What are KASH proteins?
KASH proteins (Klarsicht, ANC-1, Syne homology) are C-tail-anchored membrane proteins, which are targeted specifically to the outer membrane of the nuclear envelope. The defining feature of KASH proteins is the carboxy-terminal KASH domain that consists of a hydrophobic region spanning the outer nuclear membrane and 6–30 residues in the perinuclear space. The perinuclear domains of KASH proteins are often highly similar; for example, 13 of 20 residues are identical between Caenorhabditis elegans ANC-1 and human Syne/Nesprin-1 and -2. Other KASH proteins have shorter and/or divergent perinuclear sequences. Due to a lack of homology, additional KASH proteins likely remain to be discovered. KASH proteins also have large, non-conserved cytoplasmic domains.
What are the names of mammalian KASH proteins — Syne or Nesprin?
In 2000–2002, around the time of the discovery of KASH proteins, two mammalian KASH proteins were independently identified by at least six groups. They were originally named Syne-1 and -2 because of their roles in anchoring nuclei at the neuromuscular junction. Today, many call these proteins ‘Syne’, but the majority of the field uses the term ‘Nesprin’ (for nuclear envelope with spectrin repeats) for the proteins and SYNE for the genes. Neither name is perfect, because not all KASH proteins contain spectrin repeats. Furthermore, the term Nesprin refers exclusively to mammalian KASH proteins and excludes the functional roles elucidated from studies of KASH proteins in other model systems.
What are SUN proteins?
SUN proteins (for Sad1 and UNC-84) are integral components of the inner nuclear membrane with conserved, carboxy-terminal SUN domains that localize to the perinuclear space. SUN domains consist of approximately 175 residues and are conserved across all eukaryotes. The nucleoplasmic domains of SUN proteins are not conserved, but nonetheless interact with structural components of the nucleoskeleton; many interact directly with lamins. The presence of multiple SUN proteins in a single organism (at least five in humans), their various isoforms, and their ability to form multimers complicates the studies of their functions.
How are KASH proteins targeted to the outer nuclear membrane?
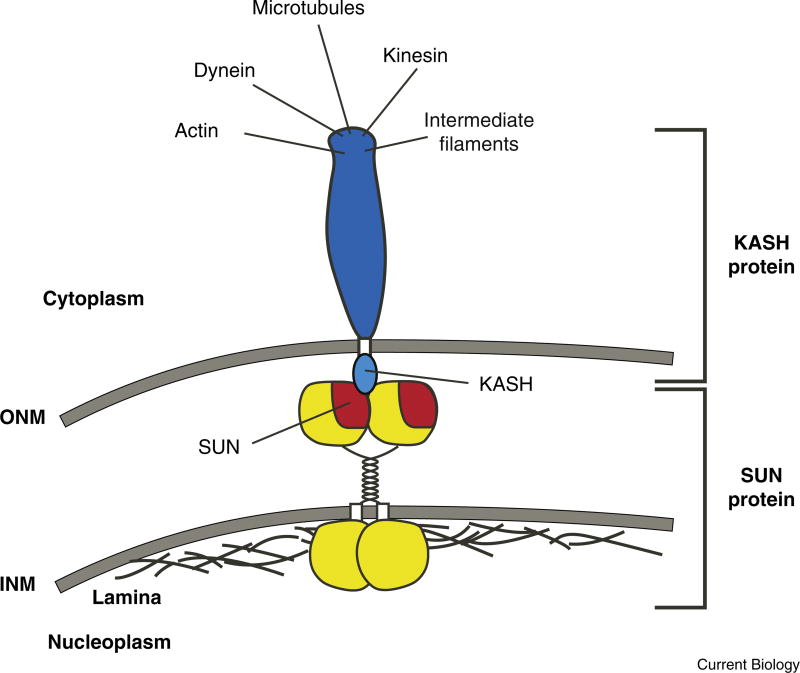
The nuclear envelope is a specialized extension of the endoplasmic reticulum, complicating trafficking of KASH and SUN proteins to specific membrane domains. The outer nuclear membrane is contiguous with the endoplasmic reticulum, and the inner and outer membranes are connected at nuclear pores. Although a KASH domain alone is sufficient to target a heterologous protein to the outer nuclear membrane, recruitment of KASH proteins to the outer nuclear membrane requires both KASH and SUN domains, as a mutation in either one blocks the targeting of KASH proteins to the outer nuclear membrane. In agreement with these genetic data, KASH and SUN proteins physically interact in the perinuclear space to connect the inner and outer nuclear membranes and to maintain the even spacing of these membranes. Together, SUN and KASH proteins form bridges that span both membranes of the nuclear envelope (Figure 1).
Figure 1. KASH and SUN proteins bridge the nuclear envelope.
KASH proteins (blue) span the outer nuclear membrane (ONM). The cytoplasmic domains of KASH proteins interact with a variety of cytoskeletal components. SUN proteins (yellow and red; shown as a dimer) span the inner nuclear membrane (INM). The nucleoplasmic domains of SUN proteins interact with lamins or other structural components of the nucleus. SUN and KASH domains interact with one another in the perinuclear space to form the central link of a bridge that spans the nuclear envelope, connecting the cytoskeleton to the nucleoskeleton.
What is the LINC complex?
LINC (linker of nucleoskeleton and cytoskeleton) complexes connect the nucleus to the cytoskeleton. The nucleoskeleton, which provides structure to the nucleus, is made of lamins, inner nuclear membrane proteins, and chromosomes. It is separated from the cytoskeleton by the nuclear envelope. Forces generated in the cytoplasm must be transferred across both membranes to the nucleoskeleton. KASH and SUN proteins are central to the transfer of this force because they form the bridge across the nuclear envelope. The cytoplasmic domains of KASH proteins interact with a variety of components of the cytoskeleton. SUN proteins, in turn, interact with the nucleoskeleton. The entire chain of proteins, from cytoskeletal elements through KASH and SUN bridges, to the nucleoskeleton, is referred to as a LINC complex.
How do KASH proteins interact with the cytoskeleton?
The large cytoplasmic domains of KASH proteins interact with a variety of cytoskeletal elements. The mammalian Syne/Nesprin-1 and -2, C. elegans ANC-1, and Drosophila MSP-300 orthologs are giant proteins (over 8,000 residues) with similarity to dystrophin. They function to connect the nucleus to the actin cytoskeleton in order to anchor or move nuclei. Drosophila Klarsicht, C. elegans UNC-83 and ZYG-12, mammalian Nesprin-4, and Schizosaccharomyces pombe Kms1 act as cargo adaptors for the microtubule motors dynein and/or kinesin-1 during nuclear migration. C. elegans ZYG-12 attaches the centrosome to the nuclear envelope. Human Nesprin-3 connects intermediate filaments to nuclei, although the functional significance of this remains unknown. Saccharomyces cerevisiae Csm4 is thought to couple actin dynamics to the nucleus. In addition, other KASH proteins function in cell-cycle regulation (C. elegans KDP-1) or as GTPase-activating proteins for the small GTPase Ran (e.g. Arabidopsis WIPs). Many functions for KASH proteins remain to be fully characterized, especially with regard to mechanotransduction and the global organization of the cytoskeleton.
When in development are SUN and KASH proteins required?
In many cell types, especially in neurons and in muscle cells, the nucleus is precisely positioned in a polarized location. KASH–SUN bridges interact with the cytoskeleton to help move nuclei to a location and to anchor nuclei in place. Forces generated by the cytoskeleton and transferred through KASH–SUN bridges are also used to move structures within the nucleus, including meiotic chromosomes. The nucleus responds to mechanical stimuli that are generated outside of the cell and are transmitted to the cell through the cytoskeleton. The nucleus also regulates the organization of the cytoskeleton in a global sense. To accomplish these important functions, the nucleus interacts with actin, microtubules, and intermediate filaments through KASH and SUN proteins.
With which diseases are SUN and KASH proteins associated?
Defects in the nuclear envelope lead to a broad spectrum of diseases termed laminopathies, a subset of which are linked to mutations in SUN or KASH proteins. Hints about the roles of SUN and KASH proteins in disease come from mouse knockout models that have dramatic nuclear positioning defects in skeletal muscle cells and nuclear migration defects in neurons of the central nervous system. Syne/Nesprin-1 knockout mice phenocopy Emery Dreifuss muscular dystrophy, and a subset of patients with this disease have mutations in Syne/Nesprin-1 or -2. Mutations in Syne/Nesprin-1 cause autosomal recessive cerebellar ataxia type 1. Low levels of KASH proteins have been linked to progression of the premature aging disease Hutchinson-Gilford progeria syndrome. Syne/Nesprin-1 has also been linked to Meckel-Gruber syndrome, which is characterized by defective ciliogenesis. Finally, mutations in Syne/Nesprin-1 and -2 have been linked to increased risk for a number of cancers. Future studies of SUN and KASH proteins are likely to find additional roles for nuclear envelope bridges in human disease and development.
Where can I find out more?
- Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 2010;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298:406–409. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]