Abstract
Objective
We have previously shown that the consumption of a low-carbohydrate ketogenic diet (KD) by mice leads to a distinct physiologic state associated with weight loss, increased metabolic rate, and improved insulin sensitivity [1]. Furthermore, we identified fibroblast growth factor 21 (FGF21) as a necessary mediator of the changes, as mice lacking FGF21 fed KD gain rather than lose weight [2]. FGF21 activates the sympathetic nervous system (SNS) [3], which is a key regulator of metabolic rate. Thus, we considered that the SNS may play a role in mediating the metabolic adaption to ketosis.
Methods
To test this hypothesis, we measured the response of mice lacking all three β-adrenergic receptors (β-less mice) to KD feeding.
Results
In contrast to wild-type (WT) controls, β-less mice gained weight, increased adipose tissue depots mass, and did not increase energy expenditure when consuming KD. Remarkably, despite weight-gain, β-less mice were insulin sensitive. KD-induced changes in hepatic gene expression of β-less mice were similar to those seen in WT controls eating KD. Expression of FGF21 mRNA rose over 60-fold in both WT and β-less mice fed KD, and corresponding circulating FGF21 levels were 12.5 ng/ml in KD-fed wild type controls and 35.5 ng/ml in KD-fed β-less mice.
Conclusions
The response of β-less mice distinguishes at least two distinct categories of physiologic effects in mice consuming KD. In the liver, KD regulates peroxisome proliferator-activated receptor alpha (PPARα)-dependent pathways through an action of FGF21 independent of the SNS and beta-adrenergic receptors. In sharp contrast, induction of interscapular brown adipose tissue (BAT) and increased energy expenditure absolutely require SNS signals involving action on one or more β-adrenergic receptors. In this way, the key metabolic actions of FGF21 in response to KD have diverse effector mechanisms.
Keywords: Ketogenic diet, Weight loss, Sympathetic nervous system, β-Adrenergic receptors
Abbreviations: BAT, brown adipose tissue; β-less, lacking β1, β2, β3 adrenergic receptors; EE, energy expenditure; FGF21, fibroblast growth factor 21; ITT, insulin tolerance test; IWAT, inguinal white adipose tissue; IP, intraperitoneal; KD, ketogenic diet; PPARα, peroxisome proliferator-activated receptor alpha; SEM, standard error of the mean; SNA, sympathetic nerve activity; SNS, sympathetic nervous system; UCP1, uncoupling protein 1
Highlights
-
•
Ketogenic diets lead to rapid increases in energy expenditure via increased sympathetic outflow to BAT. Long term the diet leads to weight loss.
-
•
β-adrenergic receptors mediate these effects; in mice lacking all three β-receptors the effects of the ketogenic diet are not observed.
-
•
Browning of subcutaneous fat by the diet is partially activated by presumed peripheral mechanisms in the absence of β-adrenergic receptors.
-
•
Sympathetic nervous system activity not required for improved insulin sensitivity and activation of fatty acid oxidation in the liver.
1. Introduction
In humans, the consumption of a high-fat, low-carbohydrate KD leads to weight-loss and improves glucose tolerance, with no adverse effects on lipid profile [4]. In mice, we have previously shown that KD feeding leads to a distinct metabolic state characterized by weight loss, increased energy expenditure, activation of BAT, increased systemic insulin sensitivity, and a distinct pattern of hepatic gene expression [1]. Subsequently, we evaluated long-term effects of KD feeding and found long-term resistance to weight-gain on this diet by WT mice with no adverse effects on morbidity and mortality [5].
Unlike in humans, hepatic expression of FGF21 increases in mice consuming KD and is a necessary mediator of the physiologic adaptations to the diet. FGF21 knockout (KO) mice gain, rather than lose weight on the diet [6]. FGF21 also activates BAT in part by increasing SNS drive [3], [7]. In addition, the ob/ob mouse, a model with a diminished SNS outflow [8], is also partially resistant to the full effects of KD; glucose tolerance is improved, but weight loss is not observed [6]. As it is known that both metabolic rate and the BAT thermogenic program are regulated by the SNS [9], we hypothesized that the SNS may play a role in the adaptation to ketogenic diets.
To test this hypothesis, we used a mouse model lacking all β-adrenergic receptors (β-less mice) [10] and measured the response to KD. In contrast to weight-loss observed in normal WT mice, β-less mice consuming KD had a distinctly different phenotype gaining rather than losing weight. This weight-gain was observed despite demonstrating a typical pattern of ketotic gene expression in the liver. Interestingly, unlike in the liver, the adipose tissue of β-less mice failed to show the expected adaptation to KD. While increased uncoupling protein 1 (UCP1) protein was observed in WT BAT, this increase was not observed in the β-less mice. Furthermore, we demonstrate that increased UCP1 expression observed in WT mice consuming KD was mediated through increased SNS drive to BAT 24-hours after switching to the KD diet. This SNS outflow profile was absent in the β-less mice. Our findings confirm that SNS activity, mediated through β-adrenergic receptors, is required for the physiologic response and adaptation to the ketogenic diet that ultimately results in weight loss.
2. Materials and methods
2.1. Mouse maintenance and diets
All experiments were carried out on 8- to 16-week old male WT (FVB/C57BL6/DBA/2/129SvJ) and β-less mice (mice homozygous for disruption of β1, β2, and β3 adrenoceptors). These mice have been used in the genetic sympathectomy studies [10] and have been maintained as distinct colonies: a WT colony and β-less colony at Beth Israel Deaconess Medical Center in the Bradford Lowell Laboratory since 2002. Parental strains of the β-less mice have the following genotypes – β1,2 receptor double knockout (Ardb1,2tm1Bkk FVB/C57BL6/DBA/2/129SvJ) and β3 receptor knockout (Ardb3tm1Lowl FVB) bred to generate the triple receptor knockout.
Mice were provided with ad libitum access to water and one of two diets; chow diet and KD. The control chow diet had a composition of 6.5% fat, 23.5% protein, and 56% carbohydrate (2.5% sucrose), (LabDiet 5008 – Pharmaserv, Framingham, MA). The KD had a composition of 78.9% fat, 9.5% protein, and 0.76% carbohydrate (0% sucrose), (Bio-Serv F3666, Frenchtown, NJ). This formulation has been demonstrated to induce ketosis in rodents [11] and was previously used by us for KD studies [1].
Mice were kept under a 12 h light: 12 h dark cycle and an ambient temperature of 22 ± 2 °C. All procedures were in accordance with National Institutes of Health Guidelines for the Care and Use of Animals and approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center (Boston MA) and The University of Iowa (Iowa City, IA).
2.2. Dynamic physiological tests
Glucose Tolerance Test: Mice were fasted for 16 h before an intraperitoneal (IP) injection d-glucose (2 g/kg body WT; Sigma, St. Louis, MO) was administered 4 h after onset of the light cycle, and glucose was measured at 0, 10, 20, 30, 60, 90, and 120 min after the injection.
Insulin Tolerance Test: Ad libitum-fed-mice were injected IP with insulin (0.75 U/kg; Lilly, Indianapolis, IN) 8 h after onset of the light cycle and glucose was measured at 0, 20, 40, 60, 80, 100, 120, and 140 min after the injection.
Glucose levels for both tests were measured using a OneTouch Ultra glucometer (Lifescan, Milpitas, CA).
2.3. Indirect calorimetry
Mouse energy expenditure was measured by indirect calorimetry using the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH). Mice were individually housed with ad libitum access to food and water. Analysis was performed at 24 °C under a 12:12-h light–dark cycle (light period 0600–1800). Mice were acclimated in the metabolic chambers for 48 h before collecting measurements used for data analysis.
2.4. Serum analysis
Serum FGF21 concentrations were measured using Quantikine mouse FGF21 ELISA (R&D Systems, Minneapolis, MN), β-hydroxybutyrate (β-hydroxybutyrate colorimetric assay, Stanbio Laboratory), and insulin (Ultra-sensitive mouse insulin ELISA; Crystalchem, Chicago, IL).
2.5. RNA extraction and quantitative real-time PCR
RNA was isolated from tissue flash-frozen in liquid nitrogen using Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA). cDNA was made from isolated RNA using oligo(dt) and random hexamer primers and reverse transcriptase (QuantiTech RT Kit; Qiagen, Germantown, MD). Quantitative PCR was performed using the 7800HT (Applied Biosystems, Foster City, CA) thermal cycler and SYBR Green master mix (Applied Biosystems, Foster City, CA). Relative mRNA abundance was calculated and normalized to levels of the housekeeping gene 36B4. Primers are included in Supplementary Table 1.
2.6. Recording of sympathetic nerve activity (SNA)
SNA to BAT was measured by multi-fiber recording. Using a dissecting microscope, the nerve subserving the BAT was identified and carefully dissected free and placed on a bipolar 36-gauge platinum-iridium electrode (A-M Systems, Carlsborg WA). When an optimum recording of SNA was obtained, the electrode was covered with silicone gel (Kwik-Sil; World Precision Instruments Inc, Sarasota FL).
Nerve electrodes were attached to a high impedance probe (HIP-511, Grass Instruments Co., Quincy, MA). The nerve signal was amplified 105 times with a Grass P5 AC pre-amplifier, filtered at a low and high frequency cutoff of 100 Hz and 1000 Hz, respectively. The amplified, filtered nerve signal was directed to speaker system and to an oscilloscope (model 54501A, Hewlett–Packard Co., Palo Alto, CA) for auditory and visual monitoring of the nerve activity and finally to a resetting voltage integrator (model B600C, University of Iowa Bioengineering) that sums the total voltage output in units of 1 s before resetting to zero. The resetting voltage integrator and the amplified, filtered neurograms were continuously routed to a MacLab analog–digital converter (model 8S, AD Instruments Castle Hill, New South Wales, Australia) for permanent recording and data analysis. To ensure that background electrical noise was excluded in the assessment of sympathetic outflow in the integrated voltage, SNA was corrected for post-mortem background activity.
2.7. Data analysis
Data are shown as means ± standard error of the means (SEM). Time course experiments were analyzed for significant differences using a two-way ANOVA with repeated measures followed by Bonferonni's post-hoc test for individual comparisons. Single point measures for four-way studies were analyzed using a one-way ANOVA followed by Bonferonni's post-hoc test for individual comparisons. Single point measures for two-way studies were analyzed using a two-tailed unpaired T-test.
3. Results
3.1. Long-term consumption of a ketogenic diet causes weight-loss in wild type mice, but not in beta-less mice
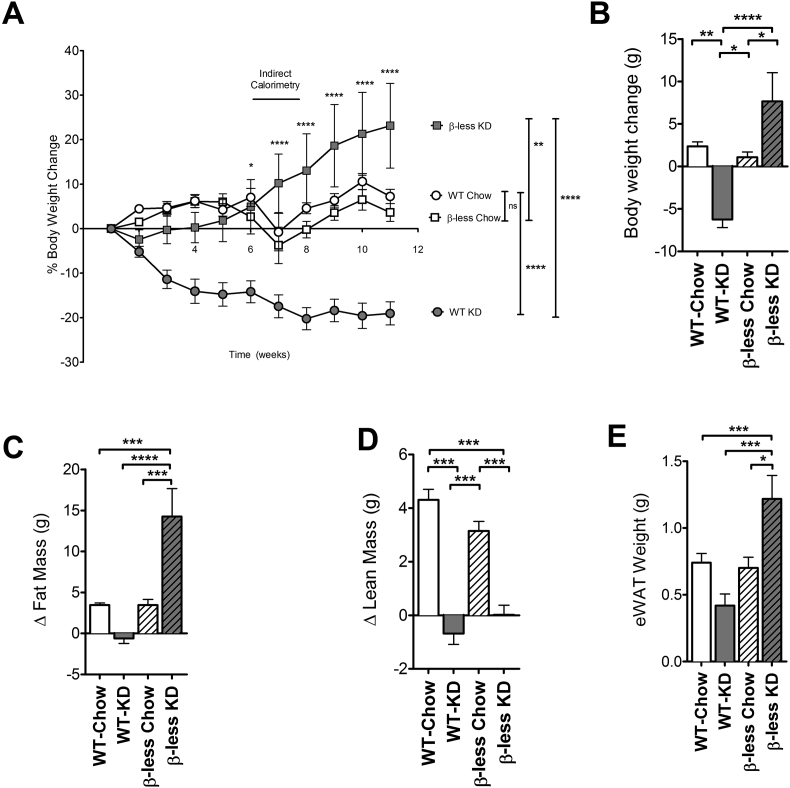
We have previously shown that the consumption of a KD by WT C57BL/6 mice leads to weight loss [1]. To investigate whether intact β-adrenergic receptors are necessary for KD induced weight loss, we chronically fed KD to mice lacking all three β-adrenergic receptors (β-less mice). Consistent with our previously published studies, WT mice consuming KD for 11 weeks lost 19% of their initial body weight (6 g). By contrast, we found that β-less mice consuming a KD for 11 weeks significantly gained weight instead of losing weight on the diet. By 11 weeks, a 23% weight gain of 7 g was seen (Figure 1A, B). WT and β-less mice on a control chow diet gained a modest 1.4 g over this interval.
Figure 1.
β-less mice are unable to lose weight on consumption of the Ketogenic Diet. β-less mice fed ketogenic diet fed gain weight compared to chow fed controls (P < 0.01), unlike WT mice that lose weight on the diet (P < 0.0001) (A, B) (Overall: Groups P < 0.0001; Time P < 0.0001; Interaction P < 0.0001). Both fat (C) and lean (D) mass are reduced in KD fed mice compared to chow fed counterparts as measured by MRI (P < 0.001). β-less mice fed KD have significantly increased epididymal fat depot mass (E) compared to chow fed counterparts (P < 0.05) and KD fed WT mice (P < 0.0001). Data represented as Mean ± SEM; n = 8–10 mice/group. Significance in weight curve was determined using two-way ANOVA with repeated measures followed by a post-hoc analysis using Bonferonni's Test. Significance in body composition was determined with one-way ANOVA with Bonferonni's post-hoc test for individual comparisons. Significance is designated by asterisks with *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The change in body weight seen in the mice was reflected in changes in fat and lean body mass. Although initial fat and lean mass were the same between wild type and β-less mice, after 11 weeks, WT mice fed KD lost body fat compared to their chow-fed counterparts, while β-less mice fed KD gained an excess of almost 10 g of body fat (Figure 1C). Lean mass was increased in the chow-fed cohorts but not the KD-fed cohorts (Figure 1D). The increase in adiposity is partly attributable to an increase in the epididymal white adipose tissue fat depot weight (Figure 1E).
3.2. Beta-less mice are unable to increase energy expenditure when consuming the ketogenic diet
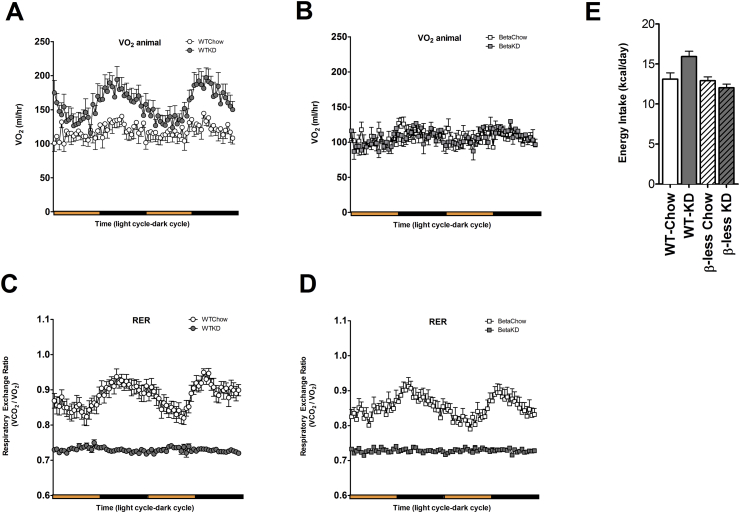
In WT mice, consumption of KD led to increased energy expenditure, as assessed by increased VO2 consumption. In contrast, β-less mice showed no increase in VO2 consumption (Figure 2A, B). Both WT and β-less mice consuming a KD had a consistently low respiratory exchange ratio (RER) between 0.70 and 0.75, demonstrating a loss in its diurnal rhythmicity, suggestive of utilizing fat as a fuel for both the fed and fasted state (Figure 2C, D). These changes were independent of caloric intake (Figure 2E).
Figure 2.
Ketogenic diet induced weight loss is due to an increase in energy expenditure. Wild-type mice fed KD for 11 weeks have a higher metabolic rate of VO2 (A) compared to chow fed counterparts (P < 0.0001) whereas β-less mice fed KD show no changes in VO2 from their chow fed counterparts (B) (Overall: Groups P < 0.0001; Time P < 0.01; Interaction P < 0.0001). The cyclic nature of the respiratory exchange ratio (RER) is lost in both WT and β-less KD-fed mice (C, D) due to constant utilization of lipid as a fuel source. There is no difference in total caloric intake between the groups (E). Data represented as Mean ± SEM; n = 8–10 mice/group. Significance in VO2 was determined using two-way ANOVA with repeated measures followed by a post-hoc analysis using Bonferonni's test for all four groups together. Significance in energy intake was determined with one-way ANOVA with Bonferonni's post-hoc test for individual comparisons. Significance designated by asterisks with *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.3. Consumption of ketogenic diet improves glucose metabolism in both wild-type and beta-less mice, but differentially alters lipid profile
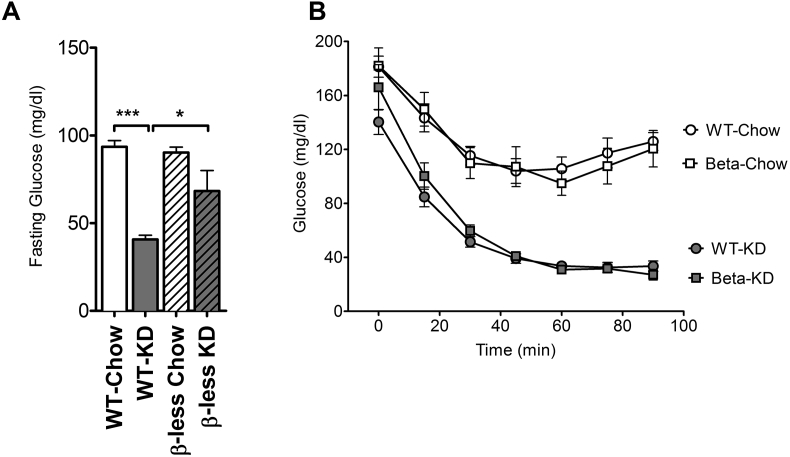
After 11 weeks of KD feeding, there was a significant decrease in fasting glucose in both WT and β-less groups (Figure 3A), although the effect was more prominent in WT mice. KD also improved insulin sensitivity in WT and β-less groups as assessed by an insulin tolerance test. Despite the absence of weight-loss while consuming KD, β-less mice fed KD were insulin sensitive with a 20% decrease in basal circulating glucose levels (Figure 3B). As previously shown [1], WT mice fed KD were also remarkably insulin sensitive with a decrease to 22% of basal levels (Figure 3B). Insulin sensitivity was observed as a consequence of the diet and was independent of genotype.
Figure 3.
Ketogenic diet feeding improves insulin sensitivity independent of the SNS. Wild-type mice fed KD have lower fasting glucose (A) and both WT and β-less mice consuming KD remain insulin-sensitive (P < 0.0001) as shown by glucose levels during an ITT (B). Data represented as Mean ± SEM; n = 8–10 mice/group. Significance in fasting glucose was determined with one-way ANOVA with Bonferonni's post-hoc test for individual comparisons. Significance in ITT was determined using two-way ANOVA with repeated measures followed by a post-hoc analysis using Bonferonni's test for all four groups together. Significance is designated by asterisks with *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.4. Ketogenic diet alters hepatic gene expression in both wild type and beta-less mice, independent of the sympathetic nervous system
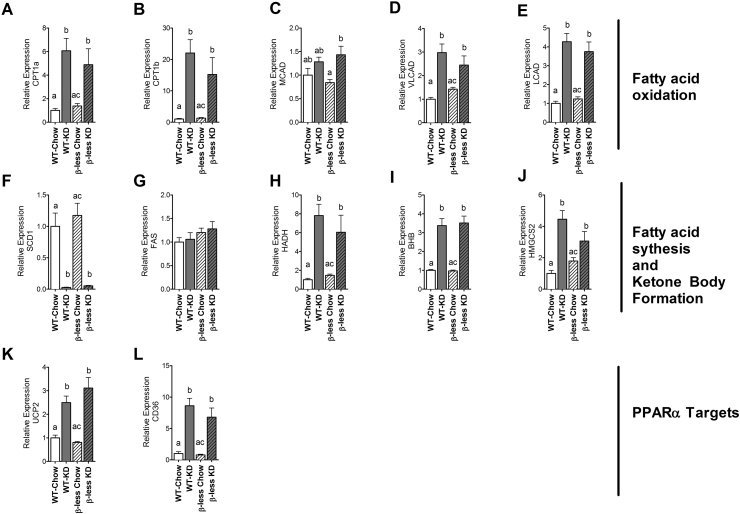
Consumption of KD leads to marked alterations in hepatic mRNA profile. We evaluated gene expression of 15 genes involved in lipid oxidation, lipid synthesis, ketone body metabolism, and PPARα targets.
Fatty acid oxidation: After 11 weeks of KD feeding, expression of genes required for fatty acid oxidation/β-oxidation was elevated. The increased gene expression was seen as function of the diet and not genotype. Carnitine palmitoyltransferase 1A, 1B (Cpt1a, Cpt1b) were elevated 4- and 20-fold, respectively, in both WT and β-less mice (Figure 4A, B). Medium-chain long-chain and very long-chain acyl-coenzyme A dehydrogenase (MCAD, LCAD, VLCAD) and 3-hydroxyacyl-coenzyme A dehydrogenase (Hadh) were all elevated 2–3 fold when feeding WT or β-less mice KD (Figure 4C–E).
Figure 4.
Ketogenic diet induced hepatic gene expression profile is independent of the SNS. Ketogenic diet feeding induces genes involved in fatty acid oxidation and ketone body formation (A, B, C, D, E, H, I, J) in both WT and β-less mice. Expression in liver of genes involved in de novo lipogenesis/triglyceride synthesis in both WT and β-less mice are either unchanged or suppressed upon KD feeding (F,G). Critical metabolic regulators remain elevated (K, L). CPT1α: carnitine palmitoyltransferase 1 alpha (P < 0.0002); CPT1β: carnitine palmitoyltransferase 1 beta (P < 0.0001); MCAD: Medium-chain acyl-CoA dehydrogenase (P < 0.0001); VLCAD: Very long-chain acyl-CoA dehydrogenase (P < 0.0001); LCAD: Long-chain acyl-CoA dehydrogenase (P < 0.0001); SCD-1: stearoyl-CoA desaturase-1(P < 0.0001); FAS: fatty acid synthase; HADH: hydroxyacyl-CoA dehydrogenase(P < 0.0002); BDH:1 3-hydroxybutyrate dehydrogenase (type 1) (P < 0.0001); Hmgcs2: 3-hydroxy-3-methylgutaryl-CoA synthase 2 (mitochondrial) (P < 0.0001); UCP2: uncoupling protein 2 (P < 0.0001); Cd36: cluster of differentiation 36 (P < 0.0001). Data represented as Mean ± SEM of fold change expression compared to WT chow group; n = 8–10 mice/group. Significance determined with one-way ANOVA with Bonferonni's post-hoc test for individual comparisons. Significance is designated by letters; means that do not share a common letter are significantly different from each other at P < 0.01 or higher significance.
Fatty acid synthesis: Enzymes in the fatty acid synthesis pathway were either suppressed or remain unchanged on KD feeding, and genotype had no additional effect on this expression – Stearoyl-CoA desaturase-1 (Scd1) expression was lower than to 3 to 4-fold of chow-fed values, while there was no change in fatty acid synthase (Figure 4A).
Ketone body formation: Genes involved in ketone body formation were all increased on KD feeding and again, this was observed as a function of the diet and not genotype. The gene encoding the mitochondrial enzyme that catalyzes the first reaction of ketogenesis, 3-hydroxy-3-methylglutaryl-coA synthase 2 (Hmgcs2) is also increased in both WT fed KD and β-less fed KD as is 3-hydroxybutyrate dehydrogenase (Bdh1), which encodes a protein that catalyzes the inter-conversion of acetoacetate and β-hydroxybutyrate (Figure 4H–J).
PPARα target genes: PPARα target genes UCP2 and CD36 were also up-regulated by KD feeding (UCP2; 2–3 fold, CD36; 7–8 fold), while there was no additional effect of genotype (Figure 4K, L).
3.5. Wild type mice on a ketogenic diet increase energy expenditure independent of browning in subcutaneous inguinal white adipose tissue
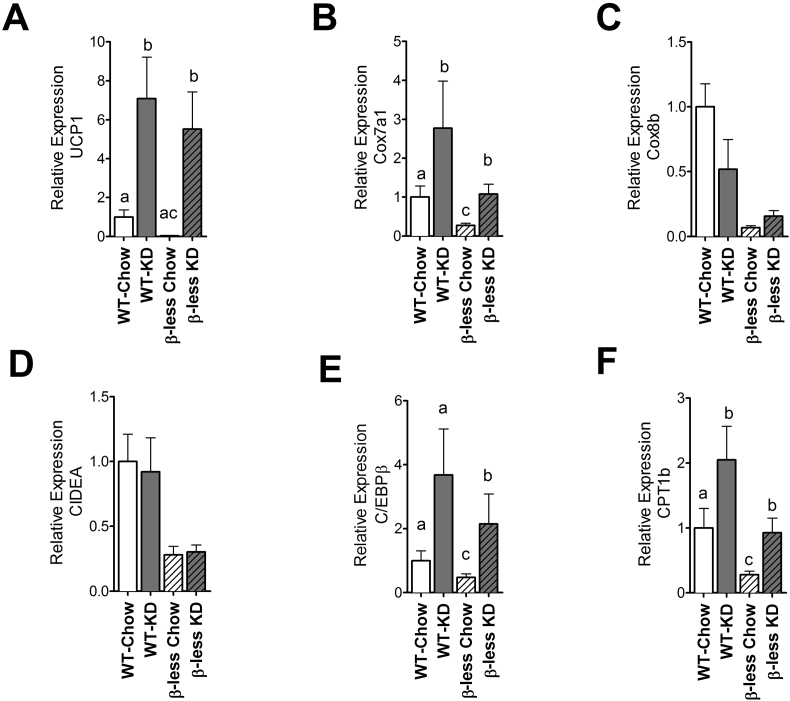
It has been previously established that cold exposed β-3-adrenoreceptor knockout mice have white adipocytes that do not express UCP1 and have reduced thermogenic markers such as PGC-1a, CIDEA, and C/EBPβ [12]. By contrast we observed browning of inguinal white adipose tissue (IWAT) in β-less mice fed KD, suggesting that the effect of KD on this process is independent of β-adrenergic receptors. Both WT mice and β-less mice on a KD showed an increase in certain thermogenic markers such as UCP1, Cox 7a1, C/EBPβ, and CPT1b (Figure 5A,B, E, F) in the Inguinal White Adipose Tissue (IWAT). However, β-less mice had blunted levels of Cox8b and CIDEA (Figure 5C, D) compared to WT mice, and KD did not induce the expression of these genes.
Figure 5.
Ketogenic diet feeding is able to partially activate the browning program in IWAT independent of intact SNS activity. Ketogenic diet feeding induced select browning marker genes in IWAT of both WT and β-less mice (A, B, E, F) while some genes remained unchanged (C, D). UCP1: uncoupling protein 1 (P < 0.007); Cox7a1: cytochrome c oxidase polypeptide 7A1 (P < 0.03); Cox8b: cytochrome c oxidase polypeptide 8b; CIDEA: Cell death-inducing DFFA-Like Effector A; C/EBPβ: CCAAT/Enhancer Binding Protein beta (P < 0.05); CPT1β: carnitine palmitoyltransferase 1 beta (P < 0.05); Data represented as Mean ± SEM of fold change expression compared to WT chow group; n = 8–10 mice/group. Significance determined with one-way ANOVA with Bonferonni's post-hoc test for individual comparisons. Significance is designated by letters; means that do not share a common letter are significantly different from each other at P < 0.05 or higher significance.
3.6. Brown adipose tissue is not activated in beta-less mice fed ketogenic diet
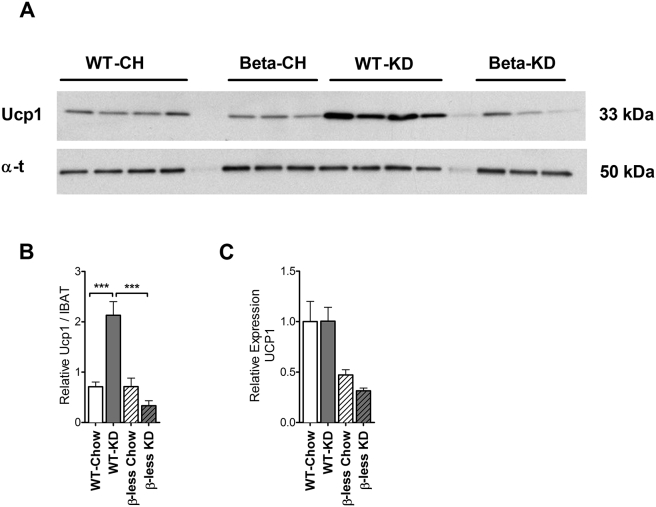
We have previously shown that UCP1 is up-regulated in BAT of WT mice consuming KD [1]. As β-less mice did not exhibit increased energy expenditure (EE), we suspected KD did not have the effect of increasing uncoupling in the BAT of these mice. We measured UCP1 protein levels in BAT and found that KD-feeding did increase UCP1 protein concentration in WT mice, but not in β-less mice (Figure 6A, B). Increased levels of UCP1 protein in BAT of WT mice was not a consequence of increased gene expression (Figure 6C).
Figure 6.
Ketogenic diet feeding induced BAT activation is dependent on intact SNS activity. Ketogenic diet feeding activates BAT at the post-translational level as indicated by elevated UCP1 protein in WT mice. This effect is absent in β-less mice (A, B) (P < 0.001). The diet does not increase UCP1 at the gene expression level in BAT (C) UCP1: uncoupling protein 1 (P < 0.001). Data represented as Mean ± SEM; n = 8–10 mice/group. Gene expression data represented as fold change expression compared to WT chow group. Significance determined with one-way ANOVA with Bonferonni's post-hoc test for individual comparisons. Significance designated by asterisks with ***P < 0.001.
3.7. Ketogenic diet increases energy expenditure in wild-type mice by increasing sympathetic nervous system outflow to brown adipose tissue 24-hours after exposure to the diet
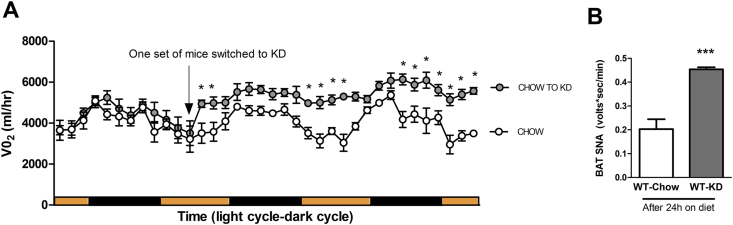
In order to determine the timing of increase in EE after KD feeding, we put WT mice in an indirect calorimeter and switched them from chow to KD. We observed that WT mice show an immediate increase in EE, as early as 24 h after a switch to KD, and a sustained increase in EE 36 h after exposure to the diet (Figure 7A). The early onset of increased EE in WT mice on KD was likely due to sympathetic nerve activation as indicated by the increased BAT SNA 24 h after exposure to the diet (Figure 7B). This experiment cannot be performed in β-less mice as they have no mechanism of increasing SNS outflow to BAT.
Figure 7.
Wild-type mice are able to acutely activate SNS outflow BAT within 24 h of consuming the ketogenic diet. Wild-type mice exposed to the ketogenic diet rapidly increase energy expenditure within 24–36 h of exposure to the diet (A). Increased energy expenditure on exposure to the diet is consistent with increased sympathetic nerve activity to BAT 24 h after exposure to the diet (B) (P < 0.001). β-less mice lack this effect due to a disrupted sympathetic nervous system. Data represented as Mean ± SEM; n = 6–8 mice/group. Significance determined with two-tailed unpaired T-test. Significance designated by asterisks with ***P < 0.001.
3.8. Circulating fibroblast growth factor 21 increases 3-fold higher in beta-less mice compared to wild-type mice consuming ketogenic diet
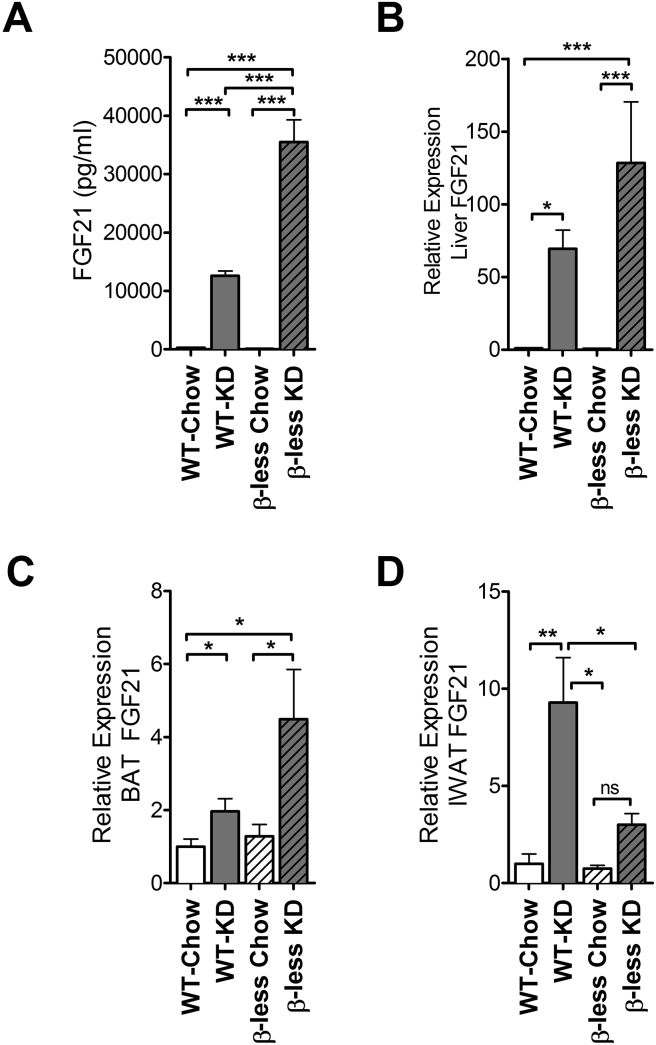
We examined circulating FGF21 levels in serum and found that WT mice fed KD had FGF21 serum levels that were 43-fold higher than WT mice fed chow. Surprisingly, β-less mice fed KD had circulating serum levels of FGF21 that were 294-fold higher than β-less mice fed chow and 121-fold higher than WT mice fed chow (Figure 8A). FGF21 gene expression in liver and BAT was induced by KD feeding in WT mice and induced to a significantly greater extent in β-less mice (Figure 8B, C). By contrast, while FGF21 expression was significantly induced in IWAT of WT mice consuming KD, this induction was blunted in β-less mice consuming KD (Figure 8D). This suggests that SNS signaling and β-adrenergic receptors are required for the activation of BAT in the context of increased systemic FGF21.
Figure 8.
Ketogenic diet feeding causes a 43-fold rise in circulating FGF21 of WT mice and 294-fold rise in circulating FGF21 of β-less mice. Ketogenic diet increases circulating FGF21 of WT mice by 43-fold compared to WT chow-fed counterparts (293 pg/ml vs 12.5 ng/ml). The diet causes a larger rise in serum FGF21 of β-less mice, 294-fold compared to β-less chow fed mice (121 pg/ml vs 35.5 ng/ml) (A) (P < 0.0001). FGF21 gene expression in the liver (P < 0.0001) and BAT (P < 0.01) follows a pattern similar to serum levels (B, C). Ketogenic diet consumption increases FGF21 gene expression in IWAT of WT mice but not β-less mice (D) (P < 0.0003). FGF21: Fibroblast growth factor 21. Data represented as Mean ± SEM; n = 8–10 mice/group. Gene expression data represented as fold change expression compared to wild-type chow group. Significance determined with one-way ANOVA with Bonferonni's post-hoc test for individual comparisons. Significance designated by asterisks with *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
4. Discussion
In humans, ketogenic diets have generated interest as a dietary intervention for both weight loss [4], [13], [14] and the management of type II diabetes [15]. These low-carbohydrate, high fat diets have also been studied in mouse models to better understand the physiologic mechanisms that mediate weight loss and to define long term consequences of the diets. KD feeding in mice induces a unique metabolic state in mice characterized by weight loss, activation of BAT, and improved insulin sensitivity [1], [6], [16]. Furthermore, mice consuming this diet demonstrate long term resistance to weight gain with no adverse effects on morbidity or mortality [5]. The data presented here demonstrate for the first time that, sympathetic nerve activity acting through β-adrenergic receptors is critical for the energy expenditure induced weight loss in mice consuming KD, but not for other long term adaptations to the diet.
We found that unlike WT mice consuming KD [1], β-less mice gain rather than lose weight due to a failure to increase energy expenditure. This is consistent with previous observations in mouse models with diminished SNS activity, such as the ob/ob mouse, that are unable to increase energy expenditure and also gain weight when consuming the diet [6], [17]. BAT is a specialized fat depot, the activation of which can lead to increased energy expenditure, heat production, and a negative energy balance in mice, limiting weight gain [18], [19], [20]. KD consumption in WT mice leads to increased EE and activation of BAT as indicated by a remarkable 5-fold increase in UCP1 protein level [1]. In contrast, β-less mice showed no increase in EE and were unable to activate BAT, suggesting that intact sympathetic tone is required for BAT activation and subsequent weight loss on KD.
Sympathetic activation of BAT is mediated through β-adrenergic receptors. In rodents, brown adipocytes are characterized by predominant expression of β3 adrenergic receptor, very low levels of the β1 receptor, and likely vascular tissue expression of β 2-receptors [21], [22]. The hormone norepinephrine, released from sympathetic nerve terminals and acting primarily through the β3 adrenergic receptor, has long been known to be a key regulator of BAT activation [23], [24]. In cold exposed β3 knockout mice, β1 receptors have been shown to be capable of mediating an increase in BAT UCP1 [25]. Therefore in this study, we used the β1, β2, β3 triple receptor knockout (β-less) model to determine the role of beta-receptors in mediating this response.
Here, we conclusively demonstrate that in the context of KD feeding, a dietary stimulant of BAT activation, an intact SNS response and its peripheral effectors are required for activation. Furthermore, our data show that in WT mice, KD rapidly increases SNS outflow to BAT. Since β-less mice on the diet are unable to lose weight, we conclude that this acute increase in SNS outflow to BAT, acting via β-adrenergic receptors, is critical for energy expenditure driven weight loss observed in WT mice feeding KD.
Ketogenic diets are more effective in promoting weight loss than conventional caloric restriction [26] however, their effect on hepatic glucose and lipid metabolism remains inconclusive. Blood glucose homeostasis is tightly regulated. In both mice and humans consuming KD, baseline blood glucose decreases and insulin falls dramatically as ketones become the primary fuel for both brain and muscle [14], [16]. As a result, systemic insulin requirements decrease in patients with diabetes [15]. Similarly, mice on KD show increased systemic insulin sensitivity as assessed by an insulin tolerance test (ITT) [5]. Therefore, by the parameter of systemic sensitivity, KD consuming mice and humans have increased insulin sensitivity. By contrast, studies in rats [27] and hyperinsulinemic-euglycemic clamp studies in mice [28] have reported that KD causes hepatic insulin resistance at the level of gluconeogenesis and suggested that the diet renders animals overall insulin resistant. Our interpretation of the somewhat conflicting results is that liver insulin resistance results from a long term inactivation of pathways involved in insulin action, which is also seen in diabetic ketoacidosis, a state of complete insulin insufficiency [29]. We speculate that in animals consuming KD, glucose is rapidly utilized by peripheral tissues such as fat and muscle in response to insulin, contributing to reduced serum glucose in the context of low circulating insulin. These beneficial effects on glucose homeostasis are seen in both WT and β-less mice consuming KD, demonstrating that the SNS, and the effects it has on body weight, are dispensable for the observed glucose lowering effects of KD.
KD feeding activates transcription factor PPARα in the liver, resulting in hepatic gene expression signatures that promote increased fatty acid oxidation and reduced fatty acid synthesis [1], [2], [6], [30]. Both WT and β-less mice consuming KD demonstrated an increase in PPARα mediated fatty acid oxidation enzymes CPT1α, CPT1β, acyl CoA dehydrogenases MCAD, VLCAD, and LCAD, and expression of enzymes involved in lipogenesis SCD and FAS. Collectively, these data indicate that the β-adrenergic receptors are dispensable for the adaptation of the liver to ketosis.
The effect of KD consumption on browning of IWAT has not been previously examined. In cold exposed β3-adrenoreceptor knockout mice, white adipocytes do not increase expression of UCP1 and have reduced browning markers such as PGC-1α, CIDEA, and C/EBPβ [12]. By contrast, here we observed that β-less mice on KD are able to partially activate the thermogenic program in white adipose tissue, increasing expression of markers such as UCP1, Cox7a, C/EBPβ, and CPT1β while expression of other browning markers such as Cox8b and CIDEA is blunted. Together, these data show that (i) chronic consumption of KD requires intact SNS action and β-adrenergic receptors to induce certain browning markers but not the entire thermogenic program and (ii) KD induced IWAT browning is not sufficient for weight loss.
Hepatic FGF21 has been established as an essential mediator of the physiologic adaptations to KD [2], and chronic consumption of the diet leads to marked induction of circulating FGF21 in both normal mice and obese mouse models [5], [16]. Here we find that in β-less mice consuming KD, this effect is exaggerated with circulating FGF21 levels three fold higher than WT mice consuming KD (12,576 pg/ml in WT KD; 35,490 pg/ml in β-less KD). Despite having three-fold higher circulating FGF21 levels compared to WT mice on the diet, β-less mice on KD are unable to increase energy expenditure and activate BAT. This suggests that circulating FGF21 may require β-adrenergic receptors to activate BAT and induce weight loss on KD, indicative of a possible regulatory feedback loop from β-adrenergic receptors to FGF21 levels.
We have previously observed that central or peripheral administration of FGF21 in β-less mice fails to induce IWAT browning [3]. By contrast, here we observe that chronic consumption of KD in β-less mice is able to partially activate the browning program in IWAT. We hypothesize that β-less mice on KD are able to brown IWAT through a cell autonomous mechanism involving a possible direct interaction between the very high circulating FGF21 levels and the KD itself. This is consistent with our previous demonstration that FGF21 can brown IWAT in a cell autonomous manner in WT mice [31], in addition to acting centrally to brown IWAT through SNS activation [3], [7].
The studies presented here demonstrate for the first time that intact sympathetic nerve activity is required or critical in mice consuming KD in order to facilitate the energy expenditure driven weight loss effects of the diet. While intact SNS outflow to BAT is critical for weight loss, we find that KD induced browning observed in IWAT can be partially activated by peripheral mechanisms in the absence of β-adrenergic receptors, but this is not sufficient for weight loss observed on the diet. In addition, the SNS and β-adrenergic receptors are also not required for KD induced improved systemic insulin sensitivity and activation of fatty acid oxidation in the liver. In conclusion, our findings demonstrate the requirement for the SNS and intact β-adrenergic receptors for the physiologic adaptation to KD that results in weight loss, but not for several other metabolic adaptations to the diet.
Acknowledgements
The authors would like to thank Dr. Bradford B. Lowell for providing us with β-less and WT breeding pairs. Funding sources: This work was supported by NIH Grant DK028082 (to E.M.F) and by an Institutional Research Training Grant NRSA 5T32DK751627 (to N.D.).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.05.017.
Conflict of interests
The authors have nothing to disclose.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Kennedy A., Pissios P., Otu H., Roberson R., Xue B., Asakura K. A high-fat, ketogenic diet induces a unique metabolic state in mice. American Journal of Physiology. Endocrinology and Metabolism. 2007;292(6):39. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 2.Badman M.K., Koester A., Flier J.S., Kharitonenkov A., Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150(11):4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douris N., Stevanovic D.M., Fisher F.M., Cisu T.I., Chee M.J., Nguyen N.L. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology. 2015;156(7):2470–2481. doi: 10.1210/en.2014-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster G.D., Wyatt H.R., Hill J.O., McGuckin B.G., Brill C., Mohammed B.S. A randomized trial of a low-carbohydrate diet for obesity. The New England Journal of Medicine. 2003;348(21):2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 5.Douris N., Melman T., Pecherer J.M., Pissios P., Flier J.S., Cantley L.C. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochimica et biophysica acta. 2015;1852(10 Pt A):2056–2065. doi: 10.1016/j.bbadis.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badman M.K., Kennedy A.R., Adams A.C., Pissios P., Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. American Journal of Physiology. Endocrinology and Metabolism. 2009;297(5):E1197–E1204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen B.M., Ding X., Morgan D.A., Coate K.C., Bookout A.L., Rahmouni K. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metabolism. 2014;20(4):670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young J.B., Landsberg L. Diminished sympathetic nervous system activity in genetically obese (ob/ob) mouse. The American Journal of Physiology. 1983;245(2):E148–E154. doi: 10.1152/ajpendo.1983.245.2.E148. [DOI] [PubMed] [Google Scholar]
- 9.Bartness T.J., Vaughan C.H., Song C.K. Sympathetic and sensory innervation of brown adipose tissue. International Journal of Obesity (2005) 2010;34(Suppl 1):42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachman E.S., Dhillon H., Zhang C.Y., Cinti S., Bianco A.C., Kobilka B.K. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297(5582):843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 11.Bough K.J., Eagles D.A. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia. 1999;40(2):138–143. doi: 10.1111/j.1528-1157.1999.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez M., Barbatelli G., Allevi R., Cinti S., Seydoux J., Giacobino J.P. Beta 3-adrenoceptor knockout in C57BL/6J mice depresses the occurrence of brown adipocytes in white fat. European Journal of Biochemistry. 2003;270(4):699–705. doi: 10.1046/j.1432-1033.2003.03422.x. [DOI] [PubMed] [Google Scholar]
- 13.Volek J., Sharman M., Gomez A., Judelson D., Rubin M., Watson G. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutrition & Metabolism. 2004;1(1):13. doi: 10.1186/1743-7075-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dashti H.M., Mathew T.C., Hussein T., Asfar S.K., Behbahani A., Khoursheed M.A. Long-term effects of a ketogenic diet in obese patients. Experimental and Clinical Cardiology. 2004;9(3):200–205. [PMC free article] [PubMed] [Google Scholar]
- 15.Yancy W.S., Jr., Foy M., Chalecki A.M., Vernon M.C., Westman E.C. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutrition & Metabolism. 2005;2:34. doi: 10.1186/1743-7075-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mobbs C.V., Mastaitis J., Isoda F., Poplawski M. Treatment of diabetes and diabetic complications with a ketogenic diet. Journal of Child Neurology. 2013;28(8):1009–1014. doi: 10.1177/0883073813487596. [DOI] [PubMed] [Google Scholar]
- 17.Breslow M.J., Min-Lee K., Brown D.R., Chacko V.P., Palmer D., Berkowitz D.E. Effect of leptin deficiency on metabolic rate in ob/ob mice. The American Journal of Physiology. 1999;276(3 Pt 1):E443–E449. doi: 10.1152/ajpendo.1999.276.3.E443. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell N.J., Stock M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 19.Lowell B.B., V S.S., Hamann A., Lawitts J.A., Himms-Hagen J., Boyer B.B. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366(6457):740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 20.Feldmann H.M., Golozoubova V., Cannon B., Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism. 2009;9(2):203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Chernogubova E., Hutchinson D.S., Nedergaard J., Bengtsson T. Alpha1- and beta1-adrenoceptor signaling fully compensates for beta3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology. 2005;146(5):2271–2284. doi: 10.1210/en.2004-1104. [DOI] [PubMed] [Google Scholar]
- 22.Rohlfs E.M., Daniel K.W., Premont R.T., Kozak L.P., Collins S. Regulation of the uncoupling protein gene (Ucp) by beta 1, beta 2, and beta 3-adrenergic receptor subtypes in immortalized brown adipose cell lines. The Journal of Biological Chemistry. 1995;270(18):10723–10732. doi: 10.1074/jbc.270.18.10723. [DOI] [PubMed] [Google Scholar]
- 23.Arch J.R., Ainsworth A.T., Cawthorne M.A., Piercy V., Sennitt M.V., Thody V.E. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- 24.Nedergaard J., Lindberg O. The brown fat cell. International Review of Cytology. 1982;74:187–286. doi: 10.1016/s0074-7696(08)61173-0. [DOI] [PubMed] [Google Scholar]
- 25.Mattsson C.L., Csikasz R.I., Chernogubova E., Yamamoto D.L., Hogberg H.T., Amri E.Z. beta(1)-Adrenergic receptors increase UCP1 in human MADS brown adipocytes and rescue cold-acclimated beta(3)-adrenergic receptor-knockout mice via nonshivering thermogenesis. American Journal of Physiology. Endocrinology and Metabolism. 2011;301(6):E1108–E1118. doi: 10.1152/ajpendo.00085.2011. [DOI] [PubMed] [Google Scholar]
- 26.Brehm B.J., Seeley R.J., Daniels S.R., D'Alessio D.A. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. The Journal of Clinical Endocrinology and Metabolism. 2003;88(4):1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 27.Kinzig K.P., Honors M.A., Hargrave S.L. Insulin sensitivity and glucose tolerance are altered by maintenance on a ketogenic diet. Endocrinology. 2010;151(7):3105–3114. doi: 10.1210/en.2010-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jornayvaz F.R., Jurczak M.J., Lee H.Y., Birkenfeld A.L., Frederick D.W., Zhang D. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. American Journal of Physiology. Endocrinology and Metabolism. 2010;299(5):E808–E815. doi: 10.1152/ajpendo.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett E.J., DeFronzo R.A., Bevilacqua S., Ferrannini E. Insulin resistance in diabetic ketoacidosis. Diabetes. 1982;31(10):923–928. doi: 10.2337/diab.31.10.923. [DOI] [PubMed] [Google Scholar]
- 30.Garbow J.R., Doherty J.M., Schugar R.C., Travers S., Weber M.L., Wentz A.E. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2011;300(6):G956–G967. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26(3):271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.