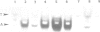Abstract
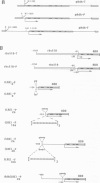
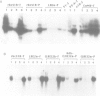
A deletion analysis of the Arabidopsis thaliana rbcS-1A promoter defined a 196 bp region (-320 to -125) sufficient to confer light-regulated expression on a heterologous Arabidopsis alcohol dehydrogenase (Adh) reporter gene in transgenic Nicotiana tabacum (tobacco) leaves. This region, which contains DNA sequences I, G and GT boxes, with homology to other ribulose-1,5-bisphosphate carboxylase small subunit (RBCS) gene promoter sequences, directed expression independent of orientation and relative position in the Adh promoter. Site-specific mutagenesis of these conserved sequences and subsequent expression analysis in transgenic tobacco showed that both G box and I box mutations in the context of the full (-1700 to +21) rbcS-1A promoter substantially reduced the expression of Adh and beta-glucuronidase (GUS) reporter genes. The G box has previously been shown to specifically bind in vitro a factor isolated from nuclear extracts of tomato and Arabidopsis. This factor (GBF) is distinct from the factor GT-1 which binds to adjacent GT boxes in the pea rbcS-3A promoter. Multiple mutations in putative Arabidopsis rbcS-1A promoter GT boxes had no pronounced affect on expression, possibly due to a redundancy of these sites. Experiments in which rbcS-1A promoter fragments were fused to truncated 35S CaMV (cauliflower mosaic virus) promoter--GUS reporter constructs showed that cis-acting CaMV promoter elements could partially restore expression to G-box-mutated rbcS-1A sequences.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Callis J., Fromm M., Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987 Dec;1(10):1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- Castresana C., Garcia-Luque I., Alonso E., Malik V. S., Cashmore A. R. Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia. EMBO J. 1988 Jul;7(7):1929–1936. doi: 10.1002/j.1460-2075.1988.tb03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Favreau M., Dunsmuir P., Bedbrook J. Confirmation of the relative expression levels of the Petunia (Mitchell) rbcS genes. Nucleic Acids Res. 1987 Jun 11;15(11):4655–4668. doi: 10.1093/nar/15.11.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., van den Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Linkage and homology analysis divides the eight genes for the small subunit of petunia ribulose 1,5-bisphosphate carboxylase into three gene families. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4964–4968. doi: 10.1073/pnas.82.15.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R., Jacobs M. Polymorphism of alcohol dehydrogenase in Arabidopsis thaliana (L.) Heynh.: genetical and biochemical characterization. Biochem Genet. 1984 Oct;22(9-10):817–838. doi: 10.1007/BF00499475. [DOI] [PubMed] [Google Scholar]
- Donald R. G., Schindler U., Batschauer A., Cashmore A. R. The plant G box promoter sequence activates transcription in Saccharomyces cerevisiae and is bound in vitro by a yeast activity similar to GBF, the plant G box binding factor. EMBO J. 1990 Jun;9(6):1727–1735. doi: 10.1002/j.1460-2075.1990.tb08296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P., Smith S., Bedbrook J. A number of different nuclear genes for the small subunit of RuBPCase are transcribed in petunia. Nucleic Acids Res. 1983 Jun 25;11(12):4177–4183. doi: 10.1093/nar/11.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R. X., Nagy F., Sivasubramaniam S., Chua N. H. Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell. 1989 Jan;1(1):141–150. doi: 10.1105/tpc.1.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Brosio P., Bond-Nutter D., Bedbrook J., Dunsmuir P. Novel cis-acting elements in Petunia Cab gene promoters. Mol Gen Genet. 1989 Jan;215(2):337–344. doi: 10.1007/BF00339739. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Pichersky E., Malik V. S., Timko M. P., Scolnik P. A., Cashmore A. R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7089–7093. doi: 10.1073/pnas.85.19.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Kay S. A., Chua N. H. Sequence-specific interactions of a pea nuclear factor with light-responsive elements upstream of the rbcS-3A gene. EMBO J. 1987 Sep;6(9):2543–2549. doi: 10.1002/j.1460-2075.1987.tb02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Yong M. H., Cuozzo M., Kano-Murakami Y., Silverstein P., Chua N. H. Binding site requirements for pea nuclear protein factor GT-1 correlate with sequences required for light-dependent transcriptional activation of the rbcS-3A gene. EMBO J. 1988 Dec 20;7(13):4035–4044. doi: 10.1002/j.1460-2075.1988.tb03297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F., Lam E., Chua N. H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989 Aug 31;340(6236):727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Kuhlemeier C., Cuozzo M., Green P. J., Goyvaerts E., Ward K., Chua N. H. Localization and conditional redundancy of regulatory elements in rbcS-3A, a pea gene encoding the small subunit of ribulose-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4662–4666. doi: 10.1073/pnas.85.13.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlemeier C., Fluhr R., Green P. J., Chua N. H. Sequences in the pea rbcS-3A gene have homology to constitutive mammalian enhancers but function as negative regulatory elements. Genes Dev. 1987 May;1(3):247–255. doi: 10.1101/gad.1.3.247. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Benfey P. N., Gilmartin P. M., Fang R. X., Chua N. H. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7890–7894. doi: 10.1073/pnas.86.20.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Chua N. H. ASF-2: a factor that binds to the cauliflower mosaic virus 35S promoter and a conserved GATA motif in Cab promoters. Plant Cell. 1989 Dec;1(12):1147–1156. doi: 10.1105/tpc.1.12.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., Jacobs J. D., Howell S. H. Functional regions of the cauliflower mosaic virus 35S RNA promoter determined by use of the firefly luciferase gene as a reporter of promoter activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4870–4874. doi: 10.1073/pnas.84.14.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Cashmore A. R. Evidence for selection as a mechanism in the concerted evolution of Lycopersicon esculentum (tomato) genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3880–3884. doi: 10.1073/pnas.83.11.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat S., Willmitzer L., Sánchez-Serrano J. J. Nuclear proteins binding to a cauliflower mosaic virus 35S truncated promoter. Mol Gen Genet. 1989 Jun;217(2-3):209–214. doi: 10.1007/BF02464883. [DOI] [PubMed] [Google Scholar]
- Schulze-Lefert P., Dangl J. L., Becker-André M., Hahlbrock K., Schulz W. Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J. 1989 Mar;8(3):651–656. doi: 10.1002/j.1460-2075.1989.tb03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger D., Kaulen H., Schell J. A CACGTG motif of the Antirrhinum majus chalcone synthase promoter is recognized by an evolutionarily conserved nuclear protein. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6930–6934. doi: 10.1073/pnas.86.18.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M., Manzara T., Pichersky E., Cashmore A., Gruissem W. Genomic organization, sequence analysis and expression of all five genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from tomato. Mol Gen Genet. 1987 Sep;209(2):247–256. doi: 10.1007/BF00329650. [DOI] [PubMed] [Google Scholar]
- Ueda T., Pichersky E., Malik V. S., Cashmore A. R. Level of expression of the tomato rbcS-3A gene is modulated by a far upstream promoter element in a developmentally regulated manner. Plant Cell. 1989 Feb;1(2):217–227. doi: 10.1105/tpc.1.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]