Abstract
Background/Objectives:
To assess the effectiveness of the SLIMMER combined dietary and physical activity lifestyle intervention on clinical and metabolic risk factors, dietary intake, physical activity, and quality of life after 12 months, and to investigate whether effects sustained six months after the active intervention period ended.
Subjects/Methods:
SLIMMER was a randomised controlled intervention, implemented in Dutch primary healthcare. In total, 316 subjects aged 40–70 years with increased risk of type 2 diabetes were randomly allocated to the intervention group (10-month dietary and physical activity programme) or the control group (usual healthcare). All subjects underwent an oral glucose tolerance test and physical examination, and filled in questionnaires. Identical examinations were performed at baseline and after 12 and 18 months. Primary outcome was fasting insulin.
Results:
The intervention group showed significantly greater improvements in anthropometry and glucose metabolism. After 12 and 18 months, differences between intervention and control group were -2.7 kg (95% confidence interval (CI): −3.7; −1.7) and −2.5 kg (95% CI: −3.6; −1.4) for weight, and −12.1 pmol l−1 (95% CI: −19.6; −4.6) and −8.0 pmol l−1 (95% CI: −14.7; −0.53) for fasting insulin. Furthermore, dietary intake, physical activity, and quality of life improved significantly more in the intervention group than in the control group.
Conclusions:
The Dutch SLIMMER lifestyle intervention is effective in the short and long term in improving clinical and metabolic risk factors, dietary intake, physical activity, and quality of life in subjects at high risk of diabetes.
Introduction
Universal consensus exists on the need to translate and implement evidence from landmark clinical trials on combined lifestyle interventions to prevent type 2 diabetes in real-world settings.1 Recent reviews on studies conducted in such settings showed limited results, with significant reductions in weight and waist circumference but inconclusive findings for metabolic indicators of diabetes risk, such as blood glucose or HbA1c.2, 3, 4 Furthermore, current evidence on sustainability and long-term clinical benefits of such interventions is limited.2, 3, 4 To date, no evidence-based diabetes prevention interventions have been effectively implemented in Dutch primary healthcare,5, 6 while the Study on Lifestyle intervention and Impaired glucose tolerance Maastricht (SLIM), conducted in an experimental setting, had earlier revealed a 47% diabetes risk reduction.7 We therefore used the SLIM intervention as a starting point for implementation and translated this into the SLIMMER intervention (SLIM iMplementation Experience Region Noord- en Oost-Gelderland). This translation was done jointly by SLIM intervention developers and local healthcare professionals.8 Pilot-testing of the adapted intervention showed its implementation was feasible in Dutch primary healthcare and that it was likely to achieve desired impact.9 These results served as input for broader implementation and evaluation of the intervention.10 In this study, we assess the effectiveness of the SLIMMER intervention on clinical and metabolic risk factors, dietary intake, physical activity (PA), and quality of life after 12 months. Moreover, the aim is to investigate whether effects sustained six months after the active intervention period ended.
Materials and methods
Study design
The SLIMMER study’s design and 10-month lifestyle intervention programme have been described in detail elsewhere.10 In short, SLIMMER was a randomised, controlled intervention study, conducted in the cities of Apeldoorn and Doetinchem (the Netherlands). It was implemented in Dutch public health and primary healthcare, involving 25 general practices—general practitioners (GPs) and their practice nurses—, 11 dieticians, 16 physiotherapists and 15 sports clubs. The study protocol was approved by the Wageningen University Medical Ethics Committee, and all subjects gave their written informed consent before the study started. The SLIMMER study is registered with ClinicalTrials.gov (Identifier NCT02094911).
Study population
Study subjects were recruited by GPs and practice nurses from their patient registration database, using either a laboratory glucose test or the Dutch Diabetes Risk Test. Inclusion criteria were (1) aged between 40 and 70 years at screening, (2) impaired fasting glucose (IFG; 6.1–6.9 mmol l−1) or an elevated/high risk of type 2 diabetes (a Diabetes Risk Test score of ⩾7 points), (3) willing and able to participate in the study for at least 1.5 years, and (4) able to speak and understand the Dutch language. Exclusion criteria were, amongst others, known diabetes and any severe cardiovascular or psychiatric disease. Criteria were checked using electronic medical records. There were no racial or gender criteria. Recruitment took place between October 2011 and September 2012 in three consecutive groups for logistical reasons.
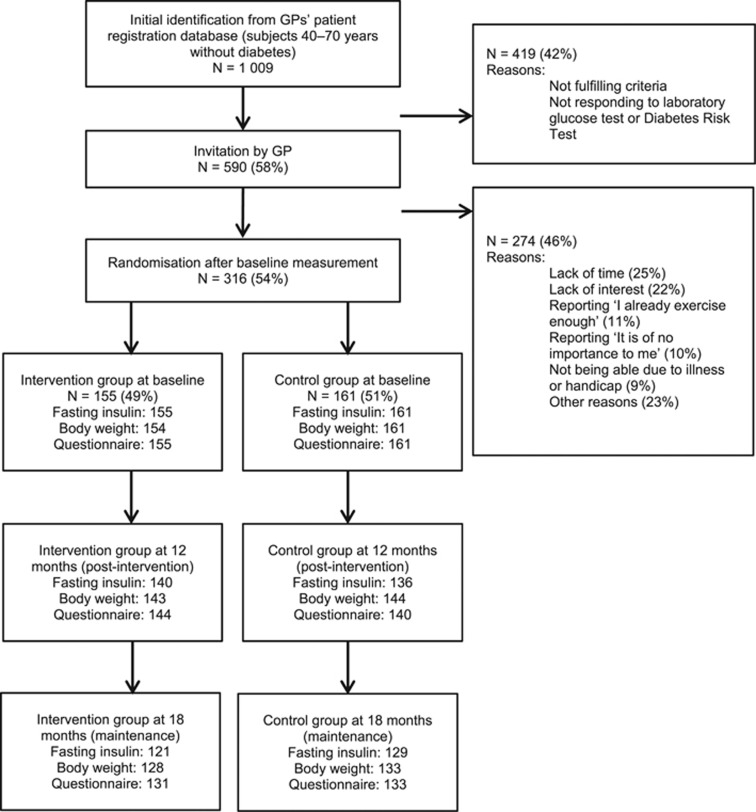
In total, 1009 individuals aged 40–70 years without diabetes mellitus were initially identified from the patient registration database (Figure 1). Of these, 590 (58%) fulfilled all criteria and were invited to participate. In total, 316 subjects (54%) were willing to participate and underwent an oral glucose tolerance test (OGTT) and physical examination at baseline.
Figure 1.
Flow diagram.
After baseline measurements, participants were randomly allocated to the intervention or control group (allocation ratio 1:1), using block randomisation at GP level (permuted blocks with size 2) and stratification for sex. Couples were allocated to the same group to avoid contamination. An independent research assistant from the Division of Human Nutrition, Wageningen University, performed the randomisation, using a computerised random number generator. Allocation concealment was ensured, as allocation was not announced until baseline measurements were completed. One of the researchers (GD) informed participants on the assignment to the intervention or control group.
Intervention
The SLIMMER combined lifestyle intervention resembled the SLIM intervention,7 which was based on the Finnish Diabetes Prevention Study,11 and consisted of a dietary and a PA component, delivered by primary healthcare professionals (GPs, practice nurses, dieticians and physiotherapists).10 Furthermore, case management and a maintenance programme were included. The dietary intervention consisted of tailored dietary advice given by a dietician, during five to eight individual consultations (average of 5.6 consultations per participant) and one group session. The aim was to adopt, step by step, a sustainable, healthy dietary pattern according to the Dutch dietary guidelines. Furthermore, the intervention aimed to help overweight participants to achieve 5–10% weight loss. The PA intervention was delivered by physiotherapists as weekly group-based combined aerobic and resistance training sessions (average of 38 sports lessons per participant), based on the Dutch guidelines for PA and type 2 diabetes.12 The aim was to obtain and maintain an active lifestyle, which includes moderate-intensity PA for at least 30 min per day on at least five days a week. Furthermore, case management was performed by practice nurses (contacting intervention participants and healthcare professionals by phone) to enhance participant compliance and feasibility of implementation.
In addition to the core dietary and PA intervention, a maintenance programme was delivered, starting in the last phase of the 10-month intervention period and lasting up to three months thereafter. This programme comprised sports clinics at local sports clubs, concluding meetings with the dietician and physiotherapist, and a return session with the physiotherapist, dietician, and the PA group.13 This programme was added to guide participants in the process of maintaining lifestyle behaviour change in an independent and sustainable manner.
Control group subjects received usual healthcare as provided by GPs and practice nurses (yearly monitoring of blood glucose, according to the guidelines of the Dutch College of General Practices).14 Furthermore, at baseline they received written information on the beneficial effects of a healthy diet and increased PA. No additional appointments were scheduled, apart from visits for follow-up measurements.
Data collection and outcome measures
Baseline measurements were taken between February and October 2012. All study subjects underwent an OGTT and physical examination, and filled in questionnaires. Identical examinations were performed at baseline, after 12 months (at the end of the intervention; between February and September 2013), and after 18 months (6 months after the end of the intervention; between September 2013 and March 2014; Supplementary Figure S1). These procedures have previously been described in detail.10
The primary outcome was fasting insulin, determined on the basis of a standard OGTT with a glucose load of 75 g, performed by trained nurses after at least 10 h of fasting. Fasting and 2-h plasma glucose levels, HbA1c, and serum lipids (cholesterol (total, HDL and LDL) and triglycerides) were determined at SHO laboratory in Velp, The Netherlands. For fasting insulin, all blood samples were analysed within one run after 18 months. An index for insulin resistance was calculated from fasting plasma glucose and insulin concentration, using the homoeostasis model assessment (HOMA-IR). Diabetes was classified based on World Health Organization recommendations15, 16 and standards of the American Diabetes Association.17 Normoglycaemia was defined as fasting glucose <6.1 mmol l−1 and 2-h glucose <7.8 mmol l−1; isolated IFG was defined as fasting glucose 6.1–6.9 mmol l−1 and 2-h glucose <7.8 mmol l−1; impaired glucose tolerance was defined as fasting glucose <7.0 mmol l−1 and 2-h glucose 7.8–11.0 mmol l−1; and diabetic values were defined as fasting glucose ⩾7.0 mmol l−1, 2-h glucose ⩾11.1 mmol l−1, HbA1c ⩾6.5%, or using diabetes medication. Clinical assessments were performed by trained research assistants in research centres in Apeldoorn and Doetinchem according to standardised procedures. BMI was calculated as the ratio of weight and height squared (kg m−2). Waist circumference was obtained at the level midway between the lowest rib and the iliac crest. Blood pressure was measured using the Omron Digital Blood Pressure Monitor HEM-907.
Socio-demographic characteristics (age, sex, education level, ethnic background, smoking and family history of diabetes) were collected by participant questionnaires. These data were collected according to standards of the national surveillance system in the Netherlands and an existing questionnaire. Self-reported medication use was determined using a questionnaire. Non-response data (age, sex, reason for non-participation, perceived health, and education level) were collected during the recruitment period by practice nurses.
Dietary intake (nutrient intake and food intake) was assessed by a validated Food Frequency Questionnaire (FFQ). FFQs were checked by trained research assistants. Average daily nutrient intakes were calculated by multiplying frequency of consumption by portion size and nutrient content per gram using the 2011 Dutch food composition table.18 Food intake behaviours were formulated based on Dutch food-based dietary guidelines and common dietician practices in the SLIMMER pilot study.9 Adherence to the Dutch dietary guidelines was calculated, based on the Dutch Healthy Diet Index (DHD-index).19 The original DHD-index consisted of 10 components representing the guidelines, whereas for the current study we adapted the index and included eight components: PA, vegetable, fruit, fibre, fish (EPA and DHA), saturated fat, trans-fatty acids, and alcohol. Two components (‘acidic drinks and foods’ and ‘sodium’) were excluded because no data were available on these components. Per component, the score ranged between 0 and 10, resulting in a total score between 0 (no adherence) and 80 (complete adherence).
PA was measured using the validated Short QUestionnaire to Assess Health-enhancing physical activity (SQUASH). The durations (minutes per week) of total and light-, moderate-, and vigorous-intensity physical activities were calculated. Level of compliance with the PA guidelines (moderate-intensity PA for at least 30 min per day on at least five days a week) was represented as inactive (0 days), semi-active (1–4 days), or norm-active (at least 5 days).20 Furthermore, physical fitness was measured as the distance covered in metres during the six-minute walk test.
Quality of life was assessed by the Short-Form Health Survey (SF-36), which has proved to be a practical, reliable, and valid tool for both general and chronic disease populations in the Netherlands. The questions were organised into one item on health transition and eight scales for, respectively, physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and general mental health. The eight scales were converted to a 0–100 scale indicating worst to best possible health. Scores were summarised into the physical component summary score and the mental component summary score.
Statistical analysis
A sample size of 145 subjects per group was required to detect differences between groups in fasting insulin, assuming an alpha of 0.05, power of 80%, two-sided test, and an expected drop-out rate of 10%. After exclusion of subjects because of missing data on fasting insulin or BMI at baseline or 12 months (n=16 in the intervention group and n=25 in the control group), data collected from 275 subjects were used for statistical analysis. Participants who dropped out were not substantially different from the completers in baseline characteristics, except that drop-outs were more often divorced than completers (25 vs 6% in the intervention group and 28 vs 5% in the control group). Furthermore, the HOMA-IR was higher in intervention drop-outs than in completers (3.1±2.8 vs 2.0±1.1, P=0.053), whereas this was lower in control drop-outs than in completers (1.5±0.6 vs 2.0±1.2, P=0.015).
Continuous variables are presented as mean±s.d. and categorical variables as percentages. Natural log transformations were used in the event of skewed distributions. Differences within groups were tested for statistical significance with paired samples t-tests for normally distributed variables and Wilcoxon signed-rank tests for non-normally distributed variables. Differences between groups were tested for statistical significance with independent samples t-tests for normally distributed variables and Mann–Whitney tests for non-normally distributed variables, χ2-tests and analysis of covariance adjusting for baseline value, sex, recruitment phase, and medication use if applicable. Additional analyses showed similar results when subjects on medication were excluded. We included an interaction term in the analysis of covariance models to test whether the association between treatment and outcome measures differed by sex. All primary analyses were performed according to the intention-to-treat principle: participants were analysed in the groups to which they were originally randomly assigned, regardless of whether or not they actually participated in the intervention. All analyses were performed using IBM SPSS Statistics version 22 (IBM Corp., Armonk, NY, USA).
Results
Baseline results
Study subjects and non-responders (those who were not willing to participate) were similar in terms of sex, age and education level. However, 90% of the non-responders perceived their health as good or even better, against 79% of the study subjects. The most important reasons for non-response were lack of time (25%), lack of interest (22%), reporting ‘I already exercise enough’ (11%), reporting ‘It is of no importance to me’ (10%), and not being able due to illness or handicap (9%).
Table 1 shows the baseline characteristics of the 275 subjects. No differences in baseline characteristics between study groups were found. On average, subjects were 61 years old, and most had a low education level, were Dutch, and had a family history of diabetes. Of the total, 48% were overweight (BMI ⩾25 and <30 kg m−2), 42% were obese (BMI⩾30 kg m−2), and 15% had a cardiovascular disease in the past (data not shown). Dietary intake was similar between groups both in terms of nutrient intake and food intake, and both groups had similar adherence to the Dutch dietary guidelines. Moderate-to-vigorous intensity PA was comparable between groups and 80% of participants were classified as norm-active. Health-related quality of life was comparable in both groups, with scores around 50 for both physical and mental component scores.
Table 1. Baseline characteristics of participants in the SLIMMER intervention (n=275)a.
| INT (n=139) | CON (n=136) | |
|---|---|---|
| Socio-demographics | ||
| Sex (n, %) | ||
| Male | 75 (54) | 69 (51) |
| Female | 64 (46) | 67 (49) |
| Age (years) | 61.1±6.1 | 61.2±6.6 |
| Education level (n, %)b | ||
| Low | 74 (53) | 73 (54) |
| Middle | 39 (28) | 26 (19) |
| High | 26 (19) | 37 (27) |
| Ethnicity (n, %) | ||
| Dutch | 123 (88) | 123 (90) |
| Western non-Dutch | 12 (9) | 9 (7) |
| Non-western non-Dutch | 4 (3) | 4 (3) |
| Family history of diabetes (n, %) | ||
| No | 46 (33) | 57 (42) |
| First degree | 66 (48) | 62 (46) |
| Second degree | 27 (19) | 17 (12) |
| Smoking status (n, %) | ||
| Yes | 21 (15) | 26 (19) |
| Ex-smoker | 86 (62) | 78 (57) |
| No, never | 32 (23) | 32 (24) |
| Clinical and metabolic risk factors | ||
| Weight (kg) | 89.5±17.0 | 87.8±15.2 |
| BMI (kg/m2) | 30.2±4.5 | 29.9±4.8 |
| Waist circumference (cm) | ||
| Male | 109.3±12.2 | 107.4±10.2 |
| Female | 101.0±12.0 | 100.0±12.9 |
| Fasting glucose (mmol l−1) | 6.6±0.6 | 6.6±0.6 |
| 2-h glucose (mmol l−1)c | 8.2±2.8 | 8.0±2.5 |
| Fasting insulin (pmol l−1) | 87.8±48.2 | 86.0±52.8 |
| HOMA-IR | 2.0±1.1 | 2.0±1.2 |
| HbA1c (% (mmol mol−1)) | 5.8±0.4 (40.3±3.9) | 5.8±0.4 (40.1±4.0) |
| Total cholesterol (mmol l−1) | 5.5±1.1 | 5.5±1.2 |
| HDL cholesterol (mmol l−1) | 1.5±0.4 | 1.4±0.4 |
| LDL cholesterol (mmol l−1) | 3.5±1.0 | 3.6±1.0 |
| Triglycerides (mmol l−1) | 1.7±0.9 | 1.6±0.8 |
| Blood pressure (mm Hg)c | ||
| Systolic | 133.0±15.5 | 131.0±13.5 |
| Diastolic | 77.5±10.7 | 75.6±8.7 |
| Dietary intake | ||
| Energy intake (kcal per day) | 1986.3±576.1 | 2040.7±634.2 |
| Saturated fat (en%) | 11.8±2.2 | 11.9±2.3 |
| Fibre (g per 1000 kcal) | 11.0±2.6 | 11.1±2.4 |
| Alcohol (en%) | 4.8±5.7 | 4.3±5.2 |
| Fruit intake incl. fruit juices (g per day) | 186.1 ±119.9 | 206.5±140.0 |
| Vegetable intake (g per day) | 149.3±96.6 | 137.8±84.7 |
| Fibre intake from total bread intake (%) | 5.6±1.0 | 5.8±1.2 |
| Fat intake from total bread spread intake (%) | 21.0±6.4 | 19.6±6.1 |
| Snack intake (kcal per day) | 278.5±208.2 | 323.0±272.7 |
| Soft drink intake (kcal per day) | 56.3±69.6 | 48.5±60.1 |
| Dutch Healthy Diet index (0–80 scale) | 58.8±9.5 | 58.7±9.0 |
| Physical activity (PA) | ||
| Total PA (min per week) | 2254±1337 | 2306±1232 |
| Light PA (min per week) | 1307±1094 | 1331±970 |
| Moderate PA (min per week) | 593±692 | 559±552 |
| Vigorous PA (min per week) | 354±427 | 417±450 |
| Physical fitness (m)c | 454.0±58.0 | 455.5±57.7 |
| Quality of life | ||
| Physical component score | 50.1±8.2 | 49.8±7.9 |
| Mental component score | 50.1±10.3 | 50.7±8.1 |
Abbreviations: BMI, body mass index; CON, Control group; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HOMA-IR, homoeostasis model assessment insulin resistance; INT, Intervention group; LDL, low-density lipoprotein; PA, physical activity.
Data are n (%) or mean±s.d.
Education level was based on the highest level of education completed and divided in three categories: low (no, primary or lower secondary school), middle (higher secondary school or intermediate vocational school), and high (higher professional education or university level).
2- h glucose INT n=138, CON n=136; systolic blood pressure INT n=138, CON n=135; diastolic blood pressure INT n=138, CON n=135; physical fitness INT n=138, CON n=136.
Results on clinical and metabolic risk factors
Table 2 summarises changes in clinical and metabolic risk factors after 12 and 18 months. Beneficial changes were observed in the intervention group compared with the control group. At 12 months, mean weight reduction was 3.4% in the intervention group and 0.3% in the control group (P<0.001). Furthermore, waist circumference reduction was greater in the intervention group. Fasting insulin declined more in the intervention group than in the control group (−12.6 vs 0.6 pmol l−1, P=0.005). Also, greater improvements were seen in fasting glucose (−0.2 vs −0.01 mmol l−1), 2- h glucose (−0.5 vs 0.2 mmol l−1), HbA1c (−0.15 vs −0.07%), and HOMA-IR (−0.29 vs 0.02) in the intervention group than in the control group (P<0.05). Compared to baseline, more subjects had normoglycaemia (19 vs 25% in the intervention group and 15 vs 20% in the control group) and fewer intervention subjects (32 vs 27%) than control subjects (28 vs 31%) had diabetic values. No significant differences in serum lipids between groups were observed. Systolic and diastolic blood pressure reduced in both groups. However, no significant differences between groups were noted.
Table 2. Changes in clinical and metabolic risk factors from baseline to 12 months (n=275) and 18 months (n=240)a.
|
From baseline to 12 months |
From baseline to 18 months |
|||||
|---|---|---|---|---|---|---|
| INT | CON | β (95% CI)b | INT | CON | β (95% CI)b | |
| n | 139 | 136 | 118 | 122 | ||
| Weight (kg) | −3.0±5.1c | −0.3±3.6 | −2.7 (−3.7; −1.7) | −2.9±5.1c | −0.4±3.7 | −2.5 (−3.6; −1.4) |
| BMI (kg m−2) | −1.0±1.7c | −0.1±1.2 | −0.9 (−1.3; −0.6) | −1.0±1.7c | −0.1±1.3 | −0.8 (−1.2; −0.5) |
| Waist circumference (cm) | ||||||
| Men | −5.4±5.1c | −2.0±4.8c | −3.2 (−4.8; −1.6) | −4.9±5.6c | −2.4±5.7c | −2.3 (−4.3; −0.3) |
| Women | −5.2±6.0c | −0.8±4.2 | −4.4 (−6.1; −2.6) | −3.8±6.7c | −0.2±4.9 | −3.4 (−5.5; −1.3) |
| Fasting glucose (mmol l−1) | −0.2±0.7c | −0.0±0.7 | −0.2 (−0.3; −0.0) | −0.6±0.6c | −0.4±0.6c | −0.2 (−0.3; −0.0) |
| 2-h glucose (mmol l−1)c | −0.5±2.2c | 0.2±2.6 | −0.8 (−1.3; −0.3) | −1.0±2.1c | −0.3±2.5c | −0.7 (−1.2; −0.1) |
| Fasting insulin (pmol l−1) | −12.6±36.9c | 0.6±38.1 | −12.1 (−19.6; −4.6) | −17.9±35.5c | −9.3±38.5c | −8.0 (−14.7; −0.53) |
| HOMA-IR | −0.29±0.85c | 0.02±0.86 | −0.28 (−0.46; −0.11) | −0.45±0.81c | −0.25±0.85c | −0.19 (−0.34; −0.02) |
| HbA1c (% (mmol mol−1)) | −0.15±0.21c | −0.07±0.23c | −0.09 (−0.14; −0.04) | −0.17±0.21c | −0.06±0.45c | −0.11 (−0.20; −0.02) |
| −1.67±2.29c | −0.74±2.49c | −0.99 (−1.55; −0.42) | −1.86±2.31c | −0.62±4.95c | −1.18 (−2.14; −0.21) | |
| Total cholesterol (mmol l−1) | −0.07±0.88 | −0.10±0.96 | 0.02 (−0.16; 0.20) | −0.17±0.83c | −0.16±1.00 | −0.04 (−0.24; 0.16) |
| HDL cholesterol (mmol l−1) | 0.02±0.18 | 0.01±0.15 | 0.01 (−0.03; 0.05) | 0.01±0.21 | 0.04±0.18c | −0.02 (−0.07; 0.02) |
| LDL cholesterol (mmol l−1) | −0.05±0.82 | −0.14±0.88 | 0.05 (−0.11; 0.21) | −0.05±0.78 | −0.06±0.95 | −0.06 (−0.25; 0.14) |
| Triglycerides (mmol l−1) | −0.08±0.67 | 0.03±0.73 | −0.09 (−0.25; 0.07) | −0.01±0.63 | 0.03±0.57 | −0.02 (−0.16; 0.13) |
| Systolic blood pressure (mm Hg)d | −2.8±11.4c | −1.8±11.4 | −0.3 (−2.8; 2.2) | −1.9±12.9 | −2.7±11.6c | 1.0 (−2.0; 3.9) |
| Diastolic blood pressure (mm Hg)d | −4.0±7.4c | −2.4±7.0c | −1.0 (−2.6; 0.6) | −2.6±8.4c | −2.7±6.9c | 0.1 (−1.7; 1.9) |
Abbreviations: BMI, body mass index; CI, confidence interval; CON, Control group; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HOMA-IR, homoeostasis model assessment insulin resistance; INT, Intervention group; LDL, low-density lipoprotein; PA, physical activity.
Data are mean±s.d. or β (95% CI).
β (95% CI) for fasting glucose, 2-h glucose, fasting insulin, HOMA-IR, HbA1c, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, systolic and diastolic blood pressure were adjusted for medication use.
Significant difference within group (P<0.05).
From baseline to 12 months: 2-h glucose: INT n=134, CON n=132; systolic and diastolic blood pressure: INT n=136, CON n=130; From baseline to 18 months: 2-h glucose: INT n=113, CON n=118; systolic and diastolic blood pressure: INT n=113, CON n=119.
No differences in outcomes were observed between subjects recruited by laboratory glucose test (n=130) or by Diabetes Risk Test (n=110), except for fasting glucose at 12 months: in subjects recruited by laboratory glucose test, fasting glucose was significantly lower in the intervention group than in the control group (β=−0.4, 95% confidence interval (CI) −0.6; −0.1), whereas there was no effect on fasting glucose in subjects recruited by diabetes risk test (β=−0.0, 95% CI −0.2; 0.2; P for interaction=0.016; Supplementary Table S1).
At 18 months, reductions in weight, waist circumference, fasting glucose, 2-h glucose, fasting insulin, HbA1c, and HOMA-IR were sustained in favour of the intervention group (Table 2). Even more subjects than at 12 months had normoglycaemia (44% in the intervention group and 38% in the control group).
Results on dietary intake and PA
At 12 months, intake of energy, fat, and saturated fat was reduced; intake of fibre increased more in the intervention group than in the control group (Table 3). No significant differences in intake of protein, carbohydrates and alcohol between groups were observed. Fruit intake increased significantly in the intervention group but decreased in the control group. No significant difference in vegetable intake between groups was found. Generally, similar results were observed when food groups were expressed per 1000 kcal. The DHD-index score improved significantly more in the intervention group than in the control group (3.6 vs 0.3, P<0.001, Table 3).
Table 3. Changes in dietary intake and physical activity from baseline to 12 months (n=272) and 18 months (n=239)a.
|
From baseline to 12 monthsb |
From baseline to 18 monthsb |
|||||
|---|---|---|---|---|---|---|
| INT | CON | β (95% CI) | INT | CON | β (95% CI) | |
| n | 139 | 134 | 117 | 122 | ||
| Dietary intake | ||||||
| Energy intake (kcal per day) | −233.5±452.5c | −178.8±538.7c | −93.2 (−186.3;−0.2) | −226.3±453.7c | −255.3±518.8c | −5.2 (−104.3; 93.9) |
| Total protein (en%) | 0.7±2.6c | 0.3±2.29 | 0.4 (−0.2; 0.9) | 0.8±2.7c | 0.7±2.6c | 0.2 (−0.4; 0.8) |
| Total fat (en%) | −1.5±5.1c | −0.4±4.5 | −1.2 (−2.2; −0.1) | −1.6±5.4c | −0.1±5.1 | −1.6 (−2.8; −0.4) |
| Saturated fat (en%) | −0.9±2.3c | −0.2±2.0 | −0.7 (−1.2; −0.3) | −1.0±2.3c | −0.1±1.9 | −0.9 (−1.4; −0.4) |
| Total carbohydrates (en%) | 1.0±5.1c | 0.3±4.7 | 0.6 (−0.4; 1.7) | 0.9±5.0 | −0.2±5.2 | 1.0 (−0.2; 2.2) |
| Fibre (g per 1000 kcal) | 1.2±2.5c | 0.5±2.1c | 0.6 (0.1; 1.1) | 1.2±2.7c | 0.3±2.0 | 0.9 (0.4; 1.5) |
| Alcohol (en%) | −0.6±2.8c | −0.3±2.7 | −0.2 (−0.8; 0.4) | −0.4±2.7c | −0.2±3.4 | −0.1 (−0.8; 0.6) |
| Fruit intake incl. fruit juices (g per day) | 20.2±116.8c | −28.5±126.6c | 38.6 (14.3; 62.9) | 7.4±125.4 | −23.8±141.6 | 23.6 (−4.0; 51.3) |
| Vegetable intake (g per day) | 6.1±83.0 | 2.6±78.7 | 8.4 (−8.6; 25.4) | 15.7±91.9c | 2.2±80.9 | 19.3 (1.0; 37.6) |
| Fibre intake from total bread intake (%) | 0.4±1.5c | −0.0±1.1 | 0.3 (0.0; 0.5) | 0.4±1.6c | 0.1±0.9 | 0.3 (0.0; 0.6) |
| Fat intake from total bread spread intake (%) | −3.3±6.2c | −0.5±5.6 | −2.1 (−3.3; −0.9) | −3.9±6.8c | −0.5±6.5 | −2.6 (−4.1; −1.1) |
| Snack intake (kcal per day) | −59.6±146.0c | −72.3±222.0c | −15.0 (−44.5; 14.6) | −45.4±175.6c | −93.1±239.8c | 14.8 (−22.0; 51.7) |
| Soft drink intake (kcal per day) | −15.6±66.9c | −13.7±55.4c | 2.2 (−9.1; 13.6) | −21.9±57.4c | −10.6±52.3c | −8.0 (−18.5; 2.6) |
| High-fat dairy intake (g per day) | −18.0±55.6c | −7.6±51.1c | −11.6 (−21.7;−1.6) | −16.3± 52.1c | −13.7±46.8c | −6.5 (−16.1; 3.1) |
| Low-fat dairy intake (g per day) | 20.8±157.0c | −12.9±158.7 | 33.7 (−0.3; 67.6) | 12.5±182.1 | −2.3±127.4 | 16.3 (−22.7; 55.2) |
| Dutch Healthy Diet index (0–80 scale)d | 3.6±7.9c | 0.3±7.7 | 3.4 (1.7; 5.0) | 3.8±7.8c | 0.3±7.8 | 3.8 (2.1; 5.5) |
| Physical activity (PA) | ||||||
| Total PA (min per week) | 152.5±1346.5 | −80.1±1225.3 | 229.9 (−47.3; 507.1) | 159.2±1552.6 | 98.8±1094.6 | 55.0 (−258.5; 368.6) |
| Light PA (min per week) | 89.7±970.4 | 26.4±997.0 | 70.4 (−139.9; 280.8) | 33.7±1270.8 | 56.7±875.2 | −21.5 (−267.9; 225.0) |
| Moderate PA (min per week) | −2.9±649.6 | −26.3±531.0 | 39.7 (−81.8; 161.2) | 17.9±684.4 | 28.2±609.1 | 0.8 (−149.3; 150.8) |
| Vigorous PA (min per week) | 65.7±415.7c | −80.2±412.7c | 118.3 (33.8; 202.8) | 107.6±429.7c | 13.9±317.3 | 82.0 (−7.9; 171.9) |
| Physical fitness (m) | 25.1±38.7c | 2.3±37.4 | 22.6 (13.5; 31.7) | 32.8±47.2c | 10.0±40.8c | 22.3 (10.7; 33.9) |
Abbreviations: CI, confidence interval; CON, Control group; INT, Intervention group; PA, physical activity.
Data are mean±s.d. or β (95% CI).
From baseline to 12 months: dietary intake: INT n=138, CON n=134; physical activity (min/week): INT n=139, CON n=133; physical fitness: INT n=137; CON n=130; From baseline to 18 months: dietary intake: INT n=116, CON n=122; physical activity (min per week): INT n=117, CON n=122; physical fitness: INT n=105, CON n=118.
Significant difference within group (P<0.05).
From baseline to 12 months: INT n=138, CON n=133.
At 18 months, no effect on energy and protein intake was found, whereas intake of fat and saturated fat reduced even more than at 12 months in the intervention group compared with the control group (Table 3), especially in men (data not shown). Furthermore, the effect on fibre intake continued at 18 months. No significant difference in fruit intake between groups was observed anymore, in contrast to vegetable intake, which was significantly higher in the intervention group than in the control group (15.7 vs 2.2 g, P=0.039). Also at 18 months, the DHD-index score was significantly higher in the intervention group than in the control group.
At 12 months, the intervention group spent more time on vigorous activities compared with baseline, whereas this decreased in the control group (65.7 vs −80.2 min per week, P=0.006; Table 3). Furthermore, the intervention group improved more on physical fitness than the control group (covered distance 25.1 vs 2.3 m, P<0.001).
At 18 months, the intervention group further increased time spent on vigorous activities. However, the control group also slightly improved (Table 3). Especially women in the intervention group spent more time on vigorous activities than women in the control group (at 12 months p for interaction=0.055, and at 18 months p for interaction=0.051). Furthermore, the improvement in physical fitness was maintained in favour of the intervention group (Table 3).
Results on quality of life
At 12 months, the item ‘health transition’ and the sub-scales ‘physical functioning’ and ‘general mental health’ improved in the intervention group compared with the control group (P<0.05; Table 4). Additional analyses showed that the sub-scale ‘role limitations due to physical health problems’ improved in women in the intervention group but not in men (P for interaction=0.014). No significant differences in physical component score or mental component score between groups were observed (Table 4).
Table 4. Changes in quality of life from baseline to 12 months (n=271) and 18 months (n=233)a.
|
From baseline to 12 months |
From baseline to 18 months |
|||||
|---|---|---|---|---|---|---|
| INT | CON | β (95% CI) | INT | CON | β (95% CI) | |
| n | 138 | 133 | 115 | 118 | ||
| Health transition | 15.8±26.5b | 0.4±23.2 | 14.5 (10.0; 18.9) | 12.2±25.5b | −0.2±21.8 | 10.9 (5.9; 16.0) |
| General health | 5.5±15.3b | 3.0±14.6b | 2.7 (−0.3; 5.7) | 7.0±15.6b | 2.4±15.8 | 4.4 (0.9; 7.9) |
| Physical functioning | 3.4±13.1b | 0.2±12.1 | 3.1 (0.2; 5.9) | 2.7±14.4b | −1.0±14.0 | 3.4 (−0.1; 6.8) |
| Role physical | 4.7±36.1 | −0.4±35.8 | 4.3 (−3.1; 11.8) | 3.5±40.3 | 2.1±36.5 | 0.3 (−7.9; 8.5) |
| Role emotional | 4.8±34.5 | 3.1±29.9 | −0.5 (−6.8; 5.8) | 9.0±37.3b | −1.6±34.6 | 8.1 (0.6; 15.5) |
| Social functioning | 2.2±20.3 | −1.0±17.1 | 2.5 (−1.4; 6.5) | 2.5±18.8 | −2.3±19.0 | 4.5 (0.3; 8.7) |
| Bodily pain | 2.6±21.2 | −0.1±19.7 | 3.7 (−0.7; 8.0) | 1.2±21.8 | 0.5±18.6 | 1.3 (−3.4; 6.0) |
| Vitality | 4.5±12.3b | 2.1±12.6 | 2.7 (−0.1; 5.5) | 3.7±11.4b | 1.2±14.5 | 3.1 (−0.2; 6.4) |
| Mental health | 2.8±10.0b | −0.6±12.1 | 3.4 (1.0; 5.7) | 3.4±11.8b | 0.9±12.4 | 2.7 (−0.2; 5.6) |
| Physical component score | 1.5±7.4b | 0.3±6.2 | 1.4 (−0.1; 2.9) | 0.9±7.7b | 0.4±7.1 | 0.5 (−1.2; 2.3) |
| Mental component score | 1.6±7.6b | 0.4±6.9 | 1.0 (−0.5; 2.5) | 2.4±8.3b | −0.1±8.7 | 2.5 (0.6; 4.5) |
Abbreviations: CI, confidence interval; CON, Control group; INT, intervention group.
Data are mean±s.d. or β (95% CI).
Significant difference within group (P<0.05).
At 18 months, the effect on health transition continued and, additionally, significant effects were found for ‘general health perceptions’, ‘role limitations due to emotional problems’, and ‘social functioning’ in favour of the intervention group (Table 4). Moreover, the mental component score significantly improved in the intervention group compared with the control group (2.4 vs −0.1), but no effect was found on the physical component score.
Discussion
The aim of this study was to assess the effectiveness and maintenance of the SLIMMER lifestyle intervention after 12 and 18 months in Dutch primary healthcare. It was shown that the SLIMMER intervention improved body weight, clinical and metabolic risk factors, dietary intake, physical activity, and quality of life. Furthermore, it was shown that most of these improvements sustained at 18 months.
It is often shown that the effectiveness of lifestyle interventions in real-world settings is limited compared with experimental settings,3 due to the real world’s complexity and limited finance and resources.21 The SLIMMER lifestyle intervention, however, showed a weight reduction of 3.0 kg after 12 months, which is comparable with that in the original SLIM study (−2.8 kg).7 Furthermore, our study found better improvements in several clinical and metabolic risk factors, such as weight, BMI, waist circumference, fasting and 2-h glucose, and HbA1c, compared with most other real-world programmes.3, 4, 5 These results were found in two primary healthcare settings that are representative of Dutch primary healthcare.
Weight reduction during our study can be indicated as modest. However, it is still relevant, as several studies have shown that even modest weight reduction can reduce the risk of diabetes.22, 23 In the US Diabetes Prevention Program, it was shown that diabetes incidence can be reduced by around 16% for each kilogram of weight lost.22 Given the weight loss seen in the SLIMMER intervention group compared with the control group, we would expect around a 43% reduction in type 2 diabetes attributable to weight loss at 12 months, and a 40% reduction at 18 months, which is comparable with the 47% risk reduction in the SLIM study.7 A review of 36 studies assessing diabetes prevention in real-world settings revealed a 26% risk reduction, which is lower than our results, possibly because of the less intensive nature of many included interventions.4
Weight reduction is maintained at 18 months. This is remarkable, as it is well known that weight regain following the end of an intensive lifestyle programme is common within five years,24 even in successful lifestyle interventions.25, 26 This result might partly be explained by the inclusion of a maintenance programme following the intensive lifestyle programme. It is suggested that such a maintenance programme could enhance intervention effectiveness because of the use of specific behaviour change techniques such as goal-setting, self-monitoring, and relapse prevention.27 However, data on weight loss maintenance in real-world trials is limited, as very few studies report outcomes beyond 12 months.4 Our study investigated maintenance after six months. However, benefits over extended follow-up should be further investigated.
Our results indicate that the SLIMMER intervention was successful in improving overall dietary patterns and several nutrient intakes, such as total fat, saturated fat, and fibre intake. Furthermore, vigorous physical activities were more improved in the intervention group than in the control group. The Dutch Aphrodite lifestyle intervention found beneficial effects only for total physical activities and fibre intake.5 A systematic review of diabetes prevention interventions in the real world, however, concluded that, overall, changes in diet and PA are poorly reported, and that more research is needed.4
Several factors in our lifestyle programme compared to others could have contributed to intervention effectiveness. First, the SLIMMER intervention was highly intensive, with weekly sports lessons and regular dietary consultations for 10 months. Several reviews found that increased intervention effectiveness was associated with higher intervention intensity.3, 4, 27 Second, lifestyle advice in our study was provided by dieticians and physiotherapists rather than by more general lifestyle coaches in other studies, such as GPs or practice nurses (or both) and lay community educators.27 Specialist professionals are more specifically educated for, and more experienced in, delivering nutritional or physical activity advice, and this may have contributed to intervention effectiveness. Moreover, several international reviews concluded that a wide range of staff can deliver effective interventions.4, 27 Third, the thorough preparation of the SLIMMER intervention may have contributed to its effectiveness.28 Much attention was paid to carefully translating the intervention programme to the real world in a joint decision-making process with intervention developers and local healthcare professionals,8 followed by pilot-testing of the adapted intervention programme9 prior to implementation and evaluation.
Risk scores might be good tools to screen people at high risk of type 2 diabetes in primary healthcare. They are non-invasive, easy, and cheap compared with fasting plasma glucose measurements. The Diabetes Risk Test used in the current study is based on the FINDRISC questionnaire, which has been shown to be capable of predicting undiagnosed diabetes and prediabetes.29 As shown in our study, intervention subjects improved weight and glucose tolerance, independent of manner of recruitment (fasting plasma glucose or Diabetes Risk Test). This is in line with the review by Ashra et al.4
Strengths and limitations
The randomised design, comprehensive evaluation approach (outcomes at several levels), and validated methods to measure dietary intake and PA allow us to draw solid conclusions on the SLIMMER intervention’s effectiveness. By investigating outcomes at several levels (dietary intake and PA alongside clinical and metabolic risk factors), we now have more insight into determinants contributing to diabetes prevention, such as intakes of fat, saturated fat and fibre, and vigorous activities. However, it should be noted that dietary intake and physical activity were based on self-reported data. Although we observed beneficial changes in quality of life, many were non-significant. Therefore, a disease-specific questionnaire might have been used rather than the SF-36 questionnaire, as such a generic instrument is less responsive to changes in health-related quality of life.30
Conclusions
In summary, this study has shown that the Dutch SLIMMER lifestyle intervention is effective in the short and long term in improving clinical and metabolic risk factors, dietary intake, physical activity, and quality of life in subjects at high risk of diabetes. More insight into longer-term effects of the intervention on maintenance and cost-effectiveness is needed and important for sustainable diabetes prevention. The results provide valuable information for primary healthcare professionals, researchers and policymakers.
Acknowledgments
We thank all participants and healthcare professionals involved in the SLIMMER study. We also thank the local steering committees of Apeldoorn and Doetinchem (Community health service, municipality, health insurer, regional supporting organisation for primary care (ROS), general practitioners, physiotherapists, and dieticians) for facilitating implementation of the study. The Netherlands Organization for Health Research and Development ZonMw (87600048, 20400.7003) and the Dutch Diabetes Research Foundation (2011.15.1462) provided funding to conduct the study. This manuscript was made available through the repository of Wageningen University and Research Library.
Footnotes
Supplementary Information accompanies this paper on the Nutrition & Diabetes website (http://www.nature.com/nutd)
The authors declare no conflict of interest.
Supplementary Material
References
- Cefalu WTA. "spoonful of sugar" and the realities of diabetes prevention!. Diabetes Care 2014; 37: 906–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R, Davidson M. The reality of type 2 diabetes prevention. Diabetes Care 2014; 37: 943–949. [DOI] [PubMed] [Google Scholar]
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations - A systematic review and meta-analysis. Diabetes Care 2014; 37: 922–933. [DOI] [PubMed] [Google Scholar]
- Ashra NB, Spong R, Carter P, Davies MJ, Dunkley A, Gillies C et al. A Systematic Review and Meta-Analysis Assessing the Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of Type 2 Diabetes Mellitus in Routine Practice. Public Health England: London, UK, 2015. [Google Scholar]
- Vermunt PWA, Milder IEJ, Wielaard F, de Vries JHM, Baan CA, van Oers JAM et al. A lifestyle intervention to reduce Type 2 diabetes risk in Dutch primary care: 2.5-year results of a randomized controlled trial. Diabet Med 2012; 29: e223–e231. [DOI] [PubMed] [Google Scholar]
- Lakerveld J, Bot SD, Chinapaw MJ, van Tulder MW, Kostense PJ, Dekker JM et al. Motivational interviewing and problem solving treatment to reduce type 2 diabetes and cardiovascular disease risk in real life: A randomized controlled trial. Int J Behav Nutr Phys Act 2013; 10: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumen C, Corpeleijn E, Feskens EJM, Mensink M, Saris WHM, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: The SLIM study. Diabet Med 2008; 25: 597–605. [DOI] [PubMed] [Google Scholar]
- Jansen SC, Haveman-Nies A, Duijzer G, Ter Beek J, Hiddink GJ, Feskens EJ. Adapting the SLIM diabetes prevention intervention to a Dutch real-life setting: Joint decision making by science and practice. BMC Public Health 2013; 13: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijzer G, Haveman-Nies A, Jansen SC, ter Beek J, Hiddink GJ, Feskens EJM. Feasibility and potential impact of the adapted SLIM diabetes prevention intervention in a Dutch real-life setting: The SLIMMER pilot study. Patient Educ Couns 2014; 97: 101–107. [DOI] [PubMed] [Google Scholar]
- Duijzer G, Haveman-Nies A, Jansen SC, Ter Beek J, Hiddink GJ, Feskens EJM. SLIMMER: a randomised controlled trial of diabetes prevention in Dutch primary health care: Design and methods for process, effect, and economic evaluation. BMC Public Health 2014; 14: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson J, Hemio K et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the finnish diabetes prevention study. Lancet 2006; 368: 1673–1679. [DOI] [PubMed] [Google Scholar]
- Praet SFE, van Uden C, Hartgens F, HHCM Savelberg, Toereppel K, de Bie RA. KNGF-standaard Beweeginterventie diabetes mellitus type 2 [Royal Dutch Society for Physical Therapy's guidelines on physical activity intervention type 2 diabetes mellitus]. Amersfoort: Koninklijk Nederlands Genootschap voor Fysiotherapie, 2009. [Google Scholar]
- Elsman E, Leerlooijer J, ter Beek J, Duijzer G, Jansen S, Hiddink G et al. Using the intervention mapping protocol to develop a maintenance programme for the SLIMMER diabetes prevention intervention. BMC Public Health 2014; 14: 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEHM Rutten, Grauw WJC De, Nijpels G, Goudswaard AN, PJM Uitewaal, FEE van der Does et al. NHG-standaard Diabetes mellitus type 2 - Tweede herziening [Dutch College of General Practitioners' guidelines on Type 2 Diabetes Mellitus - second version]. Huisarts Wet 2006; 49: 137–152. [Google Scholar]
- World Health OrganizationDefinition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva: World Health Organization, 2006. [Google Scholar]
- World Health Organization Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus Abbreviated Report of a WHO Consultation. Geneva: World Health Organization, 2011. [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes - 2015. Diabetes Care 2015; 38(Supplement 1): S1–S94. [Google Scholar]
- RIVM/Dutch Nutrition CentreNEVO-tabel, Nederlands Voedingsstoffenbestand 2011 [Dutch Food Composition Table 2011]. RIVM/Dutch Nutrition Centre: Den Haag, 2011. [Google Scholar]
- Van Lee L, Feskens EJM, Hooft van Huysduynen EJC, de Vries JHM, van 't Veer P, Geelen A. The Dutch Healthy Diet index as assessed by 24 h recalls and FFQ: associations with biomarkers from a cross-sectional study. J Nutr Sci 2014; 2: e40–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hollander EL, Zwart L, De Vries SI, Wendel-Vos W. The SQUASH was a more valid tool than the OBiN for categorizing adults according to the Dutch physical activity and the combined guideline. J Clin Epidemiol 2012; 65: 73–81. [DOI] [PubMed] [Google Scholar]
- Garfield SA, Malozowski S, Chin MH, Narayan KMV, Glasgow RE, Green LW et al. Considerations for diabetes translational research in real-world settings. Diabetes Care 2003; 26: 2670–2674. [DOI] [PubMed] [Google Scholar]
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006; 29: 2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaristo T, Moilanen L, Korpi-Hyovalti E, Vanhala M, Saltevo J, Niskanen L et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010; 33: 2146–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: The effect of dietary counseling for weight loss. Ann Intern Med 2007; 147: 41–50. [DOI] [PubMed] [Google Scholar]
- Knowler W, Fowler S, Hamman R, Christophi C, Hoffman H, Brenneman A et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 2009; 374: 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Peltonen M, Eriksson J, Ilanne-Parikka P, Aunola S, Keinanen-Kiukaanniemi S et al. Improved lifestyle and decreased diabetes risk over 13years: long-term follow-up of the randomised finnish Diabetes Prevention Study (DPS). Diabetologia 2013; 56: 284–293. [DOI] [PubMed] [Google Scholar]
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011; 11: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP et al. A tutorial on pilot studies: The what, why and how. BMC Med Res Methodol 2010; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos FX, Barengo NC, Costa B, Mundet-Tudurí X, Lindström J, Tuomilehto JO. Screening for people with abnormal glucose metabolism in the European DE-PLAN project. Diabetes Res Clin Pract 2015; 109: 149–156. [DOI] [PubMed] [Google Scholar]
- El Achhab Y, Nejjari C, Chikri M, Lyoussi B. Disease-specific health-related quality of life instruments among adults diabetic: A systematic review. Diabetes Res Clin Pract 2008; 80: 171–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.