Abstract
In the context of breast cancer, the importance of the skeleton in the regulation of primary tumour development and as a site for subsequent metastasis is well characterized. Our understanding of the contributions made by the host bone and bone marrow cells increasingly demonstrates the extent of the interaction between tumour cells and normal host cells. As a result, the need to develop and utilize therapies that can impede the growth and/or function of tumour cells while sparing normal host bone and bone marrow cells is immense and expanding. The need for these new treatments is, however, superimposed on the orthopaedic management of patients’ quality of life, where pain control and continued locomotion are paramount. Indeed, the majority of the anticancer therapies used to date often result in direct or indirect damage to bone. Thus, although the bone microenvironment regulates tumour cell growth in bone, cells within the bone marrow niche also mediate many of the orthopaedic consequences of tumour progression as well as resistance to the antitumour effects of existing therapies. In this Review, we highlight the effects of existing cancer treatments on bone and the bone marrow microenvironment as well as the mechanisms mediating these effects and the current utility of modern orthopaedic interventions.
The importance of the skeleton in the regulation of primary tumour development and as a site for subsequent tumour progression is well characterized1,2. Our increasing understanding of the specific contributions of host bone and bone marrow cells to cancer progression is revealing the dynamic nature of the interaction between tumour cells and normal bone cells. In fact, many currently available anticancer therapies often result in direct or indirect damage to bone and bone health1,2. As a result, therapies that preserve bone while eradicating the tumour urgently need to be developed and incorporated into clinical care. Additionally, an increased awareness of the response of the skeleton to these treatments from the perspective of the entire skeleton is essential for the development of improved therapies.
The consequences of anticancer treatment on the skeleton are primarily managed by a multidisciplinary team, whose interventions are largely aimed at the restoration of patient mobility and improved quality of life. In fact, the indications for surgery are frequently the presence of pathologic fractures or alterations in structural integrity of bone, to the extent that these manifestations elicit bone pain and/or increase the risk of fractures. With this caveat in mind, the central role of the skeleton in the development and regulation of bone metastasis, as well as the skeletal response to endocrine therapies (such as aromatase inhibitors and tamoxifen) and how these can be managed, are highlighted in this Review. Indeed, the skeletal consequences of bone metastasis from an orthopaedic perspective are largely under-represented at present and are specifically emphasized in this Review, which links orthopaedic consequences with cellular mechanisms of bone metastasis.
Bone microenvironment
The skeleton is a dynamic, multifunctional tissue responsible for a variety of processes that are fundamental to life. These processes include support, strength and mobility of the overall organism, protection of internal organs, as well as maintenance of calcium and phosphate homeostasis3. The dynamic nature of bone is a consequence of the constant removal of old or damaged bone by osteoclasts of the haematopoietic macrophage and/or monocyte lineage, followed by the formation of new mineralized bone matrix by osteoblasts of the mesenchymal lineage3. Moreover, bone is also an endocrine organ, whose cells are capable of regulating (and being regulated by) the central nervous system, energy and glucose metabolism, and gonadal function4.
Bone-forming osteoblasts and bone-resorbing osteo-clasts (and their precursors) reside in the bone marrow compartment throughout their maturation, along with other immune cells of the haematopoietic lineage, where they create stem-cell niches and microenvironments5 in which interdependence exists between haematopoietic stem-cell differentiation and mesenchymal stem cells6. In the context of cancer, the bone-marrow compartment provides favourable niches into which circulating tumour cells can migrate via blood vessels and proliferate, and where mesenchymal and haematopoietic progenitors at multiple stages of differentiation can influence, and be influenced by, circulating tumour cells during their colonization of bone1,2. Frequently, mesenchymal and haematopoietic progenitors are associated with the action of parathyroid hormone-related protein (PTHrP), a well-known regulator of tumour-associated bone destruction7, as well as the hypercalcaemia seen in many types of cancer8. Interestingly, PTHrP has also been implicated in the molecular basis of cancer cachexia, which although controversial is a negative risk factor for patient survival9.
The cells that orchestrate the complex bone remodelling process by coordinating the activities of both osteo-clasts and osteoblasts are osteocytes, which are terminally differentiated, bone-matrix-encased cells of the osteo-blastic lineage that comprise >95% of total bone cells10. Osteocytes function as mechanosensors (integrators and transducers) in bone via their dendritic processes that extend throughout the bone matrix in canaliculi, sensing the extent of mechanical load and matrix damage as well as changes in fluid flow in the surrounding canalicular fluid3,11. In addition, osteocyte canalicular fluid carries hormones, exchanges circulating factors and provides access to potential drug therapies5,11. Via gap junctions, osteocyte dendrites have direct contact with other osteocytes, osteoblasts and lining cells along the bone surface, as well as directly reaching into the bone marrow. Osteocytes also express classic osteoblast genes such as osteocalcin. Importantly, similar to both osteo-blasts and T cells, osteocytes also activate the differentiation and function of osteoclasts via the production of receptor activator of nuclear factor κB ligand (RANKL), which is the essential factor for osteoclast formation12,13.
The demonstration that osteocytes can produce RANKL has expanded the multifunctional role of the osteocyte to include the regulation of bone resorption as well as the maintenance of bone metabolism11. Osteocytes interact extensively with cells of the bone marrow microenvironment, which implicates these bone-entombed cells in modification of the bone marrow microenvironment, including that of metastatic tumour cells11. The interactions between host bone cells and the immune system are only just beginning to be unravelled14. Most immune cells, including B cells, T cells, natural killer (NK) cells, marrow-derived suppressor cells, macrophages and neutrophils, and immune cytokines (interleukins and interferons) are variably involved in modulating or fine tuning bone turnover. The emergence of the primary malignancy followed by its establishment and growth in bone and/or bone marrow is now recognized to be associated with major changes that divert immune cells from attacking to collaborating with the tumour14,15. Thus, although the bone microenvironment regulates tumour cell growth in bone, other cells within the bone marrow niche also seem to mediate many of the orthopaedic consequences of tumour progression in bone as well as resistance to the antitumour effects of current treatments.
Mechanisms of bone metastasis
The growth of disseminated tumour cells in subsequent metastatic locations is the primary cause of death in patients with cancer16. During the development of a primary tumour, incipient malignant cells undergo a series of changes, both genetic and epigenetic, that enable them to overcome cellular and host tumour suppressor mechanisms and provide the cells with the ability to escape the confines of the primary tumour site, enter the systemic circulation and eventually find a distant site in which cell survival and growth are facilitated17. Despite being the focus of intense investigation, the molecular and cellular mechanisms that regulate the metastatic spread of disseminated tumour cells (DTCs) remain largely unknown. Several studies have highlighted the early timing and high frequency of the dissemination process in many cancers. More than 50% of patients with early-stage breast cancer have DTCs in their bone marrow at the time of diagnosis18. Multiple studies have identified the molecular changes that drive metastasis to bone and other sites19–22; however, little consistency exists between the molecular targets identified. Once resident within the bone marrow microenvironment, DTCs are able to orchestrate an extensive series of molecular events that culminate in the eventual colonization of the skeleton (FIG. 1).
Figure 1. Cells in the bone and bone marrow microenvironment.
Circulating tumour cells, as well as normal circulating platelets and other normal haematopoietic progenitor cells, arrive in the bone marrow microenvironment from blood vessels in the highly vascularized bone marrow compartment (1). Tumour cells that arrive in the bone marrow are able to interact with the resident normal host haematopoietic and stromal cells to establish, maintain and survive in a bone marrow niche (2). Resident tumour cells are also able to drive the activity of resident bone-residing cells such as osteoclasts, osteoblasts and osteocytes (3). Activation of resident bone cells before, during or after the dissemination of metastatic tumour cells enhances the stimulation of osteolysis, osteoblast proliferation and even bone formation (4). These effects then provide additional growth factors and nutrients that continue the survival and progression of tumour cells as well as supporting resident bone and bone marrow cells (5). Activation of bone remodelling and normal marrow cells also results in the release of molecules that regulate the activity and function of bone cells (6). These combined events eventually result in tumour colonization of the skeleton.
Collectively, it seems that the original ‘seed and soil hypothesis’ of Paget23 has evolved and that tumour metastasis is the result of a series of collaborative interactions between tumour cells and normal host cells at both the primary and secondary tumour sites. However, DTCs can be eliminated or enter a phase of dormancy—the interval between arrival in the bone marrow and the development of overt bone metastases—that might extend from years to decades24. Cellular dormancy is believed to be mediated by attenuating phosphatidylinositol 3-kinase (PI3K)–protein kinase B (commonly known as AKT) signalling25 with out necessarily inhibiting signalling through mammalian target of rapamycin (mTOR)26, which leads to activation of autophagy and quiescence in DTCs. The microenvironment usually has a dormancy-permissive role that prevents the emergence of macrometastases by downregulating vascular cell adhesion protein 1 and periostin, and upregulating transforming growth factor β2 (TGF-β2), bone morphogenetic protein 4 (BMP-4) and BMP-7; however, the microenvironment can have a dormancy-restrictive role mediated by the opposite effects that leads to overt metastases24. The hypothetical major role of collaboration between immune cells and cancer cells at the primary site and in the bone marrow has gained momentum with the advent of novel checkpoint inhibitors that have proved very effective in redirecting immune effector cells against cancer cells, which leads to dramatic responses27. CD8+ T lymphocytes and to a lesser extent CD4+ T lymphocytes and NK cells maintain DTCs in dormancy, whereas type 2 T helper cells (TH2), macro-phages, myeloid-derived suppressor cells and regulatory T (TREG) cells facilitate immune escape and the development of overt metastases28. Some of the prodormancy effects of the immune system are related to the cytotoxic effects of the immune effector cells but others are cytostatic and are related to the promotion of cell growth arrest and angiogenic control (so-called angiogenic dormancy) via IFN-γ and tumour necrosis factor receptor 1 signalling, and the action of potent angiogenic inhibitors CXC motif chemokine 9 (CXCL9) and CXCL10 (REF. 29).
Metastatic tumours represent the greatest threat to the survival of patients with cancer. When breast cancer is diagnosed early and metastases are not present, 5-year survival is ~90%, yet once metastases are present, 5-year survival is diminished to ~10%30. Indeed, in patients who develop metastatic tumours due to aggressive tumour growth at the primary site, bone metastases are fairly uncommon. This finding does not suggest that the metastatic tumour cells do not have the ability to grow in the skeleton, but rather that they do not have the opportunity to do so1.
The homing to and colonization of the bone-marrow microenvironment and the skeleton by tumour cells that have escaped the primary tumour site and survived to reach the bone marrow is critical for the development of eventual bone metastases1 and requires tumour control of the bone and bone-marrow microenvironment. Two haematopoietic niches have been described in the bone marrow: the endosteal (or osteoblastic) niche and the vascular niche. Stromal cell-derived factor 1 (SDF-1; also known as CXCL12) is constitutively expressed by bone-marrow stromal cells such as osteoblasts, endo-thelial cells and fibroblasts, and helps retain haematopoietic stem cells (HSCs) in the bone marrow by binding CXC chemokine receptor type 4 (CXCR-4), which is expressed on HSCs31. A small number of cancer cells of breast, prostate and other cancers express CXCR-4 (REFS. 32,33), which might explain why bone metastases arise almost exclusively in regions of the skeleton containing red bone marrow. Indeed, the osteoblastic niche is responsible for maintaining HSC dormancy whereas the endothelial niche contains HSCs that have been induced to differentiate into mature haematopoietic cells31. Many cytokines, such as angiopoietin-1 and SDF-1, are secreted by osteoblasts and help retain HSCs and cancer cells in the niche and, by activating the Notch pathway, promote HSC and cancer cell ‘stemness’ and block their differentiation33. Drugs that target either CXCL12 or CXCR-4 are now used to mobilize HSCs for stem cell transplantation and in preclinical and clinical trials to ‘mobilize’ cancer cells out of their protective environment in the bone marrow, which decreases their invasive-ness and leaves them more vulnerable to chemotherapy and radiation therapy34.
The cancers that most commonly metastasize to bone are breast and prostate cancer, but many other cancers can spread to bone, including lung, kidney and thyroid30,35,36. Once resident in bone, metastatic tumour cells (as well as factors secreted by the primary tumour) have the ability to activate local bone and bone marrow cells (osteoblasts, osteoclasts, osteocytes and immune-suppressive cells), thereby facilitating the release of factors from bone that support tumour survival and proliferation1. Once activated, tumour-induced osteoclast activation and bone resorption can proceed at a rate that is dangerous for patients and results in the development of bone lesions that cause considerable pain and patient morbidity. For many of these patients, treatment goals include decreasing tumour burden and growth, preventing further metastases and inhibiting the development of tumour-associated bone complications, such as fracture, pain or hypercalcaemia. Clearly, the tumour microenvironment, which develops and changes in parallel with the primary tumour burden as well as with disseminated tumour cell content and the activation of host responses, is a central participant in the complex process of bone metastasis1,37 (FIG. 1).
Although common practice to refer to bone metastases as either osteolytic or osteoblastic, this definition is not accurate as osteolytic and osteoblastic bone lesions are but two extremes of a spectrum of activity that drives the overall destruction of bone. When bone resorption predominates with little or no new bone formation, focal bone destruction occurs and the metastases have a lytic appearance on radiographs (FIG. 2a). By contrast, bone metastases characterized by an increase in osteoblastic activity appear largely sclerotic (FIG. 2b). In particular, prostate cancer bone metastases are especially sclerotic; breast and lung tumour bone metastases also give rise to highly sclerotic lesions (FIG. 2b,c). Even though the dominant breast cancer bone metastasis lesion is lytic and destructive, a local bone formation response often exists, which presumably represents an attempt at local bone repair38 (FIG. 2d). Both bone homeostatic processes are tightly linked, and although bone turnover can be disrupted in cancer, bone resorption and formation are nevertheless always present39. In fact, the clinical measurement of bone biochemical markers has demonstrated that both bone resorption and formation can be increased in patients who have primary breast cancer but no clinical evidence of bone metastasis40–43.
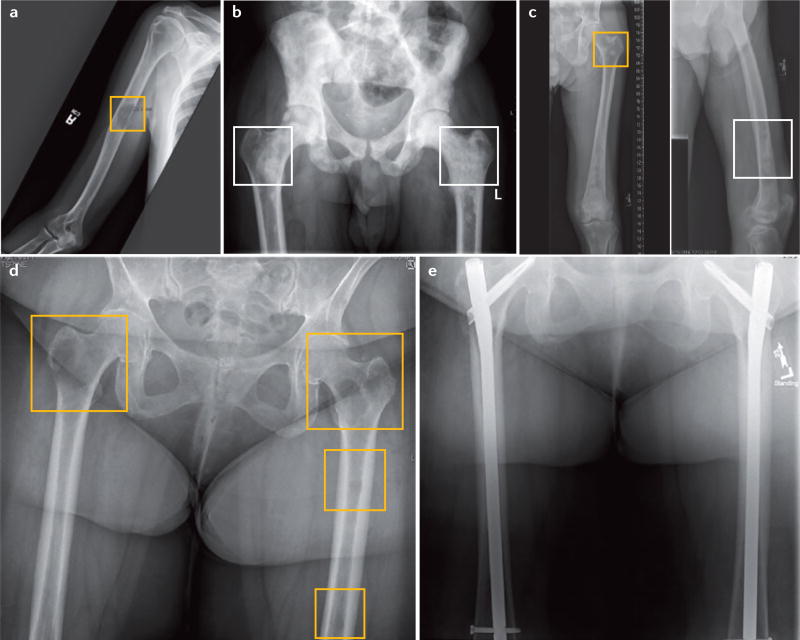
Figure 2. Presentation of lytic, blastic and mixed bone lesions.
a | Anterior–posterior radiograph of the humerus demonstrating a mid-shaft lytic lesion (yellow square), in which >50% of the cortical bone has been destroyed. The radiograph is consistent with osseous involvement by metastatic adenocarcinoma of the lung. b | Anterior–posterior radiograph of the pelvis demonstrating diffuse blastic lesions involving the pelvis and bilateral proximal femurs (white squares). The radiograph is consistent with diffuse osseous involvement by metastatic prostate cancer. c | Anterior–posterior radiograph of femur demonstrating mixed blastic (white square) and lytic lesions (yellow square) involving the proximal femur and shaft from a patient with hepatocellular carcinoma. d | Anterior–posterior radiograph of bilateral femurs demonstrating multiple lytic lesions in both femurs (yellow squares). Both femurs are at considerable risk of fracture. The radiograph is consistent with osseous involvement by metastatic breast cancer. e | Postoperative anterior–posterior radiograph of bilateral femurs (from part d) after radiotherapy and surgical treatment with intramedullary nails for prophylactic stabilization of impending fractures and improved patient quality of life.
Bone is a densely mineralized tissue with a high rigidity and modulus, which makes bone a particularly harsh environment for any tumour cell to become established in and grow44. Of note, tumour osteolysis and bone destruction, as well as tumour-induced bone formation, are entirely the result of tumour activation of resident bone cells and are not related at all to any direct action of the tumour cells themselves on the skeleton. The ability to activate resident bone and bone marrow cells is essential for tumour progression, but it is obvious that the most important phenotypic characteristic required of cancer cells to metastasize and colonize the skeleton is an ability to stimulate the activity of resident bone cells, osteoclasts and osteoblasts1,45–47. The repertoire of bone cells activated by tumour cells, once thought primarily to involve only osteoclasts and osteoblasts, must now be expanded to include matrix-embedded osteocytes11,48 along with the other components of the bone marrow1,46. Several studies have suggested that the osteocyte is a major regulator of the bone-marrow microenvironment12,13,49,50 and in the past few years these cells have been implicated in the local control of tumour proliferation51,52. That said, recognition that osteoclastic bone resorption is not only essential for normal bone remodelling53,54 but also for providing a demineralized site for tumour cell adhesion to bone matrix proteins55,56, as well as the establishment and expansion of tumour cells within the strict confines of the skeleton, is crucial.
Role of osteocytes
Several lines of evidence have demonstrated that the osteolytic process of bone metastasis involves the secretion of a plethora of tumour-cell-derived growth factors and cytokines; among these, PTHrP, TGF-β, IL-8, IL-6 and tumour necrosis factor either directly or indirectly activate the recruitment, differentiation and activity of osteoclasts and their progenitors1,2. In addition, metastatic breast cancer cells also inhibit osteoblast differentiation via the runt-related transcription factor 2 (commonly known as RUNX2)-dependent expression of sclerostin by the tumour cells57,58. Such a sequence of events disrupts the tight coupling of bone formation and bone resorption, which enables osteoclastic bone resorption to proceed unimpeded and leads to profound skeletal destruction (FIG. 2).
Osteocytes have a role in the activation and progression of osteolytic metastases in part via control of osteocyte regulation of the Wnt pathway, and by expression of dickkopf-related protein 1 (DKK-1)59 and sclerostin by tumour cells51. Multiple myeloma cells produce RANKL, increase the RANKL: osteoprotegerin ratio60 and suppress bone formation by secreting sclerostin, thus possibly overriding the ability of the osteocyte to control bone formation61. These data raise the possibility that inhibition of sclerostin action and, thus, regulation of Wnt signalling might be a potential therapeutic route for treating bone metastasis in patients with breast cancer and multiple myeloma-related bone disease57,61,62. Indeed, early clinical trials with a sclerostin antibody romosozumab in postmenopausal women with low bone mass demonstrated increased BMD and bone formation despite decreased bone resorption63,64. These promising findings suggest the potential utility of romosozumab to also increase bone mass in patients with osteolytic bone metastasis. The potential benefit of this approach will require careful study, as existing anabolic bone agents such as parathyroid hormone are contraindicated in patients with cancer65,66 and are likely to replicate the tumour progression driven by PTHrP1.
The multitude of interactions that exist between bone cells and cells in the bone marrow that are necessary to maintain endocrine homeostasis creates a variety of bone-targeted therapeutic options to treat cancer cells resident in bone. However, the need to maintain bone homeostasis and overall mass and strength of the skeleton during the necessary treatments to successfully kill tumour cells is critical. Indeed, several studies have indicated a lack of bone regeneration and/or healing after treatment of osteolytic metastases or following chemotherapy, which suggests that cancer cells (and specific treatments) might have altered the behaviour and/or differentiated activity of osteoclasts, osteoblasts and/or osteocytes67,68.
Role of microRNAs
The multiple modulatory effects of microRNAs (miRNAs) on gene expression identify them as regulatory hubs of cellular function69. Bone marrow stromal cell-derived mi RNAs can be transferred via gap junctions or exosomes to incoming cancer cells and might contribute to dormancy (Let-7c, miR-127, miR-197, miR-222, miR-223 and miR-23b) or enhance tumour progression (miR-204, miR-11, miR-379 and miR-34a), thereby providing a rationale for their use as biomarkers or therapeutic targets70–75. Once released, cell-free miRNAs circulate bound to their specific protein, Argonaute2, or to other carriers (HDL or LDL); they can also be packaged in exosomes or other microvesicles that act locally or distantly from the site of their formation and release. Circulating miRNAs are derived from both tumour and host cells and attempts at defining bone metastasis-associated signatures have proven successful. Serum from patients with breast cancer metastasis76 and animals with lung cancermeta stasis77 showed significantly increased levels of miR-16 and miR-378, and miR-326, respectively.
Managing skeletal complications of cancer
The key to managing patients with cancer-related skeletal complications is coordination between members of a multidisciplinary team of medical, surgical and radiation oncologists in addition to physical therapists and primary care physicians. In early stage cancer, the focus is on preventing metastases and treatment-related complications. In most cases of metastatic cancer, the treatment is palliative. However, in cases of oligometastatic disease, aggressive treatments can lead to prolonged remission that is similar to a cure. Regardless of the stage of cancer, the treatment is practically always a combination of cancer-directed therapy and bone-targeted therapy.
Cancer-directed therapies
After diagnosis, surgery and sometimes radiation therapy, patients are frequently offered systemic therapy (neoadjuvant or adjuvant chemotherapy, endocrine therapy or select targeted agents) with the hope that treatment will eradicate micrometastases and thereby decrease the risk of future recurrence. In the metastatic setting, patients are primarily treated with systemic chemotherapy, endocrine therapy or one of the targeted agents. In this case, achieving control of the cancer is considered a prerequisite for the healing of cancer-induced bone damage.
These treatments have been proposed to result in cure in many cases of early stage cancer, but the treatments invariably lead to marked decreases in bone integrity by either direct effects on the skeleton or indirectly via inducing gonadal failure with secondary bone deteri-oration1. In the metastatic setting, these treatments can be, and often are, associated with considerable bone destruction78,79. Chemotherapy-induced hypogonadism is frequently observed in women receiving cyclophosphamide-based chemotherapy, especially in those >40 years old (96% versus 33% in women aged 30–39 years)80. Hypogonadism is also seen in male patients receiving high cumulative doses of cisplatin for testicular cancer (>400 mg/m2) or radiation therapy for prostate cancer81,82.
Hormone-deprivation therapy is a common strategy used to control hormone-receptor-positive breast cancer and hormone-sensitive prostate cancer. Castration or gonadal suppression can be achieved by surgical, radiation or chemical means, and is frequently associated with bone loss (between 2% and 6% a year) that might in turn increase the propensity of cancer cells to grow in the bone83,84. In patients with prostate cancer, the risk of fracture increases with the number of gonadotropin-releasing hormone administrations85.
Tamoxifen, a selective estrogen receptor modulator, has a bone-preserving effect in postmenopausal women with osteoporosis86,87. Aromatase inhibitors (anastrozole, letrozole and exemestane) inhibit the conversion of androgens to estrogens in fat and muscle with an efficiency approaching 96–99% in postmenopausal women88. Due to the superiority to tamoxifen in preventing breast cancer recurrence and their lower risk of thromboembolic events and endometrial cancer, aromatase inhibitors have become the drugs of choice for postmenopausal women with hormone-receptor-positive breast cancer89. Their effects on BMD and fracture risk are greatest if treatment is started immediately after menopause and in patients with low BMD or baseline serum levels of estradiol. The increased fracture risk varies with individual agents and is between 15% and 115%90. In the adjuvant setting, complete estrogen deprivation induced by aromatase inhibitors possibly has two opposed effects on micrometastases in the bone marrow. On one side, aromatase inhibitors result in decreased proliferation or apoptosis of highly estrogen-dependent breast cancer cells. On the other side, estrogen deprivation induces high bone turnover that results in the release of many growth factors embedded in the bone, which might rescue estrogen-deprived cancer cells (insulin-like growth factors) or suppress the immune cells infiltrating and surrounding the tumour (TGF-β). The net effect is favourable in most patients, which might explain why antiresorptive therapy with bisphosphonates decreases recurrences only in postmenopausal women receiving aromatase inhibitors. This observation has been validated in an animal model91. Other systemic cancer treatments (TABLE 1) have been found to have a bone-protective effect, including the combination of axitinib and crizotinib in lung cancer, antiandrogens alone in prostate cancer and afinitor in breast cancer92–94.
Table 1.
Treatments for bone metastasis
| Drug | Mechanism of action | Indication |
|---|---|---|
| Everolimus174 | mTOR inhibition | Kidney cancer, breast cancer, pancreatic neuroendocrine tumours |
| Bevacizumab214 sorafenib215, sunitinib216 | VEGF inhibition | Colon cancer, kidney cancer, ovarian cancer, lung and liver cancers, carcinoid tumours |
| Bisphosphonates132 denosumab217,218 | Osteoclast inhibition | Breast cancer, prostate cancer, multiple myeloma, other solid tumours |
| Lapatinib219 | EGFR/ERRB2 inhibition | HER2-positive breast cancer |
| Sipuleucel-T220 ipilimumab221,222 | Immunomodulation | Prostate cancer (sipuleucel-T), melanoma (ipilimumab) |
| Gemcitabine223 pemetrexed disodium224 satraplatin225 | DNA synthesis inhibition | Solid tumours |
| Docetaxel226 cabazitaxel227 | Microtubule inhibition | Solid tumours |
| Cabozantinib166 | Tyrosine kinase inhibition (c-MET and VEGFR2) | Medullary thyroid cancer |
| 223Ra chloride104 | Alpha emitter targeting bone metastasis | Castrate-resistant prostate cancer |
| Odanacatib*160 | Cathepsin K inhibition | Osteoporosis, breast cancer |
Not yet approved.
Abbreviations: c-MET, proto-oncogene c-Met (also known as hepatocyte growth factor receptor); EGFR, epidermal growth factor receptor; ERRB2, estrogen-related receptor β; HER2, receptor tyrosine-protein kinase erbB2; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2.
Tyrosine kinase inhibitors, the newest cancer-targeting agents, have off-target class effects on cells of the osteoclast and/or osteoblast lineage, which results in a bone-sparing effect95. Mast/stem cell growth factor receptor Kit (commonly known as proto-oncogene c-KIT), platelet-derived growth factor receptor α (PDGFR-α) and PDGFR-β, macrophage colony-stimulating factor 1 receptor (commonly known as M-CSF-R), proto-oncogene tyrosine-protein kinase Src (commonly known as SRC) and rearranged during transfection (RET) induce osteoclastogenesis when stimulated by their respective ligands95. The net effect of tyrosine kinase inhibition depends on the degree of inhibition of osteoclasts and osteoblasts. In all of these cases, tyrosine kinase inhibitors have demonstrated a bone-building effect95. mTOR inhibitors have a dual effect on the cancer and osteoclasts, which results in a net bone-sparing effect.
In addition to the possibility of inducing hypo-gonadism, radiation therapy might also directly damage bone, which results in insufficiency fractures that are common in the pelvis in gynaecological, prostate, anal and rectal cancers, and in the ribs96. The development of an expanding series of radiopharmaceuticals might help to alleviate some of these important limitations.
Skeletal-related events
Regardless of their type, bone lesions compromise the integrity of the bone and result in many skeletal complications, so-called skeletal-related events (SREs)97. These events include pathologic fractures, cord compression, the need for surgery, the need for radiation therapy and hypercalcaemia1. Bone pain is very common even when not associated with fractures98. The morbidity associated with SREs is evident and their treatment undoubtedly improves patient quality of life; however, their effect on survival (hinted at from population-based cohort studies99) has not been confirmed in randomized controlled trials (RCTs), which showed no improvement in overall survival even though the number of SREs was considerably reduced as a result of treatment100–102. Some investigators have questioned the validity of SREs as an end point for assessing the benefit of bone-modifying agents and have suggested the use of symptomatic skeletal-related events (SSEs) as a more clinically relevant end point103. Two trials evaluating the effect of 223Ra and denosumab (a human monoclonal antibody that sequesters RANKL) in prostate cancer104,105 demonstrated that the experimental agents were better than the controls (placebo and zoledronic acid, respectively) at reducing the risk of SSEs.
Assessment of response
Different imaging methods are used to diagnose bone metastases and to assess the response to treatment. Although plain films and bone scans are acceptable modalities for the initial diagnosis of bone lesions or of their disappearance following treatment, they are inadequate to assess the response to treatment. Biochemical markers of bone resorption (serum C-terminal telopeptide [CTX], urinary N-terminal telopeptide [NTX] and others) and bone formation (bone-specific alkaline phosphatase [BSAP], serum N-terminal propeptide of type 1 collagen [PINP] and others) have been proposed for the diagnosis, prognosis and monitoring of bone metastases106,107. Their role in routine practice has yet to be defined with certainty, but a study evaluating the role of bone markers in the future selection of patients who might benefit from certain specific therapies is ongoing108. In addition, cross-sectional imaging techniques (CT or MRI) are considered adequate modalities to measure lytic or mixed lesions with a soft tissue component109.
Osteoclast-targeted agents
Bisphosphonates and RANKL inhibitors
The role of bisphosphonates in the treatment of cancer-related bone loss is well-established1. Much has been written regarding the inhibition of osteoclastic bone resorption as the primary treatment of choice for bone metastasis in major solid tumours and multiple myeloma. The primary mechanism of action for nitrogen-containing bisphosphonates is inhibition of the mevalonate pathway, which induces cytotoxic effects on osteoclasts, to inhibit tumour-induced bone destruction and tumour growth16,110,111. In addition to their classic action on osteoclasts, bisphosphonates have also been suggested to enhance γδ T-cell proliferation, to inhibit tumour-associated macrophages and myeloid suppressor cells and to exert a potential antiangiogenic effect111–117. However, the exact mechanisms responsible for the reported nonbone effects of bisphosphonates remain controversial and the focus of ongoing investigations110.
Early stage cancer
Bisphosphonates and denosumab have been used to prevent treatment-related bone loss and fractures in breast and prostate cancer. Using different end points, two large RCTs demonstrated that the use of zoledronic acid or denosumab in women with early stage breast cancer who were receiving endocrine therapy increased BMD118 or decreased risk of fractures119, respectively. Smaller RCTs with ibandronate and risedronate demonstrated similar benefits120,121. Likewise, the use of pamidronate in patients with prostate cancer undergoing androgen-deprivation therapy proved to be effective in preventing bone loss122, whereas treatment with denosumab resulted in gains in BMD and a considerably decreased the risk of vertebral fractures123.
Bisphosphonates have been extensively investigated for the prevention of breast cancer recurrence in patients receiving endocrine therapy118. The Austrian Breast and Colorectal Cancer Study Group trial 12 (ABCSG-12 [REF. 124]) reported a significant benefit from the addition of zoledronic acid to endocrine therapy with a relative reduction of risk of disease recurrence of 36% (absolute risk reduction of 3.2%; P = 0.01). This study enrolled premenopausal women who underwent ovarian suppression before being randomly assigned to receive either tamoxifen or anastrozole. Two large subsequent trials reached different conclusions using zoledronic acid in hormone-receptor-positive patients with breast cancer. The ZO-FAST trial118 (comparing early versus late use of zoledronic acid in postmenopausal women with breast cancer receiving letrozole) showed a statistically significant improvement in disease-free survival (by 34%) in patients who received zoledronic acid early, thus confirming the results of the ABCSG-12 trial. However, an even larger study (AZURE) showed no benefit from adding zoledronic acid to endocrine therapy in premenopausal and postmenopausal women125. The majority (55%) of patients enrolled in this study were postmenopausal and 20% of all patients had hormone-receptor-negative breast cancer. Adjuvant oral clodronate trials in patients with early stage breast cancer produced similar conflicting results. The incidence of bone metastases was significantly decreased (by 41%) in patients who received clodronate daily for 2 years126. The effect was more pronounced in patients with stage II or III disease (51% decrease). However, the NSABP B34 study, which used clodronate daily for 3 years in a similar population, showed no benefit from the addition of clodronate127 to standard therapy. These conflicting results are intriguing. Subset analyses of the negative trials showed that postmenopausal women consistently derived considerable benefits from the inclusion of a bisphosphonate in their treatment. Possibly, osteoclast-targeted therapy only helps in the context of extreme hormonal deprivation, such as in postmenopausal women receiving aromatase inhibitors.
Two randomized studies in patients with prostate cancer with no systemic metastases using zoledronic acid failed to show improvement of bone-metastases-free survival128–130. By contrast, patients with castrate-resistant prostate cancer who were given denosumab showed improvement of bone-metastasis-free survival from a median of 25.2 months on placebo to a median of 29.5 months on denosumab without any difference in overall survival131.
Treatment of bone metastases
In 2012, 34 RCTs of different bisphosphonates in the treatment of metastatic breast cancer were reviewed132. Interestingly, all bisphosphonates resulted in a significant reduction in SREs (~15%) in the treatment arms and this effect was most pronounced for intravenous zoledronate, pamidronate and ibandronate, with risk reductions of 41%, 23% and 20%, respectively, observed. Although the reduction in SREs was significant and profound, no improvement in patient survival was noted in either the metastatic or adjuvant settings. Similarly, the analysis suggested no overall benefit from the addition of a bisphosphonate to standard adjuvant treatments for early breast cancer132. However, the use of bisphosphonates (in particular zoledronic acid) does reduce bone metastases and improve disease outcome, especially in the case of women with established menopause133.
Bone-modifying agents have been used for preventive intent in patients with advanced-stage prostate cancer who did not have bone metastasis and for therapeutic purposes in those with bone metastases. The efficacy of this type of agent was only proven in patients with castrate-resistant prostate cancer with bone metastases134. Clinica l trials using first and second-generation biosphosphonates such as clodronate and pamidronate did not show any statistically significant decrease in SREs135. In the past decade, zoledronic acid has emerged as the only bisphosphonate with clinical efficacy superior to placebo in RCTs136. In a three-arm RCT of placebo and two doses of zoledronic acid (4 mg and 8 mg every 3–4 weeks, administered intravenously over 15 min), a statistically significant improvement was found in the incidence of SREs with 4 mg zoledronic acid compared with placebo, as well as an improved pain and analgesic score137. The 8 mg dose was not more efficacious and was more frequently associated with renal complications than the 4 mg dose137,138; consequently, zoledronic acid is currently used at a dose of 4 mg, given every 3–4 weeks. In another noninferiority randomized placebo-controlled multicentre study of monthly intravenous injections of 4 mg zoledronic acid versus subcutaneous injections of 120 mg denosumab in patients with castrate-resistant prostate cancer, the denosumab group had a significantly improved time to first on-study SRE, demonstrating both noninferiority and superiority compared with zoledronic acid139.
RCTs comparing zoledronic acid and denosumab have also been performed in patients with breast cancer110. When directly compared, denosumab treatment improved the median time to first SRE (zoledronic acid: 26.4 months, denosumab: not reached), superiority in the prevention of subsequent SREs (23% risk reduction) and a 4 month delay in progression to moderate and/or severe disease in patients with breast cancer139,140.
Practical considerations
Current recommendations are to initiate treatment with a bone-modifying agent at the time of diagnosis of bone metastases in patients with breast or prostate cancer regardless of the presence of symptoms141. The choice of agent (denosumab or zoledronic acid) is left for the attending physician to decide. However, patients with abnormal kidney function might be selected to receive denosumab upfront whereas those who experienced severe acute phase systemic reaction to zoledronic acid might be switched to denosumab. Zoledronic acid is contraindicated in patients with creatinine clearance <30 ml/min. Hypocalcaemia is more common with denosumab than with zoledronic acid and should be treated with calcium supplements and vitamin D. Financial considerations can have a role as cost-effectiveness analyses show no superiority of denosumab over zoledronic acid141.
The recommended duration of treatment is 2 years in patients who have achieved stable disease status or in those with worsening kidney function78. Guiding the treatment by levels of bone turnover markers is inadequate142. De-escalation studies (OPTIMIZE-2 and others) have shown that switching to every 12-week dosing resulted in a similar rate of SREs, time to first-on-study SRE and mean skeletal morbidity rates (SMRs). However, follow up was limited to 1 year, which made making final conclusions all the more difficult, as levels of bone turnover markers increased on every 12-week schedule143.
Osteoblast-targeted agents and other novel agents
Radiopharmaceuticals
Radiopharmaceuticals currently available to treat painful bone metastases include 89Sr, 153Sm and 223Ra dichloride. These agents have a special avidity for osteoblasts144–146 and are all calcium and phosphorus analogues that accumulate in areas with high osteoblastic activity following intravenous administration. Radiopharmaceuticals are administered intravenously and are used to treat multiple lesions simultaneously. As their penetration is somewhat limited, they spare important adjacent structures. Several RCTs have been conducted with these agents, which demonstrated a slightly lower (but significant) benefit for 89Sr than for 153Sm (REF. 147) with 30–40% of patients achieving complete pain control and 40–50% achieving partial pain control148. Pain control was improved with the addition of bisphosphonates (50–65% achieved complete pain relief and 20–30% achieved partial pain relief)148. 223Ra was approved by the FDA in 2013 for use in patients with castrate-resistant prostate cancer with painful bone metastases. In a large phase III study (809 patients with castrate-resistant prostate cancer and bone metastases), all patients received best standard of care and were randomly assigned in a 2:1 ratio to receive 223Ra or placebo104,149,150. Median overall survival was extended from 11.2 months in the placebo arm to 14.9 months in the 223Ra arm and this benefit was associated with improved time to first SRE104,149,150. Adverse effects are manageable and myelosuppression with leukopenia, thrombocytopenia and lymphopenia is possible but mild104,149,150. Durable bone marrow failure with persistent pancytopenia is rare (~2%)104,149,150.
Patients eligible for radiopharmaceutical therapy are those with multiple bone metastases and a demonstrated increased tracer uptake on bone scintigraphy151. A transient ‘flare response’ with exacerbation of pain might happen a few days after administration in certain patients requiring analgesics and steroids. In addition, myelosuppression can also occur with a nadir (low point in blood cell count) at 3–6 weeks followed by a recovery 3–6 weeks later151.
Endothelin-1 receptor inhibitors
The endothelin ligands (endothelin-1 [ET-1], ET-2 and ET-3) and their cognate membrane receptors (endothelin-1 receptor [ETA-R] and endothelin-2 receptor [ETB-R]) have emerged as therapeutic targets for prostate cancer152. Endothelins are pleiotropic factors that activate multiple downstream signalling pathways, which leads to osteoblast differentiation and activation in bone153. Several large phase III studies with ETA-R and/or ETB-R inhibitors (zibotentan and atrasentan) in nonmetastatic and meta-static castrate-resistant prostate cancer and in metastatic ovarian cancer were completed after promising preclinical studies. Unfortunately, none of these trials showed a statistically significant benefit154–157, which led to the discontinuation of the development of these drugs.
Cathepsin K inhibitors
Cathepsin K is the primary protease secreted by osteoclasts and has a major role in bone resorption by degrading the organic bone matrix158. Cathepsin K inhibition with odanacatib in animal models of estrogen-deficient bone loss as well as in women with osteoporosis resulted in increased bone mass and decreased levels of bone turnover markers158,159. When used in patients with breast cancer and bone metastases in a double-blind study, odanacatib induced a similar decrease in levels of urinary NTX to that resulting from zoledronic acid treatment160.
MET and VEGFR2 inhibitors
The proto-oncogene c-MET (MET; also known as hepatocyte growth factor receptor), vascular endothelial growth factor A and hepatocyte growth factor are involved in the interaction between the tumour and bone microenvironment161–164. Cabozantinib, a dual MET–vascular endothelial growth factor receptor 2 inhibitor, has activity against osteoclasts and osteoblasts, as well as cancer cells165. Cabozantinib was tested in heavily pretreated patients with metastatic breast and castrate-resistant prostate cancer and in both cases the drug was effective in decreasing tumour burden, as assessed by improving bone scans and levels of bone biomarkers, as well as decreased pain, which resulted in decreased analgesic use166.
mTOR inhibitors
Mutations in the PI3K–AKT–mTOR pathway are common in patients with breast cancer167. mTOR has been implicated in resistance to endocrine therapy168 as well as the survival and differentiation of osteoclasts169,170. Hence, inhibition of this pathway is a logical strategy to target both the cancer and the bone disease171–173. The BOLERO-2 trial compared the combination of exemestane (an aromatase inhibitor) and everolimus (an mTOR inhibitor) to exemestane + placebo in patients with metastatic breast cancer after progression on nonsteroidal aromatase inhibitors and showed superior progression-free survival in the exemestane + everolimus arm for the whole population and for patients with only bone disease174. The bone subanalysis of the BOLERO-2 trial assessed clinical outcomes and levels of bone markers at 6 weeks and 12 weeks174. Compared with baseline, levels of bone makers (BSAP, P1NP and CTX) at 6 weeks and 12 weeks decreased in the exemestane + everolimus arm and increased in the exemestane + placebo arm. The difference was statistically significant regardless of the presence of bone metastases or prior bisphosphonate therapy at baseline, which supports a direct effect of everolimus on the bone independent of its effect on the cancer.
Inhibition of Wnt signalling
The Wnt signalling pathway is a highly conserved pathway that controls multiple cellular processes, including cell fate determination, proliferation and differentiation175. Numerous studies have implicated Wnt pathway activation in tumour development in both rodents and humans, as well as in tumour progression in bone58,176,177. A critical activity for the Wnt signalling pathway involves activation of the osteoblastic lineage and the maintenance of bone homeostasis. Studies using genetically engineered mice have convincingly shown that downstream effector molecules in the canonical Wnt pathway (for example, β-catenin) as well as endogenous inhibitors of Wnt signalling (such as DKK-1 and sclerostin) are important and direct regulators of osteoblasts that can also indirectly influence osteoclasts178,179.
With the ongoing clinical development of sclerostin antibodies (romosozumab), the Wnt pathway evidently represents a new therapeutic opportunity that justifies investigation. However, as Wnt signalling is a driver of both tumorigenesis and osteoblastic bone formation180, how the Wnt pathway should be targeted and modulated as a potential bone metastasis therapy is not entirely known. Stimulation of Wnt signalling via inhibition of DKK-1 or inhibition of β-catenin increased bone formation in osteolytic lesions and decreased osteolysis and pain59,181–183. Other investigators have shown that DKK-1-mediated inhibition of Wnt signalling promotes tumour proliferation and metastasis184–188. Indeed, the extent of Wnt signalling in both the primary tumour and skeletal metastasis microenvironment seems to be tightly controlled and perhaps even specific for individual tumours. The potential utility of Wnt inhibitors in the context of treating bone metastasis, thus, remains unclear and tumours in which Wnt signalling is upregulated might represent the best potential use of Wnt inhibitors. However, concerns regarding the specificity and efficacy of these agents as well as the determination of the extent of Wnt signalling upregulation will require close scrutiny and careful trial design to ensure maximal patient benefit.
Surgery
The majority of patients with metastatic bone disease do not require operative intervention. However, when utilized, surgery is primarily palliative with the primary objective to decrease pain and improve overall function. Appropriate work-up should precede surgery to establish the diagnosis of metastatic cancer, its type and address possible complications (for example, hypercalcaemia)189,190. Indications for surgery are pathologic fractures191 and apparent alterations in the structural integrity of bone, to the extent that these elicit bone pain and/or increase the risk of fractures192. The early identification and treatment of these potential fractures provides many important clinical advantages (FIG. 2d,e). Early identification enables elective surgery and provides an easier recov-ery193–195. Currently, Mirel’s scoring system192 (based on lesion size, location, radiographic appearance and pain) for impending fractures is the most commonly utilized method for determining fracture risk in long bones and for which the results have been validated196. For solitary bone metastases (such as renal and thyroid), evidence exists supporting the value of surgical resection given the potential for improved patient survival193,195,197,198 (with or without cemented prosthetic reconstructions193,194) to improve surgical stability and immediate strength, which facilitates early weight-bearing and loco-motion193–195. Once wound healing has been achieved, radiotherapy is usually performed199. Improved functional status and implant survival has been demonstrated in patients under going surgical stabilization followed by postoperative radiation200.
The case of spine metastases
Treatment goals for spine metastases are the maintenance or re-establishment of neurologic function, pain control and cancer control. Several scoring systems exist to assess eligibility for open surgery201–203. When open surgery is not indicated, vertebroplasty and/or kyphoplasty have shown excellent pain control with minimal morbidity204. Recommendations for the management of patients with metastatic spinal disease were reviewed in 2014 (REF. 205). Following diagnosis, radiosensitive tumours (such as multiple myeloma, small cell lung cancer and lymphoma) benefit from primary radiation therapy, with or without vertebroplasty or kyphoplasty. In the case of hormone-sensitive tumours (breast and prostate) with no spinal cord compression or spinal instability, hormonal therapy is the first-line treatment with or without vertebroplasty or kyphoplasty. Finally, radioinsensitive tumours (non-small cell lung cancer, melanoma, thyroid and renal cell cancer) benefit from surgery followed by radiation therapy206. Early decompression in cases of cord compression is considered the appropriate approach for select patients.
Radiation therapy and pain management
Multiple modalities are used to deliver palliative radiation to treat cancer-related bone pain207. External beam radiation therapy remains the primary modality for the treatment of painful bone metastases, can be administered in single or multiple fractions, and can be either traditional, intensity-modulated radiation therapy or stereotactic body radiation therapy207. Indeed, radiation has been demonstrated to be effective in relieving pain in the majority of patients with metastatic carcino-mas208. The type and amount of radiation administered for metastatic bone disease are in general the same as for other sites of metastasis199,208. Stereotactic body radiation therapy and intensity-modulated radiation therapy have been adapted for spinal metastases with the goal of delivering radiation more effectively at higher doses and decreasing risk to important structures such as the spinal cord208,209. Most commonly, radiotherapy is administered in multiple fractions in the USA, but single-dose treatments have been demonstrated to be equally effective in spinal metastases208.
Guidelines developed by the American Society of Radiation Oncology in 2011 recommend a single 8-Gy fraction for targeted bone lesions210. This dose provides the same pain relief with added convenience to the patient and caregiver, but is associated with an increased need to re-treat the same area (in 20% of cases with single dose treatment versus 8% for multifractionated radiation therapy)210. In addition, surgery, kyphoplasty and/or vertebroplasty, bisphosphonate use and prior radiopharmaceuticals are not contraindications to, nor do they obviate the need for, external beam radiation therapy210. If radiation therapy is deemed necessary after surgery, it should be multifractionated due to the lack of data on single-fraction radiation therapy in this setting. The major limitations of radiation therapy are the presence of multiple symptomatic metastases and/or the proximity of the metastases to critical structures207. The development of an expanding series of radio-pharmaceuticals might help to alleviate some of these important limitations.
Conclusions
As discussed in detail in this Review, the effect of cancer cells and our current treatment modalities (both chemical and surgical) on the skeleton are complex. The fundamental role that bone has in the development and progression of primary tumours and subsequent bone metastases might explain why identifying agents that can eliminate tumour cells while leaving the skeleton intact is proving a challenge.
What does seem abundantly obvious is that the bone marrow microenvironment has a critical role in protecting metastatic and disseminated tumour cells, along with resident normal cells, during the development of subsequent bone metastases. This concept should also be extended to include the osteocyte, which is in close contact with the bone marrow11, and not simply focus on resident components of the bone marrow. Indeed, although the interaction between tumour cells and bone resorbing osteoclasts is crucial for tumour progression, interactions between tumour cells and osteo-cytes, as well as with bone marrow cells resident in the microenvironment, are probably equally important.
Once cancer cells have reached the bone marrow niche, they become exposed to influences that could result either in their eradication, dormancy or continued proliferation. High rates of bone turnover and an exhausted immune system28, as well as a high proliferative drive (due to raised levels of estrogen, increased estrogen sensitivity or oncogenic gain-of-function mutations) mediate growth of the tumour in bone, whereas the opposite leads to dormancy. Cancer treatments have both direct and indirect effects on these components. If the treatment increases bone turnover (for example, aromatase inhibitors) or suppresses the immune system (for example, chemotherapy), it might result in loss of some or all of the anticancer effects. Conversely, treatments that decrease bone turnover or reprime the immune system against the cancer might augment anticancer efficacy, while maintaining bone health. In any case, these concerns must be considered in the struggle to identify and develop new anticancer therapies.
If these ideas are correct, then current bone metastasis treatment approaches that focus almost entirely on targeting osteoclasts and their bone resorption activity require substantial revision. As discussed here, the number of anticancer therapies that, in addition to having profound antitumour efficacy, can also negatively affect the skeleton is increasing; a result that could have dire consequences for patients. The oncology field has responded to the treatment-associated negative effects on bone with a plethora of new and evolving targets that have the potential to individualize treatment and preserve bone mass and strength, while maintaining profound antitumour effects.
Perhaps not surprisingly, identification of the fundamental contribution of the osteocyte to the control of the coupled activities of bone resorption and bone formation that are the basis of normal bone physiology13,54 has fostered increased interest in the role of these cells in tumour progression and metastasis. In our view, refining the focus on characterizing the normal cellular mechanisms linking the control of bone resorption and bone formation, and how these processes are disrupted by the formation of osteolytic and/or osteoblastic bone lesions is essential.
Despite incomplete understanding, the treatment of patients with cancer to prevent bone metastases or those with bone metastasis is ongoing and evolving1,46. Many questions remain unanswered. For instance, is the ‘omics’ revolution going to produce appropriate tools to identify patients who will probably develop bone metastases? This question is important because not all patients are going to develop bone metastasis and, therefore, identifying those at the highest risk will increase the likelihood that a treatment will be effective. Will an understanding of the molecular mechanisms of cancer cell homing to the bone marrow facilitate eradicating dormant cancer cells without damaging HSCs? What is the mechanism of bone-modifying agents’ failure in the metastatic setting? The short answer to this question might be the failure of the anticancer treatment. However, a possibility exists that the failure might be the result of a treatment that does not match the primary mechanism by which the cancer drives bone disease (TABLE 1). Hence the question: is there a possibility to identify the principle underlying mechanism for every cancer and match the disease with the appropriate treatment? As bone-modifying agents have different mechanisms of action, is there a possibility that combination therapy might be more effective than single agents alone? Also, is there an optimal way to order the delivery of these agents such that patients benefit from all of them? For how long should we use bone-modifying agents? And lastly, what is the best way to monitor the treatment and what laboratory tools or imaging techniques can be used to assess the efficacy of these agents beyond what is currently possible? Normal cells of the bone and bone marrow significantly affect the survival and activation of tumour cells. Indeed, in the case of multiple myeloma, but not yet demonstrated for solid tumour bone metastasis, a positive osteoblastic response is evidently capable of inducing remission and might be an effective treatment option211–213.
Increasing evidence also supports the idea that particular disease parameters, such as DTCs, might explain cancer recurrences. Therefore, further elucidation of the features of bone and bone marrow cells responsible for the survival of these cells represent valid therapeutic targets with the potential to improve patient care. We remain optimistic that, as the mechanisms driving bone metastases are identified, new and effective therapies selectively targeting tumour progression in bone and the associated activation of bone destruction, while leaving resident bone and bone marrow cells largely untouched, will be developed. Such treatment regimens would have profound consequences for preventing the emergence of bone metastases and for the quality of life of patients with metastatic cancer, as the skeleton would be protected and capable of sustaining the activities of daily living for which it has so elegantly evolved.
Key points.
Metastatic tumours represent the greatest threat to the survival of patients with cancer
The development of therapies that impede the growth and/or function of tumour cells, while sparing normal host cells, is critical to improving the care of patients with cancer
In the case of bone metastases, cells within the bone marrow niche mediate many of the orthopaedic consequences of tumour progression as well as resistance to the antitumour effects of existing therapies
Osteocytes have a key role in the activation and progression of osteolytic metastases
Acknowledgments
The authors’ work on the mechanisms driving tumour progression and the development of bone metastases is supported by the NIH (grant R01 CA166060 to L.J.S.) and the Carl L. Nelson Endowed Chair in Orthopaedic Creativity (L.J.S.).
Footnotes
Author contributions
I.M., C.O.M., D.G. and L.J.S. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Competing interests
The authors declare no competing interests.
References
- 1.Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roodman GD. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 3.Suva LJ, Gaddy D, Perrien DS, Thomas RL, Findlay DM. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr. Osteoporos. Rep. 2005;3:46–51. doi: 10.1007/s11914-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 4.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin T, Li L. The stem cell niches in bone. J. Clin. Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ying H, et al. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone β receptor inhibits mitotic progression. J. Clin. Invest. 2006;116:2972–2984. doi: 10.1172/JCI28598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guise TA, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Invest. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mundy GR, Edwards JR. PTH-related peptide (PTHrP) in hypercalcemia. J. Am. Soc. Nephrol. 2008;19:672–675. doi: 10.1681/ASN.2007090981. [DOI] [PubMed] [Google Scholar]
- 9.Kir S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonewald LF. Osteocyte messages from a bony tomb. Cell Metab. 2007;5:410–411. doi: 10.1016/j.cmet.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell and more. Endocr. Rev. 2013;34:658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong J, et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakashima T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 14.Mori G, D’Amelio P, Faccio R, Brunetti G. Bone-immune cell crosstalk: bone diseases. J. Immunol. Res. 2015;2015:108451. doi: 10.1155/2015/108451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roato I. Interaction among cells of bone, immune system, and solid tumors leads to bone metastases. Clin. Dev. Immunol. 2013;2013:315024. doi: 10.1155/2013/315024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman RE, Gregory W, Marshall H, Wilson C, Holen I. The metastatic microenvironment of breast cancer: clinical implications. Breast. 2013;22(Suppl. 2):S50–S56. doi: 10.1016/j.breast.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Klein CA. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 19.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 21.Minn AJ, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Invest. 2005;115:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bendre MS, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002;62:5571–5579. [PubMed] [Google Scholar]
- 23.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 24.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jo H, Jia Y, Subramanian KK, Hattori H, Luo HR. Cancer cell-derived clusterin modulates the phosphatidylinositol 3’-kinase-Akt pathway through attenuation of insulin-like growth factor 1 during serum deprivation. Mol. Cell. Biol. 2008;28:4285–4299. doi: 10.1128/MCB.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schewe DM, Aguirre-Ghiso JA. ATF6α -Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc. Natl Acad. Sci. USA. 2008;105:10519–10524. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani S, Wang C, Wu K, Francis R, Pestell R. Cyclin-dependent kinase inhibitors: novel anticancer agents. Expert Opin. Investig. Drugs. 2000;9:1849–1870. doi: 10.1517/13543784.9.8.1849. [DOI] [PubMed] [Google Scholar]
- 28.Romero I, Garrido F, Garcia-Lora AM. Metastases in immune-mediated dormancy: a new opportunity for targeting cancer. Cancer Res. 2014;74:6750–6757. doi: 10.1158/0008-5472.CAN-14-2406. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Hermelink N, et al. TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol. Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Sheridan C, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol. Int. 2010;60:497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 34.Cojoc M, et al. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Onco. Targets. Ther. 2013;6:1347–1361. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon X, Quer M, Orus C, de Dios E, Recher K. Treatment of neck nodes after induction chemotherapy in patients with primary advanced tumours. Eur. Arch. Otorhinolaryngol. 2000;257:521–525. doi: 10.1007/s004050000277. [DOI] [PubMed] [Google Scholar]
- 36.Patten RM, Shuman WP, Teefey S. Metastases from malignant melanoma to the axial skeleton: a CT study of frequency and appearance. AJR Am. J. Roentgenol. 1990;155:109–112. doi: 10.2214/ajr.155.1.2112830. [DOI] [PubMed] [Google Scholar]
- 37.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart AF, et al. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J. Clin. Endocrinol. Metab. 1982;55:219–227. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- 39.Huang Q, Ouyang X. Biochemical-markers for the diagnosis of bone metastasis: a clinical review. Cancer Epidemiol. 2012;36:94–98. doi: 10.1016/j.canep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Clin. Orthop. Relat. Res. 2003;415(Suppl.):S138–S147. doi: 10.1097/01.blo.0000092979.12414.54. [DOI] [PubMed] [Google Scholar]
- 41.Lipton A, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 42.Coleman R, et al. Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat. Rev. 2008;34:629–639. doi: 10.1016/j.ctrv.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Washam CL, et al. Identification of PTHrP(12–48) as a plasma biomarker associated with breast cancer bone metastasis. Cancer Epidemiol. Biomarkers Prev. 2013;22:972–983. doi: 10.1158/1055-9965.EPI-12-1318-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin TJ. Manipulating the environment of cancer cells in bone: a novel therapeutic approach. J. Clin. Invest. 2002;110:1399–1401. doi: 10.1172/JCI17124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 46.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat. Rev. Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganguly SS, Li X, Miranti CK. The host microenvironment influences prostate cancer invasion, systemic spread, bone colonization, and osteoblastic metastasis. Front. Oncol. 2014;4:364. doi: 10.3389/fonc.2014.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonewald LF. The amazing osteocyte. J. Bone Miner. Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suva LJ. Sclerostin and the unloading of bone. J. Bone Miner. Res. 2009;24:1649–1650. doi: 10.1359/jbmr.090815. [DOI] [PubMed] [Google Scholar]
- 50.Wijenayaka AR, et al. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE. 2011;6:e25900. doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Compton JT, Lee FY. A review of osteocyte function and the emerging importance of sclerostin. J. Bone Joint Surg. Am. 2014;96:1659–1668. doi: 10.2106/JBJS.M.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou JZ, et al. Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncogene. 2015;34:1831–42. doi: 10.1038/onc.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henriksen K, Karsdal MA, Martin TJ. Osteoclast-derived coupling factors in bone remodeling. Calcif. Tissue Int. 2014;94:88–97. doi: 10.1007/s00223-013-9741-7. [DOI] [PubMed] [Google Scholar]
- 54.Xiong J, O’Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. J. Bone Miner. Res. 2012;27:499–505. doi: 10.1002/jbmr.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyde A, Jones SJ, Binderman I, Harell A. Scanning electron microscopy of bone cells in culture. Cell Tissue Res. 1976;166:65–70. doi: 10.1007/BF00215125. [DOI] [PubMed] [Google Scholar]
- 56.Jones SJ, Boyde A. Morphological changes of osteoblasts in vitro. Cell Tissue Res. 1976;166:101–107. doi: 10.1007/BF00215129. [DOI] [PubMed] [Google Scholar]
- 57.Gkotzamanidou M, et al. Sclerostin: a possible target for the management of cancer-induced bone disease. Expert Opin. Ther. Targets. 2012;16:761–769. doi: 10.1517/14728222.2012.697154. [DOI] [PubMed] [Google Scholar]
- 58.Mendoza-Villanueva D, Zeef L, Shore P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFβ-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011;13:R106. doi: 10.1186/bcr3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian E, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 60.Sezer O, Heider U, Zavrski I, Kuhne CA, Hofbauer LC. RANK ligand and osteoprotegerin in myeloma bone disease. Blood. 2003;101:2094–2098. doi: 10.1182/blood-2002-09-2684. [DOI] [PubMed] [Google Scholar]
- 61.Coluzzi F, Di Bussolo E, Mandatori I, Mattia C. Bone metastatic disease: taking aim at new therapeutic targets. Curr. Med. Chem. 2011;18:3093–3115. doi: 10.2174/092986711796391660. [DOI] [PubMed] [Google Scholar]
- 62.Terpos E, et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int. J. Cancer. 2012;131:1466–1471. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- 63.McClung MR, Grauer A. Romosozumab in postmenopausal women with osteopenia. N. Engl. J. Med. 2014;370:1664–1665. doi: 10.1056/NEJMc1402396. [DOI] [PubMed] [Google Scholar]
- 64.McClung MR, et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 65.Barbehenn EK, Lurie P, Wolfe SM. Osteosarcoma risk in rats using PTH 1–34. Trends Endocrinol. Metab. 2001;12:383. doi: 10.1016/s1043-2760(01)00489-1. [DOI] [PubMed] [Google Scholar]
- 66.Okazaki R. Osteosarcoma in rats receiving long-term PTH injection [Japanese] Clin. Calcium. 2003;13:42–44. [PubMed] [Google Scholar]
- 67.Martinez LM, et al. Changes in the peripheral blood and bone marrow from untreated advanced breast cancer patients that are associated with the establishment of bone metastases. Clin. Exp. Metastasis. 2014;31:213–232. doi: 10.1007/s10585-013-9622-5. [DOI] [PubMed] [Google Scholar]
- 68.Stine KC, et al. Cisplatin inhibits bone healing during distraction osteogenesis. J. Orthop. Res. 2014;32:464–470. doi: 10.1002/jor.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat. Rev. Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cortez MA, et al. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aleckovic M, Kang Y. Regulation of cancer metastasis by cell-free mi RNAs. Biochim. Biophys. Acta. 2015;1855:24–42. doi: 10.1016/j.bbcan.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gururajan M, et al. miR-154* and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin. Cancer Res. 2014;20:6559–6569. doi: 10.1158/1078-0432.CCR-14-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin X, Wang Z, Zhang R, Feng W. High serum microRNA-335 level predicts aggressive tumor progression and unfavorable prognosis in pediatric acute myeloid leukemia. Clin. Transl Oncol. 2015;17:358–364. doi: 10.1007/s12094-014-1237-z. [DOI] [PubMed] [Google Scholar]
- 74.Zhao FL, et al. Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J. Int. Med. Res. 2012;40:859–866. doi: 10.1177/147323001204000304. [DOI] [PubMed] [Google Scholar]
- 75.Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014;11:145–156. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 76.Ell B, et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013;24:542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valencia K, et al. miR-326 associates with biochemical markers of bone turnover in lung cancer bone metastasis. BONE. 2013;52:532–539. doi: 10.1016/j.bone.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 78.Van Poznak CH, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J. Clin. Oncol. 2011;29:1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 79.Burstein HJ, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J. Clin. Oncol. 2014;32:2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 81.Bokemeyer C, Berger CC, Kuczyk MA, Schmoll HJ. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J. Clin. Oncol. 1996;14:2923–2932. doi: 10.1200/JCO.1996.14.11.2923. [DOI] [PubMed] [Google Scholar]
- 82.Hu MI, Gagel RF, Jimenez C. Bone loss in patients with breast or prostate cancer. Curr. Osteoporos. Rep. 2007;5:170–178. doi: 10.1007/s11914-007-0013-1. [DOI] [PubMed] [Google Scholar]
- 83.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N. Engl. J. Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 84.Ottewell PD, et al. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr. Relat. Cancer. 2014;21:769–781. doi: 10.1530/ERC-14-0199. [DOI] [PubMed] [Google Scholar]
- 85.Lipton A, Smith MR, Ellis GK, Goessl C. Treatment-induced bone loss and fractures in cancer patients undergoing hormone ablation therapy: efficacy and safety of denosumab. Clin. Med. Insights Oncol. 2012;6:287–303. doi: 10.4137/CMO.S8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Love RR, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N. Engl. J. Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 87.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J. Clin. Oncol. 2006;24:675–680. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 88.Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit. Rev. Oncol. Hematol. 2009;69:73–82. doi: 10.1016/j.critrevonc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 89.Suva LJ, Makhoul I. Bone: Will breast cancer chemoprevention stand on ‘solid bone’? Nat. Rev. Endocrinol. 2015;11:138–139. doi: 10.1038/nrendo.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rizzoli R, et al. Cancer-associated bone disease. Osteoporos. Int. 2013;24:2929–2953. doi: 10.1007/s00198-013-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ottewell PD, et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin. Cancer Res. 2014;20:2922–2932. doi: 10.1158/1078-0432.CCR-13-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hadji P, Coleman R, Gnant M. Bone effects of mammalian target of rapamycin (mTOR) inhibition with everolimus. Crit. Rev. Oncol. Hematol. 2013;87:101–111. doi: 10.1016/j.critrevonc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 93.Sieber PR, Keiller DL, Kahnoski RJ, Gallo J, McFadden S. Bicalutamide 150 mg maintains bone mineral density during monotherapy for localized or locally advanced prostate cancer. J. Urol. 2004;171:2272–2276. doi: 10.1097/01.ju.0000127738.94221.da. [DOI] [PubMed] [Google Scholar]
- 94.Eswaraka J, et al. Axitinib and crizotinib combination therapy inhibits bone loss in a mouse model of castration resistant prostate cancer. BMC Cancer. 2014;14:742. doi: 10.1186/1471-2407-14-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aleman JO, Farooki A, Girotra M. Effects of tyrosine kinase inhibition on bone metabolism: untargeted consequences of targeted therapies. Endocr. Relat. Cancer. 2014;21:R247–R259. doi: 10.1530/ERC-12-0400. [DOI] [PubMed] [Google Scholar]
- 96.Oh D, Huh SJ. Insufficiency fracture after radiation therapy. Radiat. Oncol. J. 2014;32:213–220. doi: 10.3857/roj.2014.32.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lipton A, et al. Advances in treating metastatic bone cancer: summary statement for the first Cambridge conference. Clin. Cancer Res. 2006;12:6209s–6212s. doi: 10.1158/1078-0432.CCR-06-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guise TA, et al. Molecular mechanisms of breast cancer metastases to bone. Clin. Breast Cancer. 2005;5(Suppl. 2):S46–S53. doi: 10.3816/cbc.2005.s.004. [DOI] [PubMed] [Google Scholar]
- 99.Yong M, et al. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007) Breast Cancer Res. Treat. 2011;129:495–503. doi: 10.1007/s10549-011-1475-5. [DOI] [PubMed] [Google Scholar]
- 100.Hortobagyi GN, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J. Clin. Oncol. 1998;16:2038–2044. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 101.Theriault RL, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J. Clin. Oncol. 1999;17:846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 102.Rosen LS, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 103.Tombal B. Assessing the benefit of bone-targeted therapies in prostate cancer, is the devil in the end point’s definition? Ann. Oncol. 2015;26:257–258. doi: 10.1093/annonc/mdu553. [DOI] [PubMed] [Google Scholar]
- 104.Parker C, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 105.Smith MR, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann. Oncol. 2015;26:368–374. doi: 10.1093/annonc/mdu519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jung K, Lein M. Bone turnover markers in serum and urine as diagnostic, prognostic and monitoring biomarkers of bone metastasis. Biochim. Biophys. Acta. 2014;1846:425–438. doi: 10.1016/j.bbcan.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 107.Barnadas A, et al. Bone turnover markers as predictive indicators of outcome in patients with breast cancer and bone metastases treated with bisphosphonates: results from a 2-year multicentre observational study (ZOMAR study) BONE. 2014;68:32–40. doi: 10.1016/j.bone.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 108.Lara PN, Jr, et al. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J. Natl Cancer Inst. 2014;106:dju013. doi: 10.1093/jnci/dju013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 110.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. BONE. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 111.Valachis A, et al. Adjuvant therapy with zoledronic acid in patients with breast cancer: a systematic review and meta-analysis. Oncologist. 2013;18:353–361. doi: 10.1634/theoncologist.2012-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ottewell PD, et al. Combination therapy inhibits development and progression of mammary tumours in immunocompetent mice. Breast Cancer Res. Treat. 2012;133:523–536. doi: 10.1007/s10549-011-1782-x. [DOI] [PubMed] [Google Scholar]
- 113.Insalaco L, et al. Analysis of molecular mechanisms and anti-tumoural effects of zoledronic acid in breast cancer cells. J. Cell. Mol. Med. 2012;16:2186–2195. doi: 10.1111/j.1582-4934.2012.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Green J, Lipton A. Anticancer properties of zoledronic acid. Cancer Invest. 2010;28:944–957. doi: 10.3109/07357907.2010.512598. [DOI] [PubMed] [Google Scholar]
- 115.Welton JL, et al. γδ T cells predict outcome in zoledronate-treated breast cancer patients. Oncologist. 2013;18:e22–e23. doi: 10.1634/theoncologist.2013-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Foroni C, et al. Pure anti-tumor effect of zoledronic acid in naive bone-only metastatic and locally advanced breast cancer: proof from the “biological window therapy”. Breast Cancer Res. Treat. 2014;144:113–121. doi: 10.1007/s10549-014-2840-y. [DOI] [PubMed] [Google Scholar]
- 117.Welton JL, et al. Monocytes and γδ T cells control the acute-phase response to intravenous zoledronate: insights from a phase IV safety trial. J. Bone Miner. Res. 2013;28:464–471. doi: 10.1002/jbmr.1797. [DOI] [PubMed] [Google Scholar]
- 118.Coleman R, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann. Oncol. 2013;24:398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 119.Gnant M, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 120.Lester JE, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin. Cancer Res. 2008;14:6336–6342. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]
- 121.Van Poznak C, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J. Clin. Oncol. 2010;28:967–975. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 122.Smith MR, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 123.Smith MR, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gnant M, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N. Engl. J. Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 125.Coleman RE, et al. Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 126.Powles T, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Res. 2006;8:R13. doi: 10.1186/bcr1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paterson AH, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13:734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Denham JW, et al. Impact of androgen suppression and zoledronic acid on bone mineral density and fractures in the Trans-Tasman Radiation Oncology Group (TROG) 03.04 Randomised Androgen Deprivation and Radiotherapy (RADAR) randomized controlled trial for locally advanced prostate cancer. BJU Int. 2014;114:344–353. doi: 10.1111/bju.12497. [DOI] [PubMed] [Google Scholar]
- 129.Tombal B. Zometa European Study (ZEUS): another failed crusade for the holy grail of prostate cancer bone metastases prevention? Eur. Urol. 2015;67:492–494. doi: 10.1016/j.eururo.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 130.Wirth M, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS) Eur. Urol. 2015;67:482–491. doi: 10.1016/j.eururo.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 131.Smith MR, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database of Systematic Reviews. (2) doi: 10.1002/14651858.CD003474.pub3. Art. No.: CD003474 http://dx.doi.org/10.1002/14651858.CD003474.pub3. [DOI] [PubMed]
- 133.Coleman R, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 134.Todenhofer T, Stenzl A, Hofbauer LC, Rachner TD. Targeting bone metabolism in patients with advanced prostate cancer: current options and controversies. Int. J. Endocrinol. 2015;2015:838202. doi: 10.1155/2015/838202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saad F, Karakiewicz P, Perrotte P. The role of bisphosphonates in hormone-refractory prostate cancer. World J. Urol. 2005;23:14–18. doi: 10.1007/s00345-004-0472-2. [DOI] [PubMed] [Google Scholar]
- 136.Berenson JR, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 137.Saad F, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 138.Saad F, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl Cancer Inst. 2004;96:879. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 139.Fizazi K, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 140.Henry DH, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 141.Snedecor SJ, Carter JA, Kaura S, Botteman MF. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin. Ther. 2012;34:1334–1349. doi: 10.1016/j.clinthera.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 142.Gralow JR, et al. NCCN Task Force report: bone health in cancer care. J. Natl Compr. Canc. Netw. 2013;11(Suppl. 3):S1–S50. doi: 10.6004/jnccn.2013.0215. [DOI] [PubMed] [Google Scholar]
- 143.Suva LJ, Brander BE, Makhoul I. Update on bone-modifying agents in metastatic breast cancer. Nat. Rev. Endocrinol. 2011;7:380–381. doi: 10.1038/nrendo.2011.80. [DOI] [PubMed] [Google Scholar]
- 144.Serafini AN, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J. Clin. Oncol. 1998;16:1574–1581. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 145.Sartor O, Reid RH, Bushnell DL, Quick DP, Ell PJ. Safety and efficacy of repeat administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007;109:637–643. doi: 10.1002/cncr.22431. [DOI] [PubMed] [Google Scholar]
- 146.Longo J, Lutz S, Johnstone C. Samarium-153-ethylene diamine tetramethylene phosphonate, a β-emitting bone-targeted radiopharmaceutical, useful for patients with osteoblastic bone metastases. Cancer Manag. Res. 2013;5:235–242. doi: 10.2147/CMAR.S35789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Silberstein EB, Eugene L, Saenger SR. Painful osteoblastic metastases: the role of nuclear medicine. Oncology (Williston Park) 2001;15:157–163. [PubMed] [Google Scholar]
- 148.Baczyk M. Radioisotope therapy of bone metastases. Nucl. Med. Rev. Cent. East Eur. 2011;14:96–104. doi: 10.5603/nmr.2011.00023. [DOI] [PubMed] [Google Scholar]
- 149.Nilsson S, et al. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin. Genitourin. Cancer. 2013;11:20–26. doi: 10.1016/j.clgc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 150.Shirley M, McCormack PL. Radium-223 dichloride: a review of its use in patients with castration-resistant prostate cancer with symptomatic bone metastases. Drugs. 2014;74:579–586. doi: 10.1007/s40265-014-0198-4. [DOI] [PubMed] [Google Scholar]
- 151.Coleman RE, et al. Bone scan flare predicts successful systemic therapy for bone metastases. J. Nucl. Med. 1988;29:1354–1359. [PubMed] [Google Scholar]
- 152.Carducci MA, Jimeno A. Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin. Cancer Res. 2006;12:6296s–6300s. doi: 10.1158/1078-0432.CCR-06-0929. [DOI] [PubMed] [Google Scholar]
- 153.Clines GA, et al. Regulation of postnatal trabecular bone formation by the osteoblast endothelin A receptor. J. Bone Miner. Res. 2011;26:2523–2536. doi: 10.1002/jbmr.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nelson JB, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–2487. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nelson JB, et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer. 2012;118:5709–5718. doi: 10.1002/cncr.27674. [DOI] [PubMed] [Google Scholar]
- 156.Quinn DI, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 2013;14:893–900. doi: 10.1016/S1470-2045(13)70294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miller K, et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:187–192. doi: 10.1038/pcan.2013.2. [DOI] [PubMed] [Google Scholar]
- 158.Duong LT, Wesolowski GA, Leung P, Oballa R, Pickarski M. Efficacy of a cathepsin K inhibitor in a preclinical model for prevention and treatment of breast cancer bone metastasis. Mol. Cancer Ther. 2014;13:2898–2909. doi: 10.1158/1535-7163.MCT-14-0253. [DOI] [PubMed] [Google Scholar]
- 159.Bonnick S, et al. Effects of odanacatib on BMD and safety in the treatment of osteoporosis in postmenopausal women previously treated with alendronate: a randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2013;98:4727–4735. doi: 10.1210/jc.2013-2020. [DOI] [PubMed] [Google Scholar]
- 160.Jensen AB, et al. The cathepsin K inhibitor odanacatib suppresses bone resorption in women with breast cancer and established bone metastases: results of a 4-week, double-blind, randomized, controlled trial. Clin. Breast Cancer. 2010;10:452–458. doi: 10.3816/CBC.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 161.Aftab DT, McDonald DM. MET and VEGF: synergistic targets in castration-resistant prostate cancer. Clin. Transl. Oncol. 2011;13:703–709. doi: 10.1007/s12094-011-0719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang S, et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol. Cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kabbarah O, et al. Integrative genome comparison of primary and metastatic melanomas. PLoS ONE. 2010;5:e10770. doi: 10.1371/journal.pone.0010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vergani E, et al. Identification of MET and SRC activation in melanoma cell lines showing primary resistance to PLX4032. Neoplasia. 2011;13:1132–1142. doi: 10.1593/neo.111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chu GC, et al. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr. Relat. Cancer. 2014;21:311–326. doi: 10.1530/ERC-13-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith MR, et al. Cabozantinib in chemotherapy-pretreated metastatic castration-resistant prostate cancer: results of a phase II nonrandomized expansion study. J. Clin. Oncol. 2014;32:3391–3399. doi: 10.1200/JCO.2013.54.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yue W, Fan P, Wang J, Li Y, Santen RJ. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J. Steroid Biochem. Mol. Biol. 2007;106:102–110. doi: 10.1016/j.jsbmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ma J, Li M, Hock J, Yu X. Hyperactivation of mTOR critically regulates abnormal osteoclastogenesis in neurofibromatosis type 1. J. Orthop. Res. 2012;30:144–152. doi: 10.1002/jor.21497. [DOI] [PubMed] [Google Scholar]
- 170.Indo Y, et al. Metabolic regulation of osteoclast differentiation and function. J. Bone Miner. Res. 2013;28:2392–2399. doi: 10.1002/jbmr.1976. [DOI] [PubMed] [Google Scholar]
- 171.Ory B, Moriceau G, Redini F, Heymann D. mTOR inhibitors (rapamycin and its derivatives) and nitrogen containing bisphosphonates: bi-functional compounds for the treatment of bone tumours. Curr. Med. Chem. 2007;14:1381–1387. doi: 10.2174/092986707780831159. [DOI] [PubMed] [Google Scholar]
- 172.Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFα and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 173.Kneissel M, et al. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. BONE. 2004;35:1144–1156. doi: 10.1016/j.bone.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 174.Gnant M, et al. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J. Natl Cancer Inst. 2013;105:654–663. doi: 10.1093/jnci/djt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 176.Lehmann BD, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Khramtsov AI, et al. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cui Y, et al. Lrp5 functions in bone to regulate bone mass. Nat. Med. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Holmen SL, et al. Essential role of β-catenin in postnatal bone acquisition. J. Biol. Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- 180.Goldring SR, Goldring MB. Eating bone or adding it: the Wnt pathway decides. Nat. Med. 2007;13:133–134. doi: 10.1038/nm0207-133. [DOI] [PubMed] [Google Scholar]
- 181.Bu G, et al. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int. J. Cancer. 2008;123:1034–1042. doi: 10.1002/ijc.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sukhdeo K, et al. Targeting the β-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc. Natl Acad. Sci. USA. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fulciniti M, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou XL, Qin XR, Zhang XD, Ye LH. Downregulation of Dickkopf-1 is responsible for high proliferation of breast cancer cells via losing control of Wnt/β-catenin signaling. Acta Pharmacol. Sin. 2010;31:202–210. doi: 10.1038/aps.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Krause U, Ryan DM, Clough BH, Gregory CA. An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a novel cancer survival mechanism through modulation of aldehyde-dehydrogenase-1 activity. Cell Death Dis. 2014;5:e1093. doi: 10.1038/cddis.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim H, et al. Regulation of Wnt signaling activity for growth suppression induced by quercetin in 4T1 murine mammary cancer cells. Int. J. Oncol. 2013;43:1319–1325. doi: 10.3892/ijo.2013.2036. [DOI] [PubMed] [Google Scholar]
- 187.Zhang H, et al. Parathyroid hormone-related protein inhibits DKK1 expression through c-Jun-mediated inhibition of β-catenin activation of the DKK1 promoter in prostate cancer. Oncogene. 2014;33:2464–2477. doi: 10.1038/onc.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thudi NK, et al. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate. 2011;71:615–625. doi: 10.1002/pros.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. A prospective study of a diagnostic strategy. J. Bone Joint Surg. Am. 1993;75:1276–1281. doi: 10.2106/00004623-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 190.Issack PS, Kotwal SY, Lane JM. Management of metastatic bone disease of the acetabulum. J. Am. Acad. Orthop. Surg. 2013;21:685–695. doi: 10.5435/JAAOS-21-11-685. [DOI] [PubMed] [Google Scholar]
- 191.Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin. Orthop. Relat. Res. 1983;178:297–302. [PubMed] [Google Scholar]
- 192.Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin. Orthop. Relat. Res. 1989;249:256–264. [PubMed] [Google Scholar]
- 193.Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J. Bone Joint Surg. Am. 2009;91:1503–1516. doi: 10.2106/JBJS.H.00175. [DOI] [PubMed] [Google Scholar]
- 194.Scolaro JA, Lackman RD. Surgical management of metastatic long bone fractures: principles and techniques. J. Am. Acad. Orthop. Surg. 2014;22:90–100. doi: 10.5435/JAAOS-22-02-90. [DOI] [PubMed] [Google Scholar]
- 195.Ward WG, Holsenbeck S, Dorey FJ, Spang J, Howe D. Metastatic disease of the femur: surgical treatment. Clin. Orthop. Relat. Res. 2003;415(Suppl.):S230–S244. doi: 10.1097/01.blo.0000093849.72468.82. [DOI] [PubMed] [Google Scholar]
- 196.Damron TA, et al. Critical evaluation of Mirels’ rating system for impending pathologic fractures. Clin. Orthop. Relat. Res. 2003;415(Suppl.):S201–S207. doi: 10.1097/01.blo.0000093842.72468.73. [DOI] [PubMed] [Google Scholar]
- 197.Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. 2007;110:1451–1456. doi: 10.1002/cncr.22956. [DOI] [PubMed] [Google Scholar]
- 198.Baloch KG, Grimer RJ, Carter SR, Tillman RM. Radical surgery for the solitary bony metastasis from renal-cell carcinoma. J. Bone Joint Surg. Br. 2000;82:62–67. doi: 10.1302/0301-620x.82b1.9995. [DOI] [PubMed] [Google Scholar]
- 199.Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J. Clin. Oncol. 2014;32:2913–2919. doi: 10.1200/JCO.2014.55.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Townsend PW, Rosenthal HG, Smalley SR, Cozad SC, Hassanein RE. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J. Clin. Oncol. 1994;12:2345–2350. doi: 10.1200/JCO.1994.12.11.2345. [DOI] [PubMed] [Google Scholar]
- 201.Tomita K, et al. Surgical strategy for spinal metastases. Spine (Phila. Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 202.Fourney DR, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J. Clin. Oncol. 2011;29:3072–3077. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 203.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila. Pa 1976) 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 204.Berenson J, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12:225–235. doi: 10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 205.Kaloostian PE, Yurter A, Zadnik PL, Sciubba DM, Gokaslan ZL. Current paradigms for metastatic spinal disease: an evidence-based review. Ann. Surg. Oncol. 2014;21:248–262. doi: 10.1245/s10434-013-3324-8. [DOI] [PubMed] [Google Scholar]
- 206.Patchell RA, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 207.Paice JA, Ferrell B. The management of cancer pain. CA Cancer J. Clin. 2011;61:157–182. doi: 10.3322/caac.20112. [DOI] [PubMed] [Google Scholar]
- 208.Howell DD, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97–14. Cancer. 2013;119:888–896. doi: 10.1002/cncr.27616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ejima Y, Matsuo Y, Sasaki R. The current status and future of radiotherapy for spinal bone metastases. J. Orthop. Sci. 2015;20:585–592. doi: 10.1007/s00776-015-0720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lutz S, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 211.Barlogie B, et al. Treatment of multiple myeloma. Blood. 2004;103:20–32. doi: 10.1182/blood-2003-04-1045. [DOI] [PubMed] [Google Scholar]
- 212.Zangari M, et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica. 2011;96:333–336. doi: 10.3324/haematol.2010.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Roodman GD. Bone-breaking cancer treatment. Nat. Med. 2007;13:25–26. doi: 10.1038/nm0107-25. [DOI] [PubMed] [Google Scholar]
- 214.Manso L, et al. Use of bevacizumab as a first-line treatment for metastatic breast cancer. Curr. Oncol. 2015;22:e51–e60. doi: 10.3747/co.22.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Moreno-Aspitia A, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J. Clin. Oncol. 2009;27:11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Curigliano G, et al. Randomized phase II study of sunitinib versus standard of care for patients with previously treated advanced triple-negative breast cancer. Breast. 2013;22:650–656. doi: 10.1016/j.breast.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 217.Coleman RE. Risks and benefits of bisphosphonates. Br. J. Cancer. 2008;98:1736–1740. doi: 10.1038/sj.bjc.6604382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lipton A, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J. Clin. Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 219.Coombes RC, et al. An open-label study of lapatinib in women with HER-2-negative early breast cancer: the lapatinib pre-surgical study (LPS study) Ann. Oncol. 2013;24:924–930. doi: 10.1093/annonc/mds594. [DOI] [PubMed] [Google Scholar]
- 220.Small EJ, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 221.Madan RA, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.van den Eertwegh AJ, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 223.Telli ML, et al. Phase II Study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J. Clin. Oncol. 2015;33:1895–1901. doi: 10.1200/JCO.2014.57.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dittrich C, et al. A phase II multicenter study of two different dosages of pemetrexed given in combination with cyclophosphamide as first-line treatment in patients with locally advanced or metastatic breast cancer. Cancer Invest. 2012;30:309–316. doi: 10.3109/07357907.2012.658938. [DOI] [PubMed] [Google Scholar]
- 225.Smith JW, 2nd, et al. Results of a phase II open-label, nonrandomized trial of oral satraplatin in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2009;118:361–367. doi: 10.1007/s10549-009-0410-5. [DOI] [PubMed] [Google Scholar]
- 226.Dagher R, et al. Approval summary: docetaxel in combination with prednisone for the treatment of androgen-independent hormone-refractory prostate cancer. Clin. Cancer Res. 2004;10:8147–8151. doi: 10.1158/1078-0432.CCR-04-1402. [DOI] [PubMed] [Google Scholar]
- 227.Villanueva C, et al. A multicentre dose-escalating study of cabazitaxel (XRP6258) in combination with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment: a phase I/II study. Eur. J. Cancer. 2011;47:1037–1045. doi: 10.1016/j.ejca.2011.01.001. [DOI] [PubMed] [Google Scholar]