Abstract
Despite the fact that SIRT4 regulates important biological processes, its primary enzymatic activity has remained ambiguous. A recent study by Anderson, Huynh et al. has uncovered deacylase activities of SIRT4 towards newly described lysine modifications derived from reactive acyl-CoAs generated in leucine catabolism.
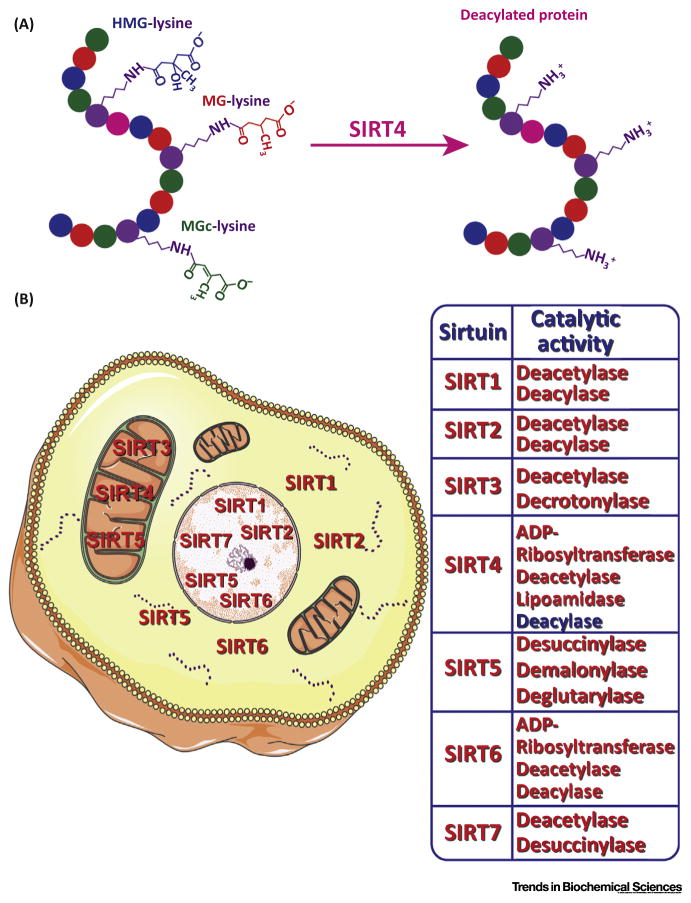
Sirtuins comprise a family of nicotinamide adenine dinucleotide (NAD+)-dependent lysine deacylases implicated in suppressing many age-associated pathologies. The seven mammalian sirtuins, SIRT1-7, show varying sub-cellular localization, enzymatic activities, and substrate specificities [1] (Figure 1). Early studies demonstrated that some sirtuins possess only very weak deacetylase activity against a histone peptide substrate. This observation implied that these sirtuins might have selective deacetylase activity against very specific substrates, and/or alternative catalytic functions altogether [2]. A growing body of literature has revealed that specific sirtuin family members catalyze removal of several novel lysine acyl modifications, providing a satisfying rationale for the evolutionary conservation of the sirtuins that possess little intrinsic deacetylase activity [1].
Figure 1.
Novel Enzymatic Activities of SIRT4, and Reported Subcellular Localization and Catalytic Activities of All Mammalian Sirtuins. (A) SIRT4 catalyzes removal of newly discovered protein modifications, 3-hydroxy-3-methylglutaryl (HMG)-lysine, 3-methylglutaryl (MG)-lysine and 3-methylglutaconyl (MGc)-lysine. (B) Mammalian sirtuin localization and reported catalytic activities. Some graphics in this figure were obtained and modified from Servier Medical Art from Servier (http://www.servier.com/Powerpoint-image-bank).
Among the seven mammalian sirtuins, SIRT1, SIRT2, and SIRT3 are robust deacetylases, and can also catalyze removal of long chain acyl groups. Lysine desuccinylation, demalonylation, and deglutarylation are catalyzed by SIRT5. SIRT6 possesses deacetylase, ADP-ribosyltransferase as well as long-chain deacylase activities, and SIRT7 catalyzes deacetylation and histone desuccinylation reactions (Figure 1) [1,3]. Important discrepancies still exist in the literature regarding activity of sirtuins against individual lysine modifications, particularly in vivo.
In contrast to other sirtuins, the catalytic activity of SIRT4 as a general matter has remained somewhat enigmatic. Biologically, SIRT4 play roles in many important processes, particularly in glutamine and fatty acid metabolism (see van de Ven et al. for references) [4]. SIRT4 interacts with glutamate dehydrogenase (GDH) in pancreatic β-cells, and was proposed to ADP-ribosylate GDH to repress its activity, thereby suppressing insulin secretion in response to the insulin secretagogue leucine. SIRT4 also suppresses glucose-stimulated insulin secretion. SIRT4 deacetylates and inhibits malonyl-CoA decarboxylase, an enzyme that catalyzes conversion of malonyl-CoA into acetyl-CoA. Malonyl-CoA allosterically inhibits the activity of carnitine palmitoyl-transferase 1 (CPT1), an enzyme that promotes mitochondrial uptake of fatty acids for fatty acid oxidation (FAO). Sirt4 KO mice showed increased FAO, associated with increased exercise capacity and resistance to diet-induced obesity. SIRT4 has also been reported to negatively regulate the activity of AMPK. AMPK phosphorylates and inhibits acetyl-CoA carboxylase, which catalyzes the production of malonyl-CoA from acetyl-CoA. AMPK also activates PGC-1α, a transcriptional co-activator of FAO genes; during fasting, SIRT4 inhibits AMPK activity, suppressing FAO. SIRT4 also inhibits activity of Pyruvate Dehydrogenase Complex, the major route by which glucose-derived carbon enters the TCA cycle [4]. In addition to its functions in normal metabolism, multiple lines of evidence implicate SIRT4 as a tumor suppressor. Reduced SIRT4 levels are associated with poor prognosis in several human cancers [1], and Sirt4 KO mice show an increased incidence of spontaneous lung tumors. Genotoxic stress or mTORC1 inhibition activate SIRT4 to suppress glutamine catabolism, a function likely to be particularly relevant in glutamine-dependent cancers, such as those driven primarily by increased MYC activity.
Although SIRT4 lacks detectable histone deacetylase activity [2], there are reports indicating that SIRT4 possesses ADP-ribosyltransferase, as well as substrate-specific deacetylase and lipoamidase activities [4]. However, the question of whether SIRT4 might target other distinct lysine modifications, analogous to SIRT5, has remained an important knowledge gap in sirtuin biology.
In a new study, Anderson, Huynh et al., have now shown that SIRT4 catalyzes removal of 3-hydroxy-3-methylglutaryl (HMG) and the related modifications, 3-methylglutaryl (MG), and 3-methylgluta-conyl (MGc), from lysine residues of target proteins (Figure 1) [5]. These modifications were discovered and characterized by the same group in a complementary study [6]. Through phylo-genetic and structural analysis, Anderson, Huynh et al. identified a highly conserved α–helical region within the catalytic pocket of SIRT4, predicted to be involved in interaction with negatively charged acyl modifications. Recombinant SIRT4 efficiently removes glutaryl-, MG-, HMG-and MGc-lysine modifications, and SIRT4-overexpressing cells show reduced glutaryl-, MG-, and HMG-lysine levels [5]. To test in vivo significance, the authors focused on methylcrotonyl-CoA carboxylase complex (MCCC), a complex involved in leucine catabolism that the authors find to be decorated with these novel modifications, previously shown to interact with SIRT4 [7]. The authors demonstrate that increased MCCC acylation, occurring in the absence of SIRT4, destabilizes MCCC, reducing leucine flux [5]. The authors also identify defects in catabolism of other branched chain amino acids in Sirt4 KO tissues. Since leucine is a well-known allosteric GDH activator, altered leucine levels likely contribute to suppression of GDH activity and glutamine catabolism by SIRT4 [4]. Indeed, Anderson, Huynh et al. find that Sirt4 KO mice show elevated insulin secretion, and age-associated glucose intolerance and hyperinsulinemia, consistent with impaired leucine catabolism, and with leucine’s known role as an insulin secretagogue [8].
This paper provides important new insights into SIRT4 function, and raises key questions for future studies. The fact that MCCC acylation inhibits its activity via complex destabilization is a very important observation. Acetylation, and perhaps other acylations as well, have been suggested to be present at low stoichiometry, especially on mitochondrial proteins such as MCCC. Mechanistically, how such low-level modifications might affect target protein function has remained somewhat mysterious [9]. Several of the MCCC acylation sites could conceivably affect stability of the entire complex. Thus, these observations provide one plausible template for how lysine modifications, individually present at low levels, might collectively exert functionally significant effects on a multimeric enzyme complex.
With regard to SIRT4, leucine is a major positive regulator of mTORC1 activity [10]. These results suggest that SIRT4 might repress mTORC1 function by reducing intracellular leucine levels. Genetic and pharmacologic studies in mammals and other organisms have demonstrated that mTORC1 promotes aging and age-associated pathologies, such as diabetes and cancer. Thus, regulation of mTORC1 signaling may help to mediate SIRT4’s known roles in tumor suppression, and in enforcing proper glucose homeostasis [4]. Finally, according to these new studies from the Hirschey group, SIRT4 and SIRT5 may possess somewhat overlapping enzymatic profiles. Hence their findings raise the potential for biological redundancy between these sirtuins. Notably, as compared with SIRT1 and SIRT6 deficiencies, neither Sirt4 nor Sirt5 single KO mice show strong phenotypes under basal conditions [1].
Acknowledgments
The authors acknowledge support from Melanoma Research Alliance, NIH (R01GM101171, 2R01HL114858, and R21AG053561), DoD (OC140123), the Glenn Foundation for Medical Research, Love Your Melon/St. Baldrick’s Foundation, the Harrington Discovery Institute, and the University of Michigan Cancer Research Committee.
References
- 1.Kumar S, Lombard DB. Mitochondrial sirtuins and their relationships with metabolic disease and cancer. Antioxid Redox Signal. 2015;22:1060–1077. doi: 10.1089/ars.2014.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North BJ, et al. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 3.Li L, et al. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun. 2016;7:12235. doi: 10.1038/ncomms12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Ven RA, et al. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol Med. 2017;23:320–331. doi: 10.1016/j.molmed.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson KA, et al. SIRT4 is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab. 2017;25:838–855. e815. doi: 10.1016/j.cmet.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner GR, et al. A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab. 2017;25:823–837. e828. doi: 10.1016/j.cmet.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirth M, et al. Mitochondrial SIRT4-type proteins in Caenorhabditis elegans and mammals interact with pyruvate carboxylase and other acetylated biotin-dependent carboxylases. Mitochondrion. 2013;13:705–720. doi: 10.1016/j.mito.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutton JC, et al. Interaction of branched chain amino acids and keto acids upon pancreatic islet metabolism and insulin secretion. J Biol Chem. 1980;255:7340–7346. [PubMed] [Google Scholar]
- 9.Lombard DB, et al. Acetyled question in mitochondrial biology? EMBO J. 2015;34:2597–2600. doi: 10.15252/embj.201592927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]