Summary
Specific neuronal types derived from embryonic stem cells (ESCs) can facilitate mechanistic studies and potentially aid in regenerative medicine. Existing induction methods, however, mostly rely on the effects of the combined action of multiple added growth factors, which generally tend to result in mixed populations of neurons. Here, we report that overexpression of specific transcription factors (TFs) in ESCs can rather guide the differentiation of ESCs towards specific neuron lineages. Analysis of data on gene expression changes 2 d after induction of each of 185 TFs implicated candidate TFs for further ESC differentiation studies. Induction of 23 TFs (out of 49 TFs tested) for 6 d facilitated neural differentiation of ESCs as inferred from increased proportion of cells with neural progenitor marker PSA-NCAM. We identified early activation of the Notch signaling pathway as a common feature of most potent inducers of neural differentiation. The majority of neuron-like cells generated by induction of Ascl1, Smad7, Nr2f1, Dlx2, Dlx4, Nr2f2, Barhl2, and Lhx1 were GABA-positive and expressed other markers of GABAergic neurons. In the same way, we identified Lmx1a and Nr4a2 as inducers for neurons bearing dopaminergic markers and Isl1, Fezf2, and St18 for cholinergic motor neurons. A time-course experiment with induction of Ascl1 showed early upregulation of most neural-specific messenger RNA (mRNA) and microRNAs (miRNAs). Sets of Ascl1-induced mRNAs and miRNAs were enriched in Ascl1 targets. In further studies, enrichment of cells obtained with the induction of Ascl1, Smad7, and Nr2f1 using microbeads resulted in essentially pure population of neuron-like cells with expression profiles similar to neural tissues and expressed markers of GABAergic neurons. In summary, this study indicates that induction of transcription factors is a promising approach to generate cultures that show the transcription profiles characteristic of specific neural cell types.
Keywords: GABAergic neurons, Dopaminergic neurons, Cholinergic neurons, Ascl1, ESCs, Gene expression profiling, miRNA
Introduction
Many neurological disorders are associated with malfunction or degeneration of specific kinds of neurons. For example, Parkinson’s disease is caused by degeneration of dopaminergic neurons (Berke and Hyman 2000; Huse et al. 2005); spinal muscular atrophy and amyotrophic lateral sclerosis are associated with degeneration of motor neurons (Crawford and Pardo 1996; Riku et al. 2014); and epilepsy, schizophrenia, autism, and Huntington’s disease are associated with degeneration of GABAergic interneurons (de Lanerolle et al. 1989; Kim et al. 2014; Benes et al. 1991; Sgado et al. 2013). Treatment of these diseases by chemical modulation of existing cells is often not satisfactory because some types of neurons may be missing, and thus, cell transplantation is a promising strategy for ameliorating neural functions. One of the challenges of this approach is generating large quantities of specific neuron types in vitro. Neuron-like cells expressing markers of specific types of neurons were successfully derived from embryonic stem cells (ESCs) following treatment with retinoic acid and/or growth factors (Chatzi et al. 2009; Nishikawa et al. 2013). However, these methods are not specific enough to generate target neuronal cell types because growth factors induce the expression of a large set of genes from various signaling pathways. An alternative approach is to manipulate individual transcription factors (TFs) which are presumably more specific in guiding cells towards certain neural fates. This approach may also yield data for the analysis of gene regulatory networks and hence elucidate the molecular mechanisms that control ESC differentiation into specific types of neurons or glia.
Many studies on cell reprogramming and gene knockout in mice showed that TFs determine specific neuron subtypes. The combinations of neural TFs can directly convert fibroblasts into neural subtypes, including dopaminergic and motor neurons (Vierbuchen et al. 2010; Caiazzo et al. 2011; Pfisterer et al. 2011, Son et al. 2011). Previous studies have shown that Lmx1a and Nr4a2 (aka Nurr1) improve the yield of dopaminergic neurons derived from ESCs (Chung et al. 2002; Kim et al. 2002; Andersson et al. 2006; Martinat et al. 2006). Overexpression of Fezf2 reprograms cortical progenitors into corticothalamic motor neurons in vivo (Rouaux and Arlotta 2010; Rouaux and Arlotta 2013). Genes from the Dlx family have been shown to be critical for interneuron specification. Dlx1/Dlx2 mutant mice show a massive decrease of neocortical GABAergic interneurons at birth (Anderson et al. 1997). Conditional knockout of Dlx5/Dlx6 decrease calretinin olfactory bulb interneurons (Wang et al. 2010). However, these data cover a small portion of all TFs in the genome and therefore are not sufficient to select prospective TFs whose manipulation may help to derive neuron types from ESCs. Although the techniques for isolating and characterizing distinct classes of neurons from CNS were developed (e.g., laser capture, FACS, and single-cell transcriptome analysis), the exact gene expression profiles of most kinds of neurons are still not known.
In this paper, we explored how the induction of individual TFs in ESCs may result in differentiation towards neural fate in general as well as towards specific types of neural cells. First, we mined the existing gene expression data on short-term effects of inducible individual TFs (Nishiyama et al. 2009; Correa-Cerro et al. 2012; Yamamizu et al. 2016) and then used prospective TFs for differentiation assays. This approach allowed us to identify TFs that facilitated the differentiation of ESCs into GABAergic, dopaminergic, and cholinergic neurons. Second, we explored mechanisms of cell differentiation towards GABAergic neurons using a time course of gene expression profiling of messenger RNA (mRNA) and microRNA (miRNA). And finally, we tested a method for enrichment of population of neuronal cells that are candidates for specific lineages, using beads carrying the anti-PSA-NCAM antibody.
Materials and Methods
Cell culture, differentiation, enrichment, and gene expression profiling
ESC lines carrying a tetracycline-regulatable TF were previously derived as described (Nishiyama et al. 2009) from MC1 (129.3) mouse ESCs which were obtained from the expanded frozen stock at Johns Hopkins University, where they were derived. Cells were cultured in DMEM with 15% FBS and 1000 u/ml leukemia inhibitory factor (LIF) on feeder cells (Nishiyama et al. 2009). For differentiation, cells were cultured in αMEM medium with 10% FBS without LIF for 3 d and were then transferred to the NeuroCult™ Differentiation medium that contained NSC Basal Medium (catalog no. 05700) and NSC Differentiation Supplements (catalog no. 05703) (Yamamizu et al. 2013). For culturing of control cells, doxycycline (Dox) was added to the medium to repress the expression of transgenic TF.
For FACS analysis, cells were harvested at 6 d of differentiation and stained with APC-conjugated PSA-NCAM antibody MoAb (Miltenyi Biotec, Bergisch Gladbach, Germany) and then analyzed using FACSCanto II (Becton Dickinson) (Zhang et al. 2010). For cell enrichment, cells were labeled with anti-PSA-NCAM MicroBeads (Miltenyi Biotec, no. 130-092-966) after 6 d of differentiation and enriched via MACS separation columns (Miltenyi Biotech), following the manufacturer’s protocol. Immediately after cell enrichment (sorting), cells were plated onto mouse laminin (10ug/ml, Sigma)-coated plates and allowed to differentiate for an additional 8 d with NeuroCult™ Differentiation medium (Yamamizu et al. 2013).
For global gene expression profiling with microarrays, cells were cultured on a gelatin-coated dish and RNA was extracted with TRIzol™ (1 ml/well; Invitrogen, Carlsbad, CA) and processed as described (Sharova et al. 2007). Additionally, we used mirVana™ kit (Thermo Fisher Scientific, Waltham, MA) for miRNA and mRNA extraction. For mRNA expression profiling, Cy3-CTP-labeled sample targets were prepared with total RNA by Low RNA Input Fluorescent Linear Amplification Kit (Agilent, Santa Clara, CA) and hybridized to NIA Mouse 44K Microarray v3.0 (Agilent, design ID 015087) (Carter et al. 2005) together with Cy5-CTP-labeled reference target, which was produced from mixture of Stratagene Universal Mouse Reference RNA and RNA from MC1 cells. The number of biologically independent replications varied from 1 to 3; a time-course experiment on Ascl1 induction was done with only one replication per time point. Because we used ANOVA, the error variance was estimated based on replicated samples and then extrapolated to non-replicated samples. Samples obtained with two mRNA extraction methods (TRIzol and mirVana) yielded slightly different gene expression results; however, relative gene expression measures (log-ratio) obtained with these methods were consistent (correlation r = 0.875) (Supplementary Fig. S1). For comparison with other data sets where RNAwas extracted with TRIzol (undifferentiated ESCs and mouse organs and tissues), we used data obtained with TRIzol for consistency. Data on PCA-NCAM(−) cells obtained with mirVana were converted to match the data obtained with TRIzol using gene-specific coefficients estimated from five pairs of identically derived samples processed with both methods. Time-course samples were obtained with mirVana, and they were compared only with each other. For miRNA expression profiling, we used Agilent-046065 Mouse miRNA V19 8x60K microarrays. Probes were labeled according to Agilent G4170-90011 miRNA Protocol 3.0. Slides were scanned with Agilent SureScan D Microarray Scanner. Microarray data are submitted to GEO/NCBI database, accession number GSE78951.
Immunohistochemistry
Immunostaining for cultured cells was carried out as described previously (Yamamizu et al. 2013). Briefly, 4% paraformaldehydefixed cells were blocked by 1% skim milk (BioLab) and incubated overnight with primary antibodies at 4°C. For immunofluorescent staining, anti-mouse, rat, rabbit, or goat IgG antibodies conjugated with Alexa488 or Alexa546 (Invitrogen) were used as secondary antibodies. Primary antibodies were as follows: mouse anti-TUJ1 (1:500; Covance, Princeton, NJ); rabbit anti-TUJ1 (1:500; Covance); mouse anti-MAP2 (1:500; Sigma-Aldrich, St. Louis, MO); mouse anti-NEUN (1:100; Millipore); rabbit anti-TH (1:500; Millipore); rat anti-DAT (1:500; Millipore); rabbit anti-VMAT2 (1:500; Millipore); rabbit anti-AADC (1:500; Abcam); rabbit anti-ALDH1A1 (1:500; Abcam); rabbit anti-GABA (1:500; Sigma-Aldrich); mouse anti-GAD67 (1:500; Millipore); mouse anti-PV (1:500; Millipore); rat anti-SST (1:500; Millipore); mouse anti-ISL1/ISL2 (1:500; DSHB); mouse anti-Hb9 (1:500; DSHB); goat anti-ChAT (1:500; Sigma-Aldrich). Differentiated neurons were photographed with inverted fluorescent microscopy (Eclipse TE300; Nikon) with the use of NIS-Elements Software (Nikon).
Data analysis
For statistical analysis of microarrays, we used ExAtlas, which uses ANOVA with error variance adjustment and estimates the false discovery rate (FDR) to account for multiple hypothesis testing (Sharov et al. 2015). Expression changes by >2-fold and FDR ≤0.05 were considered significant. ExAtlas was also used for estimating correlations between gene expression changes in two data sets and for parametric analysis of gene set enrichment (PAGE) (Kim and Volsky 2005).
Target genes with binding sites of Ascl1 in promoters and enhancers were identified as follows. First, we compiled a list of Ascl1 binding sites based on published ChIP-seq data (GEO samples: GSM1187221, GSM1187223, GSM1187225, GSM1187228, GSM1175113, GSM1347006) (Borromeo et al. 2014; Wapinski et al. 2013; Webb et al. 2013) and selected sites that matched between ≥3 studies within 500 bp. In gene expression heatmaps, we also show genes with binding sites of Ascl1 supported by two studies. These binding sites were then annotated based on transcript coordinates (RefSeq and Ensembl, genome mm9) downloaded from UCSC database (genome.ucsc.edu). Each binding site was attributed to the closest transcription start site (TSS) and to other TSSs that appear within the triple distance to the nearest TSS.
Results
ESCs acquire gene expression profiles similar to neural tissues after upregulation of specific transcription factors
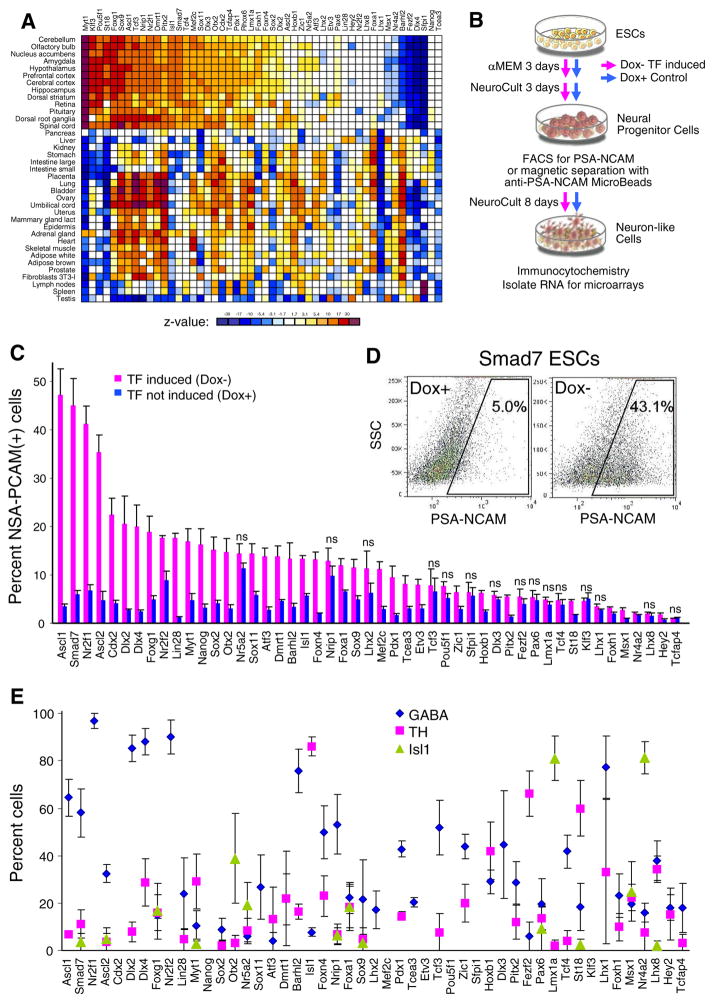
Previously, we used overexpression of 185 TFs in mouse ESCs to elucidate their effect on global gene expression profiles within 48 h (Nishiyama et al. 2009; Correa-Cerro et al. 2012; Yamamizu et al. 2016). Transgenic TFs under control of tetracycline-controlled promoters were activated by withdrawal of Dox from the culture medium. To find TFs that may facilitate neural differentiation, we explored correlations between the downstream effects of induced TFs and tissue-specific gene expression profiles from the GNF database, version 3 (Su et al. 2002; Wu et al. 2009). Pearson correlation was applied to log-transformed values of gene expression (normalized by gene-specific median) for all genes that showed significant change in expression in both data sets (ANOVA, change ≥1.5-fold for induced TFs and ≥2-fold for GNF), as described (Correa-Cerro et al. 2012; Sharov et al. 2015).1 Calculations were done using ExAtlas, where the algorithm is implemented (Sharov et al. 2015). Positive significant correlation (p ≤ 0.05) with at least some neural tissues was detected for 36 TFs; the strongest correlations were seen for Myt1, Klf3, Pou5f1, St18, Foxg1, Sox9, and Ascl1 (Fig. 1A).
Figure 1.
Induction of transcription factors (TFs) and its effect on the transcriptome and neural differentiation of ESCs. (A) Correlation of gene expression response to the induction of transcription factors (Nishiyama et al. 2009, Correa-Cerro et al. 2012, Yamamizu et al. 2016) with tissue-specific gene expression from the GNF ver. 3 database (Su et al. 2002, Wu et al. 2009); color shows z value for correlation significance, white non-significant correlation (z < 2). (B) Scheme of ESC differentiation experiment: To activate the transgenic TF, Dox was removed from the media. Cells were cultured for 3 d in αMEM and 3 d in NeuroCult medium, followed by FACS or cell enrichment by beads. For full differentiation, cells were cultured for 8 more days in NeuroCult medium and then used for immunostaining and gene expression profiling with micro-arrays. (C) Percent PSA-NCAM(+) cells identified by FACS on day 6 of neural differentiation of ESC clones with 49 individual tetracycline-repressible transgenic TFs (horizontal axis); error bars (SE) were determined from three replications; ns non-significant difference between cells cultured in Dox− and Dox+ conditions. (D) Example of FACS image used for determining the percent PSA-NCAM(+) cells. (E) Proportion of cells with GABA, TH, and ISL1/2 immunostaining among TUJ1-positive cells derived by induction of various TFs (horizontal axis) on day 14.
Because neural tissues include a large number of different cell types, we attempted to sharpen the approach by looking at TFs whose overexpression resulted in upregulation of genes that are specific to individual neural cell types. Lists of genes that are specific to individual neural and glial cell types (astrocytes, oligodendrocytes, interneurons, pyramidal neurons, motor neurons, retinal neurons, and GABAergic neurons) were compiled from the literature (Sugino et al. 2006; Al-Jaberi et al. 2013; Zeisel et al. 2015) and public data (GEO data series GSE45809, GSE52118, GSE60856, GSE19372, GSE35338, GSE5582, GSE36456, GSE11483, GSE9566, GSE17806, GSE35077, GSE2882, and GSE13379). Genes specific to GABAergic neurons, interneurons in general, and pyramidal neurons were mostly activated after overexpression of Dlx3 and Dlx2, as well as by other TFs that cause upregulation of Dlx1 and Dlx2 (e.g., Cdx2, Dmrt1, Foxl2, Foxc1, Tcf3, and Sox9) (Supplementary Fig. S2). This is consistent with previous observations that Dlx TFs are important for the GABAergic neuron fate (Anderson et al. 1997; Potter et al. 2009; Paina et al. 2011; Sellers et al. 2014). By contrast, genes specific to astrocytes and oligodendrocytes were activated after upregulation of Sox9 (Supplementary Fig. S3). This association is consistent with published observations that Sox9 determines glial fate choice in the developing spinal cord (Stolt et al. 2003).
Testing predicted potency of transcription factors to facilitate neural differentiation of ESCs
Although ESCs showed almost no change in morphology after 48 h of upregulation of induced transgenic TFs, gene expression changes towards tissue-specific patterns had already occurred, and we had found that they often correlated with the potency of specific TFs to facilitate differentiation of cells into corresponding cell types, such as myocytes, hepatocytes, and blood cells (Yamamizu et al. 2013). Here, we tested if this relationship holds for ESC differentiation into neural cell types. For neural differentiation, cells were cultured for 3 d in αMEM medium and 3 d in NeuroCult differentiation medium. On day 6, the proportion of cells with neural progenitor marker PSA-NCAM was quantified by FACS (Canto II, Becton Dickinson) (Fig. 1B). Immunostaining showed that PSA-NCAM was co-expressed with neuronal marker TUJ1 (TUBB3), and the latter one was also co-expressed with two other neuronal markers: MAP2 and NeuN (RBFOX3) (Supplementary Fig. S4A), confirming the neural nature of PSA-NCAM-positive [PSA-NCAM(+)] cells. Experiments were done with ESC clones carrying 49 individual transgenic TFs activated by Dox withdrawal. They included 35 TFs that showed significant correlation of downstream effects with gene expression profiles of some neural tissues (Fig. 1A)2; 11 additional TFs (Lin28, Msx1, Hey2, Nr2f2, Dlx4, Lhx8, Foxa1, Fezf2, Barhl2, Lhx1, and Nr4a2) that are known to promote neural differentiation or support neuronal properties; and 3 “negative control” TFs that have no apparent relation to neural differentiation (Nanog, Sfpi1, and Tcea3).3
The proportion of PSA-NCAM(+) cells differentiating towards neural lineage was generally higher for cells with induced TFs (Dox−) than in control (Dox+) (Fig. 1C, D). This difference was statistically significant for 41 TFs. Of them, 23 TFs (up to Mef2c from the left in Fig. 1C) showed a significant increase in the proportion of PSA-NCAM(+) cells compared to the average in all controls 4.06± 2.26% (±S.D.), indicating that these TFs can facilitate the differentiation of ESCs into neurons. The most potent inducers of neural differentiation appeared to be Ascl1, Smad7, Nr2f1, and Ascl2 as follows from >35% PSA-NCAM(+) cells in experiments with overexpression of these TFs (Fig. 1C). All four TFs showed high correlations between downstream gene expression changes and neural expression profiles (Fig. 1A), which further supports the idea that correlation analysis is useful for predicting the role of TFs in ESC differentiation. However, this relationship is not universal, because several TFs (e.g., Nanog, Tcea3, Dlx4, Nr2f2, and Lin28) that had no significant correlation in Fig. 1A nevertheless showed an increased proportion of PSA-NCAM(+) cells in differentiation experiments, whereas other TFs (e.g., Klf3, Tcf3, Pou5f1, and Nrip1) had significant correlation in Fig. 1A but showed no enrichment in PSA-NCAM(+) cells during differentiation. Thus, early changes in gene expression profiles after manipulation of TFs were not always indicative of the long-term differentiation potential of cells after overexpression of these TFs.
To find additional clues about early factors that control ESC differentiation towards neural fates, we searched for genes whose 48-h response to the induction of TFs was associated with high proportion of PSA-NCAM(+) cells on day 6 in differentiation experiments. This association was quantified by covariance between positive log-ratios of gene expression change after induction of each TF (negative log-ratios were counted as zeroes) with median-subtracted proportion of PSA-NCAM(+) cells. None of the four TFs that were most potent in inducing neural differentiation activated Cdh2 within 48 h, so that our data did not confirm a previous report that expression of Cdh2 is essential for efficient neural differentiation of mouse-induced pluripotent stem cells (Su et al. 2013). But among 500 genes with the highest covariance, two out of three top Gene Ontology (GO) categories appeared to be associated with Notch signaling (Supplementary Table S2). Genes, associated with Notch, included Dll1, Dll3, Dll4, Hes5, Heyl, Dner, Ascl1, Neurod4, Nrarp, Bmp2, Trp63, Mfng, Lfng, and Aph1b/c. Thus, Notch signaling may be a crucial early factor that facilitates the differentiation of ESCs towards neural fates, though how the pathway is accessed by widely different TFs remains unknown.
Neuron-like cells generated from ESCs by induction of transcription factors express markers of GABAergic, do-paminergic, and cholinergic neurons
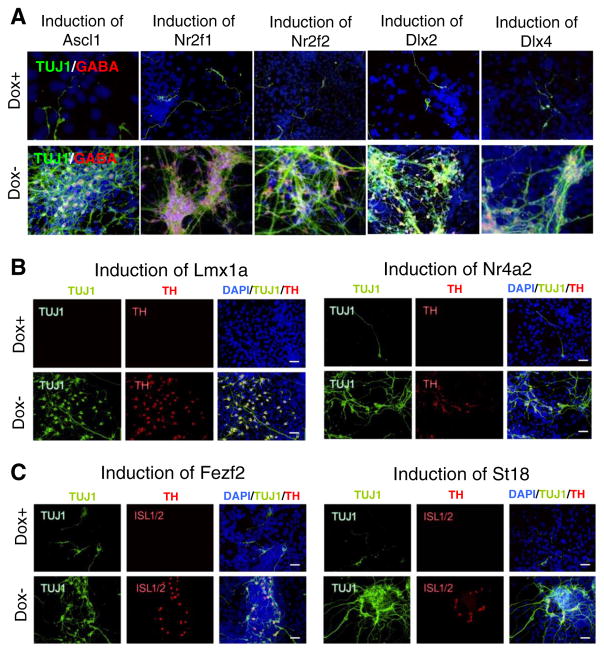
To determine which of the TFs were necessary for generating GABAergic neurons, dopaminergic neurons, and cholinergic motor neurons, we cultured cells for an additional 8 d in NeuroCult (total 14 d) and performed immunostaining with markers of these neuron types: GABA, tyrosine hydroxylase (TH), and ISL1/2, respectively (Fig. 1B). Then, we estimated the proportion of cells stained by these markers within the subpopulation of cells stained with the pan-neuronal marker TUJ1. Induction of several TFs yielded differentiation towards specific neural lineages. The majority of neuron-like cells generated by induction of Ascl1, Smad7, Nr2f1, Dlx2, Dlx4, Nr2f2, Barhl2, and Lhx1 were GABA-positive (Figs. 1E and 2A). In comparison, control cells cultured in Dox+ conditions had very few TUJ1(+) and GABA(+) cells (Fig. 2A, first row). Cells generated by induction of these eight TFs expressed other markers of GABAergic neurons, such as GAD67 (glutamine de-carboxylase 67), PV (parvalbumin), and SST (somatostatin) (see examples for Nr2f1 and Dlx2 in Supplementary Fig. S4B). Out of these eight TFs, only three (Ascl1, Smad7, and Nr2f1) produced abundant neural-like cells (Fig. 1C) and thus are most promising for practical applications.
Figure 2.
Properties of neuron-like cells derived from ESCs by the induction of TFs for 14 d. (A) Immunostaining for TUJ1 and GABA of cells derived by induction of Ascl1, Nr2f1, Nr2f2, Dlx2, and Dlx4. (B) Immunostaining for TUJ1 and TH (tyrosine hydroxylase) after induction of Lmx1a and Nr4a2 in ESCs. (C) Immunostaining for TUJ1 and ISL1/2 (ISL LIM homeobox 1/2) after induction of Fezf2 and St18 in ESCs.
High proportions of TH(+)/TUJ1(+) cells were observed in neuron-like cells obtained by induction of Lmx1a and Nr4a2 (Figs. 1E and 2B), indicating a possible capacity of these TFs to generate dopaminergic-like neurons. The inference was confirmed by testing cells for expression of other markers of dopaminergic neurons (Supplementary Fig. S5A). A high proportion of ISL1/2(+)/TUJ1(+) cells was observed in neuron-like cells obtained by induction of Isl1, Fez2, and St18 (Figs. 1E and 2C), indicating the capacity of these TFs to generate more cholinergic-like motor neurons. Cells generated with Fez2 and Isl1 expressed other markers of cholinergic neurons (Supplementary Fig. S5B). However, the total yield of neuron-like cells on day 6 was low for these TFs (Fig. 1C). Thus, it is not clear if induction of single TFs can generate enough dopaminergic and cholinergic neurons, respectively, to be useful for further studies.
Role of Ascl1 induction in the emergence of neural transcriptome
Global gene expression profiling is a powerful approach for elucidating the mechanisms of neural differentiation (Aiba et al. 2006; Abranches et al. 2009; Akanuma et al. 2012). However, little is known about transcriptome changes during neural differentiation driven by individual induced TFs. We selected Ascl1 for a detailed time-course experiment because it generated the highest proportion of cells with neural progenitor marker PSA-NCAM. The effect of Ascl1 induction was measured by comparison of gene expression in cells cultured in Dox- conditions versus that in cells cultured in Dox+ at the same time point. As additional baseline, we used gene expression in ESCs in Dox+ on day3. We were focused on the expression of neural-related genes which we compiled from published sources (GEO data sets: GSE19806, GSE10246, GSE24207, GSE49847, GSE1986, GSE8249, GSE30611) (Gallardo et al. 2007; Lindsley and Murphy 2007; Wu et al. 2009; Sharov et al. 2011; Illumina 2011; Thorrez et al. 2011; Yue et al. 2014) based on their upregulation in brain versus both ESCs and median expression in non-neural tissues. For the Illumina data, human gene symbols were converted to mouse orthologs using Homologene (NCBI). The final list of neural-related genes included 2559 genes that appeared in more than one study.
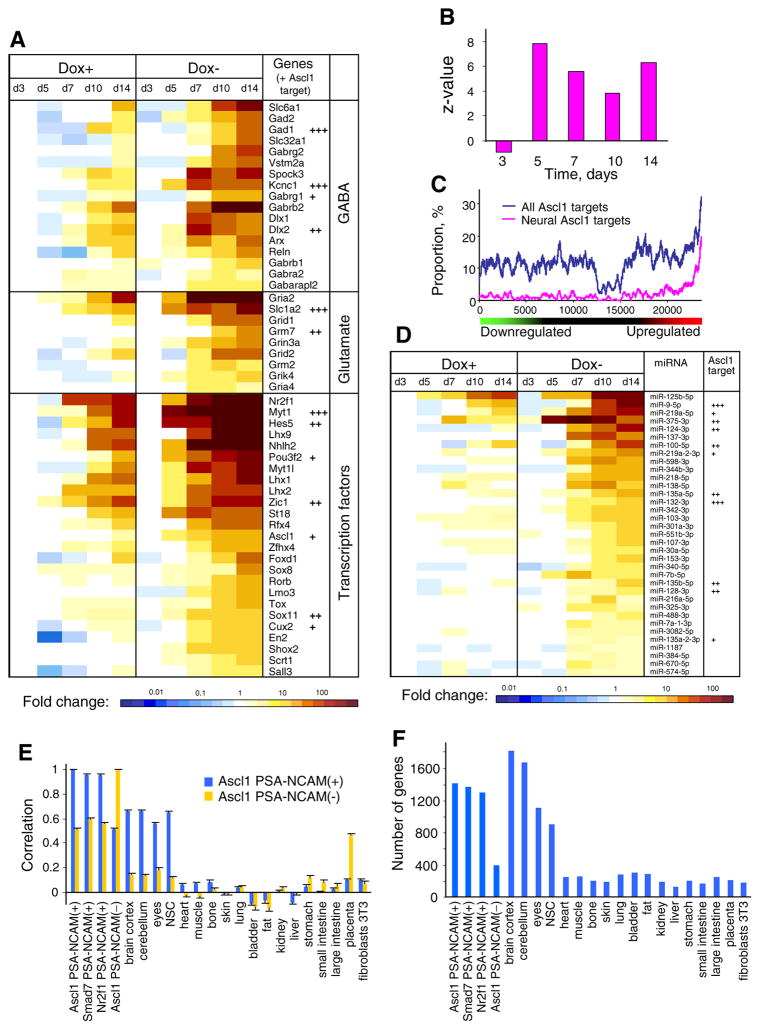
Induction of Ascl1 dramatically increased the number of neural genes that became upregulated during ESC differentiation (Supplementary Fig. S6, Dox−). The most rapid increase in the number of upregulated neural genes was observed on days 5 and 7 (N = 532 and N = 1083, respectively, 2-fold change) and then remained at that level through day 14. These upregulated genes included genes associated with GABA and glutamate signaling and transcription factors (Fig. 3A). ESCs cultured in Dox+ conditions (where transgenic Ascl1 is not induced) also show some upregulation of neural genes starting on day 5, but the number of upregulated neural genes was lower (e.g., N = 226 and N = 430, 2-fold change on days 5 and 7, respectively) than in cells cultured in Dox−conditions. We explain the activation of neural genes in Dox+ conditions by the effect of the neural differentiation medium, which alone can drive differentiation of ESCs towards neural fates (Aiba et al. 2006). In particular, Nr2f1 (i.e., one of the four top TFs with strongest potential to induce neural differentiation) was activated by 52-fold on day 7 in control cells where Ascl1 was not induced (Fig. 3A). Additional neural-related TFs (e.g., Zic1, Myt1, Lhx1, Lhx2, Lhx9, and Hes5) were activated in Dox+ conditions on day 10.
Figure 3.
Role of Ascl1, Smad7, and Nr2f1 induction in the emergence of neural transcriptome in differentiating ESCs. (A) Heatmap for expression of selected neural genes in cells with induced Ascl1 (Dox−) and control cells (Dox+) on days 3, 5, 7, 10, and 14 in culture (see conditions in Fig. 1B); baseline—gene expression in ESCs day 3 in Dox+ medium; target genes of Ascl1 are marked +++ if they had >2 binding sites within 100 kb from transcription start site (TSS) and at least one was supported by >2 data sources, ++ if they had two binding sites and at least one was supported by >2 sources, and + if they had binding sites supported by only two sources. (B) Parametric analysis of gene set enrichment (PAGE) of target genes of Ascl1 (binding sites supported by >2 data sources with 100 kb from TSS) among genes that were upregulated in cells with induced Ascl1at time points 3, 5, 7, 10, and 14 d; baseline—expression in control cells (Dox+) at the same time point. (C) Rank-plot for Ascl1 target genes (same as in (B)) in cells with induced Ascl1 on day 5; horizontal axis represents genes sorted by expression change in Dox− versus Dox+, and the graph shows the proportion of Ascl1 target genes in a sliding window of 300 genes. (D) Heatmap for expression of miRNA that were significantly upregulated in cells with induced Ascl1 (Dox−) versus control cells (Dox+) on days 3, 5, 7, 10, and 14 in culture (see conditions in Fig. 1B); baseline—same as in (A); target genes of Ascl1 are marked +++, ++, or + as in (A). (E) Correlation of gene expression profiles in neuron-like cells derived from ESCs by the induction of TFs and in various mouse tissues and cultured cells; blue bars—cells enriched by microbeads, and orange bars—remaining cells after enrichment; gene expression values were log-transformed and normalized by maximum expression of each gene in ESCs on day 3 in Dox+ and median expression in non-neural tissues. (F) Number of neural-related genes upregulated by ≥2-fold in PSA-NCAM(+) cells generated with Ascl1, Smad7, and Nr2f1 and in various mouse tissues and cultured cells; baseline—maximum expression of each gene in ESCs on day 3 in Dox+ and median expression in non-neural tissues.
To explore the role of Ascl1 in activation of its target genes, we compiled a list of reproducible binding sites of Ascl1 in the genome based on published ChIP-seq studies (see “Materials and Methods” section). Based on these binding sites, we identified 3577 prospective target genes of Ascl1 whose TSS was within 100 kb from binding sites. These target genes were strongly enriched among genes upregulated in ESCs with induced transgenic Ascl1 (i.e., in Dox− conditions) as compared to control ESCs (i.e., same clone, time, and medium, but in Dox+ conditions) (Fig. 3B), based on the parametric analysis of gene set enrichment (PAGE) (Kim and Volsky 2005). The strongest enrichment was observed on day 5 (z = 7.85, p = 4.0 × 10−14). The rank plot for day 5 shows that the proportion of targets of Ascl1 is >30% among upregulated genes and only ~10% among other genes (i.e., either downregulated or with no change) (Fig. 3C). Although binding of Ascl1 is strongly enriched within 300 bp from the TSS of target genes, these binding sites have almost no effect on the level of transcription (data not shown). Thus, regulation is achieved mainly via binding of Ascl1 to distal enhancers (>300 bp away from TSS). The proportion of Ascl1-target genes that were also classified as neural-related was ~19% among upregulated genes and only ~2% among other genes (Fig. 3C).
The majority (>50%) of neural genes that are also targets of Ascl1 were upregulated by >2-fold on day 7 in Dox− conditions, whereas only 22.5% of them were upregulated in control (i.e., in Dox− conditions) (Supplementary Fig. S6, left panels). Examples of target genes activated by Ascl1 include GABAergic-related genes Dlx2, Gad1, and Kcnc1; glutamatergic-related genes Slc1a2 and Grm7; and TFs Myt1, Hes5, Zic1, and Sox11 (Fig. 3A). Endogenous Ascl1 was also upregulated in Dox− conditions on day 7 (by 9.7-fold) and was possibly activated by the induced transgenic Ascl1.4 Many neural genes that are not Ascl1 targets were also more strongly upregulated in Dox− conditions as compared to control (Dox+) (Supplementary Fig. S6, right panels). We hypothesize that many of these genes were activated via chain response of multiple TFs that were downstream of the induced Ascl1.
In addition to the analysis of mRNA gene expression profiles, we quantified the expression of miRNA with microar-rays and identified 35 miRNAs that were significantly (FDR <0.05, change >2-fold) upregulated in cells with induced Ascl1 (Dox−) at days 10 and 14 (combined as replications) as compared to control cells (Dox+ on the same day, and Dox+ on day 3) (Fig. 3D). These include miRNAs that were previously reported in neural tissues, such as miR-124, miR-125, miR-137, miR-132, miR-99a, miR-9, and miR-128 (Babak et al. 2004; Tao et al. 2015; Zhao et al. 2012) and miR-384 which was upregulated in GABAergic neurons expressing Gad2 (He et al. 2012). Nearly a third of these upregulated miRNAs are targets of Ascl1 (Fig. 3D).
Enrichment of cells programmed by transcription factors yielded an almost pure population of neuron-like cells
Cells generated with induced transgenic TFs always included a large proportion of PSA-NCAM(−) cells that did not follow the neuronal path (Fig. 1C). To increase the purity of the differentiated cells, we used microbeads with anti-PSA-NCAM antibody to enrich the population of PSA-NCAM(+) cells generated by day 6 with three TFs: Ascl1, Smad7, and Nr2f1 that gave relatively strong differentiation into “GABAergic-like” neurons. Then, cells were replated and cultured for an additional 8 d for terminal differentiation (Fig. 1B). On day 14, PSA-NCAM(+) cells had clear neuron-like morphology and were stained by both TUJ1 and GABA, whereas PSA-NCAM(−) cells had very few neuron-like cells stained by TUJ1 and no cells stained by both TUJ1 and GABA (Supplementary Fig. S7).
To confirm that enrichment of PSA-NCAM(+) cells yielded an almost pure population of neuron-like cells, we compared global gene expression profiles of PSA-NCAM(+) and PSA-NCAM(−) cells, on day 14 with microarrays. For additional comparison, we utilized previously published data on gene expression profiles in various mouse organs and tissues, processed with the same microarray platform (Sharov et al. 2011). Log-transformed gene expression values of PSA-NCAM(+) cells generated with Ascl1 normalized by median gene expression in non-neural tissues were highly correlated with gene expression values in brain cortex and cerebellum (r = 0.66), neural stem cells (NSC) (r = 0.65), and eyes (r = 0.56) (Fig. 3E, blue bars). Correlation with gene expression in other (non-neural) tissues was <0.105. In contrast, correlation between gene expression in PSA-NCAM(−) cells generated with Ascl1 and in brain cortex and cerebellum was low (r = 0.14–0.15); instead, gene expression in PSA-NCAM(−) cells correlated with placenta (Fig. 3E, orange bars).
In addition to correlation analysis, we compared the number of neural-specific genes that were upregulated by ≥2-fold in PSA-NCAM(+) cells generated with Ascl1, Smad7, and Nr2f1 with that in various mouse organs and tissues, based on data from (Sharov et al. 2011). As baseline, we used the maximum expression of each gene in ESCs on day 3 in Dox+ and median expression in non-neural tissues. Cells generated with Ascl1, Smad7, and Nr2f1 and enriched by microbeads showed upregulation of 1307–1421 neural genes, which is close to the number of neural genes upregulated in brain cortex (N = 1824) and is higher than the number of neural genes upregulated in NSCs (N = 907) (Fig. 3F). In contrast, PSA-NCAM(−) cells generated by induction of Ascl1 had only 397 upregulated neural genes. Low expression of neural-related genes that were not upregulated in ESC-derived cells can be explained by the difference between the in vitro conditions and brain, as follows from the comparison with other published in vitro studies (Mahony et al. 2011; Burney et al. 2013; Deng et al. 2013) (Supplementary Analysis). In addition, some low-expressed genes were simply not typical for the GABAergic neurons that predominated among cells generated with these three TFs (Supplementary Analysis).
Thus, enrichment of ESC-derived cells yielded an almost pure population of neuron-like cells; however, this method was successful only for TFs that generated high proportions of PSA-NCAM(+) cells on day 6. Further work is necessary to design methods for enrichment of neuron-like cells induced by other TFs.
Discussion
This paper presents the first large-scale study of the potential of 49 individual manipulated TFs to induce neural differentiation in mouse ESCs after 6–14 d of culture. These TFs were selected from the quantitative analysis of data from a previous pilot study in which global gene expression profiles were identified after 2 d of TF induction. In total, 23 TFs show a statistically significant increase in the proportion of PSA-NCAM(+) cells compared to control (i.e., no TF induction), indicating an apparent capacity of these TFs to facilitate the differentiation of ESCs into neurons. Induction of four individual TFs (Ascl1, Smad7, Nr2f1, and Ascl2) resulted in the highest proportion of cells with neural progenitor marker PSA-NCAM, and therefore, these TFs were the most effective for neural differentiation in ESCs. The capacity of these TFs to induce neural differentiation is most likely linked to the early activation of the Notch signaling pathway, as follows from the global gene expression profiles at 2 d after induction of TFs. This hypothesis is consistent with previous observations that Notch signaling is required for neural differentiation (Halder et al. 2015). The role of Ascl1 and Nr2f1 in neural differentiation is also well established (Borello et al. 2013; Kageyama et al. 1997; Naka et al. 2008; Sommer et al. 1995; Vasconcelos and Castro 2014). The other two TFs have not been associated with the determination of neural fate. Ascl2 is mostly involved in the maintenance of giant cell precursors in the trophoblast differentiation (Scott et al. 2000); however, it shares a DNA binding motif with Ascl1 and activates a similar subset of genes after induction in ESCs (Nishiyama et al. 2009). Thus, it is not surprising that it facilitates neural differentiation in the context of ESCs. And Smad7 was reported as a factor sufficient to directly convert human ESCs to a neural fate possibly via inhibition of TGFβ (Ozair et al. 2012).
In ESC cultures, eight TFs (Ascl1, Smad7, Nr2f1, Dlx2, Dlx3, Nr2f2, Barhl2, and Lhx1) generated neural-like cells that were predominantly GABA-positive after 14 d of TF induction. The first three of them are most perspective candidates for producing GABAergic neurons because of their strong potency as inducers of neural differentiation. The capacity of Ascl1 and distal-less (Dlx) family TFs to promote GABA signaling was expected because these TFs are markers and inducers of GABAergic neurons (Potter et al. 2009; Al-Jaberi et al. 2013; Sellers et al. 2014; Smith et al. 2014). Nr2f2 is also a marker of GABAergic interneurons in fetal forebrain (Reinchisi et al. 2011) and hippocampus (Fuentealba et al. 2010). Lhx1 together with Lhx5 support the development of GABAergic interneurons in spinal cord (Pillai et al. 2007). The role of Nr2f1 and Smad7 in specification of GABAergic neurons was unknown; instead, Nr2f1 was reported to facilitate the differentiation towards motor neurons (Tomassy et al. 2010).
A few TFs increased the proportion of cells with markers of dopaminergic (Lmx1a and Nr4a2) or cholinergic (Isl1, Fezf1, and St18) neurons among neuron-like cells, but the yield of neuron-like cells was relatively low on day 6 and they have not been further studied. Judging by the further results with GABAergic-like cultures, further modification of time of induction or other conditions may provide higher efficiency; but it is also possible that more than one factor, perhaps a second TF, would be required for efficient generation of other types of neurons.
Detailed gene expression profiling of cells in the course of their differentiation assisted by the induced Ascl1 showed that this TF has a much stronger effect on the activation of neural-related genes than neural differentiation medium alone. The strongest effect of Ascl1 induction was observed by days 5 and 7, i.e., long before cells acquired typical neural morphology on day 14. Genes upregulated due to Ascl1 induction were enriched in target genes with binding of this TF to enhancers, which presents a mechanistic explanation of the effect of Ascl1. In addition, we found 35 miRNAs upregulated after the induction of Ascl1 in ESCs, and a third of them are targets of Ascl1. Six of these miRNAs are known to be expressed in neural tissues, and we hypothesize that other miRNAs from this list may also have neural functions.
Beads bearing the anti-PSA-NCAM antibody appeared efficient for enrichment of neural-like cells obtained with three TFs Ascl1, Smad7, and Nr2f1 on day 6. These cells showed a high correlation of their global gene expression profiles with neural tissues (brain cortex, cerebellum, eye) and neural stem cells (NSCs), and the number of upregulated neural genes in these cells was just a little lower than in the brain, which indicates high similarity between in vivo and in vitro cell phenotypes. Interestingly, global gene expression profiles of cells obtained with three TFs were very similar to each other (Supplementary Fig. S8) despite of the fact that these TFs belong to entirely different classes of proteins. In the short term (2 d), Ascl1 activates ca. three times more genes than Smad7 and Nr2f1, but 99 downstream genes were common between for all three TFs, and 422 downstream genes were common for at least two of these TFs. We hypothesize that this similarity is sufficient to direct ESCs towards the same stable pathway of cell differentiation. This notion is supported by the Waddington’s model of epigenetic landscape, where developmental trajectories converge due to the properties of gene regulatory networks (Waddington 1957).
In summary, we demonstrated that our approach efficiently identified TF candidates for inducing neural differentiation in ESCs based on short-term gene expression profile changes. In particular, TFs can be used for guiding cell differentiation towards specific neural cell types. Future studies should aim to identify individual TFs and their combinations for differentiating more specific and mature neural cell types from pluripotent stem cells and ultimately for possible therapeutic applications.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging, project Z01 AG000656-09. Y.T-O. was funded by Post-Baccalaureate Intramural Research Training Award. K.Y. was supported by the postdoctoral fellowships from the Kanae Foundation, Japan, Uehara Memorial Foundation, Japan, Naito Foundation, Japan, and the Japan Society for Promotion of Science (JSPS). This research was also supported in part by the Japan Science and Technology Agency (JST), CREST program. The authors declare no competing financial interests. Y.T-O., K.Y., Y.P., and L.S. performed experiments; M.A. made new ES clones; H.Y. carried out vector construction for ES clones and synthetic mRNA; D.S. and M.S.H.K supervised the project and edited the manuscript; and A.A.S. performed statistical analysis and wrote the manuscript.
Footnotes
In a previous version of this method, we used 10,000 most significant genes for each data set and then estimated correlation for common genes in these two subsets (Correa-Cerro et al. 2012).
One TF, Rhox6, was not tested, and hence the total number of TFs was 35 rather than 36.
Data on three TFs (Myt1, Isl1, and St18) was published earlier (Yamamizu et al. 2016).
There are two binding sites of Ascl1 near it own transcription start site: at +0.7 kb and −47.5 kb (mm9 genome) supported by two data sources. The latter binding site, located within the third intron of neighboring gene Pah, is more likely to be the functional enhancer because of multiple binding motifs of Ascl1 and strong evolutionary conservation, whereas the first site is within the ORF.
Electronic supplementary material
The online version of this article (doi:10.1007/s11626-016-0056-7) contains supplementary material, which is available to authorized users.
References
- Abranches E, Silva M, Pradier L, Schulz H, Hummel O, Henrique D, Bekman E. Neural differentiation of embryonic stem cells in vitro: a road map to neurogenesis in the embryo. PLoS ONE. 2009;4:e6286. doi: 10.1371/journal.pone.0006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba K, Sharov AA, Carter MG, Foroni C, Vescovi AL, Ko MS. Defining a developmental path to neural fate by global expression profiling of mouse embryonic stem cells and adult neural stem/progenitor cells. Stem Cells. 2006;24:889–895. doi: 10.1634/stemcells.2005-0332. [DOI] [PubMed] [Google Scholar]
- Akanuma H, Qin XY, Nagano R, Win-Shwe TT, Imanishi S, Zaha H, Yoshinaga J, Fukuda T, Ohsako S, Sone H. Identification of stage-specific gene expression signatures in response to retinoic acid during the neural differentiation of mouse embryonic stem cells. Front Genet. 2012;3:141. doi: 10.3389/fgene.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jaberi N, Lindsay S, Sarma S, Bayatti N, Clowry GJ. The early fetal development of human neocortical GABAergic interneurons. Cereb Cortex. 2013;25:631–645. doi: 10.1093/cercor/bht254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Babak T, Zhang W, Morris Q, Blencowe BJ, Hughes TR. Probing microRNAs with microarrays: tissue specificity and functional inference. RNA. 2004;10:1813–1819. doi: 10.1261/rna.7119904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingu-late cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Borello U, Madhavan M, Vilinsky I, Faedo A, Pierani A, Rubenstein J, Campbell K. Sp8 and COUP-TF1 reciprocally regulate patterning and Fgf signaling in cortical progenitors. Cereb Cortex. 2013;24:1409–1421. doi: 10.1093/cercor/bhs412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borromeo MD, Meredith DM, Castro DS, Chang JC, Tung KC, Guillemot F, Johnson JE. A transcription factor network specifying inhibitory versus excitatory neurons in the dorsal spinal cord. Development. 2014;141:2803–2812. doi: 10.1242/dev.105866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney MJ, Johnston C, Wong KY, Teng SW, Beglopoulos V, Stanton LW, Williams BP, Bithell A, Buckley NJ. An epigenetic signature of developmental potential in neural stem cells and early neurons. Stem Cells. 2013;31:1868–1880. doi: 10.1002/stem.1431. [DOI] [PubMed] [Google Scholar]
- Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Carter MG, Sharov AA, VanBuren V, Dudekula DB, Carmack CE, Nelson C, Ko MS. Transcript copy number estimation using a mouse whole-genome oligonucleotide microarray. Genome Biol. 2005;6:R61. doi: 10.1186/gb-2005-6-7-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C, Scott RH, Pu J, Lang B, Nakamoto C, McCaig CD, Shen S. Derivation of homogeneous GABAergic neurons from mouse embryonic stem cells. Exp Neurol. 2009;217:407–416. doi: 10.1016/j.expneurol.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Chung S, Sonntag KC, Andersson T, Bjorklund LM, Park JJ, Kim DW, Kang UJ, Isacson O, Kim KS. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci. 2002;16:1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Cerro LS, Piao Y, Sharov AA, Nishiyama A, Cadet JS, Yu H, Sharova LV, Xin L, Hoang HG, Thomas M, Qian Y, Dudekula DB, Meyers E, Binder BY, Mowrer G, Bassey U, Longo DL, Schlessinger D, Ko MS. Generation of mouse ES cell lines engineered for the forced induction of transcription factors. Sci Rep. 2012;1:167. doi: 10.1038/srep00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis. 1996;3:97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Deng T, Zhu ZI, Zhang S, Leng F, Cherukuri S, Hansen L, Marino-Ramirez L, Meshorer E, Landsman D, Bustin M. HMGN1 modulates nucleosome occupancy and DNase I hypersensitivity at the CpG island promoters of embryonic stem cells. Mol Cell Biol. 2013;33:3377–3389. doi: 10.1128/MCB.00435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Klausberger T, Karayannis T, Suen WY, Huck J, Tomioka R, Rockland K, Capogna M, Studer M, Morales M, Somogyi P. Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci. 2010;30:1595–1609. doi: 10.1523/JNEUROSCI.4199-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo TD, John GB, Shirley L, Contreras CM, Akbay EA, Haynie JM, Ward SE, Shidler MJ, Castrillon DH. Genomewide discovery and classification of candidate ovarian fertility genes in the mouse. Genetics. 2007;177:179–194. doi: 10.1534/genetics.107.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder D, Chang GE, De D, Cheong E, Kim KK, Shin I. Combining suppression of stemness with lineage-specific induction leads to conversion of pluripotent cells into functional neurons. Chem Biol. 2015;22:1512–1520. doi: 10.1016/j.chembiol.2015.10.008. [DOI] [PubMed] [Google Scholar]
- He M, Liu Y, Wang X, Zhang MQ, Hannon GJ, Huang ZJ. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron. 2012;73:35–48. doi: 10.1016/j.neuron.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S, Lenhart G. Burden of illness in Parkinson's disease. Mov Disord. 2005;20:1449–1454. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- Illumina. Gene Expression Omnibus. 2011. Illumina Human Body Map 2.0 Project. [Google Scholar]
- Kageyama R, Ishibashi M, Takebayashi K, Tomita K. bHLH transcription factors and mammalian neuronal differentiation. Int J Biochem Cell Biol. 1997;29:1389–1399. doi: 10.1016/s1357-2725(97)89968-2. [DOI] [PubMed] [Google Scholar]
- Kim EH, Thu DC, Tippett LJ, Oorschot DE, Hogg VM, Roxburgh R, Synek BJ, Waldvogel HJ, Faull RL. Cortical inter-neuron loss and symptom heterogeneity in Huntington disease. Ann Neurol. 2014;75:717–727. doi: 10.1002/ana.24162. [DOI] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC Bioinforma. 2005;6:144. doi: 10.1186/1471-2105-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley RC, Murphy KM. Gene Expression Omnibus. 2007. Global gene expression across a range of tissues. [Google Scholar]
- Mahony S, Mazzoni EO, McCuine S, Young RA, Wichterle H, Gifford DK. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 2011;12:R2. doi: 10.1186/gb-2011-12-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinat C, Bacci JJ, Leete T, Kim J, Vanti WB, Newman AH, Cha JH, Gether U, Wang H, Abeliovich A. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci U S A. 2006;103:2874–2879. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11:1014–1023. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Yanagawa N, Yuri S, Hauser P, Jo OD. Effective induction of cells expressing GABAergic neuronal markers from mouse embryonic stem cell. In Vitro Cell Dev Biol Anim. 2013;49:479–485. doi: 10.1007/s11626-013-9640-2. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Xin L, Sharov AA, Thomas M, Mowrer G, Meyers E, Piao Y, Mehta S, Yee S, Nakatake Y, Stagg C, Sharova L, Correa-Cerro LS, Bassey U, Hoang H, Kim E, Tapnio R, Qian Y, Dudekula D, Zalzman M, Li M, Falco G, Yang H, Lee S, Monti M, Stanghellini I, Islam MN, Nagaraja R, Goldberg I, Wang W, Longo DL, Schlessinger D, Ko MSH. Uncovering early response of gene regulatory networks in ES cells by systematic induction of transcription factors. Cell Stem Cells. 2009;5:420–433. doi: 10.1016/j.stem.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozair MZ, Noggle S, Warmflash A, Krzyspiak JE, Brivanlou AH. SMAD7 directly converts human embryonic stem cells to telencephalic fate by a default mechanism. Stem Cells. 2012;31:35–47. doi: 10.1002/stem.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paina S, Garzotto D, DeMarchis S, Marino M, Moiana A, Conti L, Cattaneo E, Perera M, Corte G, Calautti E, Merlo GR. Wnt5a is a transcriptional target of Dlx homeogenes and promotes differentiation of interneuron progenitors in vitro and in vivo. J Neurosci. 2011;31:2675–2687. doi: 10.1523/JNEUROSCI.3110-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Mansouri A, Behringer R, Westphal H, Goulding M. Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development. 2007;134:357–366. doi: 10.1242/dev.02717. [DOI] [PubMed] [Google Scholar]
- Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JL. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic inter-neurons. Mol Cell Neurosci. 2009;40:167–186. doi: 10.1016/j.mcn.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinchisi G, Ijichi K, Glidden N, Jakovcevski I, Zecevic N. COUP-TFII expressing interneurons in human fetal forebrain. Cereb Cortex. 2011;22:2820–2830. doi: 10.1093/cercor/bhr359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riku Y, Atsuta N, Yoshida M, Tatsumi S, Iwasaki Y, Mimuro M, Watanabe H, Ito M, Senda J, Nakamura R, Koike H, Sobue G. Differential motor neuron involvement in progressive muscular atrophy: a comparative study with amyotrophic lateral sclerosis. BMJ Open. 2014;4:e005213. doi: 10.1136/bmjopen-2014-005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nat Neurosci. 2010;13:1345–1347. doi: 10.1038/nn.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol. 2013;15:214–221. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol Cell Biol. 2000;20:530–541. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers K, Zyka V, Lumsden AG, Delogu A. Transcriptional control of GABAergic neuronal subtype identity in the thalamus. Neural Dev. 2014;9:14. doi: 10.1186/1749-8104-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgado P, Genovesi S, Kalinovsky A, Zunino G, Macchi F, Allegra M, Murenu E, Provenzano G, Tripathi PP, Casarosa S, Joyner AL, Bozzi Y. Loss of GABAergic neurons in the hippocampus and cerebral cortex of Engrailed-2 null mutant mice: implications for autism spectrum disorders. Exp Neurol. 2013;247:496–505. doi: 10.1016/j.expneurol.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Nishiyama A, Piao Y, Correa-Cerro LS, Amano T, Thomas M, Mehta S, Ko MS. Responsiveness of genes to manipulation of transcription factors in ES cells is associated with histone modifications and tissue specificity. BMC Genomics. 2011;12:102. doi: 10.1186/1471-2164-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Schlessinger D, Ko MS. ExAtlas: An interactive online tool for meta-analysis of gene expression data. J Bioinform Comput Biol. 2015:1550019. doi: 10.1142/S0219720015500195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharova LV, Sharov AA, Piao Y, Shaik N, Sullivan T, Stewart CL, Hogan BL, Ko MS. Global gene expression profiling reveals similarities and differences among mouse pluripotent stem cells of different origins and strains. Dev Biol. 2007;307:446–459. doi: 10.1016/j.ydbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Maragnoli ME, Phull PM, Tran KM, Choubey L, Vaccarino FM. Fgfr1 inactivation in the mouse telencephalon results in impaired maturation of interneurons expressing parvalbumin. PLoS ONE. 2014;9:e103696. doi: 10.1371/journal.pone.0103696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer L, Shah N, Rao M, Anderson DJ. The cellular function of MASH1 in autonomic neurogenesis. Neuron. 1995;15:1245–1258. doi: 10.1016/0896-6273(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Wang L, Huang W, Qin D, Cai J, Yao X, Feng C, Li Z, Wang Y, So KF, Pan G, Wu W, Pei D. Immediate expression of Cdh2 is essential for efficient neural differentiation of mouse induced pluripotent stem cells. Stem Cell Res. 2013;10:338–348. doi: 10.1016/j.scr.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Tao Z, Zhao H, Wang R, Liu P, Yan F, Zhang C, Ji X, Luo Y. Neuroprotective effect of microRNA-99a against focal cerebral ischemia-reperfusion injury in mice. J Neurol Sci. 2015;355:113–119. doi: 10.1016/j.jns.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Thorrez L, Laudadio I, Van Deun K, Quintens R, Hendrickx N, Granvik M, Lemaire K, Schraenen A, Van Lommel L, Lehnert S, Aguayo-Mazzucato C, Cheng-Xue R, Gilon P, Van Mechelen I, Bonner-Weir S, Lemaigre F, Schuit F. Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Res. 2011;21:95–105. doi: 10.1101/gr.109173.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassy GS, De Leonibus E, Jabaudon D, Lodato S, Alfano C, Mele A, Macklis JD, Studer M. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci U S A. 2010;107:3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos FF, Castro DS. Transcriptional control of vertebrate neurogenesis by the proneural factor Ascl1. Front Cell Neurosci. 2014;8:412. doi: 10.3389/fncel.2014.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. The strategy of the genes. George Allen & Unwin Ltd; London: 1957. [Google Scholar]
- Wang Y, Dye CA, Sohal V, Long JE, Estrada RC, Roztocil T, Lufkin T, Deisseroth K, Baraban SC, Rubenstein JL. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical inter-neurons. J Neurosci. 2010;30:5334–5345. doi: 10.1523/JNEUROSCI.5963-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski OL, Vierbuchen T, Qu K, Lee QY, Chanda S, Fuentes DR, Giresi PG, Ng YH, Marro S, Neff NF, Drechsel D, Martynoga B, Castro DS, Webb AE, Sudhof TC, Brunet A, Guillemot F, Chang HY, Wernig M. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell. 2013;155:621–635. doi: 10.1016/j.cell.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AE, Pollina EA, Vierbuchen T, Urban N, Ucar D, Leeman DS, Martynoga B, Sewak M, Rando TA, Guillemot F, Wernig M, Brunet A. FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis. Cell Rep. 2013;4:477–491. doi: 10.1016/j.celrep.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamizu K, Piao Y, Sharov AA, Zsiros V, Yu H, Nakazawa K, Schlessinger D, Ko MS. Identification of transcription factors for lineage-specific ESC differentiation. Stem Cell Reports. 2013;1:545–559. doi: 10.1016/j.stemcr.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamizu K, Sharov AA, Piao Y, Amano M, Yu H, Nishiyama A, Dawood BD, Schlessinger D, Ko MSH. Generation and gene expression profiling of 48 transcription-factor-inducible mouse embryonic stem cell lines. Sci Rep. 2016 doi: 10.1038/srep25667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, Sandstrom R, Ma Z, Davis C, Pope BD, Shen Y, Pervouchine DD, Djebali S, Thurman RE, Kaul R, Rynes E, Kirilusha A, Marinov GK, Williams BA, Trout D, Amrhein H, Fisher-Aylor K, Antoshechkin I, DeSalvo G, See LH, Fastuca M, Drenkow J, Zaleski C, Dobin A, Prieto P, Lagarde J, Bussotti G, Tanzer A, Denas O, Li K, Bender MA, Zhang M, Byron R, Groudine MT, McCleary D, Pham L, Ye Z, Kuan S, Edsall L, Wu YC, Rasmussen MD, Bansal MS, Kellis M, Keller CA, Morrissey CS, Mishra T, Jain D, Dogan N, Harris RS, Cayting P, Kawli T, Boyle AP, Euskirchen G, Kundaje A, Lin S, Lin Y, Jansen C, Malladi VS, Cline MS, Erickson DT, Kirkup VM, Learned K, Sloan CA, Rosenbloom KR, Lacerda de Sousa B, Beal K, Pignatelli M, Flicek P, Lian J, Kahveci T, Lee D, Kent WJ, Ramalho Santos M, Herrero J, Notredame C, Johnson A, Vong S, Lee K, Bates D, Neri F, Diegel M, Canfield T, Sabo PJ, Wilken MS, Reh TA, Giste E, Shafer A, Kutyavin T, Haugen E, Dunn D, Reynolds AP, Neph S, Humbert R, Hansen RS, De Bruijn M, Selleri L, Rudensky A, Josefowicz S, Samstein R, Eichler EE, Orkin SH, Levasseur D, Papayannopoulou T, Chang KH, Skoultchi A, Gosh S, Disteche C, Treuting P, Wang Y, Weiss MJ, Blobel GA, Cao X, Zhong S, Wang T, Good PJ, Lowdon RF, Adams LB, Zhou XQ, Pazin MJ, Feingold EA, Wold B, Taylor J, Mortazavi A, Weissman SM, Stamatoyannopoulos JA, Snyder MP, Guigo R, Gingeras TR, Gilbert DM, Hardison RC, Beer MA, Ren B. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- Zhang Y, De S, Garner JR, Smith K, Wang SA, Becker KG. Systematic analysis, comparison, and integration of disease based human genetic association data and mouse genetic phenotypic information. BMC Med Genomics. 2010;3:1. doi: 10.1186/1755-8794-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Huang B, Li W, Jin Y. MicroRNA expression profiling during neural differentiation of mouse embryonic carcinoma P19 cells. Methods Mol Biol. 2012;936:105–116. doi: 10.1007/978-1-62703-083-0_9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.