Abstract
Objectives
β-cell dysfunction and apoptosis associated with islet inflammation play a key role in the pathogenesis of type 2 diabetes (T2D). Growing evidence suggests that islet amyloid, formed by aggregation of human islet amyloid polypeptide (hIAPP), contributes to islet inflammation and β-cell death in T2D. We recently showed the role of interleukin-1β (IL-1β)/Fas/caspase-8 apoptotic pathway in amyloid-induced β-cell death. In this study, we used human islets in culture as an ex vivo model of amyloid formation to: (1) investigate the effects of amyloid on islet levels of the natural IL-1 receptor antagonist (IL-1Ra); (2) examine if modulating the IL-1β/IL-1Ra balance can prevent amyloid-induced β-cell Fas upregulation and apoptosis.
Methods
Isolated human islets (n = 10 donors) were cultured in elevated glucose (to form amyloid) with or without a neutralizing human IL-1β antibody for up to 7 days. Parallel studies were performed with human islets in which amyloid formation was prevented by adeno-siRNA-mediated suppression of hIAPP expression (as control). β-cell levels of IL-1Ra, Fas, apoptosis as well as islet function, insulin- and amyloid-positive areas, and IL-1Ra release were assessed.
Results
Progressive amyloid formation in human islets during culture was associated with alterations in IL-1Ra. Islet IL-1Ra levels were higher at early stages but were markedly reduced at later stages of amyloid formation. Furthermore, IL-1Ra release from human islets was reduced during 7-day culture in a time-dependent manner. These changes in IL-1Ra production and release from human islets during amyloid formation adversely correlated with islet IL-1β levels, β-cell Fas expression and apoptosis. Treatment with IL-1β neutralizing antibody markedly reduced amyloid-induced β-cell Fas expression and apoptosis, thereby improving islet β-cell survival and function during culture.
Conclusions
These data suggest that amyloid formation impairs the balance between IL-1β and IL-1Ra in islets by increasing IL-1β production and reducing IL-1Ra levels thereby promoting β-cell dysfunction and death. Restoring the IL-1β/IL-1Ra ratio may provide an effective strategy to protect islet β-cells from amyloid toxicity in T2D.
Keywords: Islet amyloid, Amylin, Islet amyloid polypeptide, β-cell apoptosis, Interleukin-1β, Interleukin-1 receptor antagonist, Islet inflammation, Type 2 diabetes
Abbreviations: BSA, bovine serum albumin; ER, endoplasmic reticulum; FBS, fetal bovine serum; hIAPP, human islet amyloid polypeptide; IL-1β, interleukin-1β; IL-1Ra, IL-1 receptor antagonist; IL-1R1, IL-1 receptor type I; KRB, Krebs–Ringer bicarbonate; nIL1β, neutralizing IL-1β; PFA, paraformaldehyde; rIAPP, rat islet amyloid polypeptide; T2D, type 2 diabetes
Highlights
-
•
Endogenous amyloid formation alters IL-1Ra levels in human islet β-cells.
-
•
Amyloid impairs islet IL-1β/IL-1Ra balance by promoting IL-1β and reducing IL-1Ra.
-
•
Restoring IL-1β/IL-1Ra ratio by blocking IL-1β protects human islets against amyloid.
1. Introduction
Progressive loss of islet β-cell mass and function is a key defect in type 2 diabetes (T2D) that leads to β-cell failure and hyperglycemia [1], [2]. Increasing evidence suggests that islet inflammation contributes to progressive β-cell dysfunction in T2D [3], [4], [5], [6]. A key regulator of islet inflammation is the pro-inflammatory cytokine interleukin-1β (IL-1β) [7], [8]. The resident macrophages [9], [10], [11], [12] and pancreatic β-cells themselves [12], [13], [14], [15] are thought to be potential sources of IL-1β in islets. IL-1β signaling is mediated through IL-1 receptor type 1 (IL-1R1), while IL-1 receptor type 2 (IL-1R2) serves as a decoy receptor [16]. The high abundance of IL-1R1 on β-cells is likely the reason for higher sensitivity of β-cells to IL-1 action as compared to other islet cells [17], [18]. The contributing factors to islet inflammation and increased IL-1β levels in T2D are still not completely understood, but it appears that multiple factors such as elevated glucose, fatty acids, angiotensin II, and amyloid formation may contribute to this process [11], [12], [13], [14], [18], [19].
Islet amyloid, a pathological characteristic of the pancreas in T2D [20], [21], also forms in human islets during culture [14], [15], [22], [23] and following transplantation [24], [25], [26]. In all three conditions, amyloid formation is associated with progressive β-cell dysfunction and death [15], [21], [22], [26], [27]. Islet amyloid forms by aggregation of human islet amyloid polypeptide (hIAPP), a normally produced β-cell hormone that is co-expressed and co-secreted along with insulin from β-cells [28]. The oligomeric form of hIAPP is considered to be more toxic than large fibrils [20], [29]. It appears that multiple mechanisms contribute to amyloid-mediated β-cell death including endoplasmic reticulum (ER) stress [30], oxidative stress [31], defects in autophagy/lysosomal pathway [32], membrane disruption and formation of ion-channel like structures [33], [34]. The different mechanisms that contribute to islet amyloid induced β-cell toxicity may share similar apoptotic signaling pathways.
Growing evidence suggests that IL-1β plays a key role in mediating amyloid-induced islet inflammation: 1. Amyloid formation in cultured human and hIAPP-expressing mouse islets induces IL-1β production and β-cell expression of the Fas cell death receptor (APO-1/CD95) [14], [15]; 2. Prevention of amyloid formation markedly reduces IL-1β production, β-cell Fas expression and apoptosis in both cultured human and hIAPP-expressing mouse islets [12], [14]; 3. Treatment with anakinra (Kineret), a recombinant human IL-1 receptor antagonist, significantly decreases hIAPP-induced β-cell Fas upregulation and apoptosis [12]; 4. Exposure to synthetic hIAPP aggregates promotes IL-1β expression in islet resident macrophages [11]; 5. Islet amyloid formation in vivo is associated with islet inflammation and elevated pro-inflammatory cytokines, including IL-1β, in transgenic hIAPP-expressing mice [11], [35].
IL-1 receptor antagonist (IL-1Ra) is a natural inhibitor of both IL-1β and IL-1α which is produced and secreted from various cell types including monocytes, macrophages and islet β-cells [36], [37]. IL-1Ra has four described isoforms, three of which are intracellular while the fourth is secreted from cells [36]. The biological role of intracellular isoforms is still not clear, while the secreted isoform is known to competitively bind to IL-1R1 (but not IL-1R2) [36], [38]. Interestingly, IL-1Ra levels are reduced in islets from patients with T2D [37], suggesting that changes in IL-1Ra may play a role in the pathogenesis of T2D, but the underlying molecular mechanisms have yet to be identified.
We previously showed that the IL-1β/Fas/caspase-8 apoptotic signaling pathway plays a significant role in mediating β-cell death induced by biosynthetic hIAPP aggregates [12], [14], [15]. In this study, we investigated the potential changes in IL-1Ra levels during amyloid formation in human islets. We further examined whether modulating the balance between IL-1β and IL-1Ra by neutralizing IL-1β signaling can prevent amyloid-induced Fas upregulation and its β-cell toxicity.
2. Materials and methods
2.1. Human islet culture
Islets isolated from 10 cadaveric pancreatic donors (18–58 years) were provided by the Ike Barber Human Islet Transplant Laboratory (Vancouver, BC, Canada) in accordance with approved procedures and guidelines of the Clinical Research Ethics Board of the University of British Columbia. Hand-picked human islets (purity >90%; assessed by dithizone staining) were cultured free-floating (55 islets/well; non-adherent 24-well plate) in CMRL (Mediatech, Herndon, VA, USA) supplemented with 11.1 mmol/L glucose, 10% fetal bovine serum (FBS), 50 U/mL penicillin, 50 μg/mL streptomycin and 50 μg/mL gentamicin (Invitrogen, Burlington, ON, Canada). Islets were cultured in humidified 5% CO2/95% air at 37 °C. The medium was replaced every 48 hours.
2.2. Adenoviral transduction and treatment of human islets
Human islets were transduced overnight with Ad-prohIAPP-siRNA, an adenovirus that delivers a human proIAPP specific small interfering RNA (siRNA) at multiplicity of infection (MOI: 20), or Ad-control-siRNA, an adenovirus that delivers a random non-specific siRNA (MOI: 20), as previously described [22]. Transduced human islets were then rinsed and cultured in CMRL (11.1 mmol/L glucose, 7 days) in the presence or absence of a human IL-1β neutralizing monoclonal antibody (1 μg/mL; InvivoGen, San Diego, CA, USA), which blocks the biological activity of IL-1β (effective at IL-1β/IL-1Ra ratio: 1:2,000–1:8,000). Human islets treated with anakinra (Kineret, 10 μg/ml; donated by Sobia Pharmaceutics, Denton, MD, USA) were used as a control.
2.3. Cell culture
Transformed rat β-cell line, INS-1 (832/13), was provided by C. Newgard (Duke University Medical Center, NC, USA) and the mouse α-cell line, αTC-1, was from the American Type Culture Collection. INS-1 β-cells and αTC-1 cells were cultured in RPMI-1640 (Invitrogen; 11 mmol/L glucose) or DMEM (Invitrogen; 25 mmol/L glucose) respectively, supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin and 50 μmol/L 2-mercaptoethanol (for RPMI-1640 only). Cells were seeded (150,000/well) into 8-well chamber slides (BD Biosciences, Mississauga, ON, Canada) and treated with IL-1R1 antibody with or without IL-1β (R&D Systems, Minneapolis, MN, USA) for 24 hours.
2.4. Islet function test and IL-1Ra release
Human islets (25 islets/condition) were pre-incubated for 1 hour at 37 °C in Krebs–Ringer bicarbonate (KRB) buffer containing 10 mmol/L HEPES (pH: 7.4), 0.25% bovine serum albumin (BSA), and 1.67 mmol/L glucose. Islets were then incubated for 1-hour in fresh KRB buffer with 1.67 mmol/L glucose (basal insulin release) followed by 1-hour incubation in 16.7 mmol/L glucose (stimulated insulin release). Human islets in each well were lysed in 1 mmol/L HCl/0.1% BSA and heated at 100 °C for 10 min. Incubation media and islet lysates were centrifuged (12,000 rpm, 10 min, 4 °C) and frozen at −20 °C until assayed. Insulin levels were measured using a human specific insulin ELISA kit (ALPCO Diagnostics, Salem, NH, USA) and normalized to islet protein levels (Pierce-ThermoFisher, Rockford, IL, USA). Islet IL-1Ra release was measured in culture medium collected on day 3 and 7 from each condition by a human specific IL-1Ra ELISA (R&D Systems).
2.5. Quantitative immunohistochemistry
Paraffin-embedded islet or tissue sections (5 μm) were dewaxed and rehydrated followed by antigen retrieval with citrate buffer. Islet sections were blocked in 2% normal goat and/or donkey serum (Vector Labs, Burlingame, CA, USA) and incubated overnight at 4 °C with primary antibodies, followed by 1-hour incubation at room temperature with appropriate secondary antibodies as detailed in Supplementary Table 1. For TUNEL staining, after immunolabeling for insulin, islet sections (or cells) were incubated with TUNEL reaction mixture (1:20, Roche Diagnostics, Laval, QC, Canada) for 30 min at 37 °C. For thioflavin S staining, sections were incubated with 0.5% (wt/vol.) thioflavin S solution (Sigma–Aldrich, Oakville, ON, Canada) for 5 min at room temperature.
Images were captured using an Olympus IX81 inverted fluorescence microscope controlled by MetaMorph advanced software (version 7.7) or an ImageXpress Micro XLS system controlled by MetaXpress high-content image acquisition and analysis software (Molecular Devices Corporation; Sunnyvale, CA, USA) with a scientific CMOS camera and a 20× Plan Apo objective (NA = 0.75, 1-6300-0196; Nikon, Tokyo, Japan). The proportions of apoptotic and Fas-positive islet β-cells were assessed by counting the number of double insulin and TUNEL-positive or insulin and Fas-positive cells in islets. Islet β/α-cell ratio was calculated as the ratio of insulin- to glucagon-positive cells in islets. The proportions of A11 (oligomer)-positive and thioflavin S (amyloid)-positive islets were calculated as the percentage of A11 or thioflavin S-positive islets to total number of islets, respectively. Islet insulin- and amyloid-positive areas were calculated as the insulin-positive or thioflavin S-positive islet area divided by total islet area using Image-Pro analyzer software (version 6.3; Media Cybernetics, MD, USA). Quantitative immunohistochemistry analyses were performed on a minimum 4 human islet preparations (15–20 islets per condition in each study).
2.6. Statistical analysis
Data are expressed as means ± SEM. Statistical analyses were performed using two-way ANOVA followed by Newman–Keuls test as appropriate. A p-value of <0.05 was considered significant. Studies were performed on 4–10 human islet preparations (4–10 donors) as indicated in each figure legend.
3. Results
3.1. Treatment with neutralizing IL-1β antibody markedly reduces IL-1β immunoreactivity and prevents amyloid induced β-cell Fas upregulation in human islets during culture
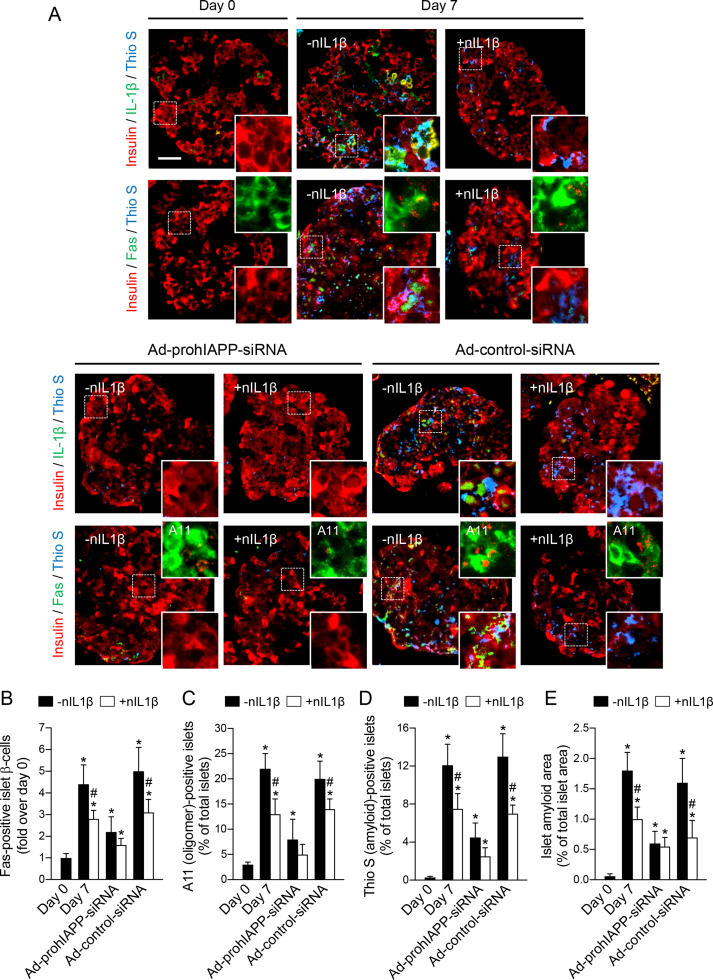
We examined the effects of blocking IL-1β action on its auto-stimulatory production and β-cell Fas expression in amyloid forming and non-forming human islets during culture. Human islets that formed islet amyloid during 7-day culture in elevated glucose (11.1 mmol/L) had markedly higher IL-1β immunoreactivity and Fas-positive β-cells than those in which amyloid formation was prevented by adeno-siRNA-mediated suppression of prohIAPP expression (Figure 1A,B). Blocking IL-1β action by treatment with a monoclonal human IL-1β neutralizing antibody markedly decreased amyloid-induced IL-1β immunoreactivity (likely by reducing auto-stimulatory effects of IL-1β) and β-cell Fas upregulation in cultured human islets. Furthermore, neutralizing IL-1β reduced the proportion of both oligomer (A11)-positive and thioflavin S (amyloid)-positive islets as well as amyloid area to total islet area during 7-day culture (Figure 1C–E). Similarly, human islets transduced with Ad-control-siRNA (which expresses a non-specific siRNA) formed amyloid during 7-day culture and had comparable IL-1β immunoreactivity and Fas-positive β-cells with non-treated human islets, both of which were markedly reduced by treatment with IL-1β antibody.
Figure 1.
Treatment with neutralizing IL-1β antibody markedly reduces islet IL-1β immunoreactivity and prevents β-cell Fas upregulation induced by amyloid formation in cultured human islets. Human islets non-transduced or transduced with Ad-prohIAPP-siRNA or Ad-control-siRNA (MOI: 20) were cultured with (+nIL1β) or without (-nIL1β) a neutralizing monoclonal human IL-1β antibody (1 μg/ml) in CMRL (11.1 mmol/L glucose) for 7 days. (A) Paraffin-embedded islet sections were immunolabeled for insulin (red), IL-1β or Fas (green) and thioflavin S (Thio S; blue) as indicated. The squares (dashed white lines) denote regions enlarged and depicted as inserts at the bottom right of each image. The top right inserts in each micrograph show immunolabeling for insulin (green) and A11 (red). Scale bar = 50 μm; inserts: ×3 (A11: ×4). (B) The proportion of Fas positive islet β-cells (fold over day 0). The percentage of (C) A11 (oligomer)-positive islets, (D) thioflavin S (amyloid) positive islets, and (E) amyloid area to total islet area. Data are presented as mean ± SEM of four independent studies (4 human islet preparations); n = 15–20 islets per condition in each study. *vs day 0; #vs corresponding non-treated group (p < 0.05, two-way ANOVA).
3.2. Neutralizing IL-1β signaling reduces amyloid-induced β-cell toxicity and enhances β-cell function in cultured human islets
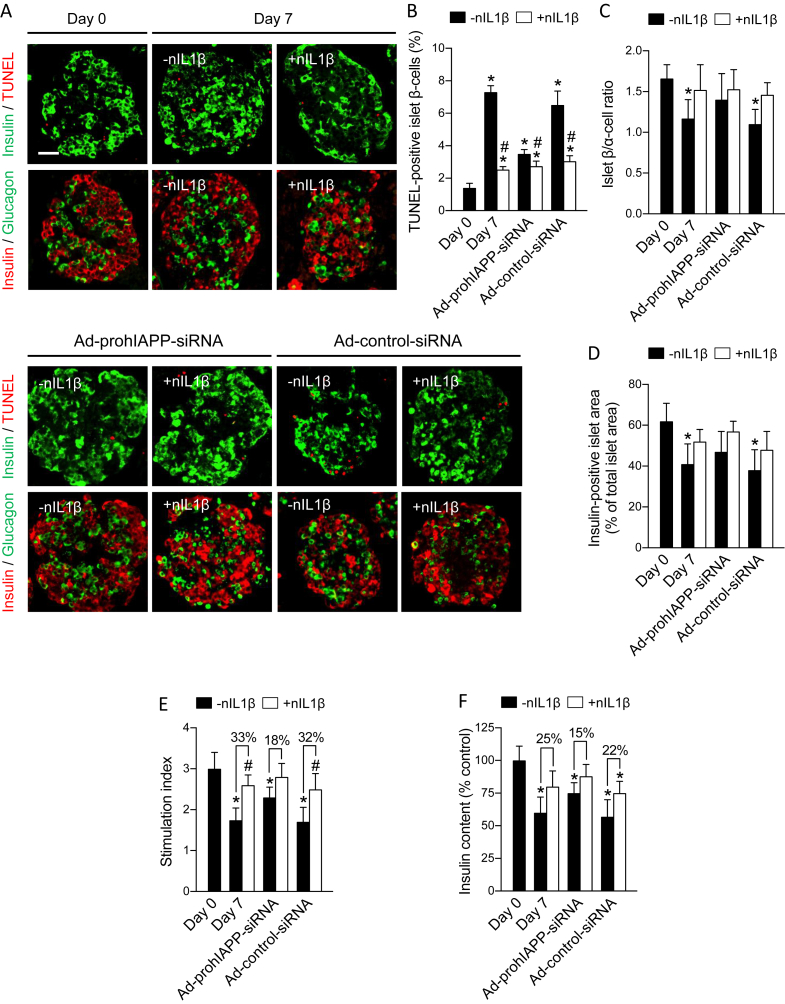
Blocking IL-1β action by treatment with IL-1β antibody markedly reduced the proportion of TUNEL-positive (apoptotic) β-cells in human islets during 7-day culture similar to that observed by adeno-siRNA-mediated inhibition of amyloid formation (Figure 2A,B). Treatment of Ad-prohIAPP-siRNA transduced islets with IL-1β antibody further reduced the number of apoptotic β-cells during 7-day culture as compared to islets treated with Ad-prohIAPP-siRNA alone. Moreover, as expected, progressive loss of islet β-cells during culture resulted in a significantly lower β/α-cell ratio and insulin-positive islet area in 7-day cultured human islets as compared to freshly isolated islets. Blocking IL-1β action increased islet β/α-cell ratio and insulin-positive area by ∼23% and ∼21%, respectively (Figure 2C,D). Finally, blocking IL-1β action improved β-cell function in 7-day cultured human islets manifested as higher islet insulin content and insulin response to elevated glucose (Figure 2E,F).
Figure 2.
Neutralizing IL-1β signaling reduces amyloid-induced β-cell apoptosis, improves islet β/α-cell ratio and β-cell area, and enhances β-cell function in cultured human islets. Human islets non-transduced or transduced with Ad-prohIAPP-siRNA (or Ad-control-siRNA) were cultured with (+nIL1β) or without (-nIL1β) neutralizing IL-1β antibody (1 μg/ml) for 7 days. (A) Paraffin-embedded islet sections were immunolabeled for insulin (green) and TUNEL (red) or insulin (red) and glucagon (green) as indicated. Scale bar = 50 μm. (B) The proportion of TUNEL-positive islet β-cells, (C) islet β/α-cell ratio, and (D) insulin-positive area to total islet area. (E) Islet insulin response to elevated glucose (stimulation index) and (F) islet insulin content were measured in each condition. Data are presented as mean ± SEM of 6–8 independent studies (6–8 donors); n = 15–20 islets per condition in each study. Glucose stimulation test was performed on 10 human islet preparations (15 islets per condition in each study). *vs day 0; #vs corresponding non-treated group (p < 0.05, two-way ANOVA).
3.3. Islet IL-1Ra immunoreactivity is elevated in oligomer-positive human islets but is reduced in thioflavin S-positive human islets
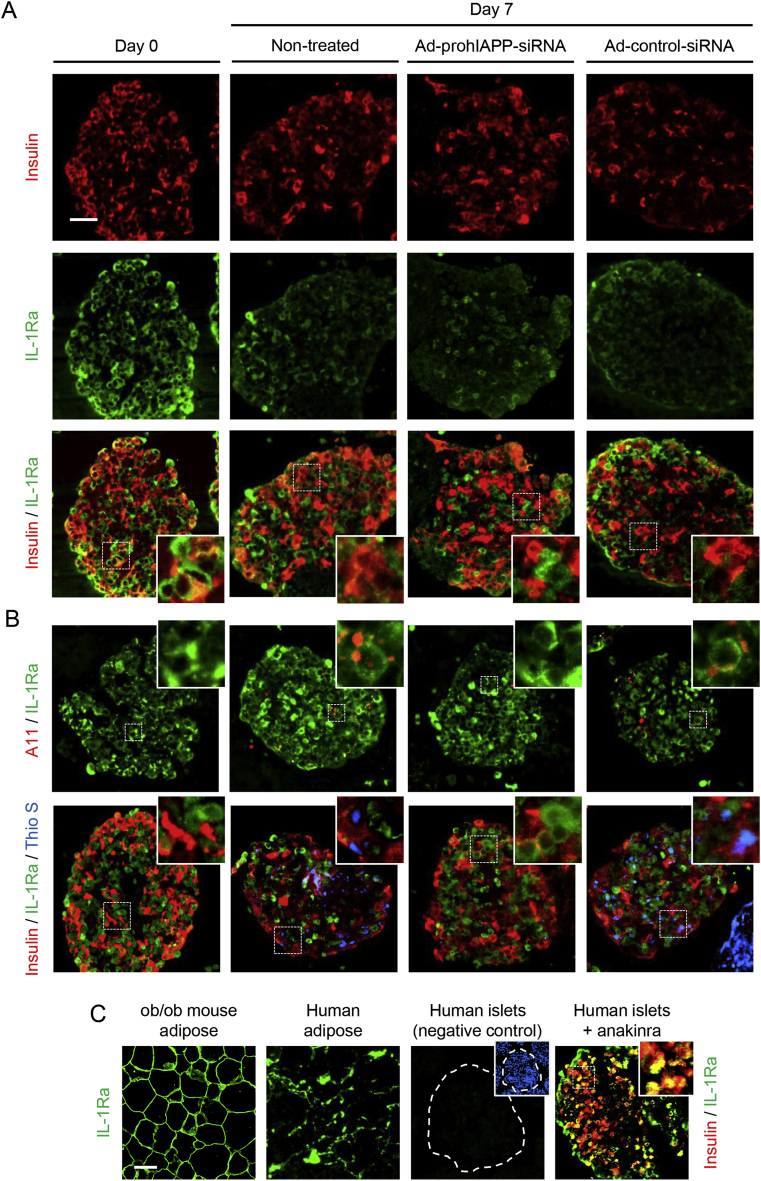
We next examined the effects of amyloid formation at early and late stages of this process on IL-1Ra levels during islet culture. Human islets following 7-day culture in elevated (11.1 mmol/L) glucose had two distinguished populations of IL-1Ra-positive cells, those with low or high IL-1Ra immunoreactivity (Figure 3A). This pattern of IL-1Ra expression was observed mainly in amyloid forming cultured islets and to a much lesser extent in Ad-prohIAPP-siRNA transduced islets in which amyloid formation was suppressed (Figure 3) or in islets cultured in normal (5.5 mmol/L) glucose (Supplementary Figure 1). Interestingly, islet areas which had stronger IL-1Ra immunoreactivity closely correlated with islet areas containing small hIAPP aggregates (oligomers), while islet areas which had faint IL-1Ra immunoreactivity closely correlated with thioflavin S-positive islet areas containing large hIAPP fibrils (Figure 3B). Islet IL-1Ra immunoreactivity was also detectable in human islets treated with anakinra, a recombinant IL-1Ra that binds to and therefore blocks IL-1R1 on islet cells, suggesting that islet IL-1Ra immunoreactivity, at least partially, is due to its production in human islet cells (Figure 3C).
Figure 3.
IL-1Ra levels are increased in oligomer-positive islet areas and reduced in thioflavin S-positive islet areas. (A) Paraffin embedded sections from 7-day cultured (11.1 mmol/L glucose) non-transduced or transduced with Ad-prohIAPP-siRNA (or Ad-control-siRNA) islets were immunolabeled for insulin (red) and IL-1Ra (green). The squares (dashed white lines) denote enlarged regions shown as inserts. (B) Human islets were immunolabeled for IL-1Ra (green) and A11 (red; top panel) or insulin (red), IL-1Ra (green) and thioflavin S (Thio S; blue; bottom panel). (C) Paraffin-embedded sections from (left to right): ob/ob mouse and human adipose tissue immunolabeled for IL-1Ra (green; positive control); human islets incubated with secondary antibody alone (negative control) and anakinra-treated human islets immunolabeled for insulin (red) and IL-1Ra (green). Scale bar = 50 μm; inserts: ×3 (A11: ×4). Micrographs are representative of four independent studies (4 human islet preparations).
3.4. Protein expression of β-cell specific genes Pdx-1 and MafA are not markedly changed during 7-day culture
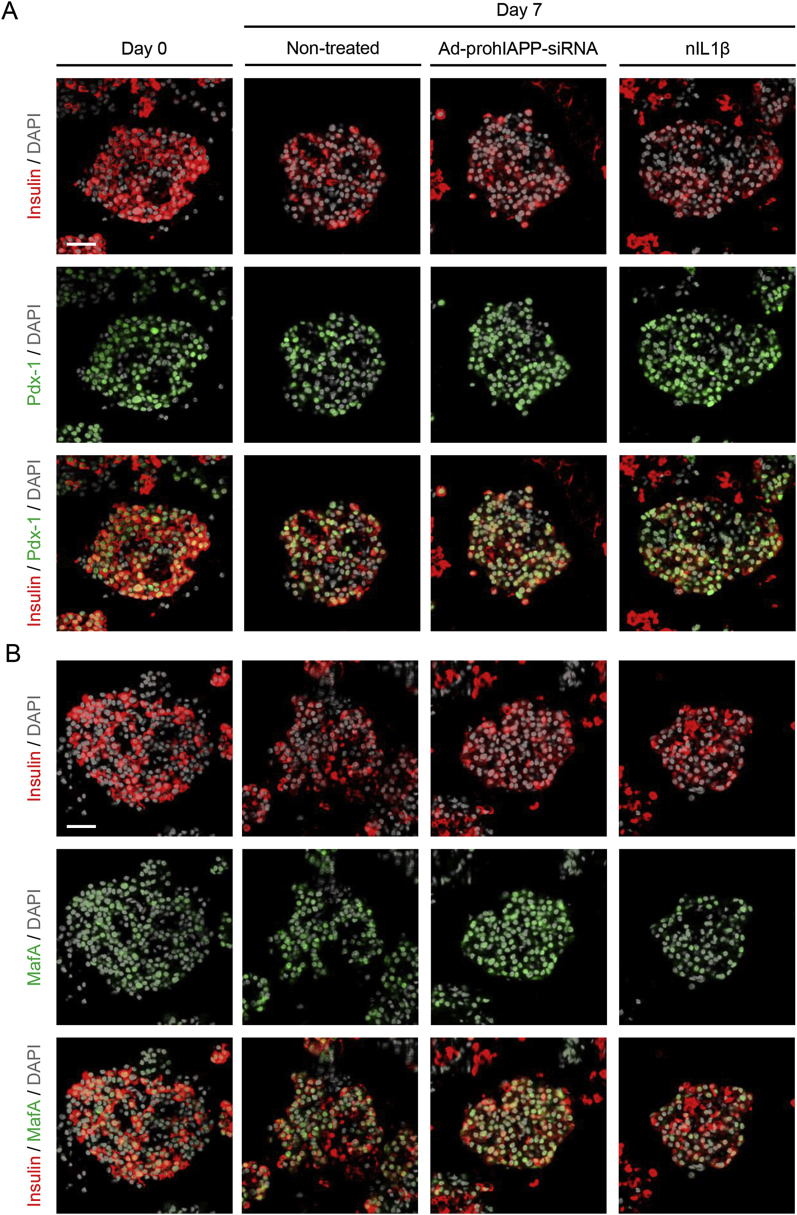
To examine whether IL-1Ra downregulation observed in amyloid forming human islets during culture in elevated glucose is specific to IL-1Ra or is due to a global decrease in β-cell protein expression associated with culture period, we assessed Pdx-1 and MafA, two β-cell specific proteins, in pre-culture and 7-day cultured human islets in different conditions (Figure 4). Following 7-day culture, Pdx-1 and MafA immunoreactivity in human islets were mildly decreased or comparable with pre-culture islets, suggesting that 7-day islet culture does not have any significant effect on their protein expression. Moreover, there was no detectable difference in Pdx-1 and MafA immunoreactivity between non-treated, Ad-prohIAPP-siRNA transduced, or neutralizing IL-1β antibody treated cultured islets.
Figure 4.
Expression of β-cell specific proteins in pre-culture and 7-day cultured human islets. Human islets non-treated (control), transduced with Ad-prohIAPP-siRNA or treated with neutralizing IL-1β antibody (nIL1β; 1 μg/ml) were cultured (11.1 mmol/L glucose) for 7 days. Paraffin-embedded islet sections were immunolabeled for (A) insulin (red), Pdx-1 (green), and DAPI (gray) or (B) insulin (red), MafA (green), and DAPI (gray) as indicated. Scale bar = 50 μm. Micrographs are representative of four independent studies (4 human islet preparations).
3.5. IL-1Ra immunoreactivity is detectable in human islet α-cells
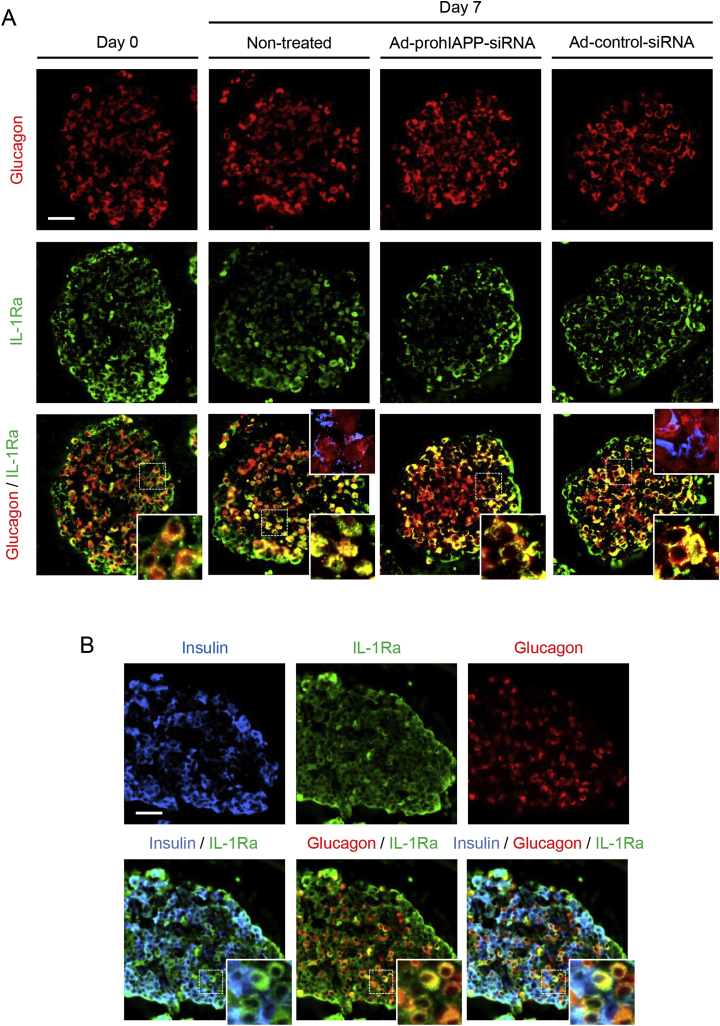
We also performed double glucagon and IL-1Ra immunolabeling on pre-culture and 7-day cultured human islets, to examine if IL-1Ra-positive islet cells that were not insulin-positive (Figure 3A) might be α-cells. Interestingly, we found IL-1Ra immunoreactivity in a significant number of glucagon-positive islet cells in both pre-culture and 7-day cultured human islets (Figure 5). These immunolabeling studies were further validated using a different IL-1Ra antibody (Supplementary Figure 2). Moreover, treatment of transformed αTC-1 cells with IL-1β increased IL-1Ra immunoreactivity similar to that observed in transformed INS-1 β-cells, although αTC-1 cells had much lower basal IL-1Ra immunoreactivity as compared to INS-1 β-cells (Supplementary Figure 3). Also, the effective IL-1β concentration required to induce IL-1Ra in αTC-1 cells was higher than that in INS-1 β-cells.
Figure 5.
IL-1Ra immunoreactivity is detectable in human islet α-cells. (A) Paraffin-embedded sections from human islets transduced with Ad-prohIAPP-siRNA (or Ad-control-siRNA) and cultured (11.1 mmol/L glucose) for 7 days were immunolabeled for glucagon (red) and IL-1Ra (green). (B) Triple immunostaining of pre-culture human islets for insulin (blue), glucagon (red) and IL-1Ra (green). The squares (dashed white lines) denote regions enlarged and depicted as inserts at the bottom right of the corresponding images. Amyloid formation during islet culture is shown as an insert in merged micrographs (top right, insulin; red and thioflavin S; blue). Scale bar = 50 μm; inserts: ×3. Micrographs are representative of four independent studies (4 human islet preparations).
3.6. IL-1Ra release from human islets is reduced during 7-day culture
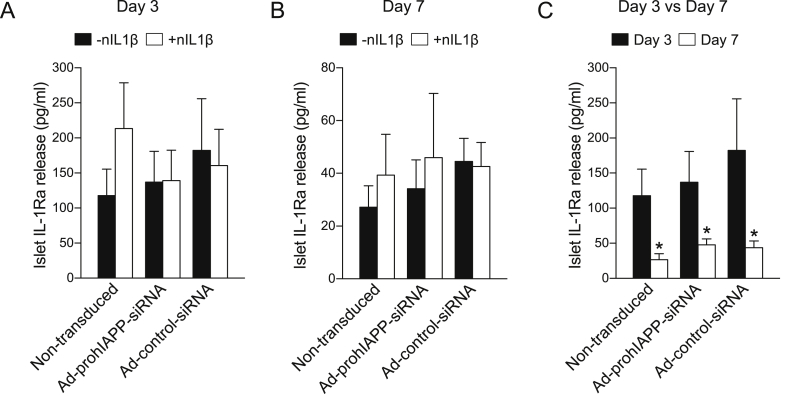
IL-1Ra was measured by ELISA in the culture medium collected on day 3 and day 7 from non-transduced, Ad-prohIAPP-siRNA, or Ad-control-siRNA transduced human islets with or without treatment with neutralizing IL-1β antibody. IL-1Ra release from human islets was reduced during 7-day culture in a time-dependent manner (Figure 6), which correlated with progressive amyloid formation (Figure 1) and loss of β-cells during culture (Figure 2). There was no significant difference in IL-1Ra levels detected in the culture medium from different groups.
Figure 6.
IL-1Ra release from Ad-prohIAPP-siRNA transduced and non-transduced human islets cultured with or without neutralizing IL-1β antibody. (A, B) IL-1Ra release from human islets transduced with Ad-prohIAPP-siRNA or Ad-control-siRNA following 3 or 7 days culture (11.1 mmol/L glucose) in the presence (+nIL1β) or absence (-nIL1β) of neutralizing IL-1β antibody. (C) Comparison between islet IL-1Ra release following 3 and 7 days culture in each condition. Data are expressed as mean ± SEM of four independent studies (4 human islet preparations) performed in duplicate. *vs corresponding day 3 group (p < 0.05, two-way ANOVA).
4. Discussion
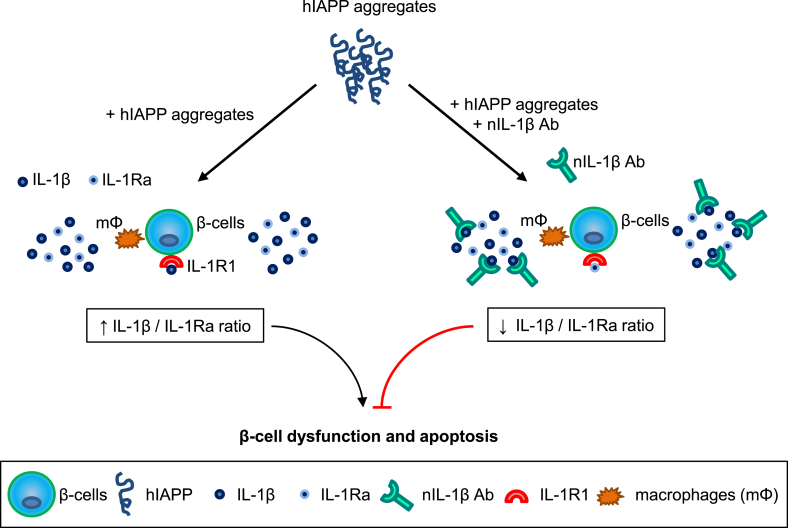
Increasing evidence from recent studies suggests that amyloid formation contributes to islet inflammation in T2D [11], [12], [14], [15], [35], [39] but the underlying molecular mechanisms are largely unknown. Our recent studies have shown that the IL-1β/Fas/caspase-8 apoptotic pathway plays an important role in amyloid-induced β-cell death in human islets [12], [14], [15]. In this study, we demonstrate that production of IL-1Ra, the key natural inhibitor of IL-1β, is reduced in amyloid-positive human islets. We further show that restoring amyloid-induced impairment in IL-1β/IL-1Ra balance by neutralizing IL-1β can prevent Fas upregulation and β-cell apoptosis mediated by amyloid formation. Thus, it appears that amyloid formation disrupts the balance between islet IL-1β and IL-1Ra by promoting IL-1β and reducing IL-1Ra production, leading to β-cell dysfunction and death (Figure 7).
Figure 7.
A proposed mechanism for islet amyloid-induced islet inflammation and β-cell apoptosis. Amyloid formation promotes islet IL-1β production while reducing islet IL-1Ra levels resulting in increased IL-1β/IL-1Ra ratio thereby contributing to islet inflammation, β-cell dysfunction, and apoptosis. Blocking IL-1β signaling or preserving islet IL-1Ra levels to restore IL-1β/IL-1Ra balance may provide effective potential strategies to protect islet β-cells from amyloid toxicity in pathologic conditions associated with islet amyloid formation.
Elevated islet IL-1β levels [13], [40] associated with increased number of islet resident macrophages [9] and reduced islet IL-1Ra levels [37] has been reported in patients with T2D, raising the idea that the balance between IL-1β and IL-1Ra may play a key role in the pathogenesis of T2D. The factor(s) that contribute to changes in IL-1β/IL-1Ra ratio during T2D are still not well understood. One of the key findings of our study was to show that endogenous amyloid formation alters IL-1Ra levels in human islets. Islet IL-1Ra immunoreactivity was higher at early stages of amyloid formation but was markedly reduced at later stages of amyloid formation in cultured human islets. Furthermore, IL-1Ra release from human islets was significantly reduced during culture. The decrease in IL-1Ra levels in human islets cultured in elevated glucose (which formed amyloid) was much more profound than those cultured in normal glucose (which formed no or little amyloid) and was markedly improved by adeno-siRNA-mediated suppression of amyloid formation.
These changes in islet IL-1Ra production and release during amyloid formation in culture adversely correlated with increased islet IL-1β levels. The higher IL-1Ra levels observed at early stages of amyloid formation suggest a local protective mechanism in islets against β-cell apoptotic factors. The decrease in islet IL-1Ra levels at later stages of amyloid formation might be due, at least partially, to progressive β-cell dysfunction and inefficient protein synthesis associated with amyloid toxicity during islet culture. However, expression of β-cell specific proteins, Pdx-1 and MafA, was mildly changed during 7-day culture period therefore other mechanisms, in addition to global decrease in β-cell protein expression, may also contribute to reduced IL-1Ra levels in amyloid forming human islets.
Consistent with our previous findings [12], [14] amyloid formation in human islets during culture in elevated glucose promoted IL-1β production and β-cell (but not α-cell) Fas upregulation, leading to increased β-cell apoptosis. Blocking IL-1β action by treatment of human islets with a neutralizing IL-1β antibody, which specifically blocks IL-1β (but not IL-1α) action, markedly reduced islet IL-1β levels and prevented amyloid-induced Fas upregulation and β-cell death. Moreover, adeno-siRNA-mediated prevention of amyloid formation or specific blockade of IL-1β were both able to efficiently reduce β-cell Fas upregulation and apoptosis in human islets during 7-day culture. Blocking IL-1β action in human islets also enhanced β-cell function and reduced amyloid formation. This finding further supports our previous studies in which we showed that enhancing β-cell function either by glucagon-like peptide-1 (GLP-1) agonists [23] or IL-1 receptor antagonists [12] can improve processing of prohIAPP, the IAPP precursor, thereby reducing amyloid formation.
Resident macrophages [9], [10], [11], [12] and islet β-cells [12], [13], [14], [15] are two potential sources of IL-1β in islets. Therefore, reduced IL-1β immunoreactivity in β-cells of human islets treated with neutralizing IL-1β antibody is likely mediated by two different mechanisms. IL-1β has been shown to increase its own production via auto-stimulation [40]. Thus, blocking IL-1β with a neutralizing antibody can ultimately reduce its production in islet macrophages and β-cells (or other islet cells) by inhibition of IL-1β auto-stimulatory action. Moreover, IL-1β immunoreactivity in islet β-cells might be partially derived from IL-1β that is produced by islets or islet resident macrophages due to amyloid formation and bound to IL-1R1 on β-cells. So prevention of IL-1β binding to IL-1R1 in islet cells by IL-1β antibody treatment may potentially reduce islet IL-1β immunoreactivity.
Interestingly, we also observed IL-1Ra immunoreactivity in the majority of human islet α-cells. This finding suggests that primary human α-cells, same as β-cells, produce IL-1Ra and that elevated IL-1β levels associated with islet culture and amyloid formation may promote IL-1Ra production in α-cells. This notion is supported by our in vitro studies, which showed that IL-1β can induce IL-1Ra production in both transformed INS-1 β-cells and αTC-1 cells; albeit IL-1Ra immunoreactivity was much lower in αTC-1 cells and inducing IL-1Ra production in αTC-1 cells required a higher concentration of IL-1β as compared to INS-1 β-cells. It is also possible that IL-1Ra produced and released from islet resident macrophages and β-cells binds to IL-1R1 on α-cells resulting in positive IL-1Ra immunoreactivity in these cells.
Taken together, our findings suggest that modulating IL-1β/IL-1Ra balance by neutralizing IL-1β can prevent amyloid-induced β-cell dysfunction and death. Therefore, restoring the IL-1β/IL-1Ra ratio by treatment strategies that focus on specific blockade of IL-1β or increasing IL-1Ra production in islets may provide an effective therapeutic approach to protect β-cells from amyloid toxicity as well as other factors that mediate islet IL-1β production in T2D. Furthermore, the findings of this study may also be applicable to other amyloid-associated pathologic conditions such as Alzheimer's disease in which amyloidogenic Aβ peptide contributes to neural inflammation by promoting IL-1β maturation and release [41]. In support, IL-1Ra is normally produced in human neural cells, and its expression and release are altered in patients with Alzheimer's disease [42], [43].
In summary, our studies suggest that amyloid formation impairs the balance between islet IL-1β and IL-1Ra both by increasing IL-1β and reducing IL-1Ra production, thereby promoting β-cell dysfunction and death. Pharmacological strategies to reduce the IL-1β/IL-1Ra ratio may effectively protect β-cells from amyloid toxicity in conditions associated with islet amyloid formation such as T2D and human islet grafts in T1D.
Funding
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) to L. Marzban (MOP-126204). Infrastructure support was provided by a grant from the Canadian Foundation for Innovation to L. Marzban (13391). Q. Hui was recipient of a CIHR studentship.
Contribution statement
All authors contributed to the conception and design, or analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content. All authors gave final approval of the version to be published.
Acknowledgments
Human islets for these studies were provided by the Ike Barber Human Islet Transplant Laboratory (Vancouver, BC, Canada). We gratefully acknowledge the outstanding technical assistance of I. Barta (Histology Service Laboratory, Biomedical Research Centre, University of British Columbia, Vancouver, Canada).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2017.05.016.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1. IL-1Ra immunoreactivity in human islets cultured in low or high glucose. Paraffin-embedded sections from human islets cultured in 5.5 or 11.1 mmol/L glucose for 7 days were immunolabeled for insulin (red), IL-1Ra (green), and thioflavin S (Thio S; blue). The squares (dashed white lines) denote enlarged regions shown as inserts. Scale bar = 50 μm; inserts: ×3.
Supplementary Figure 2. IL-1Ra immunoreactivity in human islets. Paraffin embedded human islet sections from 7-day cultured (11.1 mmol/L glucose) non-transduced or transduced with Ad-prohIAPP-siRNA (or Ad-control-siRNA) were immunolabeled for (A) insulin (red) and IL-1Ra (green) or (B) glucagon (red) and IL-1Ra (green). (C) Paraffin-embedded sections from mouse and human adipose tissue immunolabeled for IL-1Ra (green; positive control). IL-1Ra immuno-labeling was performed using a polyclonal rabbit anti-IL-1Ra antibody (ThermoFisher; PA5-21776). The squares (dashed white lines) denote enlarged regions shown as inserts. Scale bar = 50 μm; inserts: ×3.
Supplementary Figure 3. IL-1Ra immunoreactivity in INS-1 β-cells and αTC-1 cells. (A) INS-1 β-cells and (B) αTC-1 cells treated with recombinant IL-1β, neutralizing IL-1R1 (nIL-1R1) antibody, or both were immunolabeled for insulin or glucagon (red) and IL-1Ra or Fas (green). Scale bar = 50 μm. The proportion of TUNEL-positive β-cells (C) and α-cells (D) was quantified by counting the number of double insulin (or glucagon) and TUNEL-positive cells in a minimum of five microscopic fields each containing 300–400 INS-1 β-cells or αTC-1 cells. *vs corresponding IL-1β non-treated group; #vs corresponding nIL1R1 non-treated group (p < 0.05, two-way ANOVA).
References
- 1.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 2.Kahn S.E., Cooper M.E., Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 4.Quan W., Jo E.K., Lee M.S. Role of pancreatic beta-cell death and inflammation in diabetes. Diabetes Obesity Metabolism. 2013;15(Suppl. 3):141–151. doi: 10.1111/dom.12153. [DOI] [PubMed] [Google Scholar]
- 5.Donath M.Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nature Reviews Drug Discovery. 2014;13(6):465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 6.Donath M.Y. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia. 2016;59(4):679–682. doi: 10.1007/s00125-016-3873-z. [DOI] [PubMed] [Google Scholar]
- 7.Ehses J.A., Lacraz G., Giroix M.H., Schmidlin F., Coulaud J., Kassis N. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proceedings of the Natural Academy of Sciences of the United States of America. 2009;106(33):13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello C.A., Donath M.Y., Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Current Opinion in Endocrinology, Diabetes, and Obesity. 2010;17(4):314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 9.Ehses J.A., Perren A., Eppler E., Ribaux P., Pospisilik J.A., Maor-Cahn R. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56(9):2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 10.Masters S.L., Dunne A., Subramanian S.L., Hull R.L., Tannahill G.M., Sharp F.A. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nature Immunology. 2010;11(10):897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westwell-Roper C.Y., Ehses J.A., Verchere C.B. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1beta production and beta-cell dysfunction. Diabetes. 2014;63(5):1698–1711. doi: 10.2337/db13-0863. [DOI] [PubMed] [Google Scholar]
- 12.Park Y.J., Warnock G.L., Ao Z., Safikhan N., Meloche M., Asadi A. Dual role of IL-1beta in islet amyloid formation and its beta-cell toxicity: implications for type 2 diabetes and islet transplantation. Diabetes, Obesity and Metabolism. 2017;19(5):682–694. doi: 10.1111/dom.12873. [DOI] [PubMed] [Google Scholar]
- 13.Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H.I., Spinas G.A. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. Journal of Clinical Investigation. 2002;110(6):851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park Y.J., Lee S., Kieffer T.J., Warnock G.L., Safikhan N., Speck M. Deletion of Fas protects islet beta cells from cytotoxic effects of human islet amyloid polypeptide. Diabetologia. 2012;55(4):1035–1047. doi: 10.1007/s00125-012-2451-2. [DOI] [PubMed] [Google Scholar]
- 15.Park Y.J., Woo M., Kieffer T.J., Hakem R., Safikhan N., Yang F. The role of caspase-8 in amyloid-induced beta cell death in human and mouse islets. Diabetologia. 2014;57(4):765–775. doi: 10.1007/s00125-013-3152-1. [DOI] [PubMed] [Google Scholar]
- 16.Garlanda C., Dinarello C.A., Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarim A.L., Arnush M., Hill J.R., Marshall C.A., Baldwin A., McDaniel M.L. Evidence for the presence of type I IL-1 receptors on beta-cells of islets of Langerhans. Biochimica et Biophysica Acta. 1997;1361(3):313–320. doi: 10.1016/s0925-4439(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 18.Boni-Schnetzler M., Boller S., Debray S., Bouzakri K., Meier D.T., Prazak R. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150(12):5218–5229. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 19.Sauter N.S., Thienel C., Plutino Y., Kampe K., Dror E., Xu S. Angiotensin II induces IL-1beta-mediated islet inflammation and beta-cell dysfunction independently of vasoconstrictory effects. Diabetes. 2015;64(4):1273–1283. doi: 10.2337/db14-1282. [DOI] [PubMed] [Google Scholar]
- 20.Westermark P., Andersson A., Westermark G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiological Reviews. 2011;91(3):795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 21.Jurgens C.A., Toukatly M.N., Fligner C.L., Udayasankar J., Subramanian S.L., Zraika S. Beta-cell loss and beta-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. The American Journal of Pathology. 2011;178(6):2632–2640. doi: 10.1016/j.ajpath.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzban L., Tomas A., Becker T.C., Rosenberg L., Oberholzer J., Fraser P.E. Small interfering RNA-mediated suppression of proislet amyloid polypeptide expression inhibits islet amyloid formation and enhances survival of human islets in culture. Diabetes. 2008;57(11):3045–3055. doi: 10.2337/db08-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park Y.J., Ao Z., Kieffer T.J., Chen H., Safikhan N., Thompson D.M. The glucagon-like peptide-1 receptor agonist exenatide restores impaired pro-islet amyloid polypeptide processing in cultured human islets: implications in type 2 diabetes and islet transplantation. Diabetologia. 2013;56(3):508–519. doi: 10.1007/s00125-012-2802-z. [DOI] [PubMed] [Google Scholar]
- 24.Westermark G., Westermark P., Eizirik D.L., Hellerstrom C., Fox N., Steiner D.F. Differences in amyloid deposition in islets of transgenic mice expressing human islet amyloid polypeptide versus human islets implanted into nude mice. Metabolism. 1999;48(4):448–454. doi: 10.1016/s0026-0495(99)90102-6. [DOI] [PubMed] [Google Scholar]
- 25.Westermark G.T., Westermark P., Berne C., Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. The New England Journal of Medicine. 2008;359(9):977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 26.Potter K.J., Abedini A., Marek P., Klimek A.M., Butterworth S., Driscoll M. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4305–4310. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Udayasankar J., Kodama K., Hull R.L., Zraika S., Aston-Mourney K., Subramanian S.L. Amyloid formation results in recurrence of hyperglycaemia following transplantation of human IAPP transgenic mouse islets. Diabetologia. 2009;52(1):145–153. doi: 10.1007/s00125-008-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn S.E., D'Alessio D.A., Schwartz M.W., Fujimoto W.Y., Ensinck J.W., Taborsky G.J., Jr. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39(5):634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 29.Haataja L., Gurlo T., Huang C.J., Butler P.C. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocrine Reviews. 2008;29(3):303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C.J., Lin C.Y., Haataja L., Gurlo T., Butler A.E., Rizza R.A. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56(8):2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 31.Zraika S., Hull R.L., Udayasankar J., Aston-Mourney K., Subramanian S.L., Kisilevsky R. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52(4):626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera J.F., Gurlo T., Daval M., Huang C.J., Matveyenko A.V., Butler P.C. Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic beta-cells: protective role of p62-positive cytoplasmic inclusions. Cell Death and Differentiation. 2011;18(3):415–426. doi: 10.1038/cdd.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demuro A., Mina E., Kayed R., Milton S.C., Parker I., Glabe C.G. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. The Journal of Biological Chemistry. 2005;280(17):17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J., Hu R., Sciacca M.F., Brender J.R., Chen H., Ramamoorthy A. Non-selective ion channel activity of polymorphic human islet amyloid polypeptide (amylin) double channels. Physical Chemistry Chemical Physics. 2014;16(6):2368–2377. doi: 10.1039/c3cp53345j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westwell-Roper C.Y., Chehroudi C.A., Denroche H.C., Courtade J.A., Ehses J.A., Verchere C.B. IL-1 mediates amyloid-associated islet dysfunction and inflammation in human islet amyloid polypeptide transgenic mice. Diabetologia. 2014;58(3):575–585. doi: 10.1007/s00125-014-3447-x. [DOI] [PubMed] [Google Scholar]
- 36.Arend W.P., Malyak M., Guthridge C.J., Gabay C. Interleukin-1 receptor antagonist: role in biology. Annual Review of Immunology. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 37.Maedler K., Sergeev P., Ehses J.A., Mathe Z., Bosco D., Berney T. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8138–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Symons J.A., Young P.R., Duff G.W. Soluble type II interleukin 1 (IL-1) receptor binds and blocks processing of IL-1 beta precursor and loses affinity for IL-1 receptor antagonist. Proceedings of the Natural Academy of Sciences of the United States of America. 1995;92(5):1714–1718. doi: 10.1073/pnas.92.5.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier D.T., Morcos M., Samarasekera T., Zraika S., Hull R.L., Kahn S.E. Islet amyloid formation is an important determinant for inducing islet inflammation in high-fat-fed human IAPP transgenic mice. Diabetologia. 2014;57(9):1884–1888. doi: 10.1007/s00125-014-3304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boni-Schnetzler M., Thorne J., Parnaud G., Marselli L., Ehses J.A., Kerr-Conte J. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta-cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. The Journal of Clinical Endocrinology and Metabolism. 2008;93(10):4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasuhara O., Matsuo A., Terai K., Walker D.G., Berger A.E., Akiguchi I. Expression of interleukin-1 receptor antagonist protein in post-mortem human brain tissues of Alzheimer's disease and control cases. Acta Neuropathologia. 1997;93(4):414–420. doi: 10.1007/s004010050633. [DOI] [PubMed] [Google Scholar]
- 43.Tarkowski E., Liljeroth A.M., Nilsson A., Minthon L., Blennow K. Decreased levels of intrathecal interleukin 1 receptor antagonist in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2001;12(5):314–317. doi: 10.1159/000051276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.