Abstract
The impact of treatment on subsequent fertility and the safety of childbearing are major complicating factors for young women diagnosed with breast cancer. As national data indicate women are postponing first pregnancy to older ages; therefore, many young patients are seeking clinical guidance regarding the safety of conception and treatment options that may not prevent subsequent pregnancy. Newly developed chemotherapy protocols of brief duration have improved life expectancy enabling some women to consider childbearing. This study was conducted to compare prognosis among breast cancer patients with and without a subsequent pregnancy. Medical record review of female members of a Northern California prepaid health care plan enabled the identification of 107 women with one or more subsequent pregnancies and 344 cases without a pregnancy, who were diagnosed between 1968 and 1995. Sets were matched on age, year and stage at diagnosis, months of survival and recurrence status at conception. Among the matched sets, neither risk of recurrence nor death differed significantly by subsequent pregnancy history during an average 12 years of follow-up (adjusted hazard ratio [HR] recurrence: 1.2 [0.8, 2.0]; adjusted HR death: 1.0 [0.6, 1.9]). Women interested in preserving their fertility and considering pregnancy are a self-selected population; therefore, to reduce potential bias, cases were matched on recurrence status at time of conception. Although the number of cases was limited, subgroup analyzes indicated a small, nonsignificant adverse effect among women who conceived within 12 months of diagnosis. This analysis of carefully matched cases provides reassurance that long-term prognosis was not adversely affected by subsequent pregnancy.
Keywords: breast cancer, long-term prognosis, subsequent pregnancy
Breast cancer diagnosis at a young age, especially during childbearing years, can be devastating; however, recent advances in diagnostic procedures and treatment options have improved survival providing young patients with the possibility of subsequent pregnancy. In the past, some clinicians recommended young women delay or avoid childbearing to reduce any potential hormonal stimulation of metastases (1–3). However, newly developed therapeutic options provide young patients with shorter more intensive therapy enabling consideration of childbearing. These women expect clinical guidance regarding maintenance of fertility associated with various treatment options and the prognostic impact of subsequent pregnancy. In recognition of their concerns the American Society of Clinical Oncology (ASCO) has published guidelines for oncologists caring for premenopausal breast cancer patients (4). Influenced by ASCO and in response patients’ requests, many clinicians now discuss family planning and fertility preservation concerns with their patients prior to recommending definitive cancer treatment (5).
The American Cancer Society data estimates more than 55,000 women younger than age 50 will be diagnosed with invasive or in situ breast cancer during 2009; approximately 1 in 208 will be <40 years (6). As the mean age at first birth in the USA has increased (7), thousands of young women are developing breast cancer before beginning or adding to their families. For many young survivors having a child after breast cancer is a major life affirming decision (8,9). A recent web-based survey of young women treated for breast cancer, members of the Young Survival Coalition, noted interest in future childbearing had influenced their decisions regarding systemic treatment (10).
Although numerous studies have assessed the impact of subsequent pregnancy on breast cancer prognosis; most investigations have lacked appropriate comparison groups, while others did not control adequately for potential self-selection bias (11–18). This study was designed to assess prognosis by controlling for potential survival bias through careful matching breast cancer cases with and without subsequent pregnancy.
PATIENTS AND METHODS
Data Sources
Kaiser Permanente of Northern California (KPNC), a prepaid health care plan, includes a racially diverse population representing approximately 30% of the breast cancer cases reported to the Northern California Cancer Center Surveillance, Epidemiology and End Result (SEER) site for counties in the San Francisco Bay Area. The KPNC data base provided a unique opportunity to identify cases diagnosed at age 45 or younger between 1968 and 1995 who were treated similarly within standard clinical protocols. KPNC trained medical record abstractors, using a study-specific data collection instrument, recorded clinical information including: reproductive history, primary and adjuvant treatment, subsequent pregnancy (including miscarriages and induced abortions), and dates of recurrence. Date and cause of death were obtained from KPNC records and confirmed for all state residents from the California Automated Mortality Linkage and Information System. Women who were pregnant at the time of diagnosis were excluded.
Between 1968 and 1995, breast cancer prognosis has improved as a result of greater diversity of chemo-therapeutic agents. Technological advancements have enabled detection of tumor markers and targeted adjuvant treatment. To address these changing clinical protocols and insure appropriate comparisons, cases were matched within ±5 years of diagnosis. Some tumor characteristics such as measured size, estrogen, and progesterone receptors (PR) were not systematically assessed and recorded during earlier years.
This study was approved by both the KPNC and Columbia University IRBs. Informed consent was not required or obtained for these analyses of stored data. To protect the confidentiality of the study subjects, all personal identifying information was removed and each case was assigned a unique ID number before the data was provided to authors not affiliated with KPNC.
Study Design
This breast cancer survival analysis compared cases discordant for subsequent pregnancy matched on the following five criteria: (1) SEER stage at diagnosis (local [including in situ] or regional [positive axillary lymph nodes]); (2) age at diagnosis (±5 years); (3) months of survival from date of diagnosis to last menstrual period (LMP) prior to conception (determined through chart review and estimated gestational age of the fetus); (4) recurrence status at time of conception of first subsequent pregnancy; and (5) year of diagnosis (±5 years). Criteria three and four, comparable survival intervals from date of diagnosis to LMP and cancer status at conception within matched sets, were essential to avoid survival bias among women having a subsequent pregnancy. The detailed KPNC records provided recurrence status and survival intervals for the full study population and enabled estimation of date of conception of women with subsequent pregnancy. The fifth criteria, year of diagnosis, insured comparability of clinical treatment protocols available for cases treated within the KPNC network. Four comparison cases without a history of subsequent pregnancy were sought for each woman who had one or more pregnancies after diagnosis; however, the availability of cases satisfying the strict matching criteria provided 107 matched sets: 8 with a ratio of 2:1 (7.5%), 68 with a ratio of 3:1 (63.6%), and 31 with a ratio 4:1 (29.0%).
Statistical Analysis
Kaplan–Meier curves were used to graphically assess the impact of subsequent pregnancy on recurrence and death due to breast cancer. Sites of recurrence included local, regional or distant metastases and excluded the diagnosis of a second primary breast cancer. A stratified Cox model was applied to account for the correlations induced by the matched design and 95% confidence limits were computed (19). Subsequent pregnancy status was treated as a time dependent covariate (defined as positive after date of first conception following breast cancer diagnosis). Adjusted models additionally controlled for age at diagnosis (continuous), adjuvant chemotherapy (yes/no), and radiation therapy (yes/no). The proportionality assumption for each covariate was checked through time dependent variables (19,20) using the PROC PHREG procedure (SAS 9.1; SAS Institute, Cary, NC). All tests were two-sided.
Estrogen receptor (ER) and PR were not available for 33.3% and 59.0% respectively for women diagnosed before these tumor markers were routinely assessed; therefore, receptor status was not controlled in the analyzes. Similarly, race and tumor size were excluded due to missing data. Other newly identified tumor markers such as Her2 status were not among the prognostic factors available for these KPNC breast cancer cases.
Follow-up began on the date of diagnosis. Cases were considered free of recurrence if asymptomatic at last KPNC medical visit, at end of membership, or at time of death due to causes other than breast cancer. Recurrence was noted from medical records as date metastases were first detected. Three women who died of breast cancer lacked recorded recurrence information; date of death was used as date of recurrence. Additional cases excluded from recurrence analyzes were three women alive with recurrent disease lacking a date of detection and five matched sets in which metastatic disease developed prior to conception. Follow-up for mortality analyses ended at death due to breast cancer. Cases were censored at date of last KPNC medical visit, date of last membership renewal, or date of death due to causes other than breast cancer. Information on membership status was available through July 2002 and last chart review was completed in December 2002.
Cases with either invasive or in situ breast cancer were included, although separate analyzes were conducted after excluding the 11 sets diagnosed exclusively with ductal carcinoma in situ (DCIS). Although the number of matched sets was limited, subgroup analyses were conducted by disease stage at diagnosis, (local [including in situ disease] and regional [with positive axillary nodes]); interval between diagnosis and first conception (≤12, >12 months) and outcome of first subsequent pregnancy: (term birth or interrupted: miscarriage, induced abortion and ectopic pregnancy).
RESULTS
The 107 cases identified with one or more pregnancies after breast cancer diagnosis were matched to 344 cases without a subsequent pregnancy. Characteristics of these 451 women are compared in relation to their postdiagnosis pregnancy status (Table 1). Women without a subsequent pregnancy were slightly older, more likely to have had a pregnancy prior to diagnosis and to have received adjuvant therapy.
Table 1.
Characteristics of Unmatched Study Population by Subsequent Pregnancy Status
| Subsequent pregnancy (n = 107) | No subsequent pregnancy (n = 344) | |
|---|---|---|
| Mean age at diagnosis (SD) | 32.0 (4.0) | 34.1 (3.9) |
| Stage of disease | No. (%) | No. (%) |
| Carcinoma in situ | 11 (10.3) | 37 (10.8) |
| Local | 65 (60.8) | 200 (58.1) |
| Regional (positive axillary nodes) | 31 (29.0) | 107 (31.1) |
| Parous before diagnosis* | 68 (64.2) | 254 (74.1) |
| Surgery type* | ||
| Mastectomy | 76 (71.0) | 248 (72.7) |
| Lumpectomy | 31 (29.0) | 93 (27.3) |
| Axillary dissection† | 93 (96.9) | 302 (98.4) |
| Adjuvant therapy* | ||
| Chemotherapy | 41 (38.7) | 170 (50.2) |
| Radiation | 34 (32.1) | 119 (35.2) |
| Hormonal | 3 (2.8) | 35 (10.4) |
Percentage estimates exclude missing values in each category: prior parity 2 (<1%), surgery type 3 (<1%), axillary dissection 1 (1%), chemotherapy 6 (1.3%), radiation 7 (1.6%), hormonal therapy 8 (1.8%).
Numbers and percentages exclude DCIS cases.
DCIS, ductal carcinoma in situ.
Within each matched set, stage of disease at diagnosis (in situ, local and regional [positive axillary nodes]) and disease status at time of conception of subsequent pregnancy (recurrence, no recurrence) were the same for cases with and without subsequent pregnancy. As required by our matching criteria, all cases without a postdiagnosis pregnancy survived at least as long as the interval from diagnosis to estimated date of conception of their matched case; five sets included cases who developed metastatic disease before conception. In total, 96% of cases without a subsequent pregnancy were diagnosed during a concurrent 5 year interval of their matched case. The median follow-up interval from diagnosis for cases with a subsequent pregnancy was 12.7 years, slightly longer than for cases without a subsequent pregnancy 11.4 years. Thirty-three women were diagnosed with a second primary breast cancer including 6 cases (5.6%) who had a pregnancy and 27 (8.1%) without subsequent pregnancy; 15 of these cases developed metastatic disease, 11 concurrent with or after detection of the second primary.
Conception occurred within 12 months of diagnosis for 46 women (43%) and 33 cases (30.9%) had two or more subsequent pregnancies (Table 2). The outcome of the first subsequent pregnancy was a live birth for 53%; 46% were interrupted either by miscarriage or induced abortion and one woman experienced an ectopic pregnancy. Among the six women with subsequent pregnancy who developed a second primary, three were diagnosed following a term birth and three after an interrupted pregnancy.
Table 2.
Characteristics of Breast Cancer Cases with Subsequent Pregnancy History
| No. (%) Subsequent pregnancy (n = 107) |
|
|---|---|
| Interval from diagnosis to estimated date of conception | |
| ≤12 months | 46 (43.0) |
| 13–24 months | 24 (22.4) |
| 25–48 months | 21 (19.6) |
| ≥49 months | 16 (15.0) |
| Outcome of first subsequent pregnancy | |
| Live birth | 57 (53.3) |
| Abortion | 38 (35.5) |
| Miscarriage | 11 (10.3) |
| Ectopic | 1 (<1) |
| Number of subsequent pregnancies | |
| 1 | 74 (69.1) |
| 2 | 17 (15.9) |
| 3+ | 16 (15.0) |
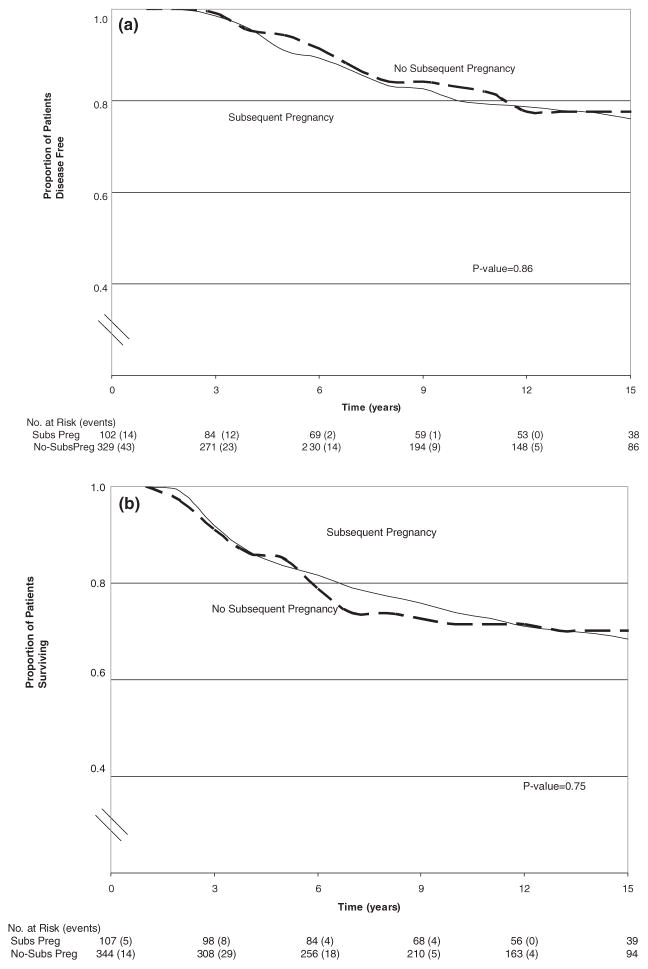
The Kaplan–Meier curves, Figure 1a,b, reflect individual breast cancer cases, not matched sets. Having a subsequent pregnancy did not influence the risk of recurrence or death from breast cancer.
Figure 1.
Kaplan–Meier estimates of disease free survival (a) and survival (b) according to subsequent pregnancy status.
Analyses of matched sets by Cox proportional hazards models are presented in Table 3; no significant prognostic differences were observed in the hazard ratios (HR) (adjusted HR for recurrence = 1.2; 95% CI, 0.8–2.0; adjusted HR for death = 1.0; 95% CI, 0.6–1.9). To assess risk specifically among cases with invasive disease at diagnosis, the 11 matched sets with only DCIS were excluded. Similar HRs for recurrence (1.1; 95% CI, 0.7–1.9) and death (1.0; 95% CI, 0.6–1.9) were observed. Although the number of matched sets was limited, the effect of subsequent pregnancy was examined among subgroups of the study population noted in Tables 4 and 5. In contrast to matched cases with in situ or local breast cancer, subsequent pregnancy was associated with a nonsignificant increased risk among the 31 matched sets with positive axillary lymph nodes at diagnosis (adjusted recurrence HR = 1.6; 95% CI, 0.7–3.4; adjusted mortality HR = 1.3, 95% CI, 0.5–3.4). Compared to their matched cases, women who conceived within 12 months of diagnosis experienced slightly worse survival outcomes, while those who delayed pregnancy tended to have improved survival. However, these results were not significant (Table 5). Pregnancy outcome did not significantly affect survival (term birth: adjusted recurrence HR = 1.2; 95% CI, 0.6–2.4; adjusted mortality HR = 1.4, 95% CI, 0.5–3.5; interrupted pregnancy: adjusted recurrence HR = 1.3; 95% CI, 0.6–2.6; adjusted mortality HR = 0.8, 95% CI, 0.4–1.9).
Table 3.
Multivariate Analyzes of the Effect of Subsequent Pregnancy on the Risk of Recurrence and Death
| Total no. of cases | Unadjusted HR (95% CI) | Full model* HR (95% CI) | |
|---|---|---|---|
| Recurrence | |||
| No subsequent pregnancy | 329 | 1 | 1 |
| Subsequent pregnancy | 102 | 1.1 (0.7, 1.7) | 1.2 (0.8, 2.0) |
| Survival | |||
| No subsequent pregnancy | 344 | 1 | 1 |
| Subsequent pregnancy | 107 | 0.8 (0.4, 1.3) | 1.0 (0.6, 1.9) |
Adjusted for age at diagnosis (continuous), chemotherapy (yes/no), and radiation therapy (yes/no).
Table 4.
Multivariate Analyzes of the Effect of First Pregnancy After Breast Cancer on Risk of Recurrence and Death by Stage at Diagnosis
| Total no. of cases | Unadjusted HR (95% CI) | Adjusted model* HR (95% CI) | |
|---|---|---|---|
| Recurrence | |||
| Regional disease (positive axillary nodes) | |||
| No subsequent pregnancy | 98 | 1 | 1 |
| Subsequent pregnancy | 28 | 1.3 (0.7,2.6) | 1.6 (0.7,3.4) |
| Local disease† | |||
| No subsequent pregnancy | 231 | 1 | 1 |
| Subsequent pregnancy | 74 | 1.0 (0.5, 1.7) | 1.1 (0.5, 2.0) |
| Death | |||
| Regional disease (positive axillary nodes) | |||
| No subsequent pregnancy | 107 | 1 | 1 |
| Subsequent pregnancy | 31 | 1.0 (0.4, 2.1) | 1.3 (0.5, 3.4) |
| Local disease† | |||
| No subsequent pregnancy | 237 | 1 | 1 |
| Subsequent pregnancy | 76 | 0.6 (0.3, 1.4) | 0.8 (0.4, 2.0) |
Adjusted for age at diagnosis (continuous), chemotherapy (yes/no), radiation therapy (yes/no).
Includes local disease and DCIS.
DCIS, ductal carcinoma in situ.
Table 5.
Multivariate Analyzes of the Effect of First Pregnancy After Breast Cancer on Risk of Recurrence and Death by Time Interval Since Diagnosis
| Total no. of cases | Unadjusted HR (95% CI) | Adjusted model* HR (95% CI) | |
|---|---|---|---|
| Recurrence | |||
| ≤12 months between diagnosis and conception | |||
| No subsequent pregnancy | 144 | 1 | 1 |
| Subsequent pregnancy | 44 | 1.5 (0.8,2.6) | 1.4 (0.8,2.7) |
| >12 months between diagnosis and conception | |||
| No subsequent pregnancy | 185 | 1 | 1 |
| Subsequent pregnancy | 58 | 0.7 (0.4, 1.5) | 1.1 (0.5, 2.4) |
| Death | |||
| ≤12 months between diagnosis and conception | |||
| No subsequent pregnancy | 151 | 1 | 1 |
| Subsequent pregnancy | 46 | 1.1 (0.5, 2.2) | 1.5 (0.7, 3.6) |
| >12 months between diagnosis and conception | |||
| No subsequent pregnancy | 193 | 1 | 1 |
| Subsequent pregnancy | 61 | 0.5 (0.2, 1.2) | 0.6 (0.2, 1.7) |
Adjusted for age at diagnosis (continuous), chemotherapy (yes/no), radiation therapy (yes/no).
DISCUSSION
Our analyses indicate that pregnancy subsequent to breast cancer diagnosis does not adversely affect prognosis. The study population of carefully matched cases controlled for potential survival biases; results should be reassuring to young patients concerned with long-term survival following a subsequent pregnancy. With increasing numbers of young breast cancer patients and survey data indicating the concern that many newly diagnosed patients express about their future fertility and prognosis (4,10), the data from this study contributes to an important body of research.
Skilled KPNC abstractors collected detailed data pertaining to diagnosis, treatment, and recurrence status of women at the time of conception. Prognostic factors known to influence breast cancer survival, including stage, age, and year of diagnosis were controlled either in the design or analysis. Results from subgroup analyses were similar to our main findings.
Although other comparative studies have reported similar findings, most publications did not adequately control for the comparative health status of women at the time of conception potentially biasing their conclusions (11–18). A recent similarly designed study by Velentgas et al. included matched cases younger than age 40 with invasive disease at diagnosis. No difference in survival, HR = 0.8 (CI, 0.3–2.3), was observed, when the 53 with subsequent pregnancy were compared to the 265 women without a postdiagnosis pregnancy (21). A study by Gelber et al. (22) selected cases from several European chemotherapy trials matched on recurrence status at conception; however, the authors compromised their results by adding to their study sample women with subsequent pregnancy identified from their clinical practices (22). Therefore, their findings of improved survival following subsequent pregnancy may be biased.
Although population based studies are often preferable for unbiased analyzes, the difficulty of overcoming selection and survival bias from analyses relying on large data bases with limited clinical information was noted by Kroman et al. in their 2008 publication which extended the follow-up of their earlier series and revealed a statistically significant reduced risk of death 10 years after diagnosis among cases with a subsequent pregnancy (15,18). These authors reported that the number of expected pregnancies was significantly lower and the number of abortions was higher than expected among age matched women in the Danish population; they noted, “…women with poor prognosis are believed to avoid pregnancies…” Several previous studies assessed only women with term pregnancies, while the KPNC records provided the opportunity to assess the effect on prognosis of miscarriage and induced abortion outcomes. When our data were restricted to only those sets with term births (n = 57), no significant survival differences were noted.
Our subgroup analysis, based on very limited numbers of matched sets, revealed nonsignificant increased risks for both recurrence (28 sets) and breast cancer mortality among cases diagnosed with node positive disease (31 sets). Although results from other similar investigations by nodal status at diagnosis were inconsistent, Velentgas et al. (21) observed findings similar to ours: a nonsignificant increase in risk among cases with involved lymph nodes at diagnosis (RR = 1.4; 95% CI, 0.4–5.2). As positive lymph nodes at diagnosis are associated with increased risk of distant metastases, the potential adverse effect of the hormonal stimulation associated with pregnancy may be of greatest concern among cases diagnosed with regional disease. However, recent advances in adjuvant chemotherapy have improved disease free survival for young women with lymph node positive disease while preserving their fertility. Therefore, more breast cancer survivors may be able to consider subsequent pregnancy.
Given the possibility of childbearing, patients have asked about a safe interval of delay before conception. Our study noted a nonsignificant increased risk of recurrence and death associated with conception within 12 months of diagnosis (46 sets). Our results concur with others who reported improved, though nonsignificant, survival with a longer interval between diagnosis and pregnancy (11,16,23).
While we were able to examine receipt of chemotherapy, radiation, and hormone therapy, KPNC records did not indicate treatment options offered to cases nor their recommended duration. The nonsignificant elevated HRs found among cases who conceived within 1 year of diagnosis may reflect an interruption or delay in treatment associated with pregnancy. We also lacked information regarding their fertility status after treatment or patients’ interest in childbearing. Future studies should collect pathologic and biochemical tumor characteristics known or suspected to impact prognosis and guide systemic treatment decision-making. By prospectively recording details of systemic treatment and family planning considerations, future studies will have more complete information available to guide premenopausal breast cancer patients.
Although this study is one of the largest matched analyzes to examine the effects of subsequent pregnancy while controlling for the health status at conception, the number of available cases was limited in the extensive KPNC records, especially for subgroup analyses. Changing technology and treatment options over several decades have provided clinical advances during the interval between diagnosis of our earliest cases (1968) and those most recently treated (1995). To address any potential prognostic influences associated with changes over time, we successfully matched 96% of cases on diagnosis within a 5 year interval. However, our study as well as other recent reports lacked tumor markers, such as ER and PR receptor status, for cases treated in earlier decades before these characteristics were considered an essential component for standard of care.
The extended follow-up for these matched sets lends credence to the results of the study. KPNC medical records provided an invaluable resource of extensive clinical data for cases treated over several decades enabling the identification of a larger study population with pregnancies subsequent to diagnosis providing greater power for statistical analyses.
Our study found no significant increase in risk of breast cancer recurrence or decreased survival associated with subsequent pregnancy. Additionally, the 10 year recurrence free survival for this population was approximately 70%. While our results are encouraging, the long-term commitment to childbearing must be weighed against patient-specific prognostic factors. The nonsignificant tendency toward elevated risk among subgroups suggests that the prognostic effect of subsequent pregnancy may vary according to stage at diagnosis, histologic characteristics, disease free interval prior to subsequent pregnancy, and other factors potentially including genetic modulation of the hormonal milieu.
Newer adjuvant chemotherapy protocols may avoid premature ovarian failure enabling more women to achieve pregnancy after diagnosis. A recent report suggests ovarian stimulation may be another option for breast cancer patients desiring childbearing (5). Therefore, physicians must consider the psychosocial needs of patients and their partners when discussing treatment options. Through collaborative research efforts by clinicians caring for young breast cancer patients, optimum treatment protocols that preserve fertility will be identified. Additional larger studies of subsequent pregnancy are required to assess new treatment protocols especially among young breast cancer patients at increased risk due to a genetic mutation.
Acknowledgments
We acknowledge Jeanne A. Petrek for her role in study design. Dr. Petrek died on April 11, 2005, before analyzes were completed. We thank Leo B. Hurley, MPH, Ralph S. Vogel, PhD, and staff of Kaiser Permanente Northern California for data collection and organization.
This study was funded by the U.S. Army Medical Research and Materiel Command, DAMD17-96-1-6122. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, nor approval of the manuscript.
References
- 1.Danforth DN. How subsequent pregnancy affects outcome in women with a prior breast cancer. Oncology. 1991;5:23–30. [PubMed] [Google Scholar]
- 2.Gemignani ML, Petrek JA. Pregnancy after breast cancer. Cancer Control. 1999;6:272–6. [PubMed] [Google Scholar]
- 3.Calhoun K, Hansen N. The effect of pregnancy on survival in women with a history of breast cancer. Breast Disease. 2005–2006;23:81–6. doi: 10.3233/bd-2006-23111. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 5.Azim AA, Constantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–5. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 7.Mathews TJ, Hamilton BE. Mean age of mother, 1970–2000. Nalt Vital Stat Rep. 2002;51:1–13. [PubMed] [Google Scholar]
- 8.Petrek JA. Pregnancy safety after breast cancer. Cancer Suppl. 1994;74:528–31. doi: 10.1002/cncr.2820741342. [DOI] [PubMed] [Google Scholar]
- 9.Upponi SS, Ahmad F, Whitaker IS, et al. Pregnancy after breast cancer. Eur J Cancer. 2003;39:736–41. doi: 10.1016/s0959-8049(02)00870-5. [DOI] [PubMed] [Google Scholar]
- 10.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 11.Sankila R, Heinavaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: ‘healthy mother effect’. Am J Obstet Gynecol. 1994;170:818–23. doi: 10.1016/s0002-9378(94)70290-x. [DOI] [PubMed] [Google Scholar]
- 12.von Schoultz E, Johansson H, Wilking N, et al. Influence of prior and subsequent pregnancy on breast cancer prognosis. J Clin Oncol. 1995;13:430–4. doi: 10.1200/JCO.1995.13.2.430. [DOI] [PubMed] [Google Scholar]
- 13.Lethaby AE, O’Neill MA, Mason BH, et al. Overall survival from breast cancer in women pregnant at or after diagnosis. Int J Cancer. 1996;67:751–5. doi: 10.1002/(SICI)1097-0215(19960917)67:6<751::AID-IJC1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Malamos NA, Stathopoulos GP, Keramopoulos A, et al. Pregnancy and offspring after the appearance of breast cancer. Oncology. 1996;53:471–5. doi: 10.1159/000227622. [DOI] [PubMed] [Google Scholar]
- 15.Kroman N, Jensen MB, Melbye M, et al. Should women be advised against pregnancy after breast cancer treatment? The Lancet. 1997;350:319–22. doi: 10.1016/S0140-6736(97)03052-3. [DOI] [PubMed] [Google Scholar]
- 16.Mueller BA, Simon MS, Deapen D, et al. Childbearing and survival after breast carcinoma in young women. Cancer. 2003;98:1131–40. doi: 10.1002/cncr.11634. [DOI] [PubMed] [Google Scholar]
- 17.Blakely LJ, Buzdarm AU, Lozada JA, et al. Effects of pregnancy after treatment for breast carcinoma on survival and risk of recurrence. Cancer. 2004;100:465–9. doi: 10.1002/cncr.11929. [DOI] [PubMed] [Google Scholar]
- 18.Kroman N, Jensen MB, Wohlfahrt J, et al. Pregnancy after treatment of breast cancer—a population based study on behalf of Danish Breast Cancer Cooperative Group. Acta Oncol. 2008;47:545–9. doi: 10.1080/02841860801935491. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S. Applied Survival Analysis. New York, NY: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 20.Allison PD. Survival Analysis Using SAS A Practical Guide. Cary, NC: SAS Institute Inc; 1995. [Google Scholar]
- 21.Velentgas P, Daling JR, Malone KE. Pregnancy after breast carcinoma. Cancer. 1999;85:2424–32. doi: 10.1002/(sici)1097-0142(19990601)85:11<2424::aid-cncr17>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Gelber S, Coates AS, Goldhirsch A. Effects of pregnancy on overall survival after the diagnosis of early-stage breast cancer. J Clin Oncol. 2001;19:1671–5. doi: 10.1200/JCO.2001.19.6.1671. [DOI] [PubMed] [Google Scholar]
- 23.Ives A, Saunders C, Bulsara M, et al. Pregnancy after breast cancer: population based study. BMJ. 2007;334:194–198. doi: 10.1136/bmj.39035.667176.55. [DOI] [PMC free article] [PubMed] [Google Scholar]