Abstract
Objective
Pulmonary hypertension (PH) is characterized by progressive elevation of pulmonary vascular resistance, right ventricular failure, and ultimately death. We have shown that in rodents, hypoxia-induced mitogenic factor (HIMF; also known as FIZZ1 or RELMα) causes PH by initiating lung vascular inflammation. We hypothesized that hypoxia-inducible factor-1 (HIF-1) is a critical downstream signal mediator of HIMF during PH development.
Approach and Results
In this study, we compared the degree of HIMF-induced pulmonary vascular remodeling and PH development in wild-type (HIF-1α+/+) and HIF-1α heterozygous null (HIF-1α+/−) mice. HIMF-induced PH was significantly diminished in HIF-1α+/− mice and was accompanied by a dysregulated VEGF-A–VEGF receptor 2 pathway. HIF-1α was critical for bone marrow-derived cell migration and vascular tube formation in response to HIMF. Furthermore, HIMF and its human homolog, resistin-like molecule-β (RELMβ), significantly increased IL-6 in macrophages and lung resident cells through a mechanism dependent on HIF-1α and, at least to some extent, on nuclear factor κB.
Conclusions
Our results suggest that HIF-1α is a critical downstream transcription factor for HIMF-induced pulmonary vascular remodeling and PH development. Importantly, both HIMF and human RELMβ significantly increased IL-6 in lung resident cells and increased perivascular accumulation of IL-6–expressing macrophages in the lungs of mice. These data suggest that HIMF can induce HIF-1, VEGF-A, and interleukin-6, which are critical mediators of both hypoxic inflammation and PH pathophysiology.
Keywords: Pulmonary hypertension, hypoxia-inducible factor 1α, hypoxia-induced mitogenic factor/FIZZ1/RELMα, macrophage, interleukin-6
INTRODUCTION
Pulmonary hypertension (PH) is characterized by remodeling of distal pulmonary arteries as well as vasoconstriction and in situ thrombosis that lead to enhanced pulmonary vascular resistance and pressure, progressive right-sided heart failure, and ultimately death. In humans, severe pulmonary arterial hypertension is characterized by plexiform lesions that contain phenotypically altered pulmonary smooth muscle cells (PSMCs) and endothelial cells (ECs).1 Despite major advances in diagnosis and treatment of this disease over the last several decades, the underlying mechanisms of PH and its etiology are poorly understood. Growing evidence indicates that inflammation plays a key role in triggering and maintaining pulmonary vascular remodeling.
Our group has shown that hypoxia-induced mitogenic factor (HIMF, also known as FIZZ1 or RELMα), a member of the resistin family of proteins, is dramatically upregulated in the proliferative phase of a hypoxia-induced PH model. Conversely, inhibition of the HIMF pathway prevents development of hypoxia-induced PH.2 HIMF has proinflammatory actions3–5 and is known to be persistently upregulated in animal lungs in models of allergic inflammation,6 bleomycin-induced pulmonary fibrosis,7 and herpes virus-induced pulmonary fibrosis.8 HIMF exhibits chemotactic actions on bone marrow derived (BMD) cells in part by binding Bruton’s tyrosine kinase.9 Interestingly, genetic transfer of HIMF into rodent lung induces the vascular remodeling and hemodynamic changes of PH in rats and promotes BMD cell recruitment to the remodeled pulmonary vasculature in mice.10
HIMF (FIZZ1) is well known as a marker for alternatively activated (M2) macrophages.11 It has been suggested that this phenotype is positively associated with tissue remodeling and vascular growth response in chronic inflammatory conditions, including PH.12, 13 We also have shown that HIMF expression in the remodeled pulmonary vasculature, but not in airway epithelial cells, positively correlates with increased mean pulmonary arterial pressure in experimental PH.14 Based on these data, it is reasonable to speculate that macrophage-derived HIMF (FIZZ1) protein plays a critical role in PH development.
We have recently established a mouse model of PH in which a single systemic injection of recombinant (r)HIMF protein causes early lung inflammation (day 7) and PH development (day 30) that are dependent on the VEGF and T helper (Th) type 2 cytokine IL-4 pathways.3, 4 Using this HIMF-injection model, we also have shown that macrophage recruitment to the lung and lung vascular inflammation in response to HIMF are completely suppressed in IL-4 knockout mice.5 It has been suggested that HIMF recruits and binds BMD macrophages, dendritic cells, and T cells.15, 16 Moreover, we have proven that HIMF can activate pulmonary ECs and EC apoptosis to trigger pulmonary vascular inflammation.5 Although HIMF is thought to be a shared mediator of both hypoxic and Th2 inflammation,17, 18,14 the underlying mechanism by which HIMF induces downstream mediators critical for PH development is unknown.
The two human homologs of HIMF are resistin-like molecule β (RELMβ) and resistin (hRETN). RELM proteins are 105–114 amino acids in length, and their C-terminal signature sequence contains 10 cysteines that are highly conserved and constitute nearly half of the molecule.19 RELMβ is upregulated in lungs of patients with scleroderma-associated PH, strongly suggesting an etiologic role of resistin family proteins in PH.20 On the other hand, hRETN is expressed by myeloid cells, especially macrophages, and its expression pattern shows a greater similarity to that of murine HIMF (RELMα) than to that of murine resistin.21 We have recently shown that, like HIMF, hRETN induces endothelial activation, apoptosis, and release of growth factors for smooth muscle cells (SMCs), suggesting that it has a critical role in EC–SMC crosstalk under inflammatory conditions.5
In the present study, we dissected the mechanism by which HIMF induces mediators that contribute to PH development. Accumulating evidence suggests that hypoxia inducible factor-1 (HIF-1) is a critical transcription factor in both hypoxic inflammation and Th2 immune activation in the lung.22, 23 Because we have shown previously that HIMF upregulates angiogenic/proinflammatory mediators in the lung that are downstream of HIF-1, such as VEGF, we hypothesized that HIF-1 is a critical downstream mediator of HIMF during pulmonary vascular remodeling and development of PH. In this context, we investigated (1) whether HIF-1 mediates HIMF-induced pulmonary vascular remodeling and PH development in vivo; (2) whether HIF-1 mediates HIMF-induced EC apoptosis; (3) whether HIF-1 mediates BMD cell migration/recruitment in response to HIMF; (4) whether HIF-1 is critical to inducing proinflammatory mediators in macrophages and lung resident cells; and lastly (5) whether the human homolog of HIMF mediates HIF-1 activation as a downstream signaling pathway in human lung resident cells.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
RESULTS
HIMF increases expression of HIF-1α, but not HIF-2α, in the lung
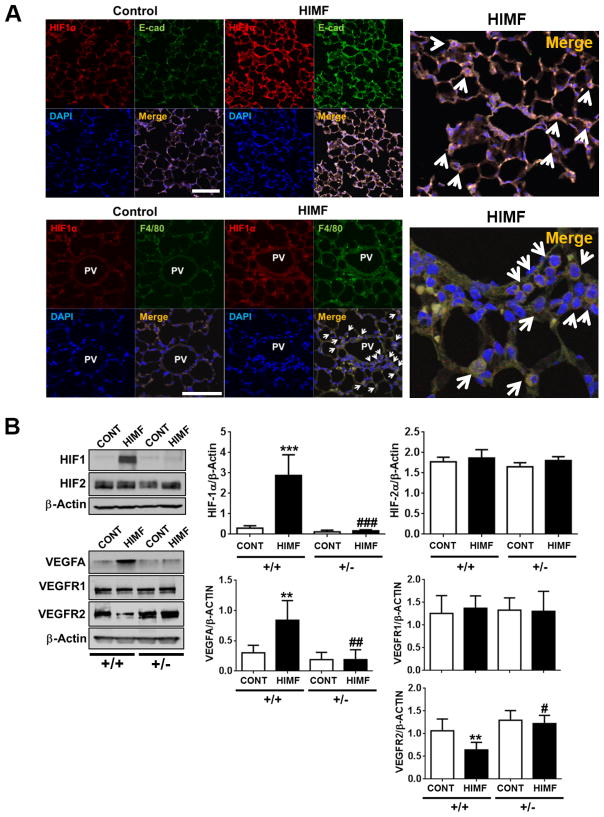
We and others have shown that HIMF is the critical shared mediator for hypoxia and Th2 inflammation in the lung.14, 17, 18 Both hypoxia and inflammation also stimulate the transcription factor HIF-1.23 Therefore, we examined whether HIF-1 is activated in HIMF-induced pulmonary vascular remodeling and PH development. In HIMF-injected mice,5 we observed that HIF-1α expression in lung epithelial cells and macrophages was increased during the PH development phase (Day 30, Figure 1A). We also analyzed HIF protein expression in response to HIMF injection in HIF-1α+/+ and HIF-1α+/− mice. Interestingly, HIMF treatment increased HIF-1α protein expression, but not HIF-2α expression, in lung tissue of HIF-1α+/+ mice (Figure 1B). However, this HIMF-induced HIF-1α expression was abolished in HIF-1α+/− mice. Next, we examined the expression of VEGF-A and its receptor (VEGFR) in the lung because we have shown previously that VEGF-A is increased and VEGFR2 dysregulated in this animal model, suggesting that HIMF-induced lung inflammation occurs through a VEGFR2-dependent mechanism.3 Indeed, HIMF caused significant VEGF-A induction and decreased VEGFR2 expression in HIF-1α+/+ mice; however, these responses were abolished in HIF-1α+/− mice (Figure 1B). Interestingly, HIMF treatment did not change VEGFR1 expression in either genotype. These data suggest that HIMF stimulates HIF-1–VEGF-A–VEGFR2 signaling in the lung.
Figure 1. HIMF induces HIF-1α and HIF-1α–dependent VEGF-VEGFR2 signaling in the lung.
A, HIF-1α–positive cells were detected by red fluorescence in the lungs of mice 30 days after injection with HIMF, but not after injection with vehicle control. Double staining showed that these HIF-1α–positive cells were lung epithelial cells (E-cad–positive cells; green, 200x magnification) and macrophages (F4/80–positive cells; green, 400x magnification) surrounding remodeled small pulmonary vessels (PV). Arrows indicate E-cad– or F4/80–positive cells that co-localize with HIF-1α expression. Scale bars, 100 μm. Magnified merged images are shown on the right. B, Representative Western blots show lung expression of HIF-1α, HIF-2α, VEGF-A, VEGFR1, and VEGFR2 30 days after injection with HIMF or vehicle. Quantification of protein expression is shown as a ratio of each protein to β-actin. HIMF induced HIF-1α and VEGF-A, but not HIF-2α, in HIF-1α+/+ mouse lung. Data are presented as mean ± SEM. **P < 0.01, ***P < 0.001 vs HIF-1α+/+ control; #P < 0.05, ##P < 0.01 reflects genotype differences of HIMF injection (n=3–6 animals per group). These data reflect at least 3 independent in vivo experiments.
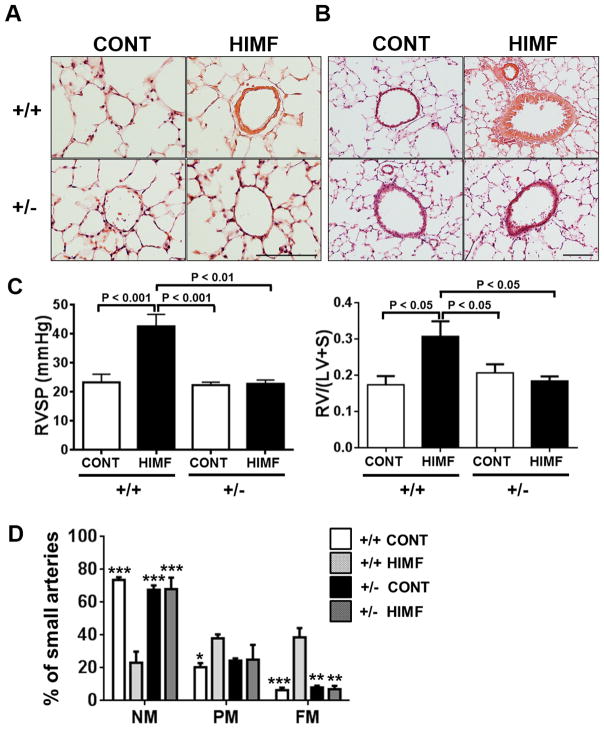
HIMF causes HIF-1α–dependent pulmonary vascular remodeling and PH development
To determine the importance of HIF-1 as a downstream signaling mediator of HIMF in the development of PH, we induced PH by injecting HIMF into HIF-1α+/+ and HIF-1α+/− mice as previously described.5 At 30 days after HIMF injection (PH development phase), medial thickening was evident in small pulmonary vessels in HIF-1α+/+ mice but not in HIF-1α+/− mice (Figure 2A). Similarly, HIMF injection significantly increased airway thickness in HIF-1α+/+ mice, but this phenomenon was markedly diminished in HIF-1α+/− mice (Figure 2B). Furthermore, HIMF-injected HIF-1α+/+ mice exhibited significant right heart hypertrophy and had higher right heart pressure than did vehicle-injected HIF-1α+/− mice (Figure 2C). These hemodynamic changes were absent in HIF-1α+/− mice, though no baseline differences were apparent between genotypes. Lastly, we compared HIMF-induced pulmonary vascular remodeling in each genotype. The two genotypes exhibited no baseline differences; however, HIMF injection caused a significant decrease in the number of non-muscularized vessels, and increases in the number of partially muscularized and fully muscularized vessels in HIF-1α+/+ mice. This HIMF-induced pulmonary vascular remodeling was significantly diminished in HIF-1α+/− mice (Fig. 2D). These data suggest that HIMF causes pulmonary vascular remodeling and development of PH by a HIF-1-dependent mechanism in this animal model.
Figure 2. HIMF injection causes HIF-1α–dependent pulmonary vascular remodeling and pulmonary hypertension.
A–B, Hematoxylin-and-eosin–stained small pulmonary vessels (A; Scale bar: 100 μm) and pulmonary airway epithelium (B; Scale bar: 200 μm) from HIF-1α +/+ and HIF-1α +/− mice 30 days after intravenous injection with HIMF or vehicle. C, Hemodynamics and right ventricular hypertrophy were analyzed 30 days post-injection. RVSP, right ventricular end systolic pressure (left, n=4–5 per group); RV/(LV+S), right ventricular weight/(left ventricular + septal weight) (right, n=4–7 per group). Data are shown as mean ± SEM. D, Comparison of HIMF-induced pulmonary vascular remodeling in HIF-1α+/+ and HIF-1α+/− mice. Bar graphs show the percentage of small pulmonary arteries in HIF-1α+/+ and HIF-1α+/− mice that were non-muscular (NM), partially muscular (PM), or fully muscular (FM). *P < 0.05, **P < 0.01, ***P < 0.001 vs. HIF-1α+/+ + HIMF. At least 500 vessels were counted in each group (3–5 animals per group).
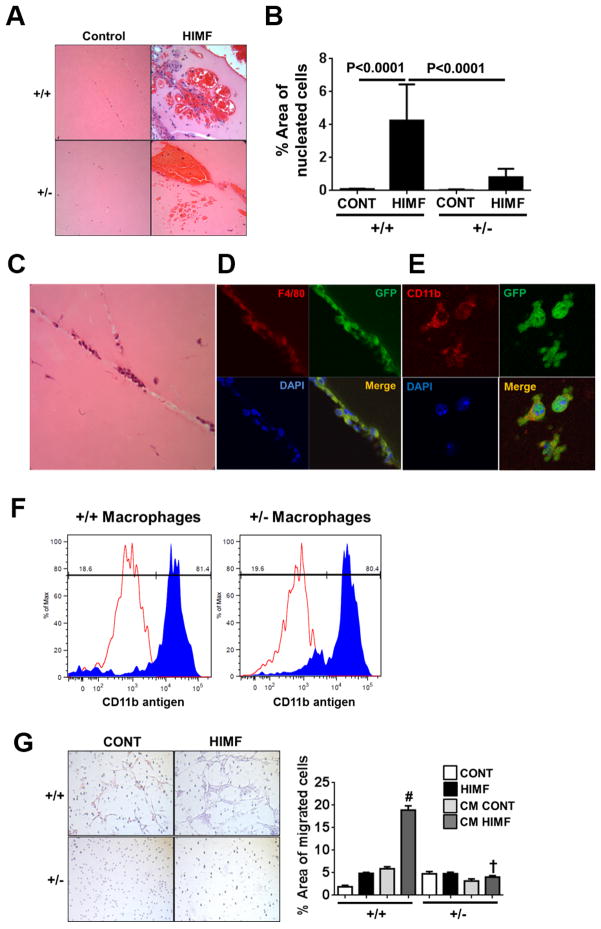
HIMF recruits BMD macrophages, and HIMF-mediated in vivo vascular formation is significantly diminished in HIF-1α+/− mice
Vasculogenesis describes the process of de novo blood vessel formation from vascular precursor cells. It has been suggested that BMD cells, especially BMD macrophages, play a central role in postnatal vessel growth as a source of proangiogenic paracrine factors.24 We have previously shown that HIMF can induce vascular tube formation in vivo as well as vascular growth response in the lung.3 We have also shown significant macrophage recruitment to the lungs of HIMF-treated mice.4 BMD macrophages are known to be recruited at the initiation stage of vascular formation during neovascularization of subcutaneous Matrigel plugs.25 In this context, we examined whether HIF-1 mediates HIMF-induced vascular tube formation in vivo using HIF-1α+/+ and HIF-1α+/− mice. HIMF caused strong vascular tube formation in the Matrigel plugs of HIF-1α+/+ mice (Figure 3A). HIMF-induced vascular tube formation is usually observed in the presence of red blood cells (RBC) in Matrigel, as shown in Figure 3A. Notably, HIMF treatment caused massive RBC recruitment in the Matrigel plugs of HIF-1α+/− mice; however, we observed significantly fewer of the nucleated cells that can form vascular tubes in HIF-1α+/− mice than in HIF-1α+/+ controls (Figure. 3B). Because BMD macrophages are thought to be recruited to the area of neovascularization to form vascular tubes and stabilize vascular networks by releasing the factors necessary for vascular growth response,25 we analyzed whether HIMF recruits BMD macrophages to the area of vascular formation using a BM transplant technique in which lethally irradiated mice were rescued with BM transplanted from GFP-positive transgenic mice, as we have previously reported (Figure 3C).10 We were able to identify BMD cells using anti-GFP antibody. GFP-positive BMD cells in the newly formed vascular tube colocalized with macrophage marker F4/80 and CD11b (Figure 3D and E). To determine whether the baseline number of macrophages differed in each genotype, we also compared the ability of BMD cells to differentiate into a macrophage phenotype in response to macrophage colony-stimulating factor (M-CSF, also known as CSF-1). M-CSF is a cytokine required for the differentiation of monocyte lineage cells to macrophages and promotes the formation of high-density vessel networks in pathological angiogenesis.26 Our results showed that BMD cells from HIF-1α +/− mice and HIF-1α +/+ controls differentiated to macrophages (CD11b-positive) in response to M-CSF to a similar degree (Figure 3F). Thus, the pathophysiologic differences in response to HIMF are not based on a difference in baseline numbers of macrophages in each genotype. These results suggest that HIMF recruits macrophages to the area of vascular formation and that this phenomenon is mediated by HIF-1 as a downstream transcription factor of HIMF.
Figure 3. HIF-1–dependent bone marrow cell recruitment and vascular formation in response to HIMF.
A–B, HIMF-induced vascular formation in Matrigel plugs from HIF-1α+/+ and HIF-1α+/− mice. A, Representative hematoxylin-and-eosin images of vascular formation in Matrigel plugs after HIMF (50 nM) administration to HIF-1α+/+ and HIF-1α+/− mice (40x magnification). B, Bar graph represents mean value of nucleated cell areas in the vascular formation from 5–10 images per mouse (n=3–4 mice per group). C, Representative hematoxylin-and-eosin image of vascular formation in a Matrigel plug from a bone marrow-transplanted mouse injected with HIMF (400x magnification). D–E, Colocalization of bone marrow-derived (BMD) cells (GFP-positive, green) with macrophage markers F4/80 (D, red, 630x magnification) or CD11b (E, red, 1000x magnification). F, Comparison of the differentiation of BMD cells to macrophages in each genotype in response to macrophage colony-stimulating factor (M-CSF). Equivalent numbers of BMD cells from each genotype were cultured for 10 days in the presence of M-CSF (20 ng/mL), harvested, stained with antibody against mouse CD11b (filled histograms) or the corresponding rat IgG2a K isotype controls (open histograms), and analyzed by flow cytometry. G, Mouse BMD cell migration in response to HIMF or conditioned media (CM) from pulmonary endothelial cells treated with or without HIMF. Images of migrated cells (left, 100x magnification) and quantified results (right) are shown. Data are presented as mean ± SEM. *P < 0.001 vs control or CM control from HIF-1α+/+ cells; #P < 0.001 reflects genotype differences of CM HIMF treatment (n=3 membranes per group).
We have previously reported that HIMF and its human homolog hRETN stimulate EC activation and apoptosis in pulmonary microvascular ECs (PMVECs).5 Furthermore, these HIMF- or hRETN-stimulated ECs produce growth factors and chemokines that enhance perivascular immune cell recruitment and SMC growth response.5 Here, we characterized EC-derived human SMC growth factors in response to hRETN and found that serpin E1 (also known as plasminogen activator inhibitor-1), tissue inhibitor of metalloproteinase-1 (TIMP-1), and urokinase plasminogen activator (uPA), were released from ECs in response to hRETN. These molecules are known to promote SMC growth response (Supplemental Figure 1). 27–29
We also have previously reported that HIMF recruits BMD cells to the area of remodeled pulmonary vasculature.10 In a cell culture system, we have shown that HIMF directly causes migration of myeloid cells through the action of Bruton’s tyrosine kinase, a functional binding partner of HIMF.9 To investigate whether HIF-1 mediates HIMF-induced BMD cell migration, we isolated BMD cells from HIF-1α+/+ and HIF-1α+/− mice and analyzed HIMF-induced BMD migration as previously described9 using rHIMF protein (20 nM) alone or conditioned medium from HIF-1α+/+ PMVECs treated with or without rHIMF (20 nM, 24 hours). BMD cells from HIF-1α+/+ mice migrated toward rHIMF as expected. To our surprise, HIF-1α+/+ rHIMF-treated PMVEC conditioned medium caused a 3.87-fold increase in BMD migration as compared to that of the untreated control groups (Figure 3G). However, BMD cells from HIF-1α+/− mice did not migrate in response to either rHIMF or rHIMF-treated PMVEC conditioned medium. These results suggest that HIMF can stimulate PMVECs to produce chemokines that cause BMD cell migration. The results also show that HIF-1α activity in BMD cells is critical to HIMF-induced myeloid cell migration.
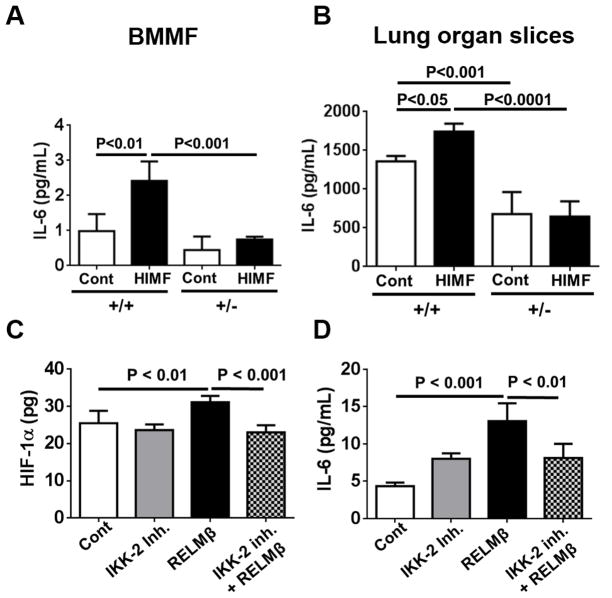
HIMF increases IL-6 production in macrophages in vitro and in lung organ slices ex vivo in a HIF-1α–dependent manner
Macrophage accumulation is not only a hallmark of PH but also a critical component of pulmonary vascular remodeling associated with PH. Recent studies have shown that macrophage activation is critical in experimental PH.12, 30 Macrophages are also known to be responsible for producing a large spectrum of chemokines and proinflammatory and vascular growth factors. In this context, we performed angiogenesis cytokine array analysis using culture medium from BMD macrophages and lung organ slices from each genotype treated with or without HIMF. We found that HIMF significantly induced IL-6 protein expression in BMD macrophages (Figure 4A) and in lung resident cells (Figure 4B). However, this effect was completely suppressed in cells from HIF-1α+/− mice (Figure 4A&B).
Figure 4. HIMF and human RELMβ induce IL-6 expression in macrophages and lung resident cells.
A, HIMF significantly increased IL-6 in bone marrow macrophages (BMMF) from HIF-1α+/+ mice but not in those from HIF-1α+/− mice. BMMF were isolated from each genotype and cultured. After 10 days, the cells were stimulated with recombinant mouse HIMF (20 nM) for 24 hours. Cell medium was collected for ELISA analysis. Results are shown as mean ± SEM (n=6 per group). B, HIMF significantly increased IL-6 in lung organ slices from HIF-1α+/+ mice but not in those from HIF-1α+/− mice. Ex vivo lung organ slices were prepared and treated with or without HIMF (10 nM) for 24 hours. Then culture medium was collected for IL-6 analysis. Data are mean ± SEM of IL-6 level/mg of lung tissue (n = 4–6 lung slices per mouse, 3 mice per group). C–D, Human RELMβ induces HIF-1α (C) and IL-6 (D) expression in human lung fibroblasts (HLF) through an NF-κB–dependent mechanism. HIF-1α protein expression and secreted IL-6 level in the medium were determined by ELISA assay after HLF were stimulated for 20 hours with hRELMβ (100 ng/mL) in the presence or absence of IKK-2 inhibitor (500 nM) (C, mean± SEM of HIF-1α level/100 μg of total protein, n = 6 per group; D, mean ± SEM of IL-6 level, n = 4–6 per group).
Human RELMβ increases HIF-1α and IL-6 expression through an NF-κB–mediated mechanism in human primary lung fibroblasts (HLF)
In our previous study, we showed that RELMβ expression was elevated in the remodeling vasculature of patients with PH, particularly those with scleroderma-associated PH.20 hRELMβ is also known to increase fibroblast proliferation and differentiation that results in deposition of extracellular matrix proteins.31 Fibroblast differentiation is a major component of vascular remodeling in PH.32 Furthermore, some RELM proteins in humans and mice mediate NF-κB signaling in multiple cell types.33, 34 In this context, we examined whether hRELMβ mediates HIF-1 and NF-κB signaling in HLF. We observed a statistically significant increase in HIF-1α protein expression in HLF that was significantly suppressed by the inhibition of IKK-β, a critical upstream mediator of NF-κB activation (Fig. 4C). Furthermore, hRELMβ significantly increased IL-6 production in HLF, but this increase was significantly attenuated in the presence of IKK-β inhibitor (Fig. 4D). These data suggest that the IKK-β– NFκB–HIF-1 axis is required for hRELMβ-induced production of proinflammatory cytokine IL-6 in HLF.
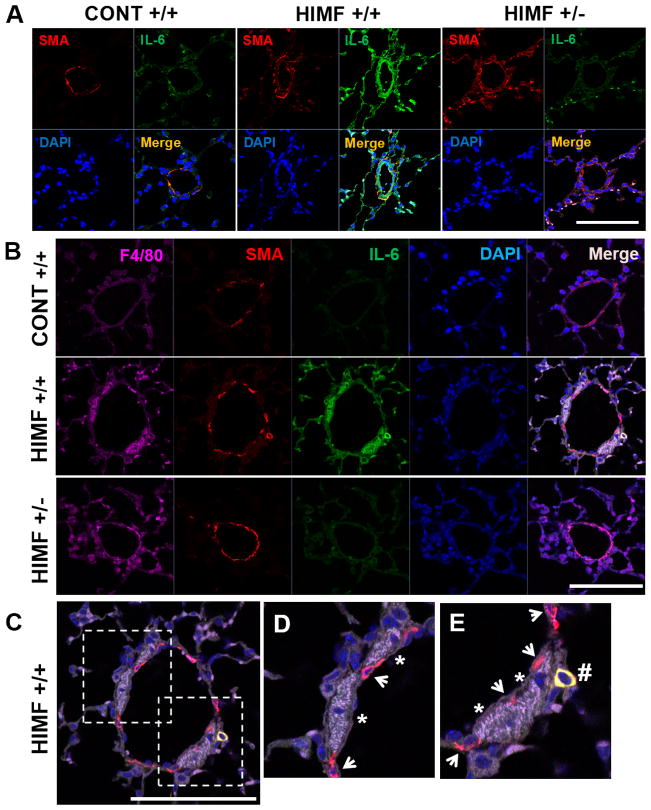
HIMF increases IL-6 production in perivascular macrophages and SMC in a HIF-1α-dependent manner in vivo
Involvement of IL-6 signaling has been strongly implicated in clinical and experimental PH.35–38 We further examined IL-6 induction in response to HIMF in the remodeled pulmonary vasculature in vivo. HIMF caused massive IL-6 expression in the remodeled vascular media (α-SMA-positive cells) and in infiltrating inflammatory cells of HIF-1α+/+ mice but not in those of HIF-1α+/− mice (Figure 5A). Next, we analyzed whether those IL-6–expressing inflammatory cells in the remodeled small pulmonary vessels are macrophages by using the marker for macrophage F4/80. We observed that IL-6–expressing macrophages (F4/80+ cells) are recruited to the media of remodeling small pulmonary vessels in HIMF-treated HIF-1α+/+ mice but not in HIF-1α+/− mice (Figure 5B). Notably, in addition to those IL-6–expressing macrophages, we observed the recruitment of IL-6–expressing α-SMA-positive cells that were distinct from resident SMC in the remodeling vasculature (Figure 5D and E). These data suggest that HIMF stimulates the recruitment of IL-6–expressing macrophages and α-SMA-positive cells to the pulmonary vessels via a HIF-1–dependent mechanism.
Figure 5. HIMF induces IL-6 expression in macrophages and smooth muscle cells (SMC) in vivo.
A, HIMF treatment increased IL-6 expression in the small pulmonary vessels of HIF-1α+/+ mice but not in those of HIF-1α+/− mice. HIMF increased α-smooth muscle actin–positive cells (α-SMA+, red, identifying vascular media) as well as IL-6 expression (green) in the remodeled pulmonary vasculature. IL-6 expression colocalized with α-SMA+ cells and inflammatory cells that infiltrated into the vasculature. These increases were strongly suppressed in HIF-1α+/− mice. B, IL-6–expressing macrophages (F4/80+, far red) were recruited to the remodeling small vessels in HIMF-treated HIF-1α+/+ mice but not in HIF-1α+/− mice. C–E, Magnified merged images of IL-6–expressing macrophages (*) and α-SMA+ cells (#) are shown. Arrows indicate resident α-SMA+ cells without IL-6 expression (Scale bars, 100 μm).
DISCUSSION
We have shown previously that HIMF induces proinflammatory and pro-angiogenic cytokines (e.g., VEGF) that are critical to hypoxic inflammation and known to lie downstream in the signaling pathway of transcription factor HIF-1. Thus, we hypothesized that HIF-1 is a critical downstream signal mediator of HIMF-induced pulmonary vascular remodeling and development of PH. In this study, we showed that HIMF can induce expression of HIF-1α, but not HIF-2α, in the lung. Moreover, HIF-1α was required for HIMF-induced vascular remodeling, vascular tube formation, BM cell recruitment, and IL-6 induction in macrophages and lung resident cells. Importantly, HIMF significantly increased lung VEGF-A expression and downregulated VEGFR2 in a HIF-1α–dependent manner. VEGFR2 dysfunction is a hallmark of the severe pathophysiology observed in human and experimental PH.39 In addition, increased HIF-1 as well as VEGF expression is observed in plexiform lesions in patients with severe PH.1 Our results are in accordance with human conditions and suggest that the mechanism of HIMF-induced PH is mediated, at least in part, by HIF-1–VEGF-A–VEGFR2 signaling in the lung.
We have shown previously that in mice, HIMF causes PH by initiating pulmonary EC apoptosis and mediating lung vascular inflammation in a Th2 cytokine IL-4–dependent mechanism.5 Indeed, HIMF/RELMα was upregulated in the lungs of animals exposed to either ovalbumin (OVA) immunization or chronic hypoxia, suggesting that it is a common mediator in both hypoxic PH and Th2 lung inflammation.14, 18 Several investigators have reported that Th2-skewed stimulation causes severe pulmonary vascular muscularization, indicating that pulmonary vascular remodeling is under the control of the immune system.18 However, a model of PH development and right heart failure after Th2 antigen challenge alone has not been reported. Mizuno et al.22 determined factors that are critical to PH development by using a rat model of PH created with a combination of VEGFR2 tyrosine kinase inhibitor Sugen 5416 and Th2 immune system activation (Su-OVA). In that study, the authors found that the lung expression of HIF-1α, VEGF, and IL-6 was significantly increased after treatment with both OVA immunization alone and Su-OVA. Notably, IL-6 protein expression in the lung was positively correlated with the mean pulmonary artery pressure,22 suggesting that the increased HIF-1α and IL-6 in lung may be a critical component of the pathogenesis of PH as a consequence of Th2 inflammation.
In our present study, we showed that HIMF treatment induced IL-6 expression in cultured BM macrophages, ex vivo lung organ slices, and in vivo pulmonary vasculature by a HIF-1α–dependent mechanism. IL-6 is elevated in several lung diseases and has been suggested to contribute to the pathogenesis of asthma and chronic obstructive pulmonary disease, as well as PH.35, 40, 41 IL-6 is an important regulator of effector CD4 T cell differentiation;42 it promotes IL-4 production during Th2 differentiation and inhibits Th1 differentiation.43 IL-6 also is known to promote Th17 cell differentiation in a manner dependent on TGF-β.44 It is produced by inflammatory cells such as macrophages but primarily by lung resident cells (e.g., epithelial cells, ECs, SMCs, and fibroblasts) in response to a variety of stimuli such as allergens, viruses, and hypoxia.30, 45 We used an ex vivo lung organ culture system that allows epithelial, interstitial, and microvascular cells to interact in the alveolar wall without systemic circulatory influences. Our finding that IL-6 expression in response to HIMF was more robust in lung resident cells than in macrophages in a circulation-free system is consistent with the observation that lung resident cells are the primary source of IL-6 production. Notably, we did not observe a significant IL-6 increase in pulmonary vascular SMCs in response to HIMF or hRETN in vitro (data not shown). However, in vivo, we observed that IL-6–expressing macrophages and α-SMA–positive cells were recruited to the remodeling vasculature of HIF-1α+/+ mice but not HIF-1α+/− mice. These results suggest that HIMF can induce IL-6 in the lung resident cells and recruit circulating IL-6–expressing cells (such as macrophages and α-SMA–positive cells) to the remodeling vasculature through a HIF-1α–dependent mechanism. It also has been suggested that lung resident cells, especially epithelial cells and SMCs, contribute to the Th2/Th17 type of immune response by secreting cytokines that can differentiate native T cells to Th2 and Th17 cells.46 We have published previously that HIMF amplifies Th2 inflammation, as evidenced by increased IL-4 in the lung.4 Furthermore, we have shown that IL-17 expression in the lung is significantly suppressed in global HIMF/RELMα knockout mice as compared with that in wild-type mice during allergic inflammation.47 It is reasonable to speculate that an increase in IL-6 in lung resident cells in response to HIMF can induce a sequential Th2/Th17 response. In accordance with our results, a recent study by Jang et al.48 showed that hRETN preferentially binds myeloid cells such as monocytes and macrophages in the lung during parasitic infections, and promotes proinflammatory cytokine expression. They also reported that increased circulating hRETN expression correlated positively with IL-6, CCL2, and TNF-α in human patients infected with soil-transmitted helminths or filarial nematodes.48 Because these cytokines, particularly IL-6, are PH markers in the context of inflammation and immunity,49 their findings support our current observation that RELM proteins can induce proinflammatory cytokines in humans during inflammation.
The mechanism by which hypoxia induces HIMF has been investigated, at least in part, by Vergadi et al.12 They reported that HIMF/FIZZ1 was induced in alveolar macrophages that were transiently recruited to the lung after 4 days of hypoxia and that inhibition of HIMF/FIZZ1-expressing M2 macrophages ameliorated hypoxic PH. It has been previously suggested that HIMF/FIZZ1/RELMα is induced by Th2 cytokines IL-4 and IL-13 via the STAT6 pathway in both macrophages and lung epithelial cell during allergic inflammation; however, we have published that hypoxia-induced HIMF expression in the lung occurred through a pathway independent of IL-4 or STAT6, suggesting that this protein can be induced by other pathways, such as hypoxia signaling.4, 32 In support of this observation, recent work by Colegio et al.50 showed that tumor-derived lactic acid can lead to HIMF/FIZZ1 expression in tumor-associated macrophages by a HIF-1α-dependent mechanism. This finding suggests that HIF-1α may be an upstream regulator of HIMF/FIZZ1 expression in macrophages under certain conditions. The data also suggest that HIMF is induced by alveolar macrophages in response to hypoxia and that increased perivascular HIMF/FIZZ1 can further activate lung resident cells and BMD macrophages to contribute directly to pulmonary vascular remodeling. In our study, hypoxia stimulation did not cause HIMF induction in naïve BMD macrophages in vitro (data not shown). A recent study by El Kasmi et al.30 showed that adventitial fibroblasts derived from hypertensive pulmonary arteries produce IL-6, which activates naïve BM macrophages to a pro-PH phenotype that is distinct from classical M1 or M2 macrophage phenotypes. Interestingly, that study showed that paracrine IL-6 activates STAT3, HIF-1α, and C/EBPβ in macrophages and that these transcription factors are necessary for macrophage activation and polarization. It is possible that HIMF/FIZZ1 is produced by alveolar macrophages in response to hypoxia-produced cytokines in the pulmonary vasculature and thus, IL-6 induced by HIMF may stimulate additional macrophage activation to a pro-PH phenotype and promote a proinflammatory feed-forward mechanism in the remodeling vasculature. In accordance with our observations, recent data showed that IL-6, which is increased in hypoxia-induced PH, also stimulates the increase of HIMF/FIZZ1-expressing M2 macrophages, supporting a positive feed-forward mechanism between IL-6 and HIMF/FIZZ1-expressing M2 macrophages in the development of PH.51
We have previously shown that HIMF triggers pulmonary EC activation and apoptosis by activating caspase-3 and stress-activated kinases such as JNK and p38 MAPK. To determine whether HIF-1α regulates HIMF-induced EC apoptosis and activation, we compared HIMF-mediated caspase-3 and stress-activated kinase activation in PMVECs from HIF-1α+/+ and HIF-1α+/− mice. However, PMVECs from the two genotypes showed similar degrees of caspase-3 and stress-activated kinase activation (data not shown), suggesting that HIF-1α does not mediate this phenomenon. We have shown previously that conditioned media from hRETN-treated PMVECs causes significant SMC proliferation5 and have identified endothelin-1 as one of the growth factors released from ECs in response to hRETN. Endothelin-1 is known to induce a PSMC-specific increase in HIF-1α level by upregulating HIF-1α synthesis and downregulating HIF prolyl hydroxylase 2-mediated degradation.52 In our study, we identified additional human SMC growth factors that are released from ECs in response to hRETN, including serpin E1, TIMP-1, and uPA. Similar to endothelin-1, serpin E1 promotes HIF-1α–dependent SMC growth response.28 hRETN is considered to be an important modulator of chronic inflammatory diseases such as obesity and atherosclerosis.53 Therefore, we speculate that in humans, PSMC HIF-1α expression is increased by PSMC growth factors from hRETN-induced PMVECs as a result of EC-SMC crosstalk during inflammation and vascular remodeling.
Another human homolog of HIMF, hRELMβ, has been reported to be increased in lung epithelial cells, fibroblasts, alveolar macrophages, and desquamated alveolar cells of patients with scleroderma-associated PH and idiopathic pulmonary fibrosis, suggesting a pro-fibrotic role in the lung.20, 54 Both HIMF/FIZZ1 and hRELMβ have been suggested to cause differentiation of fibroblasts into myofibroblasts.54, 55 These activated fibroblasts modulate vascular function and remodeling either directly or indirectly through secretion of a variety of cytokines, chemokines, growth factors, and matricellular proteins.32 HIF-1 is known to promote extracellular matrix remodeling under hypoxic conditions in fibroblasts.56 Previous studies have shown that RELM proteins mediate NF-κB signaling in multiple cell types. 33, 34 In the present study, we show for the first time that hRELMβ significantly increases HIF-1α expression and IL-6 production and that these effects are significantly suppressed by inhibition of IKK-β, a critical upstream mediator of HIF-1α that it is required for HIF-1α protein accumulation.57 These data suggest that hRELMβ induces the production of proinflammatory cytokine IL-6 via the IKK-β– NFκB–HIF-1 axis in HLF. Interestingly, we did not observe HIF-1α induction by hRETN in HLF (data not shown), indicating separate and distinct roles of these proteins in humans. Thus, RELMβ may play a more direct role than hRETN in matrix protein deposition during tissue and vascular remodeling by activating local fibroblasts.
In this study, HIF-1α+/− mice exhibited significantly less pulmonary vascular remodeling and PH development than did HIF-1α+/+ mice in response to HIMF injection. Additionally, a previous study showed that digoxin, a HIF-1 inhibitor, could prevent and reverse hypoxia-induced pulmonary vascular remodeling, right ventricle hypertrophy, and PH.58 HIF-1α has been reported to have a cell type-specific role in cardiovascular diseases such as cardiac hypertrophy. For example, HIF-1α in ECs likely plays a protective role,59 whereas HIF-1α in cardiomyocytes plays a pathological role in cardiac hypertrophy and dysfunction.60 Additional studies are needed to determine the cell-specific role of HIF-1α in HIMF- and hRETN-mediated right ventricular hypertrophy and failure.
In conclusion, we have shown that HIF-1α is a critical downstream mediator for HIMF-induced pulmonary vascular remodeling and development of PH. Our data show that HIMF-induced PH is mediated, at least in part, by HIF-1–VEGF-A–VEGFR2 signaling in the lung, and that HIMF acts by increasing the expression of IL-6 in both inflammatory and lung resident cells. HIMF also displayed a HIF-1α–dependent chemotactic effect on BMD cells and on BM macrophages. Because we observed HIMF-induced HIF-1α–dependent IL-6 expression in macrophages and lung resident cells, future study is needed to identify which HIF-1α–expressing cell type induces proinflammatory and immune-activating mediators in response to HIMF and its human homologs. Our results suggest that blocking the RELM protein and its downstream signaling pathway may be an effective immunosuppressive therapy for the treatment of PH in humans.
Supplementary Material
Significance.
PH is characterized by elevated pulmonary artery pressure that leads to progressive right-sided heart failure and ultimately death. The exact mechanisms that underlie PH development are unknown, but growing evidence indicates that inflammation plays a key role in triggering and maintaining pulmonary vascular remodeling. We have shown that in a mouse model of PH, HIMF administration causes PH by initiating pulmonary endothelial cell activation and mediating lung vascular inflammation. In this study, we found that HIF-1 is a critical downstream signal mediator of HIMF and that HIMF can induce IL-6, a critical proinflammatory mediator of both hypoxic inflammation and PH pathophysiology. Blocking the HIMF family protein and its downstream signaling pathway may be an effective immunosuppressive therapy for the treatment of PH in humans.
Acknowledgments
The authors thank John T. Skinner and Segun Bernard for technical assistance, and Claire F. Levine for editing the manuscript.
Sources of Funding
This work was supported by a PHA/ATS/Pfizer Research Fellowship in Pulmonary Arterial Hypertension (to K.Y.-K.), National Institutes of Health (NIH) Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases (CADET) I (P50HL107182) and CADET II (5UH2HL123827-02) grants (to R.A.J.), and an NIH Shared Instrumentation Grant S10OD016374 (to the JHU Microscope Facility).
Nonstandard Abbreviations and Acronyms
- BMD
bone marrow derived
- HIF
hypoxia inducible factor
- HIMF
hypoxia-induced mitogenic factor
- HLF
human primary lung fibroblast
- OVA
ovalbumin
- PH
pulmonary hypertension
- PMVEC
pulmonary microvascular endothelial cells
- PSMCs
pulmonary smooth muscle cells
- RBC
red blood cells
- RELMβ
resistin-like molecule β
- TIMP-1
tissue inhibitor of metalloproteinase-1
- uPA
urokinase plasminogen activator
- VEGFR
VEGF receptor
Footnotes
Disclosures
None.
References
- 1.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: Evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 2.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC, Crow MT, Johns RA. Hypoxia-induced mitogenic factor (himf/fizz1/relmalpha) induces the vascular and hemodynamic changes of pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology. 2009;296:L582–593. doi: 10.1152/ajplung.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaji-Kegan K, Su Q, Angelini DJ, Champion HC, Johns RA. Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via vegf and vegf receptor-2. American journal of physiology. Lung cellular and molecular physiology. 2006;291:L1159–1168. doi: 10.1152/ajplung.00168.2006. [DOI] [PubMed] [Google Scholar]
- 4.Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (himf/fizz1/relmalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an il-4-dependent mechanism. Journal of immunology. 2010;185:5539–5548. doi: 10.4049/jimmunol.0904021. [DOI] [PubMed] [Google Scholar]
- 5.Yamaji-Kegan K, Takimoto E, Zhang A, Weiner NC, Meuchel LW, Berger AE, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (fizz1/relmalpha) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology. 2014;306:L1090–1103. doi: 10.1152/ajplung.00279.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr, Shelton DL, Hebert CC. Fizz1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. Embo J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: Role of il-4/il-13 and mediation via stat-6. Journal of immunology. 2004;173:3425–3431. doi: 10.4049/jimmunol.173.5.3425. [DOI] [PubMed] [Google Scholar]
- 8.Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. American journal of respiratory cell and molecular biology. 2006;35:466–473. doi: 10.1165/rcmb.2006-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su QN, Zhou YF, Johns RA. Bruton’s tyrosine kinase (btk) is a binding partner for hypoxia induced mitogenic factor (himf/fizz1) and mediates myeloid cell chemotaxis. Faseb J. 2007;21:1376–1382. doi: 10.1096/fj.06-6527com. [DOI] [PubMed] [Google Scholar]
- 10.Angelini DJ, Su Q, Kolosova IA, Fan C, Skinner JT, Yamaji-Kegan K, Collector M, Sharkis SJ, Johns RA. Hypoxia-induced mitogenic factor (himf/fizz1/relm alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature. PloS one. 2010;5:e11251. doi: 10.1371/journal.pone.0011251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn TA, Barron L. Macrophages: Master regulators of inflammation and fibrosis. Seminars in liver disease. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Poloczek A, El-Haddad H, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (himf/fizz1/relmalpha) in chronic hypoxia- and antigen-mediated pulmonary vascular remodeling. Respiratory research. 2013;14:1. doi: 10.1186/1465-9921-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madala SK, Edukulla R, Davis KR, Schmidt S, Davidson C, Kitzmiller JA, Hardie WD, Korfhagen TR. Resistin-like molecule alpha1 (fizz1) recruits lung dendritic cells without causing pulmonary fibrosis. Respiratory research. 2012;13:51. doi: 10.1186/1465-9921-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. Alternatively activated macrophage-derived relm-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng X, Li D, Champion HC, Johns RA. Fizz1/relmalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1067. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- 18.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, Grunig G. Pulmonary arterial remodeling induced by a th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, Lazar MA. A family of tissue-specific resistin-like molecules. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Teng X, Hassoun PM, Yang SC, Champion HC, Tuder RM, Johns RA. Resistin-like molecule-beta in scleroderma-associated pulmonary hypertension. American journal of respiratory cell and molecular biology. 2009;41:553–561. doi: 10.1165/rcmb.2008-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: Lessons drawn from helminth infection and allergy. Journal of immunology. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuno S, Farkas L, Al Husseini A, Farkas D, Gomez-Arroyo J, Kraskauskas D, Nicolls MR, Cool CD, Bogaard HJ, Voelkel NF. Severe pulmonary arterial hypertension induced by su5416 and ovalbumin immunization. American journal of respiratory cell and molecular biology. 2012;47:679–687. doi: 10.1165/rcmb.2012-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoda LA, Semenza GL. Hif and the lung: Role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183:152–156. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood. 2011;117:5264–5272. doi: 10.1182/blood-2011-01-330720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tigges U, Hyer EG, Scharf J, Stallcup WB. Fgf2-dependent neovascularization of subcutaneous matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 26.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-csf inhibition selectively targets pathological angiogenesis and lymphangiogenesis. The Journal of experimental medicine. 2009;206:1089–1102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepetit H, Eddahibi S, Fadel E, Frisdal E, Munaut C, Noel A, Humbert M, Adnot S, D’Ortho MP, Lafuma C. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. The European respiratory journal. 2005;25:834–842. doi: 10.1183/09031936.05.00072504. [DOI] [PubMed] [Google Scholar]
- 28.Diebold I, Djordjevic T, Hess J, Gorlach A. Rac-1 promotes pulmonary artery smooth muscle cell proliferation by upregulation of plasminogen activator inhibitor-1: Role of nfkappab-dependent hypoxia-inducible factor-1alpha transcription. Thrombosis and haemostasis. 2008;100:1021–1028. [PubMed] [Google Scholar]
- 29.Menshikov M, Plekhanova O, Cai H, Chalupsky K, Parfyonova Y, Bashtrikov P, Tkachuk V, Berk BC. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:801–807. doi: 10.1161/01.ATV.0000207277.27432.15. [DOI] [PubMed] [Google Scholar]
- 30.El Kasmi KC, Pugliese SC, Riddle SR, et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. Journal of immunology. 2014;193:597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang CL, Yin LJ, Sharma S, Kierstein S, Wu HF, Eid G, Haczku A, Corrigan CJ, Ying S. Resistin-like molecule-beta (relm-beta) targets airways fibroblasts to effect remodelling in asthma: From mouse to man. Clin Exp Allergy. 2015;45:940–952. doi: 10.1111/cea.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: From cellular mechanisms to clinical phenotypes. American journal of physiology. Lung cellular and molecular physiology. 2015;308:L229–252. doi: 10.1152/ajplung.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, Lee S, Kim JY, Lee J, Yang HM, Mook-Jung I, Nam KY, Chung J, Lazar MA, Kim HS. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metabolism. 2014;19:484–497. doi: 10.1016/j.cmet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong Q, Zheng L, Lin L, Li B, Wang D, Huang C, Li D. Vegf is upregulated by hypoxia-induced mitogenic factor via the pi-3k/akt-nf-kappab signaling pathway. Respiratory research. 2006;7:37. doi: 10.1186/1465-9921-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 36.Savale L, Tu L, Rideau D, Izziki M, Maitre B, Adnot S, Eddahibi S. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respiratory research. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serag AR, Hazaa SM, Afifi IK, Ghoname NF, Serag AR. Regulated upon activation, normal t-cell expressed and secreted chemokine and interleukin-6 in rheumatic pulmonary hypertension, targets for therapeutic decisions. Eur J Cardiothorac Surg. 2010;37:853–858. doi: 10.1016/j.ejcts.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. 228p–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the vegf receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. Faseb J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama A, Kohno N, Sakai K, Kondo K, Hirasawa Y, Hiwada K. Circulating levels of soluble interleukin-6 receptor in patients with bronchial asthma. American journal of respiratory and critical care medicine. 1997;156:1688–1691. doi: 10.1164/ajrccm.156.5.9610070. [DOI] [PubMed] [Google Scholar]
- 41.Chaouat A, Savale L, Chouaid C, Tu L, Sztrymf B, Canuet M, Maitre B, Housset B, Brandt C, Le Corvoisier P, Weitzenblum E, Eddahibi S, Adnot S. Role for interleukin-6 in copd-related pulmonary hypertension. Chest. 2009;136:678–687. doi: 10.1378/chest.08-2420. [DOI] [PubMed] [Google Scholar]
- 42.Dienz O, Rincon M. The effects of il-6 on cd4 t cell responses. Clinical immunology. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diehl S, Chow CW, Weiss L, Palmetshofer A, Twardzik T, Rounds L, Serfling E, Davis RJ, Anguita J, Rincon M. Induction of nfatc2 expression by interleukin 6 promotes t helper type 2 differentiation. The Journal of experimental medicine. 2002;196:39–49. doi: 10.1084/jem.20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector th17 and regulatory t cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 45.Hirano T. Interleukin 6 and its receptor: Ten years later. International reviews of immunology. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 46.Rincon M, Irvin CG. Role of il-6 in asthma and other inflammatory pulmonary diseases. International journal of biological sciences. 2012;8:1281–1290. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan C, Meuchel LW, Su Q, Angelini DJ, Zhang A, Cheadle C, Kolosova I, Makarevich OD, Yamaji-Kegan K, Rothenberg ME, Johns RA. Resistin-like molecule alpha in allergen-induced pulmonary vascular remodeling. American journal of respiratory cell and molecular biology. 2015;53:303–313. doi: 10.1165/rcmb.2014-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang JC, Chen G, Wang SH, Barnes MA, Chung JI, Camberis M, Le Gros G, Cooper PJ, Steel C, Nutman TB, Lazar MA, Nair MG. Macrophage-derived human resistin is induced in multiple helminth infections and promotes inflammatory monocytes and increased parasite burden. PLoS Pathog. 2015;11:e1004579. doi: 10.1371/journal.ppat.1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashimoto-Kataoka T, Hosen N, Sonobe T, et al. Interleukin-6/interleukin-21 signaling axis is critical in the pathogenesis of pulmonary arterial hypertension. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E2677–2686. doi: 10.1073/pnas.1424774112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pisarcik S, Maylor J, Lu W, Yun X, Undem C, Sylvester JT, Semenza GL, Shimoda LA. Activation of hypoxia-inducible factor-1 in pulmonary arterial smooth muscle cells by endothelin-1. American journal of physiology. Lung cellular and molecular physiology. 2013;304:L549–561. doi: 10.1152/ajplung.00081.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Filkova M, Haluzik M, Gay S, Senolt L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Liu T, Baek HA, Yu H, Lee HJ, Park BH, Ullenbruch M, Liu J, Nakashima T, Choi YY, Wu GD, Chung MJ, Phan SH. Fizz2/relm-beta induction and role in pulmonary fibrosis. Journal of immunology. 2011;187:450–461. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. Fizz1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164:1315–1326. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (hif-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing p4ha1, p4ha2, and plod2 expression in fibroblasts. The Journal of biological chemistry. 2013;288:10819–10829. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. Nf-kappa b links innate immunity to the hypoxic response through transcriptional regulation of hif-1 alpha. Nature. 2008;453:807–U809. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, Sylvester JT, Semenza GL, Shimoda LA. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1239–1244. doi: 10.1073/pnas.1120385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei H, Bedja D, Koitabashi N, Xing D, Chen J, Fox-Talbot K, Rouf R, Chen S, Steenbergen C, Harmon JW, Dietz HC, Gabrielson KL, Kass DA, Semenza GL. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of tgf-beta signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E841–850. doi: 10.1073/pnas.1202081109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T, Krek W. Activation of a hif1alpha-ppargamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell metabolism. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.