Abstract
The objective of this study was to perform a meta-analysis to evaluate the efficacy and toxicity of gefitinib and docetaxel in treated patients with non-small-cell lung cancer (NSCLC). Methods. A literature search was performed using PubMed and CNKI databases for relevant keywords and the Medical Subject Headings. After further full-text screening, 10 clinical trials were included in the final meta-analysis. Specific odds ratios (OR) and confidence intervals were calculated. Results. The outcomes of treatment efficacy included disease control rates, quality-of-life improvement rates, 3~4 grade adverse events. Comparing gefitinib to docetaxel for NSCLC patients, the pooled odds ratios (OR) of disease control rates was 1.09, (95% confidential index [CI] = 0.84–1.43), the pooled OR of quality-of-life improvement rates was 2.49, (95% CI = 1.77–3.49), the pooled OR of 3~4 grade adverse events was 0.49, (95% CI = 0.32–0.75). Conclusion. Gefitinib was found to significantly improve patients’ quality-of-life and obviously decrease patients’ adverse events of 3~4 grade.There is no difference of disease control rates between gefitinib and docetaxel.
Keywords: Docetaxel, Gefitinib, Meta-analysis, NSCLC
1. Introduction
Lung cancer remains the most lethal cancer, which is a serious harm to human health [1].
Non-small-cell lung cancer (NSCLC) accounted for more than 80% of lung cancer, and advanced NSCLC is still an incurable disease [2]. As the standard second-line treatment of NSCLC, docetaxel and ifosfamide, vinorelbine, can significantly prolong the median survival, but their hematologic toxicity was significant [3, 4]. Pemetrexed as a second-line treatment compared with docetaxel, their efficacy is comparable and toxicity of pemetrexed is significantly reduced, but it is still cytotoxic drugs [5].The results of a series of clinical trials showed that the efficiency of chemotherapy was only 20% to 40% for advanced non-small-cell lung cancer and one year survival rate is only 35% to 45% [6, 7]. Gefitinib is an oral targeted molecular that inhibits tumor cell growth by targeting a specific enzyme on the epidermal growth factor receptor [8]. At present, gefitinib has been applied to clinical, but there is still a big controversy. This study aims to perform a meta-analysis to evaluate the clinical efficacy and safety of gefitinib and traditional therapeutic drug docetaxel in the treatment of advanced non-small cell lung cancer.
2. Methods
2.1. Searching method
We searched for relevant studies in PubMed and CNKI databases that were published between 2005 and 2017. The search terms and keywords used included “Docetaxel”, “gefitinib”, “NSCLC” and “non-small-cell lung cancer”. Duplicate articles and unpublished studies from international meetings were excluded.
2.2. Inclusion criteria
Eligible references were selected carefully based on the following criteria: (1) studies comparing the efficacy and toxicity of gefitinib with docetaxel in the treatment of non-small cell lung cancer (NSCLC) randomized controlled trials; (2) patients with non-small-cell lung cancer and liver and kidney function, hematology, ECG no obvious abnormalities; (3) information collected including disease control rates, quality-of-life improvement rates, 3~4 grade adverse events.
2.3. Excluding standard
Non-clinical randomized controlled trials; Non-lung cancer research; To accept other anti-cancer treatment; Data description is not clear; data repeated reports of the larger sample size.
2.4. Data extraction
Two researchers selected independently the relevant literature, and then download and extracted all the data with the use of standardized data-abstraction forms. The study design of the literature according to the above inclusion criteria was included in the evaluation of the patients, the intervention measures and the observation results. The data were extracted from the first author, year of publication, disease control rates, quality-of-life improvement rates, 3~4 grade adverse events.
2.5. Statistical analysis
The summary odds ratios (ORs) with 95% confidence intervals (CIs) were calculated by using RevMan (version 5.3). A random-effects (EF) model was adopted. We defined significant heterogeneity as being that of the chi-square test p value <0.1 or an I2 measure >50%, based on a statement from the Cochrane Handbook {Higgins, 2011 #32}.
3. Results
3.1. Literature searches and study characteristics
Figure 1 shows the study selection process. After a preliminary screening of the retrieved literature and further screening, we obtained 10 studies. The basic information for the 10 eligible studies is summarized in Table 1. The funnel plots for each outcome did not show any publication bias (data not shown).
Figure 1.
The study selection process
Table 1.
Baseline characeristics of included studies
| Study | Intervention | Participants | Gender(M/F) | Age(range) |
|---|---|---|---|---|
| Cufer, 2006 [15] | Gefitinib | 68 | 47/21 | 34~85 |
| Docetacel | 75 | 51/22 | 29~83 | |
| Li, 2010 [16] | Gefitinib | 50 | 30/20 | 28~79 |
| Docetacel | 48 | 29/19 | ||
| Liu, 2012 [17] | Gefitinib | 40 | Not reported | 28~79 |
| Docetacel | 38 | Not reported | ||
| Kim, 2008 [18] | Gefitinib | 733 | 466/267 | 27~84 |
| Docetacel | 733 | 488/245 | 20~84 | |
| Xiong, 2008 [19] | Gefitinib | 26 | Not reported | 33~72 |
| Docetacel | 25 | Not reported | ||
| Zhong, 2009 [20] | Gefitinib | 44 | 21/23 | 32~74 |
| Docetacel | 34 | 18/16 | 28~78 | |
| Lee, 2010 [21] | Gefitinib | 82 | 55/27 | 21~74 |
| Docetacel | 79 | 45/34 | 20~73 | |
| Morere, 2010 [22] | Gefitinib | 43 | 38/5 | 45~79 |
| Docetacel | 42 | 33/9 | 30~79 | |
| Zhang, 2009 [23] | Gefitinib | 26 | 12/14 | 34~84 |
| Docetacel | 28 | 20/8 | 40~79 | |
| Maruyama, 2008 [24] | Gefitinib | 245 | 151/94 | Not reported |
| Docetacel | 244 | 151/93 | Not reported |
3.2. Quality assessment
The Jadad scale, a 5-point scale system, was used to evaluate the methodological quality of trials, which assessed randomization (0–2 points), double-blind (0–2 points), and follow-up (0–1 points) [9]. The Jadal scale has total scores ranged from 0 to 5, and clinical trials are defined as “good” when the score is from 3 to 5. The quality of these enrolled trials was scored according to Jadad’s scale and listed in Table 2.
Table 2.
Quality assessment of included studies
| Study | Period | Jadad’s quality scores |
|---|---|---|
| Cufer, 2006 | October 2003-June 2004 | 3 |
| Li, 2010 | Not reported | 3 |
| Liu,2012 | January 2007 - August 2011 | 3 |
| Kim, 2008 | March 2004 - February 2006 | 2 |
| Xiong, 2008 | May 2004 - February 2006 | 2 |
| Zhong, 2009 | April 2002 - June 2006 | 3 |
| Lee, 2010 | September 2005-September 2006 | 2 |
| Morere, 2010 | December 2004-June 2007 | 2 |
| Zhang, 2009 | October 2006 - December 2007 | 1 |
| Maruyama, 2008 | September 2003-January 2006 | 2 |
3.3. Meta-analysis of the disease control rates of gefitinib and docetaxel
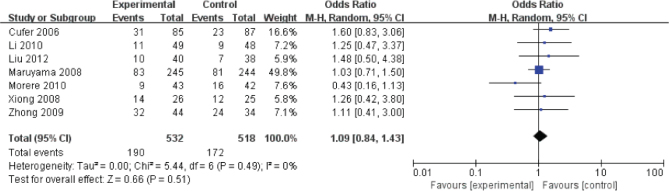
Disease control rates were reported in seven studies (Fig. 2). The pooled OR from these 9 studies was 1.09 (95% CI, 0.84-1.43). The I2 estimate of the variance between the studies is 0% and P = 0.49, which showed low heterogeneity. According to our analysis, the disease control rates between gefitinib and docetaxel was not significant (P = 0.51).
Figure 2.
The comparison for the disease control rates between geftinib and docetaxel group
3.4. Meta-analysis of the quality-of-life improvement rates of gefitinib and docetaxel
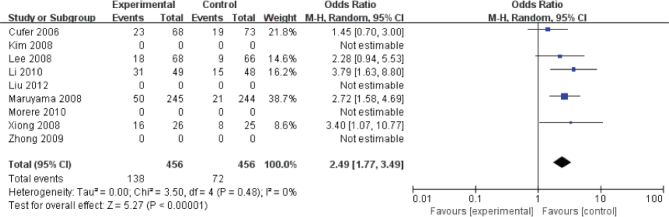
The quality-of-life improvement rates were reported in five studies (Fig. 3). The pooled OR from these 5 studies was 2.49 (95% CI, 1.77-3.49). The I2 estimate of the variance between the studies is 0% and P = 0.48, which showed low heterogeneity. According to our analysis, the quality-of-life improvement rates between gefitinib and docetaxel was significant (P < 0.01).
Figure 3.
The comparison for the quality-of-life improvement rates between geftinib and docetaxel group
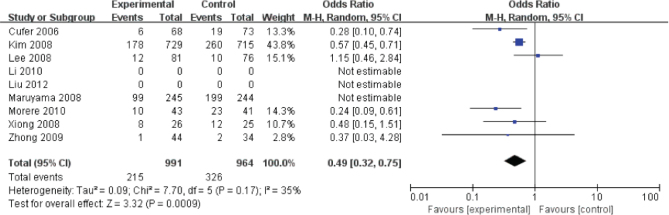
Figure 4.
The comparison for the 3~4 grade adverse events between geftinib and docetaxel group
3.5. Meta-analysis of the 3~4 grade adverse events of gefitinib and docetaxel
The 3~4 grade adverse events was reported in six studies (Fig. 3). The pooled OR from these 6 studies was 0.49 (95% CI, 0.32-0.75). The I2 estimate of the variance between the studies is 35% and P = 0.17, which showed low heterogeneity. According to our analysis, the 3~4 grade adverse events between gefitinib and docetaxel was significant (P < 0.01).
4. Discussion
As human epithelial cell cancer show that the epidermal growth factor receptor (EGFR) family is aberrantly activated, and high expression of EGFR and poor prognosis of lung cancer is closely related [9]. Therefore, EGFR is an important molecular target. Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) has become a hot topic in recent years. Gefitinib is a molecular targeted drug in the treatment of non-small cell lung cancer. Gefitinib competes with adenosine triphosphate at the ATP binding site in epithelial cells, blocking its tyrosine kinase activity, so EGFR signaling pathway were inhibited, and can induce tumor cell apoptosis [10]. The results of this study indicated that gefitinib had no inferior efficacy to docetaxel with an improvement of disease control rates. In this meta-analysis, we showed that gefitinib significantly improved life quality of patient. Besides, gefitinib decrease 3~4 grade adverse events significantly. Because disease control and quality of life were affected by subjective factors, we were not able to rule out the selection bias. Because of existence of bias, we still need high-quality research to prove its effectiveness. The study also indicated that safety and tolerability of gefitinib is superior to docetaxel group for non-small cell lung cancer. Most of the studies have confirmed that safety and tolerance of gefitinib is better than docetaxel group. In addition, some studies have shown that high expression of EGFR is also considered a sensitive factor to gefitinib [11]. The results of intervention test failed to demonstrate that EGFR over-expression factor was more sensitive to gefitinib, and of course there were some different findings [12, 13]. Studies of EGFR mutations and case characteristics have also been reported in the literature [14], and their relationship with the efficacy of gefitinib remains to be studied further.
In summary, gefitinib has certain advantages for treatment of non-small cell lung cancer. It can be treated as non-small cell lung cancer conventional drugs. However, due to the use of gefitinib by the economic conditions and other effects and the quality of included literature in this study is uneven, we still need high-quality clinical research and economic evaluation support to evaluate clinical application of gefitinib.
Footnotes
Conflict of interest statement
Authors state no conflict of interest
Reference
- [1].Esfahani K., Cohen V.. HSP90 as a novel molecular target in non-small-cell lung cancer. Lung Cancer (Auckl) 2016;7:11–17. doi: 10.2147/LCTT.S60344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Singhal S., Vachani A., Antinozerkis D., Kaiser L.R., Albelda S.M.. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: a review. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2005;11:3974. doi: 10.1158/1078-0432.CCR-04-2661. [DOI] [PubMed] [Google Scholar]
- [3].Shepherd F.A., Dancey J., Ramlau R., Mattson K., Gralla R., O’Rourke M.. et al. Prospective Randomized Trial of Docetaxel Versus Best Supportive Care in Patients With Non–Small-Cell Lung Cancer Previously Treated With Platinum-Based Chemotherapy. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- [4].Mani A., Franco S.X., Wang G., Abramson N., Schwartzberg L.S., Jakub J.. et al. A phase II tolerability trial of neoadjuvant docetaxel with carboplatin and capecitabine in locally advanced breast cancer. Community Oncology. 2011;8:209–215. [Google Scholar]
- [5].Hanna N., Shepherd F.A., Fossella F.V., Pereira J.R., De M.F., Von P.J.. et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2004;22:1589. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- [6].Schiller J.H., Harrington D., Belani C.P., Langer C., Sandler A., Krook J.. et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. New England Journal of Medicine. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- [7].Rosell R., Gatzemeier U., Betticher D.C., Keppler U., Macha H.N., Pirker R.. et al. Phase III randomised trial comparing paclitaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non-small-cell lung cancer: a cooperative multinational trial. Ann. Oncol. 2002;13:1539–1549. doi: 10.1093/annonc/mdf332. [DOI] [PubMed] [Google Scholar]
- [8].Cohen M.H., Williams G.A., Sridhara R., Chen G. Jr. MGW., Morse D.. et al. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2004;10:1212–128. doi: 10.1158/1078-0432.ccr-03-0564. [DOI] [PubMed] [Google Scholar]
- [9].Amann J., Kalyankrishna S., Massion P.P., Ohm J.E., Girard L., Shigematsu H.. et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Research. 2005;65:226–235. [PubMed] [Google Scholar]
- [10].Dhillon S.. Gefitinib: a review of its use in adults with advanced non-small cell lung cancer. Targeted Oncology. 2015;10:153–170. doi: 10.1007/s11523-015-0358-9. [DOI] [PubMed] [Google Scholar]
- [11].Wu Y.L., Zhong W.Z., Li L.Y., Zhang X.T., Zhang L., Zhou C.C.. et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. Journal of Thoracic Oncology. 2007;2:430–439. doi: 10.1097/01.JTO.0000268677.87496.4c. [DOI] [PubMed] [Google Scholar]
- [12].Socinski M.A.. Update on taxanes in the first-line treatment of advanced non-small-cell lung cancer. Current Oncology. 2014;21:e691–703. doi: 10.3747/co.21.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hirsch F.R., Varellagarcia M., Bunn P.A., Franklin W.A., Dziadziuszko R., Thatcher N.. et al. Molecular Predictors of Outcome With Gefitinib in a Phase III Placebo-Controlled Study in Advanced Non–Small-Cell Lung Cancer. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2006;24:5034. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- [14].Brandes J.C., Amin R., Khuri F., Dong M.S.. Prevention of Lung Cancer: Future Perspective with Natural Compounds. Tuberculosis & Respiratory Diseases. 2010;6:9. [Google Scholar]
- [15].Cufer T., Vrdoljak E., Gaafar R., Erensoy I., Pemberton K., Group S.S., Phase I.I.. open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anti-cancer drugs. 2006;17:401–409. doi: 10.1097/01.cad.0000203381.99490.ab. [DOI] [PubMed] [Google Scholar]
- [16].Li H.M., Wang X.M., Hua F.. Clinical study of gefitinib or docetaxel in the treatment of non-small cell lung cancer. zhongguozhongliulinchuang. 2010;37:16–18. [Google Scholar]
- [17].Liu H.L., Lei H., Deng Q.E., Hou J.. Clinical observation of gefitinib and docetaxel in the treatment of advanced non-small cell lung cancer. Zhongwaiyixueyanjiu. 2012;10:7–9. [Google Scholar]
- [18].Kim E.S., Hirsh V., Mok T., Socinski M.A., Gervais R., Wu Y-L.. et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. The Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- [19].Xiong H.H., Zou Y.M., Xia S., Yu S.Y.. Comparison of the efficacy of gefitinib and docetaxel in the treatment of non-small cell lung cancer. Lichuangneikezazhi. 2008;25:537–539. [Google Scholar]
- [20].Zhong W., Wang M.Z., Zhang L., Zhang X.T., Li L.Y.. Treatment of advanced non-small cell lung cancer with gefitinib and docetaxel. Zhongliuxuezazhi. 2009;15:508–511. [Google Scholar]
- [21].Lee D.H., Park K., Kim J.H., Lee J-S., Shin S.W., Kang J-H.. et al. Randomized phase III trial of gefitinib versus docetaxel in non– small cell lung cancer patients who have previously received platinum-based chemotherapy. Clinical cancer research. 2010;16:1307–1314. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- [22].Morère J-F., Bréchot J-M., Westeel V., Gounant V., Lebeau B., Vaylet F.. et al. Randomized phase II trial of gefitinib or gemcitabine or docetaxel chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2 or 3 (IFCT-0301 study) Lung Cancer. 2010;70:301–307. doi: 10.1016/j.lungcan.2010.03.003. [DOI] [PubMed] [Google Scholar]
- [23].Zhang Y.. Comparison of Iressa and Docetaxel in the Treatment of Recurrent Non-small Cell Lung Cancer. Shouduyiyao. 2009;16:46–47. [Google Scholar]
- [24].Maruyama R., Nishiwaki Y., Tamura T., Yamamoto N., Tsuboi M., Nakagawa K.. et al. Phase III Study, V-15-32, of Gefitinib Versus Docetaxel in Previously Treated Japanese Patients With Non– Small-Cell Lung Cancer. Journal of Clinical Oncology. 2008;26:4244–4252. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]