Abstract
Introduction
During the six month period following chemoradiotherapy, gliomas frequently develop new areas of contrast enhancement, which are due to treatment effect rather than tumor progression. We sought to characterize this phenomenon in oligodendrogliomas (OG) and mixed oligoastrocytomas (MOA).
Method
We reviewed the imaging findings from 143 patients with a WHO grade II or III OG or MOA for evidence of pseudoprogression (PsP) or early tumor progression. We characterized these cases for 1p/19q codeletions by FISH, IDH1 R132H mutation by immunohistochemistry, and TP53, ATRX, and EGFR mutations by next generation sequencing. We then reviewed the pathologic specimens of the patient cases in which a re-resection was performed.
Results
We found that OG and MOA that are 1p/19q intact developed PsP at a higher rate than tumors that are 1p/19q codeleted (27% vs. 8%). Moreover, IDH1 wild-type (WT) tumors developed PsP at a higher rate than IDH1 R132H cases (27% vs. 11%). Patients with ATRX or TP53 mutations developed PsP at an intermediate rate of 21%. Ten patients in our cohort underwent a re-resection for early contrast enhancement; these tumors were predominantly 1p/19q intact (90%) and had a low rate of IDH1 R132H mutation (50%). Eight of 10 tumors demonstrated primarily treatment effects, while the remaining 2 of 10 demonstrated recurrent/residual tumor of the same grade.
Conclusion
Early contrast enhancement that develops during the first 6 months after chemoradiotherapy is typically due to PsP and occurs primarily in OG and MOA that are 1p/19q intact and IDH WT.
Keywords: Diffuse glioma, oligodendroglioma, mixed oligoastrocytoma, astrocytoma, pseudoprogression, and radiation necrosis
Introduction
Pseudoprogression (PsP) is a radiographic phenomenon defined as new areas of contrast enhancement that occur within 6 months after radiotherapy (RT) and is thought to be augmented by concurrent chemotherapy. PsP, in malignant gliomas, likely represents a robust inflammatory reaction, which disrupts the blood brain barrier [1]. PsP is challenging to differentiate from true tumor progression and remains an outstanding problem in neuro-oncology that has considerable implications in patient management, as it can result in the premature discontinuation of effective therapy and can lead to unnecessary surgery.
Previous reports on PsP have been based primarily on studies performed on high-grade astrocytomas, especially glioblastoma (GBM), where PsP occurs in 28–66% of patients during the immediate post-chemo-RT period [2–4]. MGMT promoter methylation is thought to increase the risk of PsP in GBM; in one study, 21 of 23 tumors (91%) with MGMT promoter methylation developed PsP [1]. This high rate of PsP in methylated GBM is thought to be due in part to a higher sensitivity of these tumors to the alkylating effects of temozolomide [5].
The literature on PsP in oligodendroglioma (OG) and mixed oligoastrocytoma (MOA) is more limited. We previously reported that OG and MOA are unexpectedly less likely to develop PsP when they harbor 1p/19q codeletions, a marker of increased sensitivity to treatment and better prognosis [6]. We sought to confirm this finding by enlarging the cohort [6] and performing additional molecular testing to further define the molecular characteristics of OG and MOA that are at higher risk of developing PsP.
Methods
Institutional review board approval was obtained to review a pathologic database for all tumors diagnosed as either OG or MOA, WHO grade II or III, from 2001 to 2014. These cases were further queried for tumors that were treated with RT—during a period in which the standard of care was fractionated photon irradiation with or without chemotherapy—and serially imaged using standard of care contrast-enhanced brain MRI.
A total of 143 patients with OG or MOA were identified. PsP was diagnosed as most likely based on pathologic grounds, when a re-resection was performed and histologic analysis showed at least 50% RT-related changes without a change in WHO grade, and/or radiographically, when the clinical MRI report identified new contrast enhancement within the treatment area during the first 6 months after the completion of RT, which then spontaneously resolved, improved, or remained stable without escalation of therapy, especially bevacizumab treatment. Early progression was defined as the presence of new contrast enhancement within the treatment area during the first 6 months after the completion of RT that continued to increase on serial imaging. Alternatively, progression could be diagnosed on pathology examination, when proliferating tumor cells accounted for at least 50% of tumor cellularity.
Depending on the availability, quantity and quality of banked tumor samples from the 143 patients, the status of 1p/19q codeletions in all 143 patients, the IDH1 R132H mutational status in 87 patients, and the TP53, ATRX, and EGFR mutational status in 73 patients were determined using FISH, immunohistochemistry (IHC) and next generation sequencing, respectively. Additionally, of this patient cohort, 24 patients underwent a re-resection for an enhancing abnormality that developed after RT. Ten patients developed new contrast enhancement within 6 months after RT, and 14 patients developed new contrast enhancement over 6 months after RT.
The percent RT-related changes were retrospectively quantified by a neuro-pathologist when the surgical specimen was available for review. RT-related changes were determined based on the presence of hyalinized and thickened vessels with or without fibrinoid necrosis of the walls, ischemic/infarct-type necrosis of tumor, radiation-associated atypia, and lower cellularity with decreased mitotic activity and proliferative index in areas populated with tumor cells.
Results
Molecular Characteristics of Tumors that Developed PsP
Of the 143 patients, 59 were diagnosed with a WHO grade II and 84 with a WHO grade III OG or MOA. All patients received fractionated radiotherapy, and 89% received adjuvant chemotherapy (of which, 92% received temozolomide concomitantly with RT). 1p/19q codeletions were detected in 53 of 143 patients (37%). IDH1 R132H mutation was found in 61 of the 87 tumors tested by IHC (70%). Independent of the IDH1 mutational status, TP53 or ATRX mutations were found in 42 of the 73 sequenced tumors (58%), and EGFR mutations were present in 5 of the 73 (7%). Of these EGFR mutated tumors, only one carried IDH1 R132H, the remaining four were IDH1 WT.
Twenty-eight patients (20%) developed PsP, 8 patients (6%) demonstrated early progression, and 107 patients (75%) had no early contrast enhancement. WHO grade II and grade III OG and MOA had similar rates of PsP—17% vs. 21% (Figure 1). The frequency of PsP and progression, when the cohort is stratified by molecular characteristics, is shown in Table 1 and Figure 2. Patients with tumors that are 1p/19q intact developed PsP at a higher rate than tumors that are 1p/19q codeleted (27% vs. 8%). Patients with tumors that were negative for the IDH1 R132H mutation by IHC had a higher rate of PsP than tumors with positive IHC, 27% vs. 11%. OG/MOA that harbor TP53 or ATRX mutations developed PsP at an intermediate rate of 21%. There were no recurrent mutations in TP53 or ATRX that were associated with the development of PsP. Among the tumors with EGFR mutations, 1 of 5 demonstrated PsP following RT.
Figure 1.
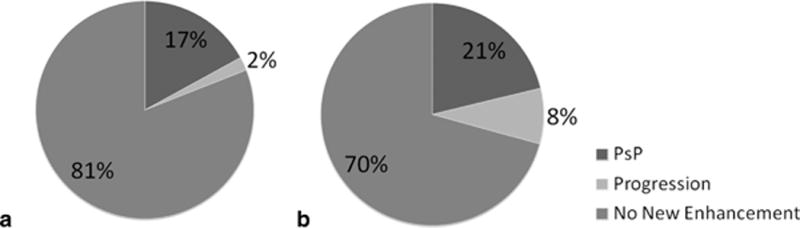
Prevalence of PsP and early progression by WHO grade: (a) grade II and (b) grade III
Table 1.
Rate of PsP and early progression by molecular characteristics
| No early enhancement | PsP | Early Progression | |
|---|---|---|---|
| IDH1 R132H | 49 (80%) | 7 (11%) | 5 (8%) |
| IDH1 wild-type | 18 (69%) | 7 (27%) | 1 (4%) |
| 1p/19q codeleted | 46 (87%) | 4 (8%) | 3 (6%) |
| 1p/19q intact | 61 (68%) | 24 (27%) | 5 (6%) |
| TP53 and ATRX WT | 27 (87%) | 3 (10%) | 1 (3%) |
| TP53 or ATRX mutant | 31 (74%) | 9 (21%) | 2 (5%) |
Figure 2.
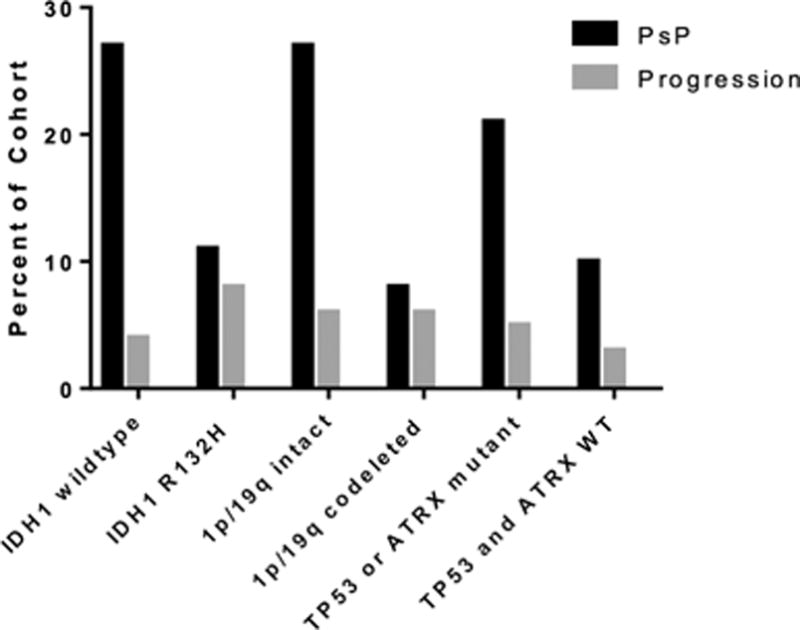
Rate of PsP and early progression by molecular characteristics
Pathologic Review of Resected Areas of Post-RT Contrast Enhancement
Twenty-four patients underwent second look surgery to resect a new enhancing abnormality that was found on surveillance brain MRI after a course of RT. Of these, 10 patients (2 WHO grade II and 8 WHO grade III tumors) developed new contrast enhancement during the first 6 months following RT and underwent a repeat surgical resection. Eight out of 10 patients had at least 50% RT-related changes (Figure 3). Nine patients received chemotherapy concurrently with radiation (8 with temozolomide and 1 with PCV). Of these 10 patients, only 1 had 1p/19q codeletions, and 4 (out of the 8 tumors for which IDH1 status is known) were IDH1 R132H mutant.
Figure 3.
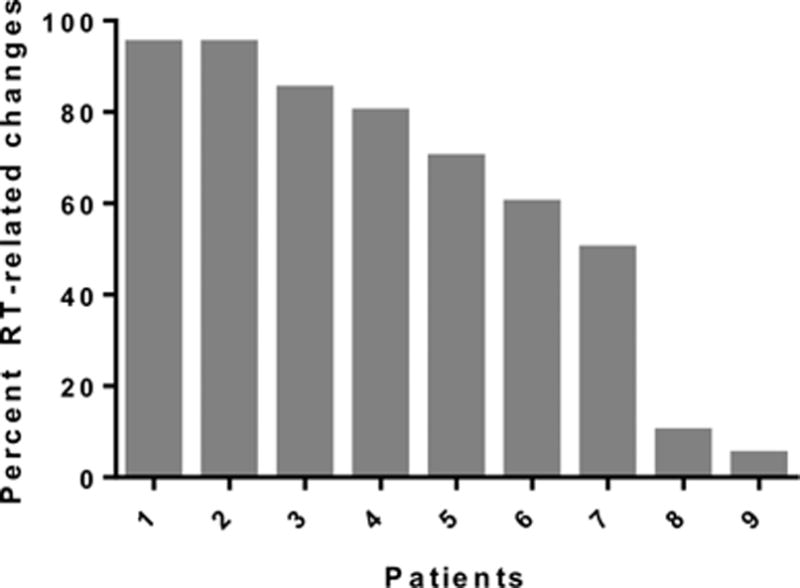
RT-related changes in patients with second-look resections for early contrast enhancement (first 6 months after RT)
There were no cases of progression to a higher grade among these 10 cases of early contrast enhancement. Eight patients had at least 50% treatment-related effects consistent with radiation necrosis, i.e. our definition for PsP, and 5 of 8 demonstrated extensive treatment effects (i.e. 80% or more treatment-related effects). One of the 2 WHO grade II tumors and 7 of 8 WHO grade III tumors had PsP. In the 2 patient cases with minimal treatment-related effects, the residual tumors had the same appearance as the original diffuse glioma with no evidence of transformation to a higher grade.
The other 14 patients developed new areas of contrast enhancement more than 6 months following the completion of RT. Eleven of 14 patients received concomitant chemotherapy, predominantly temozolomide (10 of 14). Within this group, 8 transformed to a higher grade—including 6 secondary GBM—3 had recurrent tumor of the same grade with minimal signs of treatment related changes, and only 2 had predominantly treatment-related changes.
Discussion
In GBM, PsP appears to correlate with treatment responsiveness, as it occurs more frequently in GBM with MGMT promoter methylation [7]. This study suggests that in OG and MOA, PsP occurs most frequently in 1p/19q intact, IDH WT tumors. WHO grades II and III gliomas that are 1p/19q intact and TP53 and ATRX mutated, are predominantly IDH mutated, which likely accounts for the development of PsP at an intermediate rate [8]. The finding that IDH mutants have a lower rate of PsP is unexpected, given that IDH1 R132H mutated gliomas have a higher rate of MGMT promoter methylation [9,10]. The higher rate of PsP in IDH WT tumors may be due to the fact that these tumors are molecularly similar to GBM, with frequent EGFR mutations, and follow a clinical course that is similar to GBM.
The patients in our cohort who developed new areas of contrast enhancement during the first 6 months after chemo-RT, who underwent re-resection, and had at least 50% RT-related changes on pathologic review, are molecularly similar to the larger cohort of diffuse gliomas that developed PsP. They were rarely 1p/19q codeleted, frequently IDH1 WT by IHC, and particularly aggressive in that they progressed at higher rates within the first year of follow-up. Of importance, the pathologic diagnosis was not upgraded by the second-look surgery for areas of early contrast enhancement. In stark contrast, patients who developed areas of contrast enhancement over 6 months after RT were more likely to have had disease progression and transformation to a higher WHO grade. Taken together, these results suggest that new areas of contrast enhancement that develop within 6 months of RT with or without chemotherapy is predominantly due to PsP rather than tumor progression. While these results may call into question the utility of re-resections during the first 6 months after RT, there may still be a role for these surgeries; for instance, when PsP causes significant vasogenic edema, resulting in a neurologic deterioration, and rapid decompression is required.
There are clear limitations to our study, the most significant being the retrospective design and the small population size. A prospective study in which IDH status is evaluated by sequencing, and PsP is evaluated by advanced imaging techniques (MRI perfusion, MR spectroscopy, and PET) at explicitly defined time points is needed to confirm these results [11–14]. Another limitation is that we only included tumors that were OG or MOA. For that reason, this analysis does not capture a large cohort of diffuse gliomas with a molecular astrocytoma profile (i.e. 1p/19q intact and frequently harboring TP53 and ATRX mutations).
The overall lack of information regarding the nature of new contrast enhancement in OG and MOA tumors following radiation is where this report is helpful in patient management. This retrospective study suggests that PsP occurs at a higher rate in tumors with a less favorable molecular profile, i.e. tumors that are IDH WT and 1p/19q intact. It also establishes that new contrast enhancement that develops within 6 months of completing radiotherapy is much more likely to represent PsP and that the clinician should carefully consider this possibility prior to recommending a second-look surgery or escalation to salvage therapy.
Acknowledgments
The authors would like to thank David Carrell and Lisa Snipes at Washington University School of Medicine for their technical assistance.
Funding: This work was supported by Washington University Institute of Clinical and Translational Services, which is in part supported by the NIH/National Center for Advancing Translational Sciences (NCATS), CTSA Grant UL1TR0004489 (ICTS JIT # 312 awarded to S.D.).
Footnotes
Compliance with Ethical Standards
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
References
- 1.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 2.Fink J, Born D, Chamberlain MC. Pseudoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol. 2011;12(3):240–252. doi: 10.1007/s11864-011-0157-1. [DOI] [PubMed] [Google Scholar]
- 3.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 4.Roldan GB, Scott JN, McIntyre JB, Dharmawardene M, de Robles PA, Magliocco AM, Yan ES, Parney IF, Forsyth PA, Cairncross JG, Hamilton MG, Easaw JC. Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci. 2009;36(5):617–622. doi: 10.1017/s0317167100008131. [DOI] [PubMed] [Google Scholar]
- 5.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 6.Lin AL, Liu J, Evans J, Leuthardt EC, Rich KM, Dacey RG, Dowling JL, Kim AH, Zipfel GJ, Grubb RL, Huang J, Robinson CG, Simpson JR, Linette GP, Chicoine MR, Tran DD. Codeletions at 1p and 19q predict a lower risk of pseudoprogression in oligodendrogliomas and mixed oligoastrocytomas. Neuro Oncol. 2014;16(1):123–130. doi: 10.1093/neuonc/not142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 8.Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372(26):2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leu S, von Felten S, Frank S, Vassella E, Vajtai I, Taylor E, Schulz M, Hutter G, Hench J, Schucht P, Boulay JL, Mariani L. IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro Oncol. 2013;15(4):469–479. doi: 10.1093/neuonc/nos317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceccarelli M, Barthel FP, Malta TM, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016;164(3):550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prager AJ, Martinez N, Beal K, Omuro A, Zhang Z, Young RJ. Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol. 2015;36(5):877–885. doi: 10.3174/ajnr.A4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology. 2013;55(3):361–369. doi: 10.1007/s00234-012-1127-4. [DOI] [PubMed] [Google Scholar]
- 13.Barker FG, 2nd, Chang SM, Valk PE, Pounds TR, Prados MD. 18-Fluorodeoxyglucose uptake and survival of patients with suspected recurrent malignant glioma. Cancer. 1997;79(1):115–126. [PubMed] [Google Scholar]
- 14.Zhang H, Ma L, Wang Q, Zheng X, Wu C, Xu BN. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: a systematic review and meta-analysis. Eur J Radiol. 2014;83(12):2181–2189. doi: 10.1016/j.ejrad.2014.09.018. [DOI] [PubMed] [Google Scholar]


