Abstract
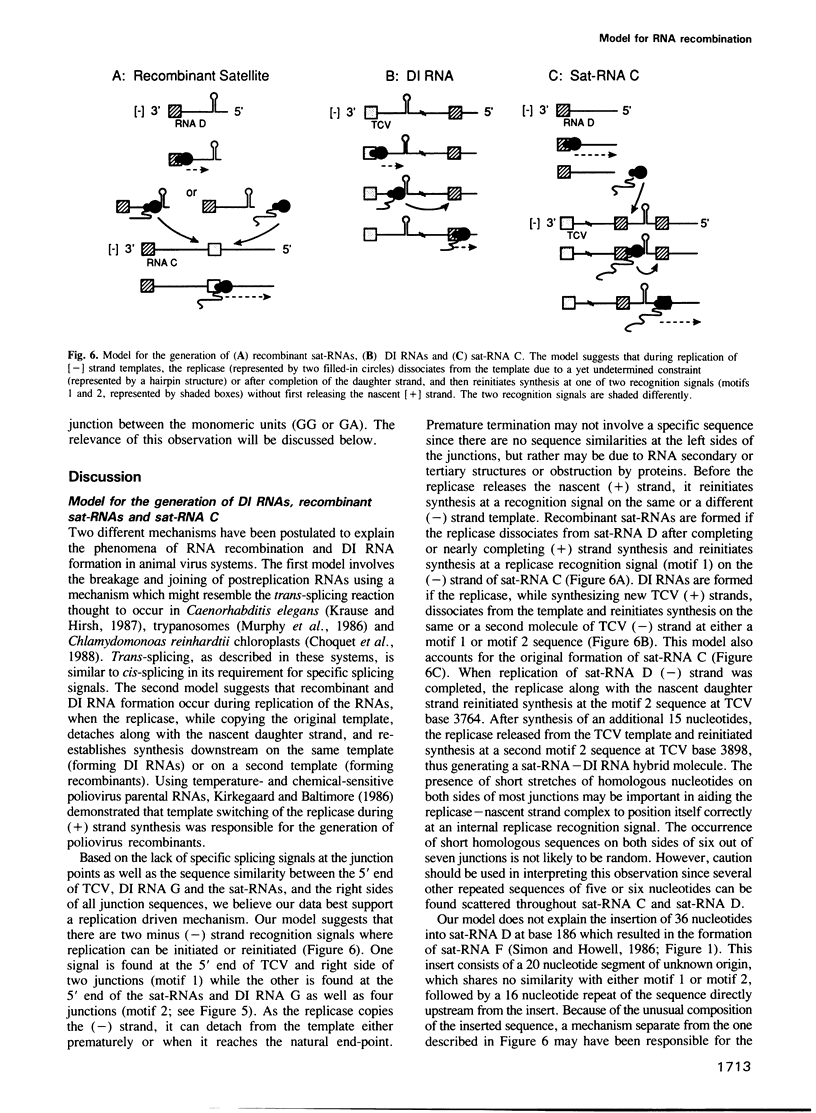
Turnip crinkle virus (TCV) is associated with satellite (sat) RNAs (sat-RNA D, sat-RNA F), defective interfering (DI) RNAs (DI RNA G, DI1 RNA), and one RNA with properties of both sat-RNAs and DI RNAs (sat-RNA C). When plants were inoculated with TCV, sat-RNA D and in vitro sat-RNA C transcripts containing non-viable mutations in the 5' domain, recombinant sat-RNAs were recovered. These recombinants were composed of sat-RNA D at the 5' end and sat-RNA C sequences at the 3' end. Analysis of 20 independent recombination junctions revealed that unequal crossing-over had occurred in planta in a region of sequence similarity between the two sat-RNAs which resulted in the duplication of 3-16 nucleotides. Thirty percent of the sat-RNA recombinants also had one to three additional nucleotides inserted at the crossover junctions which did not correspond to either sat-RNA C or sat-RNA D sequence. The right side of the recombination junctions always began with one of three consecutive nucleotides of sat-RNA C. Based on the similarity between this sequence of sat-RNA C, the right side junction of DI RNA G and the 5' end of TCV, as well as the sequence similarity between right side junctions of DI1 RNA and sat-RNA C and the 5' end of the sat-RNAs, a replicase-driven copy choice mechanism is proposed.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenent G. C., Posthumus E., Brederode F. T., Bol J. F. Genome structure of tobacco rattle virus strain PLB: further evidence on the occurrence of RNA recombination among tobraviruses. Virology. 1989 Jul;171(1):271–274. doi: 10.1016/0042-6822(89)90537-0. [DOI] [PubMed] [Google Scholar]
- Baric R. S., Shieh C. K., Stohlman S. A., Lai M. M. Analysis of intracellular small RNAs of mouse hepatitis virus: evidence for discontinuous transcription. Virology. 1987 Feb;156(2):342–354. doi: 10.1016/0042-6822(87)90414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebricher C. K., Eigen M., Gardiner W. C., Jr Kinetics of RNA replication. Biochemistry. 1983 May 10;22(10):2544–2559. doi: 10.1021/bi00279a036. [DOI] [PubMed] [Google Scholar]
- Biebricher C. K., Eigen M., Luce R. Kinetic analysis of template-instructed and de novo RNA synthesis by Q beta replicase. J Mol Biol. 1981 Jun 5;148(4):391–410. doi: 10.1016/0022-2836(81)90183-2. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. 1986 May 29-Jun 4Nature. 321(6069):528–531. doi: 10.1038/321528a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. D., Simon A. E. Simplified RNA sequencing using dideoxy chain termination. Biotechniques. 1990 Jan;8(1):26–27. [PubMed] [Google Scholar]
- Carrington J. C., Heaton L. A., Zuidema D., Hillman B. I., Morris T. J. The genome structure of turnip crinkle virus. Virology. 1989 May;170(1):219–226. doi: 10.1016/0042-6822(89)90369-3. [DOI] [PubMed] [Google Scholar]
- Choquet Y., Goldschmidt-Clermont M., Girard-Bascou J., Kück U., Bennoun P., Rochaix J. D. Mutant phenotypes support a trans-splicing mechanism for the expression of the tripartite psaA gene in the C. reinhardtii chloroplast. Cell. 1988 Mar 25;52(6):903–913. doi: 10.1016/0092-8674(88)90432-1. [DOI] [PubMed] [Google Scholar]
- Copper P. D., Steiner-Pryor A., Scotti P. D., Delong D. On the nature of poliovirus genetic recombinants. J Gen Virol. 1974 Apr;23(1):41–49. doi: 10.1099/0022-1317-23-1-41. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- King A. M., McCahon D., Saunders K., Newman J. W., Slade W. R. Multiple sites of recombination within the RNA genome of foot-and-mouth disease virus. Virus Res. 1985 Nov;3(4):373–384. doi: 10.1016/0168-1702(85)90437-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M., McCahon D., Slade W. R., Newman J. W. Recombination in RNA. Cell. 1982 Jul;29(3):921–928. doi: 10.1016/0092-8674(82)90454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K., Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986 Nov 7;47(3):433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Saito I., Nomoto A. Primary structure of poliovirus defective-interfering particle genomes and possible generation mechanisms of the particles. J Mol Biol. 1986 Dec 5;192(3):473–487. doi: 10.1016/0022-2836(86)90270-6. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Baric R. S., Makino S., Keck J. G., Egbert J., Leibowitz J. L., Stohlman S. A. Recombination between nonsegmented RNA genomes of murine coronaviruses. J Virol. 1985 Nov;56(2):449–456. doi: 10.1128/jvi.56.2.449-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H., Heaton L. A., Morris T. J., Simon A. E. Turnip crinkle virus defective interfering RNAs intensify viral symptoms and are generated de novo. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9173–9177. doi: 10.1073/pnas.86.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst H. J., Kaper J. M. Replication of peanut stunt virus and its associated RNA 5 in cowpea protoplasts. Virology. 1984 Dec;139(2):317–329. doi: 10.1016/0042-6822(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Makino S., Shieh C. K., Soe L. H., Baker S. C., Lai M. M. Primary structure and translation of a defective interfering RNA of murine coronavirus. Virology. 1988 Oct;166(2):550–560. doi: 10.1016/0042-6822(88)90526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier E., Harmison G. G., Keene J. D., Schubert M. Sites of copy choice replication involved in generation of vesicular stomatitis virus defective-interfering particle RNAs. J Virol. 1984 Aug;51(2):515–521. doi: 10.1128/jvi.51.2.515-521.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. S., Schlesinger S. RNAs from two independently isolated defective interfering particles of Sindbis virus contain a cellular tRNA sequence at their 5' ends. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3279–3283. doi: 10.1073/pnas.80.11.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Perrault J. Origin and replication of defective interfering particles. Curr Top Microbiol Immunol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Re G. G., Morgan E. M., Kingsbury D. W. Nucleotide sequences responsible for generation of internally deleted Sendai virus defective interfering genomes. Virology. 1985 Oct 15;146(1):27–37. doi: 10.1016/0042-6822(85)90050-9. [DOI] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5' poly(A) leader. Cell. 1987 Jul 17;50(2):163–169. doi: 10.1016/0092-8674(87)90212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. E., Engel H., Johnson R. P., Howell S. H. Identification of regions affecting virulence, RNA processing and infectivity in the virulent satellite of turnip crinkle virus. EMBO J. 1988 Sep;7(9):2645–2651. doi: 10.1002/j.1460-2075.1988.tb03117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. E., Howell S. H. Synthesis in vitro of infectious RNA copies of the virulent satellite of turnip crinkle virus. Virology. 1987 Jan;156(1):146–152. doi: 10.1016/0042-6822(87)90445-4. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Howell S. H. The virulent satellite RNA of turnip crinkle virus has a major domain homologous to the 3' end of the helper virus genome. EMBO J. 1986 Dec 20;5(13):3423–3428. doi: 10.1002/j.1460-2075.1986.tb04664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., Lamb R. A., Paterson R. G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988 Sep 9;54(6):891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V., Taylor P., Desselberger U. Crossover regions in foot-and-mouth disease virus (FMDV) recombinants correspond to regions of high local secondary structure. Arch Virol. 1988;102(1-2):131–139. doi: 10.1007/BF01315570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Lazzarini R. A. Analysis of the recombination event generating a vesicular stomatitis virus deletion defective interfering particle. J Virol. 1983 Feb;45(2):766–772. doi: 10.1128/jvi.45.2.766-772.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]