Abstract
Stroke is one of the most dreaded complications of left ventricular assist device therapy in patients with end-stage congestive heart failure. There is strong evidence linking anticoagulation and infection with ischemic and hemorrhagic strokes, though recent data has emerged regarding the importance of elevated blood pressure. In the recently completed Heartware Ventricular Assist Device studies, a mean arterial pressure greater than 90 mmHg was associated with greater stroke risk, particularly the hemorrhagic subtype. In this review, we discuss recent evidence regarding deleterious effects of uncontrolled hypertension in patients with left ventricular devices, and propose measurement and management strategies.
Keywords: Stroke, Hypertension, Left ventricular assist device
Introduction
The use of left ventricular assist devices (LVADs) worldwide has grown rapidly with the increased prevalence of end-stage congestive heart failure (CHF) and improved technological advances in mechanical circulatory support [1•]. For an increasing number of patients, LVADs represent a temporary treatment while awaiting cardiac transplantation (“bridge to transplant,” BTT), while in others it is definitive therapy if ineligible for cardiac transplant (“destination therapy,” DT). Over the last three decades, technological improvements have extended the life-span of patients with end-stage systolic heart failure through the use of these devices to include both “bridge to transplant” and “destination therapy,” the latter serving as an alternative to cardiac transplantation. Several clinical trials have demonstrated improved survival and quality of life among patients treated with LVADs [2••], though one of the most feared complications is stroke reported in 7–15 % of cases [3••, 4–7]. Stroke, particularly hemorrhagic subtype, is a leading cause of morbidity and mortality after an LVAD and a reason for some patients becoming ineligible for transplantation. Several risk factors for stroke have been identified across studies which center on thrombus formation within the LVAD itself [8], anticoagulation treatment, or infection [9–12]. Recent reports have pointed to the importance of hypertension as a risk factor for stroke [3••, 13], with a recent United States Food and Drug Administration recommendation for a mean arterial pressure (MAP) <90 mmHg for patients with a Heartware ventricular assist device (HVAD). This review will focus on hypertension as a risk factor for stroke in patients with LVADs, and management of hypertension after stroke.
Hypertension as a Risk Factor for Stroke
Several primary prevention trials in hypertension have demonstrated a robust decline in the rate of stroke [14]. Worldwide hypertension represents a consistent and strong risk factor for all stroke subtypes: ischemic stroke and hemorrhagic types (intracerebral hemorrhage and aneurysmal subarachnoid hemorrhage) [15]. Given the high prevalence of hypertension worldwide, hypertension has one of the highest population-attributable risks for stroke [16]. The impact of treating hypertension, particularly on hemorrhagic stroke which is more likely to be fatal, is likely to be even higher [17]. The mechanisms by which hypertension may lead to stroke are several-fold, including direct damage to cerebral arteries (intracranial plaque formation, aneurysm formation and growth, hypertensive damage to small subcortical vessels) or indirect processes (cardiac dysfunction leading to embolic events, extra-cranial atherosclerosis) [15]. To what degree these processes are relevant to patients with LVADs remains poorly defined.
Mechanisms of Stroke in Left Ventricular Assist Devices
In order to understand the impact that hypertension may have on stroke in LVADs, further description of stroke subtyping is needed. Several manuscripts have addressed the risk of stroke after LVAD implantation, though interpretation of the data was initially limited by further description of ischemic and hemorrhagic strokes. Ischemic strokes may occur due to an embolus forming within the LVAD or myocardium, though the correlation between clinical pump thrombosis and ischemic stroke appears less robust [3••, 8, 9]. Patients with embolic sources can develop ischemic strokes from different causes given their burden of cardiovascular disease risk factors, namely due to atrial fibrillation, artery to artery emboli from large artery atherosclerotic disease, or lacunar infarcts due to lipohyalinosis of small cerebral arteries. To what degree hypertension could predispose to thrombus formation within the myocardium or LVAD is not clear, though treating hypertension is an important component of primary prevention of stroke in patients with atrial fibrillation [18], and poorly controlled hypertension may decrease LVAD flow and thereby contribute to device thrombosis.
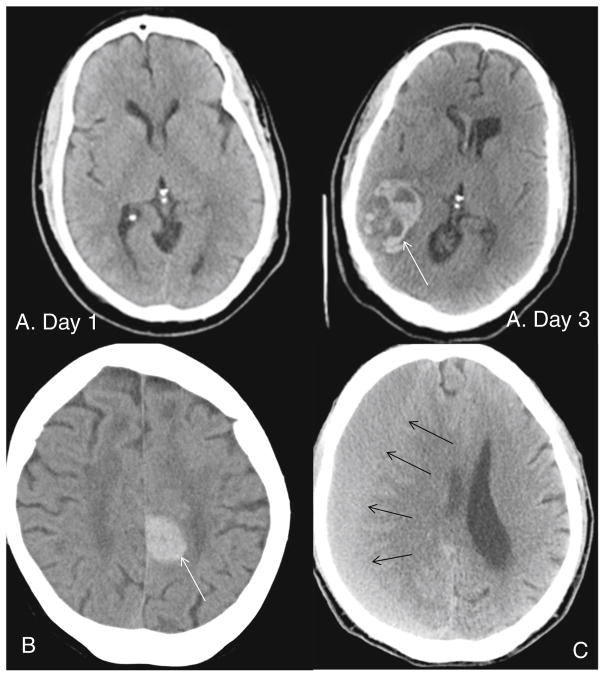
Damage to small subcortical arteries and intracranial aneurysm formation and rupture are both accelerated with hypertension and are causative precursors to subcortical intracerebral hemorrhage and subarachnoid hemorrhage (primary hemorrhage) [19]. Hypertension is increasingly also recognized as a risk factor for lobar intracerebral hemorrhage (located near the cortical surface) and likely influences other degenerative processes in small arterioles such as cerebral amyloid angiopathy [17]. Hemorrhagic strokes have been typically attributed to the anti-thrombotic treatment, though an elevated international normalized ratio (INR) is neither necessary nor sufficient to lead to hemorrhagic strokes [5]. In clinical practice, patients may frequently have supra-therapeutic INR’s without developing a hemorrhagic stroke, and conversely this stroke subtype may be seen in patients with sub-therapeutic INR’s. Coagulopathy on the other hand leads to poorer outcomes among patients in whom the hemorrhagic stroke is related to hypertension [17]. Hypertension may also contribute to hemorrhagic conversion of an ischemic stroke [20], a frequent complication of large infarcts already observed presentation [21], and which on imaging may be difficult to discern from a primary hemorrhage (Fig. 1). In the case of hemorrhagic transformation, the primary pathological process is cerebral ischemia from a cerebral embolus, followed by lysis of the embolus and restoration of flow into infarcted tissue without the presence of the blood–brain barrier. In our experience, further characterization of hemorrhagic strokes after adjudication from a vascular neurologist leads to at least one third being reclassified as hemorrhagic transformation. Lastly, the mechanism for non-traumatic subdural hematomas, not typically defined as stroke by neurologists [17] but included in the Interagency Registry for Mechanically Assisted Circulatory Support dataset [1•], is poorly understood and it is unknown if it manifests as acute or chronic in LVAD patients. Subdural hematoma (Fig. 1) is believed to arise from tears in bridging veins across the meningeal to the dura, an area not exposed to the arterial circulation. Over time, the subdural hematoma liquefies and becomes chronic, during which time it may expand in size and become symptomatic. This process is incompletely understood, though vascularization of the outer membrane has been well described and treatment with anti-hypertensives may lower the risk of expansion of a chronic subdural hematoma [22, 23].
Fig. 1.
Computed tomography scan of an ischemic stroke (day 1 without acute findings) with subsequent hemorrhagic conversion (white arrow) (day 3) (panels a), primary hemorrhagic stroke patient (panel b), subdural hematoma (black arrow) (panel c)
Hypertension and Stroke in Left Ventricular Assist Devices
The hypothesis that hypertension would lead to stroke and adverse events in LVADs [24•] emerged from data on the HVAD in a recent publication, where an MAP of greater than 90 was reported as a risk factor for stroke [3••]. In these reports, the MAP was examined as follows: on or before stroke, within 7 days before stroke, before the stroke. In analyses, all available MAP were summarized as means and then compared in stroke and non-stroke patients; MAP was also described as the mean of all measures within 7 days of stroke (including and excluding the blood pressure at time of stroke). Among 382 participants, 6.8 % developed ischemic stroke (7 out of 26 peri-operative) and 8.4 % developed hemorrhagic stroke (16 out of 32 intracerebral). In these reports, the subtyping of stroke allowed for the exclusion of patients with hemorrhage due to falls or treatment with lytics, as well as peri-operative stroke. In multi-variable models, a MAP >90 mmHg before the event was associated with a higher risk of ischemic stroke, and a MAP >90 mmHg 7 days before the event including this time, the day of the event, was associated with a higher risk of hemorrhagic stroke The latter was an important point since patients often have elevated blood pressures at the time of stroke regardless of stroke subtype. However, in support of a direct role of hypertension in hemorrhagic strokes, the authors also reported that the incidence was lower in enrolling sites which had a strict blood pressure monitoring protocol. One possible explanation is that patients who had a MAP >90 mmHg reflect a population with a higher burden of hypertension which led to long-standing cerebral arteriolar injury starting even before LVAD implantation. Arguing against this is that the average MAP was only 2 mmHg higher in those with a history of hypertension. On the other hand, the blood pressures reported in the HVAD registry were not sufficiently high to have acutely triggered the stroke within 7 days. A high blood pressure in this case might rather be an indicator of sustained damage to already fragile cerebral arterioles from chronically elevated blood pressure not only preceding but also following LVAD implantation. Investigators at Washington University have recently reported that a systolic blood pressure above the mean (>100 mmHg) 48 h before discharge from the hospital after implantation was associated with a higher risk of ischemic and hemorrhagic stroke, a mean of 16 months later without a clear difference by device type [25••]. Otherwise, strokes may also reflect a systemic process leading to hemorrhage that is independent of the cerebral vasculature.
The processes occurring in the cerebral circulation after continuous flow LVAD implantation merit some attention and have been a rare subject of research. In some patients, persistent hypertension after LVAD implantation could potentially accelerate the non-cardioembolic processes leading to ischemic stroke, for example making it more likely that patients would develop a lacunar stroke or artery to artery emboli from large artery atherosclerosis. Arguing against this process is that hypertension was not a risk factor for ischemic stroke in the HVAD trial and registry. In our experience, none of the ischemic strokes have been due to non-cardioembolic etiologies such as large artery atherosclerosis or lacunes. A stronger argument can be made for the role of cerebral arterial pathology in patients who had a primary hemorrhagic stroke, which are a substantial proportion of hemorrhages in published series and are more strongly associated with poor outcomes. One hypothesis is that the loss of pulsatile flow may lead to endothelial dysfunction due to loss of endothelial nitric oxide synthase [26]. Endothelial dysfunction could lead to the loss of at least two protective mechanisms the brain has against hemorrhage: the blood–brain barrier and cerebral auto-regulation. This was recently investigated in a series of patients with continuous flow LVADs, pulsatile flow LVADs, and healthy controls who underwent an exercise challenge while undergoing transcranial Doppler (TCD) ultrasonography. In this study, investigators found that changes in TCD were similar in patients with LVADs and controls arguing against the loss of cerebral auto-regulation [27•]. Other more advanced measures of cerebral auto-regulation, such as direct cerebral auto-regulation with a hypotensive or post-occlusive hyperemic challenge [28], have not been established in this patient population. Lastly, little data is known on whether arteriolar degenerative processes, such as accumulation of αβ-amyloid in cerebral amyloid angiopathy among those older than 55, interacts with blood pressure to increase the risk of ischemic or hemorrhagic stroke as it does in non-LVAD patients [29]. Further characterization of hemorrhagic strokes as lobar versus subcortical in location may shed further light into this process. It is likely that a hemorrhagic stroke, similar to patients without an LVAD, is due to an accumulation of multiple processes of which hypertension is an integral component. Unfortunately, post-mortem examinations of cerebral cortex, which may shed light into the pathophysiology of stroke in LVAD patients, are underutilized and/or underreported.
Measurement and Management of Blood Pressure after Left Ventricular Assist Device
Non-invasive blood pressure measurement by standard cuffs has been problematic due to reduced pulsatile flow in patients on continuous flow LVAD support. Doppler ultrasound with sphygmomanometer is routinely used to assess blood pressure in this patient population. Although Doppler is highly reliable and valid for systolic blood pressure measurement, Doppler blood pressure approximates MAP only in a setting of reduced pulse pressure (PP) [30•, 31]. Thus, the common knowledge that Doppler blood pressure accurately measures of MAP in all LVAD patients is inadequate and possibly misleading. For example, in the HVAD trial and registry cited above, the methodology used for MAP measurement is not specified. If Doppler was mainly used, a blood pressure >90 mmHg would accurately measure systolic blood pressure rather than MAP, thus, leading to different conclusions and recommendations with respect to blood pressure management in LVAD patients based on the results of this study.
We recently reported on the usefulness of combining the two currently available methodologies (i.e., Doppler and standard automated blood pressure monitor) to assess systolic blood pressure and MAP in LVAD patients. The approach was based on the observation that the probability of obtaining successful blood pressure readings with standard monitors increases the higher the pulse pressure becomes. Based on prior research, we adopted a two-step approach for the assessment of blood pressure among patients with an LVAD:
Step 1: Obtain initial blood pressure measurement by automated monitor three times.
Interpretation of Results
If at least two out of three are obtainable with an automated cuff, the average of the blood pressure readings is interpreted as valid and the use of Doppler-assisted measurements is not needed.
-
If one or no readings are obtained using the automated cuff, we proceed to step 2.
Step 2: Obtain Doppler blood pressure measurement.
Interpretation of Results
Doppler blood pressure provides valid measure of systolic blood pressure in all cases.
Doppler blood pressure also approximates MAP in case none of the three automated readings is successful, as pulse pressure is substantially reduced in these patients.
Management of patients with continuous flow LVADs can be complex in sites without significant expertise in measuring blood pressure [30•, 31]. Intensive hypertension management appears to be beneficial. In the HVAD registry and trial, those sites that used an improved BP monitor protocol (at least weekly blood pressure monitoring via telephone and/or in person visits to achieve target levels, goal MAP 90 mmHg, and use of an escalating regimen driven by the patient’s blood pressure) had a statistically significant lower hemorrhagic stroke rate (8.4 vs. 2.6 %) [3••, 13]. Blood pressure control to a target MAP ≤80 mmHg can be achieved effectively on one or two medications with classes used widely in clinical practice (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and beta blockers).
Management of Hypertension after Stroke
The management of hypertension after stroke is likely to be substantially different in patients with an LVAD compared to all others. Treatment of hypertension in ischemic stroke patients may be more challenging given the concern for expansion of the infarct due to hypo-perfusion, most often seen in patients with large artery atherosclerosis and lacunar strokes. Current recommendations for ischemic stroke patients advocate against treating acute hypertension in an ischemic stroke patient unless there is evidence of end-organ damage such as renal failure or CHF [32, 33]. None of the data used to derive these recommendations included patients with LVADs, and patients with advanced class CHF were also excluded from the studies informing the guidelines. It is likely that auto-hypertension would not be beneficial in patients with LVADs given that there is a low prevalence of non-cardioembolic etiologies. Conversely, there is potential risk for allowing blood pressures to remain high in patients with LVADs including hemorrhagic conversion of an ischemic stroke. For these reasons, the blood pressure targets in LVAD patients with ischemic stroke in our institution are driven by indications for other organ systems, though from a neurological perspective we avoid an MAP >90 mmHg. In hemorrhagic stroke, maintaining an MAP <90 mmHg is likely to be protective against hematoma expansion and therefore poor outcomes in both LVAD and non-LVAD patients [17]. Though there are also no data on long-term secondary stroke prevention for LVAD patients, maintaining the same blood pressure targets as for primary prevention is reasonable.
Conclusions
Hypertension is a major driving force behind the risk of stroke worldwide in both ischemic and hemorrhagic stroke. There is compelling data that a higher blood pressure is a consistent risk factor for stroke in LVAD, and that starting blood pressure control soon after implantation is likely to be beneficial. A new algorithm for BP assessment that combines Doppler and standard automated BP monitor may overcome the limitation of individual technologies as it offers reliable measurement of SBP and MAP in the majority of patients on CF-LVAD support. Frequent BP monitoring in clinic and when feasible at home should be considered in all LVAD patients. The mechanisms via which hypertension contribute to stroke risk are not well understood, and future research is required to better understand and possibly reduce the burden of stroke in patients treated with LVADs.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Dr. Willey is a consultant for Heartware and is on the Clinical Endpoint Committee for Reliant Heart. Drs. Boehme, Castagna, Yuzefpolskaya, Garan, Topkara, and Colombo declare no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1•.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33(6):555–64. doi: 10.1016/j.healun.2014.04.010. This manuscript reviews recent trends in implantation and complications after placement of LVAD. [DOI] [PubMed] [Google Scholar]
- 2••.Estep JD, Starling RC, Horstmanshof DA, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP study. J Am Coll Cardiol. 2015;66(16):1747–61. doi: 10.1016/j.jacc.2015.07.075. This up to date manuscript provides a description of improved survival and quality of life with LVAD despite a greater risk of complications. [DOI] [PubMed] [Google Scholar]
- 3••.Teuteberg JJ, Slaughter MS, Rogers JG, et al. The HVAD left ventricular assist device: risk factors for neurological events and risk mitigation strategies. JACC Heart Fail. 2015;3(10):818–28. doi: 10.1016/j.jchf.2015.05.011. The comprehensive overview of hypertension as a complicating factor in HVAD associated strokes is included in this manuscript. [DOI] [PubMed] [Google Scholar]
- 4.Harvey L, Holley C, Roy SS, et al. Stroke after left ventricular assist device implantation: outcomes in the continuous-flow era. Ann Thorac Surg. 2015;100(2):535–41. doi: 10.1016/j.athoracsur.2015.02.094. [DOI] [PubMed] [Google Scholar]
- 5.Boehme AK, Pamboukian SV, George JF, et al. Predictors of thromboembolic events in patients with ventricular assist device. ASAIO J. 2015;61(6):640–7. doi: 10.1097/MAT.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willey JZ, Demmer RT, Takayama H, Colombo PC, Lazar RM. Cerebrovascular disease in the era of left ventricular assist devices with continuous flow: risk factors, diagnosis, and treatment. J Heart Lung Transplant. 2014;33(9):878–87. doi: 10.1016/j.healun.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Morgan JA, Brewer RJ, Nemeh HW, et al. Stroke while on longterm left ventricular assist device support: incidence, outcome, and predictors. ASAIO J. 2014;60(3):284–9. doi: 10.1097/MAT.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 8.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 9.Whitson BA, Eckman P, Kamdar F, et al. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg. 2014;97(6):2097–103. doi: 10.1016/j.athoracsur.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Stulak JM, Lee D, Haft JW, et al. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant. 2013;33(1):60–4. doi: 10.1016/j.healun.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Stulak JM, Deo S, Schirger J, et al. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg. 2013;96(6):2161–7. doi: 10.1016/j.athoracsur.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Kato TS, Schulze PC, Yang J, et al. Pre-operative and postoperative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant. 2012;31(1):1–8. doi: 10.1016/j.healun.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagani F, Milano CA, Tatooles A, et al. HeartWare HVAD for the treatment of pateints with advanced heart failure ineligible for cardiac transplantation: results from the ENDURANCE destination therapy trial. J Heart Lung Transplant. 2015;34:S9. [Google Scholar]
- 14.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754–832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 16.Park TH, Ko Y, Lee SJ, et al. Identifying target risk factors using population attributable risks of ischemic stroke by age and sex. J Stroke. 2015;17(3):302–11. doi: 10.5853/jos.2015.17.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 18.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 19.Johansson BB. Hypertension mechanisms causing stroke. Clin Exp Pharmacol Physiol. 1999;26(7):563–5. doi: 10.1046/j.1440-1681.1999.03081.x. [DOI] [PubMed] [Google Scholar]
- 20.Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA Neurol. 2014;71(9):1181–5. doi: 10.1001/jamaneurol.2014.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon BK, Saver JL, Prabhakaran S, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke. 2012;43(9):2293–9. doi: 10.1161/STROKEAHA.112.660415. [DOI] [PubMed] [Google Scholar]
- 22.Weigel R, Hohenstein A, Schlickum L, Weiss C, Schilling L. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 2007;61(4):788–92. doi: 10.1227/01.NEU.0000298907.56012.E8. discussion 792–783. [DOI] [PubMed] [Google Scholar]
- 23.Laviv Y, Rappaport ZH. Risk factors for development of significant chronic subdural hematoma following conservative treatment of acute subdural hemorrhage. Br J Neurosurg. 2014;28(6):733–8. doi: 10.3109/02688697.2014.918578. [DOI] [PubMed] [Google Scholar]
- 24•.Wasson LT, Yuzefpolskaya M, Wakabayashi M, et al. Hypertension: an unstudied potential risk factor for adverse outcomes during continuous flow ventricular assist device support. Heart Fail Rev. 2014;20(3):317–22. doi: 10.1007/s10741-014-9458-3. This is an important review article highlighting the potential mechanisms by which hypertension may lead to complications with LVAD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Nassif ME, Tibrewala A, Raymer DS, et al. Systolic blood pressure on discharge after left ventricular assist device insertion is associated with subsequent stroke. J Heart Lung Transplant. 2015;34(4):503–8. doi: 10.1016/j.healun.2014.09.042. This study of the effects of elevated blood pressure even before hospital discharge has a deleterious effect on stroke risk and provides additional data that the risk from hypertension may not be due to only an acute reaction at time of stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uzarski JS, Scott EW, McFetridge PS. Adaptation of endothelial cells to physiologically-modeled, variable shear stress. PLoS One. 2013;8(2):e57004. doi: 10.1371/journal.pone.0057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Cornwell WK, 3rd, Tarumi T, Aengevaeren VL, et al. Effect of pulsatile and nonpulsatile flow on cerebral perfusion in patients with left ventricular assist devices. J Heart Lung Transplant. 2014;33(12):1295–303. doi: 10.1016/j.healun.2014.08.013. This physiologic study highlights that patients with continuous flow VAD’s still have preserved cerebral autoregulation. [DOI] [PubMed] [Google Scholar]
- 28.Ortega-Gutierrez S, Petersen N, Masurkar A, et al. Reliability, asymmetry, and age influence on dynamic cerebral autoregulation measured by spontaneous fluctuations of blood pressure and cerebral blood flow velocities in healthy individuals. J Neuroimaging. 2013;24(4):379–86. doi: 10.1111/jon.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reijmer YD, van Veluw SJ, Greenberg SM. Ischemic brain injury in cerebral amyloid angiopathy. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Colombo PC, Lanier GM, Orlanes K, Yuzefpolskaya M, Demmer RT. Usefulness of a standard automated blood pressure monitor in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015 doi: 10.1016/j.healun.2015.07.008. The appropriate strategies for measuring hypertension in patients with LVAD is reviewed in this manuscript. [DOI] [PubMed] [Google Scholar]
- 31.Lanier GM, Orlanes K, Hayashi Y, et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ Heart Fail. 2013;6(5):1005–12. doi: 10.1161/CIRCHEARTFAILURE.112.000186. [DOI] [PubMed] [Google Scholar]
- 32.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 33.Powers WJ, Derdeyn CP, Biller J, et al. American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020–35. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]