings have been reported in sheep exposed to 100% oxygen at 1ATA for three-four days 18. Tadalafil administration had no significant effect on PaCO2, but significantly increased arterial pH to 7.18, presumably due to an increase of bicarbonate. SaO2 was significantly decreased in Br2-exposed pregnant mice and was restored by tadalafil administration (Figure 3F).
Br2 inhalation causes systemic hypertension in pregnant mice
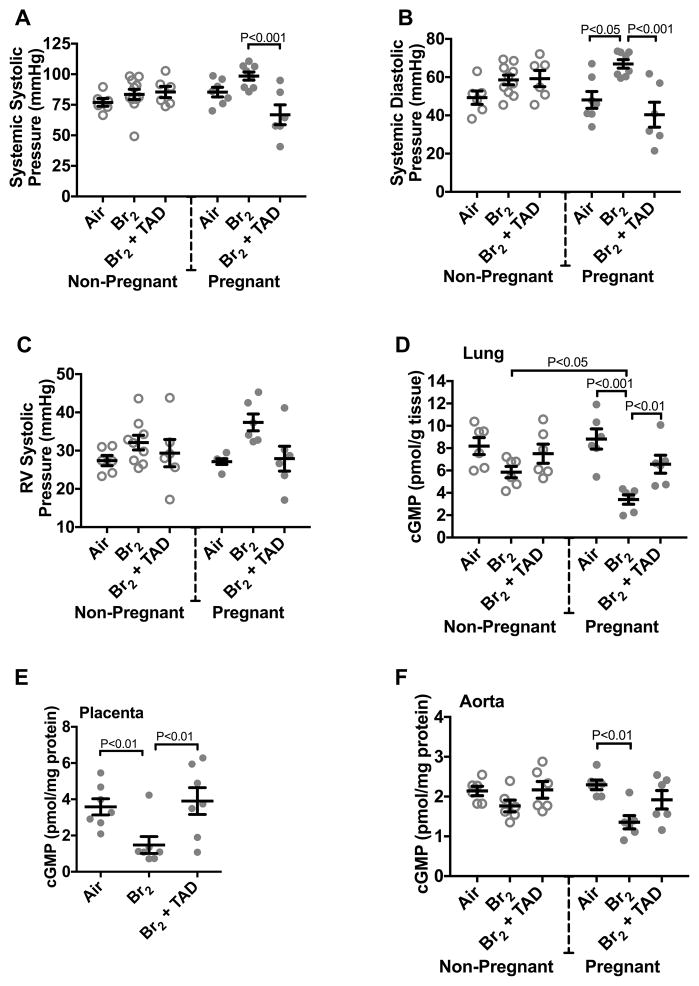
A life threatening complication of pregnancy is severe hypertension, the etiology of which is driven by placental inflammation and injury as well as production of anti-angiogenic mediators such as sFLT-1. Both systemic systolic and diastolic blood pressures of non-pregnant mice exposed to Br2 were unchanged as compared to non-pregnant air controls. Systolic pressures in pregnant mice exposed to Br2 were not significantly increased (Figure 4A). However, there was a 40% increase of systemic diastolic blood pressures in pregnant mice at E18.5. Additionally, these values returned to baseline in tadalafil treated mice (Figure 4B). Right ventricle pressures were unchanged in Br2-exposed pregnant mice (Figure 4C and S2: please see http://hyper.ahajournals.org).
Figure 4. Exposure to Br2 increases the systemic blood pressure of pregnant mice and decreases cGMP in the lungs, placentas and aortas.
Non-pregnant and pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air, then administered tadalafil (TAD) or vehicle. At E18.5, a pressure-transducer catheter was inserted into either the aortic arch via the carotid or into the right ventricle through the jugular vein in anesthetized mice. A–B) Br2 has no significant effect on systemic systolic or diastolic blood pressures in non-pregnant mice (NP). However, systemic diastolic pressures were increased in pregnant mice (P). Both systemic systolic and diastolic pressures were decreased to their corresponding air controls following tadalafil administration; n=6–11; ANOVA. C) Right ventricular (RV) systolic pressures remained unchanged in pregnant mice exposed to Br2. D–F) Lung tissue, placenta, and aorta cGMP levels of Br2-exposed pregnant mice decreased significantly. Lung and placenta cGMP levels were restored following tadalafil administration; n=6–7 per group; ANOVA. All data are means±S.E.M.
Br2 inhalation causes decreased cyclic guanosine monophosphate (cGMP) levels in pregnant mice
We hypothesized that changes in lung and aortic cGMP levels may account for the systemic hypertension seen in pregnant mice exposed to Br2. cGMP levels in pregnant mice were decreased significantly as compared to air controls in the lung, placenta, and aorta (Figure 4D–F). Treatment with tadalafil increases cGMP values in the lung and placenta.
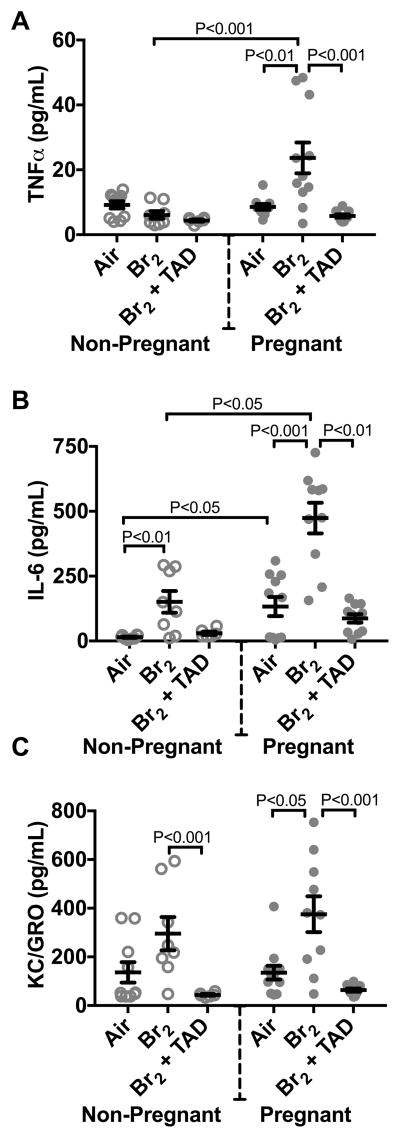
Br2 increases circulating inflammatory cytokines
At E18.5, circulating levels of tumor necrosis factor alpha (TNFα) and keratinocyte chemoattractant/ growth related oncogene (KC/GRO) (the murine functional equivalent of human interleukin-8) were increased only in the pregnant, Br2-exposed mice. Interleukin-6 (IL-6) was increased in both non-pregnant and pregnant mice exposed to Br2. These cytokines have been shown to be associated with the onset of preeclampsia 12, 19–24. Tadalafil therapy subsequently reduced these levels to those similar to air controls (Figure 5A–C).
Figure 5. Pregnant mice exposed to Br2 demonstrated systemic inflammation at E18.5.
Non-pregnant and pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air, then administered tadalafil (TAD) or vehicle. A–C) Plasma TNFα, IL-6, and KC/GRO increased in pregnant mice exposed to Br2 but only IL-6 increased in non-pregnant mice post-Br2. All three cytokines in pregnant mice exposed to Br2 returned to air control values following tadalafil administration; n=6–12 per group; ANOVA. All data are means±S.E.M.
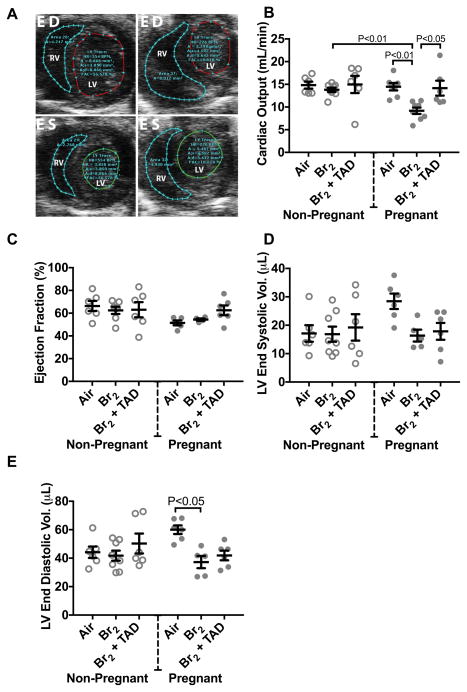
Br2 inhalation decreases cardiac function in pregnant mice that is reversed by tadalafil
At E18.5, cardiac output calculated from echo-Doppler analysis (Figure 6A) was diminished in pregnant mice exposed to Br2 as compared to air-exposed control pregnant mice (Figure 6B). Administration of tadalafil restored cardiac output. Ejection fraction (Figure 6C), fractional shortening, and heart rate were unaffected in any of the experimental groups, but stroke volume was reduced in Br2-exposed pregnant mice. Left ventricle (LV) end diastolic volumes were decreased in Br2-exposed pregnant mice (Figure 6E), explaining the decrease of LV cardiac output despite the preserved ejection fraction. These findings are consistent with the presence of heart failure with preserved ejection fraction, historically referred to as diastolic heart failure.
Figure 6. Br2-exposed pregnant mice exhibit diminished cardiac function at E18.5.
Non-pregnant and pregnant (E14.5) mice were exposed to air or 600ppm Br2 for 30 minutes, returned to room air, then administered tadalafil (TAD) or vehicle. A) Representative echosonography LV and RV traces at E18.5 of pregnant mice exposed to air or Br2 with demarcation of ventricular sizes. HR = Heart rate, A = Area, A;s = Area systole, A;d = Area diastole, FAC = Fractional area change. B) Cardiac output (LV) determined by echosonography was decreased in Br2-exposed pregnant mice, which increased following administration of tadalafil; ANOVA: n=6–8. C) Ejection fraction (LV) was unchanged in all groups; ANOVA. D) LV end-systolic volume was similar in all groups. E) However, LV end-diastolic volume was diminished in Br2-exposed pregnant mice; n=6–8; ANOVA. All data are means±S.E.M.
DISCUSSION
We show for the first time that exposure of pregnant mice to the oxidant and electrophile gas Br2, in concentrations likely to be encountered in the vicinity of industrial accidents, results in significant mortality and morbidity as compared to male and non-pregnant female mice exposed to identical concentrations of Br2. Pregnant mice experienced severe injury to their placentas resulting in the release of inflammatory cytokines and the anti-angiogenic molecule sFLT-1 in the plasma, fetal growth restriction, as well as increased systemic diastolic pressures and non-cardiogenic pulmonary edema. These symptoms are reminiscent of those seen in pregnant women with preeclampsia.
One factor that may account for this preeclampsia-like condition is the observed decrease of cGMP in lungs, aorta, and placenta. This may be caused by increased breakdown of cGMP by PDE5 or decreased production due to the systemic inactivation of eNOS as seen in mice and rats following exposure to Cl2 25. Phosphodiesterase 5 inhibitors (PDE5i) have shown therapeutic benefit in animal models of preeclampsia, 26, 27 and the most widely used PDE5i, sildenafil, is in human clinical trials to treat preeclampsia 15, 16. These protective effects are most likely due to the inhibition of cGMP breakdown by PDE5 and prevention of hypertension. We chose to administer the PDE5i tadalafil due to its long half-life (17.5 h) as well as its specificity. For example, tadalafil is more than 700x more potent for PDE5 than for PDE6 and at least 9,000x more potent for PDE5 than PDE1-4 and PDE7-10 28.
However, the protective effects of restoring cGMP may be due to a variety of additional mechanisms. cGMP has also been shown to maintain endothelial barrier function 29 and increase sodium-dependent alveolar fluid clearance via regulation of epithelial Na+ 30 and K+ channels 31. This may explain the restoration of blood gas permeability and resolution of pulmonary edema seen in pregnant mice that received tadalafil post-Br2 exposure. In the placenta, which lacks significant smooth muscle, cGMP-dependent mechanisms have been shown to participate in maintaining trophoblast survival and invasiveness 32. Therefore, it is plausible that some of the beneficial effects of tadalafil therapy were mediated by direct effect on the trophoblasts. Furthermore, within the limits of this study, we were unable to exclude any off-target effects (e.g. inhibition of other PDEs) of tadalafil.
Exposure to Br2 represents a clear danger to public health. World production of Br2 exceeds 300,000 tons per year; it is used in the manufacturing of medicinal compounds, flame-retardants, agricultural chemicals, gasoline additives, dyes, photographic chemicals, bleaching agents, and water disinfectants. It is produced in central facilities and transported by trains or trucks. A major accident recently occurred during railway Br2 transport in the city of Chelyabinsk, Russia (pop.1.1 million), resulting in 42 people being hospitalized and over 200 individuals seeking medical attention 33. A study conducted on human volunteers demonstrated that a small exposure to 0.9 ppm bromine (Br2) for five minutes results in cough, headache, and irritation of the eyes, nose, and upper respiratory tract 34. Accidental exposure to higher doses of Br2 and its ensuing respiratory complications can lead to severe morbidity and mortality, yet there is a lack of human and animal data available on Br2 toxicity, especially during pregnancy. This is particularly pertinent, since, according to the US census bureau, four percent of women in the United States are pregnant at any given time. Interestingly, two epidemiological studies in western Australia and the southern United States reported associates among levels of halogenated intermediates in drinking water (used as disinfectants) and premature births 35 and birth defects 36.
The exact mechanism by which Br2 inhalation induces placental injury remains unclear. Br2 and HOBr are strong oxidants, likely to react with targets on the plasma membranes of lung epithelial cells (such as plasmalogens), forming secondary, longer living intermediates, such as bromopalmitaldehyde (2-BrPALD) and 2-bromostearaldehyde (2-BrSALD) which may be oxidized to 2-bromopalmitic acid (2-BrPA) and 2-bromostearic acid (2-BrSA) capable of transducing injury to distal sites 37. 2-BrPA is a potent inhibitor of mitochondrial fatty acid oxidation and of protein palmitoylation 38, 39. In addition, in eosinophils, the catalytic action of eosinophil peroxidase on hydrogen peroxide and bromide generates 2-BrFALD 39. Normal physiological concentrations of bromide are in the range of 20–150 μM and will most likely rise in the mM range following inhalation of Br2 gas. Thus, production of 2-BrFALD by inhaled Br2 and inflammatory cell myeloperoxidase is likely to occur. Recently Ford et al. showed that the formation of 2-chloropalmitaldehyde (2-ClPALD), 2-chlorostearaldehyde (2-ClSALD), and their oxidized products, free and esterified 2-chloropalmitic acid (2-ClPA) and 2-chlorostearic acid could be detected in the lungs and plasma of mice and rats for up to 72 h post-exposure to 400 ppm Cl2 for 30 min 40. Furthermore, intranasal administration of 2-ClPA or 2-ClPALD resulted in increased distal lung permeability and inflammation and systemic endothelial dysfunction characterized by loss of eNOS-dependent vasodilation. Thus reactive intermediates formed during halogen inhalation are capable of causing distal organ injury and may be responsible both for placental and cardiac injury. In another study, Zaky et al. demonstrated that perfusion of isolated hearts with chloramines (another long term intermediate formed during Cl2 inhalation) resulted in severe cardiac dysfunction 41.
Placental injury can be induced by a variety of agents that induce endothelial dysfunction, including antagonists of nitric oxide synthesis 42, genetic ablation of endothelial nitric oxide synthase (eNOS) in gene-targeted mice 43, exogenous administration of the vascular endothelial growth factor (VEGF) antagonist short variant of FMS-like tyrosine kinase 1 (sFLT-1) 44, or the short form of endoglin 45. In these models, placental injury or decreased uterine/placental perfusion leading to placental ischemia, causes placental inflammation and the production of anti-angiogenic molecules which, in turn, aggravate systemic endothelial dysfunction and worsen vasoconstriction that further aggravates placenta injury 46. In Br2-exposed pregnant mice we were able to clearly demonstrate placental injury by evaluating placenta development based on the size of the junctional zone and by detecting inflammation and increased production of short-fms-like tyrosine kinase (sFLT-1) by quantifying their respective mRNA levels. We also detected increased circulating sFLT-1 and decreased vascular and tissue cGMP levels that is considered to be a diagnostic sign of decreased VEGF signaling. Acting as a decoy receptor for VEGF, sFLT-1 inhibits maternal VEGF signal transduction resulting in endothelial dysfunction, vasoconstriction and hypertension.
Similarly, inflammatory cytokines may play an important role in injuring the placenta. TNFα and IL-6 have been well established as circulating biomarkers in human preeclampsia and have been shown to be causative in pathogenesis in experimental animal models of preeclampsia 20, 47, 48. Recently IL-8 has been shown to be associated with preeclampsia in humans 49. Indeed, our analysis of plasma cytokines determined that pregnant mice exposed to Br2 exhibited significant elevation of all three of these pro-inflammatory mediators whereas there were no significant differences in cytokine or chemokine levels (except IL-6) at this time point in non-pregnant mice. These data indicate the presence of systemic inflammation in pregnant mice post-Br2, which may contribute to the observed injuries to cardiac and pulmonary systems.
An additional intriguing factor in our studies is the development of severe respiratory acidosis in the pregnant mice, which is caused by alveolar hypoventilation. Respiratory acidosis contributed to pulmonary hypertension, cardiac dysfunction and placenta hypoperfusion and injury 50, 51. Interestingly, tadalafil administration did not decrease PaCO2; however, it had a significant therapeutic effect by normalizing the arterial pH.
In human clinical studies, left ventricular diastolic dysfunction has been observed in preeclampsia 52. In cases when preeclampsia was accompanied with pulmonary edema, diastolic dysfunction was highly prevalent 53. We observed decreased cardiac output in pregnant mice exposed to Br2, and this was accompanied with a decreased filling of the left ventricle under diastole but also accompanied with preserved ejection fraction. In addition to pulmonary edema, we also observed increased BAL fluid protein, indicating that both barriers at the air-blood interface have been compromised.
CONCLUSIONS
Our data shows conclusively that pregnant C57BL/6 mice exposed to Br2 at E14.5 exhibit increased mortality as compared to non-pregnant mice and display signs of placental damage accompanying symptoms reminiscent of preeclampsia. Potential mechanisms involved are shown in Figure S3 (please see http://hyper.ahajournals.org). Tadalafil, the orally available, long-acting PDE5i, administered following Br2 exposure, increases survival exclusively in pregnant mice and eliminates signs of placental damage. The key mechanistic driver of pathology, similar to models of preeclampsia, appears to be a positive feedback loop between endothelial dysfunction and the placenta via the anti-angiogenic sFLT-1. The resulting increased vascular resistance is likely combined with additional cGMP-mediated mechanisms in non-vascular tissues such as the damage to alveolar epithelium and trophoblasts in the placenta. Damage to the placenta as well as eNOS is likely mediated by long acting mediators formed by the interaction of inhaled Br2 with plasmalogens, as recently reported in Cl2-exposed mice, as well inflammatory cytokines and chemokines. This is the first demonstration that a brief exposure of pregnant mice to an oxidant and electrophile gas (Br2) results in extensive injury to the placenta, initiating a cycle of events leading to significant morbidity and mortality.
PERSPECTIVES
In summary, we have determined exposure to Br2 causes preeclampsia-like signs and increased mortality in pregnant mice. These signs include, hypertension, placental injury, and fetal growth restriction that are accompanied by increased pulmonary and cardiac injury. We demonstrate that Br2 decreased cGMP in multiple tissues including the vasculature. Administering the PDE5i tadalafil restores cGMP levels and reduces signs of injury seen in pregnant mice exposed to Br2.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1) WHAT IS NEW?
We demonstrate that exposure of pregnant mice to Br2 damages the placenta, causes fetal growth restriction, systemic hypertension, cardiac injury and lung injury. Furthermore, we show tadalafil, administered post-exposure, alleviates all pregnancy-specific injury, and increases survival in pregnant mice from 36% to 80%.
2) WHAT IS RELEVANT?
Four % of American women are pregnant at any given time. Currently, there are no studies assessing injury to pregnant animals exposed to halogens, such as Br2.
3) SUMMARY
We demonstrate that following Br2 exposure, pregnant mice display signs reminiscent of preeclampsia with fetal growth restriction. Additionally, we documented increased cardiac and pulmonary injuries in pregnant mice exposed to Br2. Post-exposure administration of tadalafil in pregnant mice, decreases mortality and morbidity.
Acknowledgments
Sadis Matalon and Tamas Jilling were responsible for the main conceptual design of these studies, They jointly directed the project and finalized the manuscript.
James A. Lambert and Matthew A. Carlisle performed the majority of experiments, contributed to experimental design and drafted the manuscript.
Wayne E. Bradley and Tamas Jilling catheterized mice and performed and analyzed echosonography.
Louis Dell’Italia directed the design and interpretation of cardiac function studies.
Saurabh Aggarwal, Namasivayam Ambalavanan, David Ford, and Rakesh Patel contributed to experimental design, critical interpretation of the data and editing of the manuscript.
Adam Lam, Stephen Doran, Changchun Ren contributed to the execution of the experimental plan and the drafting of the manuscript.
SOURCES OF FUNDING
This research was supported by the CounterACT Program, NIH, Office of the Director, the National Institute of Environmental Health Sciences, Grant Numbers 5U01ES026458 02 (SM), 1 U01 ES027697 01 (SM and TJ) and U01ES023759 (RPP). James Lambert was supported in part by the Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant, T32HL007918.1
Footnotes
Sadis Matalon receives a stipend from the American Physiological Society for his role as Editor-in-Chief of the American Journal of Physiology-Lung Cellular and Molecular Physiology. Sadis Matalon is the Principal Investigator of a grant from Heart Biotech Holdings, LLC, entitled: Testing the Efficacy of Hydrogel-based Nanoparticles, Encapsulating Nitrite and Nitric Oxide in the Treatment of Acute Lung Injury and Pulmonary Hypertension. Drs. Dell’Italia and Patel are co-investigators in this grant. Neither of them received a stipend and funds from this grant were not used to support the research reported in this paper.
References
- 1.Malmqvist E, Liew Z, Kallen K, Rignell-Hydbom A, Rittner R, Rylander L, Ritz B. Fetal growth and air pollution - a study on ultrasound and birth measures. Environ Res. 2017;152:73–80. doi: 10.1016/j.envres.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen M, Stayner L, Slama R, Sorensen M, Figueras F, Nieuwenhuijsen MJ, Raaschou-Nielsen O, Dadvand P. Ambient air pollution and pregnancy-induced hypertensive disorders: A systematic review and meta-analysis. Hypertension. 2014;64:494–500. doi: 10.1161/HYPERTENSIONAHA.114.03545. [DOI] [PubMed] [Google Scholar]
- 3.Wang A, Zsengeller ZK, Hecht JL, Buccafusca R, Burke SD, Rajakumar A, Weingart E, Yu PB, Salahuddin S, Karumanchi SA. Excess placental secreted frizzled-related protein 1 in maternal smokers impairs fetal growth. J Clin Invest. 2015;125:4021–4025. doi: 10.1172/JCI80457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twum C, Zhu J, Wei Y. Maternal exposure to ambient pm2.5 and term low birthweight in the state of georgia. Int J Environ Health Res. 2016;26:92– 100. doi: 10.1080/09603123.2015.1061110. [DOI] [PubMed] [Google Scholar]
- 5.Amiri A, Turner-Henson A. The roles of formaldehyde exposure and oxidative stress in fetal growth in the second trimester. J Obstet Gynecol Neonatal Nurs. 2017;46:51–62. doi: 10.1016/j.jogn.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Carlisle M, Lam A, Svendsen ER, Aggarwal S, Matalon S. Chlorine- induced cardiopulmonary injury. Ann N Y Acad Sci. 2016;1374:159–167. doi: 10.1111/nyas.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackie E, Svendsen E, Grant S, Michels JE, Richardson WH. Management of chlorine gas-related injuries from the graniteville, south carolina, train derailment. Disaster Med Public Health Prep. 2014;8:411– 416. doi: 10.1017/dmp.2014.81. [DOI] [PubMed] [Google Scholar]
- 8.Agabiti N, Ancona C, Forastiere F, Di Napoli A, Lo Presti E, Corbo GM, D’Orsi F, Perucci CA. Short term respiratory effects of acute exposure to chlorine due to a swimming pool accident. Occup Environ Med. 2001;58:399–404. doi: 10.1136/oem.58.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song W, Yu Z, Doran SF, Ambalavanan N, Steele C, Garantziotis S, Matalon S. Respiratory syncytial virus infection increases chlorine-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2015;309:L205–210. doi: 10.1152/ajplung.00159.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. Heme attenuation ameliorates irritant gas inhalation-induced acute lung injury. Antioxid Redox Signal. 2016;24:99–112. doi: 10.1089/ars.2015.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of O Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the american college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 13.Radulescu C, Bacarea A, Hutanu A, Gabor R, Dobreanu M. Placental growth factor, soluble fms-like tyrosine kinase 1, soluble endoglin, il-6, and il-16 as biomarkers in preeclampsia. Mediators of inflammation. 2016;2016:3027363. doi: 10.1155/2016/3027363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George EM, Granger JP. Endothelin: Key mediator of hypertension in preeclampsia. American journal of hypertension. 2011;24:964–969. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, Baker PN. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertension in pregnancy. 2009;28:369–382. doi: 10.3109/10641950802601278. [DOI] [PubMed] [Google Scholar]
- 16.Trapani A, Jr, Goncalves LF, Trapani TF, Vieira S, Pires M, Pires MM. Perinatal and hemodynamic evaluation of sildenafil citrate for preeclampsia treatment: A randomized controlled trial. Obstet Gynecol. 2016;128:253–259. doi: 10.1097/AOG.0000000000001518. [DOI] [PubMed] [Google Scholar]
- 17.Jani DD, Reed D, Feigley CE, Svendsen ER. Modeling an irritant gas plume for epidemiologic study. Int J Environ Health Res. 2016;26:58–74. doi: 10.1080/09603123.2015.1020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matalon S, Nesarajah MS, Farhi LE. Pulmonary and circulatory changes in conscious sheep exposed to 100% o2 at 1 ata. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:110–116. doi: 10.1152/jappl.1982.53.1.110. [DOI] [PubMed] [Google Scholar]
- 19.Gillis EE, Mooney JN, Garrett MR, Granger JP, Sasser JM. Sildenafil treatment ameliorates the maternal syndrome of preeclampsia and rescues fetal growth in the dahl salt-sensitive rat. Hypertension. 2016;67:647–653. doi: 10.1161/HYPERTENSIONAHA.115.06071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: Effect of tumor necrosis factor blockade. J Hypertens. 2011;29:1203–1212. doi: 10.1097/HJH.0b013e3283468392. [DOI] [PubMed] [Google Scholar]
- 21.Guven MA, Coskun A, Ertas IE, Aral M, Zencirci B, Oksuz H. Association of maternal serum crp, il-6, tnf-alpha, homocysteine, folic acid and vitamin b12 levels with the severity of preeclampsia and fetal birth weight. Hypertension in pregnancy. 2009;28:190–200. doi: 10.1080/10641950802601179. [DOI] [PubMed] [Google Scholar]
- 22.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and il-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Mao D, Cai Y, Tan W, Hao Y, Li L, Liu W. Association between higher expression of interleukin-8 (il-8) and haplotype -353a/-251a/+678t of il-8 gene with preeclampsia: A case-control study. Medicine (Baltimore) 2016;95:e5537. doi: 10.1097/MD.0000000000005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Wang A, Zhao C, et al. Mir-125b enhances il-8 production in early-onset severe preeclampsia by targeting sphingosine-1-phosphate lyase 1. PLoS One. 2016;11:e0166940. doi: 10.1371/journal.pone.0166940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honavar J, Samal AA, Bradley KM, Brandon A, Balanay J, Squadrito GL, MohanKumar K, Maheshwari A, Postlethwait EM, Matalon S, Patel RP. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am J Respir Cell Mol Biol. 2011;45:419–425. doi: 10.1165/rcmb.2010-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke SD, Zsengeller ZK, Khankin EV, et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin ii sensitivity in preeclampsia. J Clin Invest. 2016;126:2561–2574. doi: 10.1172/JCI83918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2013;305:R397–403. doi: 10.1152/ajpregu.00216.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuan J, Brock G. Selective phosphodiesterase type 5 inhibition using tadalafil for the treatment of erectile dysfunction. Expert opinion on investigational drugs. 2002;11:1605–1613. doi: 10.1517/13543784.11.11.1605. [DOI] [PubMed] [Google Scholar]
- 29.Rentsendorj O, Mirzapoiazova T, Adyshev D, Servinsky LE, Renne T, Verin AD, Pearse DB. Role of vasodilator-stimulated phosphoprotein in cgmp-mediated protection of human pulmonary artery endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2008;294:L686–697. doi: 10.1152/ajplung.00417.2007. [DOI] [PubMed] [Google Scholar]
- 30.Han DY, Nie HG, Su XF, Shi XM, Bhattarai D, Zhao M, Zhao RZ, Landers K, Tang H, Zhang L, Ji HL. 8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate-na stimulates human alveolar fluid clearance by releasing external na+ self-inhibition of epithelial na+ channels. Am J Respir Cell Mol Biol. 2011;45:1007–1014. doi: 10.1165/rcmb.2011-0004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nara M, Dhulipala PD, Ji GJ, Kamasani UR, Wang YX, Matalon S, Kotlikoff MI. Guanylyl cyclase stimulatory coupling to k(ca) channels. Am J Physiol Cell Physiol. 2000;279:C1938–1945. doi: 10.1152/ajpcell.2000.279.6.C1938. [DOI] [PubMed] [Google Scholar]
- 32.Bolnick JM, Kilburn BA, Bolnick AD, Diamond MP, Singh M, Hertz M, Dai J, Armant DR. Sildenafil stimulates human trophoblast invasion through nitric oxide and guanosine 3′,5′-cyclic monophosphate signaling. Fertil Steril. 2015;103:1587–1595. e1581–1582. doi: 10.1016/j.fertnstert.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers JV, Price JA, Wendling MQ, Perry MR, Reid FM, Kiser RC, Graham JS. An assessment of transcriptional changes in porcine skin exposed to bromine vapor. Journal of biochemical and molecular toxicology. 2011;25:252–262. doi: 10.1002/jbt.20383. [DOI] [PubMed] [Google Scholar]
- 34.Rupp H, Henschler D. effect of low chlorine and bromine concentrations on man. Int Arch Arbeitsmed. 1967;23:79–90. [PubMed] [Google Scholar]
- 35.Horton BJ, Luben TJ, Herring AH, Savitz DA, Singer PC, Weinberg HS, Hartmann KE. The effect of water disinfection by-products on pregnancy outcomes in two southeastern us communities. J Occup Environ Med. 2011;53:1172–1178. doi: 10.1097/JOM.0b013e31822b8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chisholm K, Cook A, Bower C, Weinstein P. Risk of birth defects in australian communities with high levels of brominated disinfection by- products. Environ Health Perspect. 2008;116:1267–1273. doi: 10.1289/ehp.10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albert CJ, Thukkani AK, Heuertz RM, Slungaard A, Hazen SL, Ford DA. Eosinophil peroxidase-derived reactive brominating species target the vinyl ether bond of plasmalogens generating a novel chemoattractant, alpha-bromo fatty aldehyde. J Biol Chem. 2003;278:8942–8950. doi: 10.1074/jbc.m211634200. [DOI] [PubMed] [Google Scholar]
- 38.Draper JM, Smith CD. Palmitoyl acyltransferase assays and inhibitors (review) Mol Membr Biol. 2009;26:5–13. doi: 10.1080/09687680802683839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chase JF, Tubbs PK. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme a and carnitine esters. Biochem J. 1972;129:55–65. doi: 10.1042/bj1290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, Matalon S, Patel RP. Formation of chlorinated lipids post-chlorine gas exposure. J Lipid Res. 2016;57:1529–1540. doi: 10.1194/jlr.M069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaky A, Bradley WE, Lazrak A, Zafar I, Doran S, Ahmad A, White CW, Dell’Italia LJ, Matalon S, Ahmad S. Chlorine inhalation-induced myocardial depression and failure. Physiological reports. 2015:3. doi: 10.14814/phy2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993;169:1316–1320. doi: 10.1016/0002-9378(93)90299-x. [DOI] [PubMed] [Google Scholar]
- 43.Kulandavelu S, Qu D, Adamson SL. Cardiovascular function in mice during normal pregnancy and in the absence of endothelial no synthase. Hypertension. 2006;47:1175–1182. doi: 10.1161/01.HYP.0000218440.71846.db. [DOI] [PubMed] [Google Scholar]
- 44.Stanley JL, Andersson IJ, Hirt CJ, Moore L, Dilworth MR, Chade AR, Sibley CP, Davidge ST, Baker PN. Effect of the anti-oxidant tempol on fetal growth in a mouse model of fetal growth restriction. Biol Reprod. 2012;87:25, 21–28. doi: 10.1095/biolreprod.111.096198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 47.Xu L, Lee M, Jeyabalan A, Roberts JM. The relationship of hypovitaminosis d and il-6 in preeclampsia. Am J Obstet Gynecol. 2014;210:149 e141–147. doi: 10.1016/j.ajog.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Founds SA, Powers RW, Patrick TE, Ren D, Harger GF, Markovic N, Roberts JM. A comparison of circulating tnf-alpha in obese and lean women with and without preeclampsia. Hypertension in pregnancy. 2008;27:39–48. doi: 10.1080/10641950701825838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cemgil Arikan D, Aral M, Coskun A, Ozer A. Plasma il-4, il-8, il-12, interferon-gamma and crp levels in pregnant women with preeclampsia, and their relation with severity of disease and fetal birth weight. J Matern Fetal Neonatal Med. 2012;25:1569–1573. doi: 10.3109/14767058.2011.648233. [DOI] [PubMed] [Google Scholar]
- 50.Neville E, Bateman NT, Ward JP. Increased resistance to acute respiratory acidosis in isolated cardiac muscle following chronic hypoxia- induced hypertrophy. Cardiovasc Res. 1996;31:739–746. doi: 10.1016/0008-6363(96)00056-9. [DOI] [PubMed] [Google Scholar]
- 51.Uemura K, McClaine RJ, de la Fuente SG, Manson RJ, Campbell KA, McClaine DJ, White WD, Stamler JS, Eubanks WS, Reynolds JD. Maternal insufflation during the second trimester equivalent produces hypercapnia, acidosis, and prolonged hypoxia in fetal sheep. Anesthesiology. 2004;101:1332–1338. doi: 10.1097/00000542-200412000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Dennis AT. Transthoracic echocardiography in women with preeclampsia. Curr Opin Anaesthesiol. 2015;28:254–260. doi: 10.1097/ACO.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 53.Desai DK, Moodley J, Naidoo DP, Bhorat I. Cardiac abnormalities in pulmonary oedema associated with hypertensive crises in pregnancy. Br J Obstet Gynaecol. 1996;103:523–528. doi: 10.1111/j.1471-0528.1996.tb09800.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.