Abstract
Recent studies have demonstrated the importance of flavonoid intake and disease risk, however the association between flavonoid intake and obesity has not been evaluated in a nationally representative sample of US adults. The objective of the study was to evaluate the association between flavonoid consumption and established risk factors for obesity and obesity-related inflammation. Data from a nationally representative sample of 9551 adults who participated in the 2005–2008 National Health and Nutrition Examination Survey (NHANES) were analyzed. Flavonoid consumption was inversely associated with obesity in both men and women in multivariate models. Adults in the highest quartile of flavonoid intake had significantly lower body mass index and waist circumference than those in the lowest quartile of flavonoid intake (P<0.03 and P<0.04, respectively), and flavonoid intake was inversely related to C-reactive protein levels in women (p-trend, 0.01). These findings support a growing body of laboratory evidence that flavonoid consumption may be beneficial for disease prevention.
Introduction
Over the past few decades, the rates of obesity have risen markedly.1 Overweight and obesity have been identified as risk factors for several diseases, including cardiovascular disease, diabetes and cancer. Survey data from large, nationally representative samples of US individuals can provide insight into trends that have potentially contributed to this increase in obesity. Data from the National Health and Nutrition Examination Surveys (NHANES) have shown that although there have been no substantial changes in eating frequency, snacking behaviors or overall energy intake among US adults, there have been significant changes in macronutrient distribution and beverage consumption, suggesting that specific dietary patterns, such as increased intake of carbohydrates and sugar-sweetened beverages, may influence weight status.2, 3, 4
Flavonoid consumption has been linked to a decrease in risk for: stroke;5 cardiovascular disease;6, 7 asthma;8 and some cancers.9, 10, 11, 12, 13, 14 The majority of studies on the relationship between flavonoid intake and obesity have focused on intake of specific flavonoids subtypes and body mass index or weight gain15, 16 or lipid metabolism;17 with very few assessing the relationship between overall flavonoid intake and obesity. The objective of this study was to determine the relationship between flavonoid consumption and obesity in a representative sample of 9551 US adults. Using NHANES data, the association of flavonoid consumption and multiple markers for obesity including: body mass index (BMI), waist circumference and C-reactive protein were explored.
Materials and methods
Data source
NHANES is a large, cross-sectional survey conducted by National Center for Health Statistics. NHANES is designed to monitor the health and nutritional status of non-institutionalized civilians in the US; nationally representative survey and physical data are collected on a continual basis and released in two-year increments. Complete details regarding the NHANES sampling methodology, data collection, and interview process are available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm). Written consent is obtained from all NHANES participants. Data from the 2005–2008 survey cycles were combined for this study. The present study was approved by the Institutional Review Board at the Pennsylvania State University.
Anthropometric and biomarker data
In both cycles of NHANES, height and weight were measured by trained examiners using standardized protocols and calibrated equipment during the physical examination component of the study. Adults were classified as lean (BMI ⩽24.9 kg m−2), overweight (BMI of 25.0–29.9) or obese (BMI ⩾30) using CDC cut points (https://www.cdc.gov/obesity/adult/defining.html). For this analysis, underweight (BMI <18.5) participants were included in the lean category. Blood samples were collected on a smaller subset of the population. Non-fasting samples were obtained for C-reactive protein (CRP), a marker for inflammation.
Assessment of flavonoid intake
Flavonoid content of the diet was assessed using the USDA Flavonoid database version 3.0,18 which includes flavonols, flavones, flavanones, flavan-3-ols and anthocyanidins. The database contains a list of foods and the currently available flavonoid compound data by flavonoid class. The food data were aggregated where possible to match USDA National Nutrient Databank (NDB) standard reference (SR) codes.18 The SR codes were used to identify and match foods from the flavonoid database to corresponding FNDDS codes in order to identify foods that contain bioactive compounds (for example, a smoothie containing pomegranate juice). Using the FNDDS ingredient file, the gram weight of each flavonoid-containing component of a dish was determined; SR codes were used to re-link flavonoid content to corresponding 8-digit USDA food codes in the present NHANES database. The USDA has since released an expanded Flavonoid database, version 3.2, which contains data for 29 individual flavonoid compounds in six subclasses of flavonoids for every food in a subset of 2926 food items which provide the basis for the newer Food and Nutrient Database for Dietary Studies which can be used with later iterations of NHANES.19
Statistical analysis
For the present analyses, we initially included all adults age 18 and older that had complete dietary and anthropometric data. Individuals who were currently following a weight-loss diet, individuals with implausible or very unusual dietary recall (for example, reporting no beverages during the 24-h recall period) and women who were pregnant or lactating were excluded, resulting in a full sample of 9551 adults. The 2005–2006 NHANES dietary data includes a food-frequency questionnaire (FFQ) in addition to 24-h recalls. In this analytic sample, only FFQ data for 4296 adults was available. Data from the FFQ was used to assess the relationship between regular consumption of flavonoid-containing foods and C-reactive protein, an inflammatory marker.
Age at the time of exam, education level, smoking status (current, former, never smoker), physical activity (measured in minutes of physical activity at a specific MET level), race and socioeconomic status were all provided in the NHANES data set. Socioeconomic status was quantified as a continuous variable using poverty–income ratio (PIR), or the ratio of family income to family-size specific poverty threshold.
All data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Specific survey procedures were used in the analysis to account for sample weights, unequal selection probability, and clustered design. Multivariate regression was used to evaluate the association of flavonoid intake with obesity (for example, body mass index, waist circumference), and markers for inflammation (C-reactive protein). Sex-specific analysis was conducted to take into account the natural differences in body composition and caloric needs between men and women. All models were adjusted for age, race, education, physical activity, smoking status, income (measured by PIR), dieting status, total alcohol intake, and caloric intake with significance determined at P<0.05.
Results
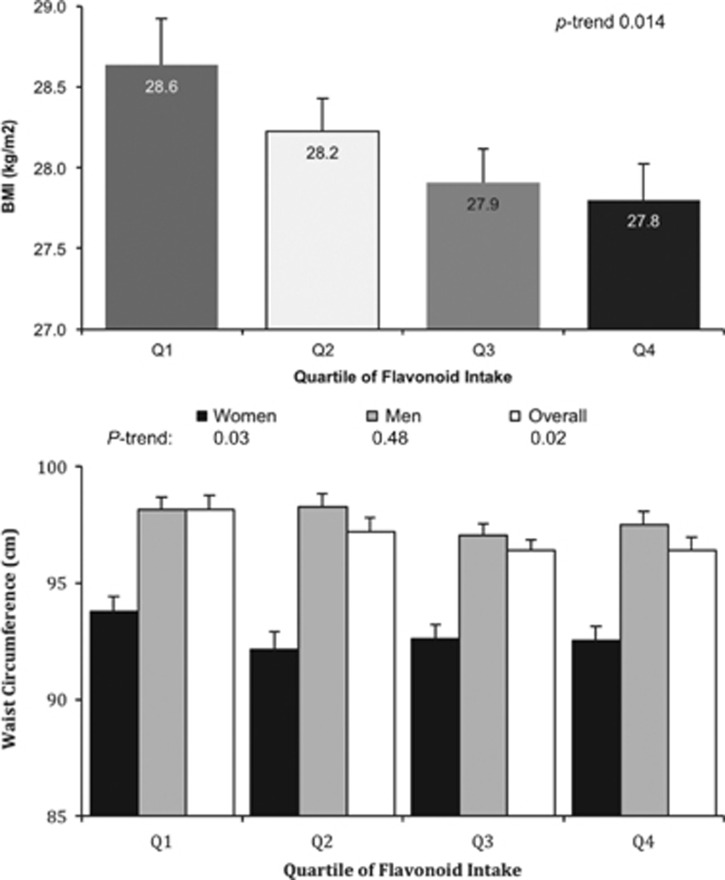
Demographic characteristics are presented in Table 1. The population sample contained equal percentages of men and women; approximately half of the sample had a history of smoking, and over 90% of the population reported consumption of a flavonoid- containing foods. An inverse association between total flavonoid intake and BMI was observed (p-trend, 0.013) after adjusting for age, sex, race, education, physical activity, smoking status, poverty:income ratio, total alcohol intake, total fat intake, and dietary energy density. (Figure 1). When evaluating flavonoid intake and waist circumference, a similar association was observed (p-trend 0.02, Figure 1). Table 2 demonstrates the relationship between regular consumption of flavonoid-containing foods and C-reactive protein levels, after controlling for the same cofactors. Across all food categories, it was noted that higher intake of flavonoid-containing foods was associated with lower CRP levels. When evaluating the association between overall flavonoid intake and CRP levels, an overall inverse association was observed (p-trend 0.01), but mean CRP levels did not differ between specific quartiles after controlling for relevant covariates.
Table 1. Demographic characteristics of US Adults: NHANES 2005–2008.
| Sample na | Percentb | |
|---|---|---|
| Sex | ||
| Female | 4587 | 50.0 |
| Male | 4964 | 50.0 |
| Age group | ||
| 18–30 | 2270 | 23.5 |
| 31–50 | 2972 | 37.6 |
| 51–70 | 2712 | 27.7 |
| >70 | 1597 | 11.2 |
| Racec | ||
| NH-White | 4502 | 71.0 |
| NH-Black | 2156 | 11.5 |
| Mex-Am | 1802 | 8.3 |
| Other | 1091 | 9.3 |
| Education | ||
| HS or less | 2931 | 19.8 |
| High School Grad/GED | 2427 | 26.5 |
| Some College or AA degree | 2545 | 30.0 |
| College Graduate or above | 1642 | 23.7 |
| Incomed | ||
| PIR <130% | 3356 | 24.9 |
| 130<PIR<350% | 3483 | 34.4 |
| PIR >350% | 2712 | 40.7 |
| Smoking status | ||
| Never smoker | 4540 | 50.9 |
| Current smoker | 2029 | 25.1 |
| Ever smoker (>100 cigarettes) | 2246 | 23.9 |
| Weight statuse | ||
| Lean (BMI <25) | 3259 | 36.2 |
| Overweight (BMI 25–30) | 3183 | 32.9 |
| Obese (BMI >30) | 3109 | 30.9 |
| Survey cycle | ||
| 2005–2006 | 4296 | 48.2 |
| 2007–2008 | 5255 | 51.8 |
| Flavonoid consumer | ||
| No | 434 | 4.9 |
| Yes | 9482 | 95.6 |
Abbreviations: BMI, body mass index; PIR, poverty–income ratio.
Sample n represents raw participant counts.
Population percentages based on NHANES survey weights and represents that population of non-institutionalized US adult residents.
Race categories: NH-White, Non-Hispanic white; NH-Black, Non-Hispanic black, Mex-Amer, Mexican American; Other.
Adjusted income level based on poverty:income ratio adjusted for household size and
Weight status categorized by body mass index, measured in kg m−2.
Figure 1.
Body mass index and waist circumference by flavonoid intake quartile. Least-squared means calculated with adjustment for age, sex, race, education, physical activity, smoking status, poverty–income ratio, total alcohol intake, total fat intake and dietary energy density.
Table 2. Adjusted mean C-reactive protein level (mg dl−1) by consumption category.
| Consumption category | Onions | Tofu | Apples | Grapes |
|---|---|---|---|---|
| Non-consumer | 4.10 | 4.10 | 4.81 | 5.63 |
| Infrequent (<1 × /month) | 4.37 | 3.24 | 4.16 | 3.83 |
| Monthly consumer (<3 × /month) | 3.84 | 3.41 | 3.81 | 3.56 |
| Weekly consumer (1–2 × /week) | 3.66 | 2.65 | 3.28 | 4.09 |
| Regular consumer (>3 × /week) | 3.85 | 2.80 | 2.91 | 2.78 |
| p-trend | 0.002 | 0.004 | 0.001 | 0.02 |
| Wine | Hot tea | |||
| Non-consumer | 4.30 | 5.71 | ||
| Infrequent (1c/week or less) | 3.44 | 4.43 | ||
| Weekly, but not daily (2–6c/week) | 3.39 | 3.84 | ||
| Daily consumer (1c/day) | 2.79 | 3.81 | ||
| Multiple cups/day | 4.09 | 3.26 | ||
| p-trend | 0.04 | 0.0002 |
CRP least-squared means presented are adjusted for sex, age, race, smoking status, SES, education, physical activity, BMI and total caloric intake.
Discussion and conclusion
In this nationally representative sample of US adults, intake of dietary flavonoids was inversely associated with obesity and CRP. Few studies have evaluated or identified specific dietary patterns that are related to obesity and markers for Metabolic syndrome.19 The association between intake of a specific flavonoid-containing food (that is, tea, soy) and obesity has been previously demonstrated in a nationally representative sample of US adults,20, 21 has been inversely correlated to longitudinal weight gain in larger cohort studies22 and has also been recently correlated with lower levels of inflammatory markers in a cohort sample from the Framingham Offspring Study.23 However the present study takes a novel approach to assessing the relationship between total dietary flavonoid intake and markers for obesity by applying the USDA Flavonoid database to NHANES survey data. These findings support a growing body of laboratory evidence that flavonoid consumption may be beneficial for disease prevention.
Acknowledgments
JAV performed the analysis and wrote the first draft of the manuscript, JDL provided critical revisions to the manuscript before submission. Both authors read, reviewed and approved the final version of this manuscript. This study was funded by a grant from the National Institutes of Health (AT004678) to JDL.
Footnotes
The authors declare no conflict of interest.
References
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010; 303: 235–241. [DOI] [PubMed] [Google Scholar]
- Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971-1975 to NHANES 1999-2002. Am J Clin Nutr 2006; 84: 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971-2006. Am J Clin Nutr 2011; 93: 836–843. [DOI] [PubMed] [Google Scholar]
- Bleich SN, Wang YC, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988-1994 to 1999-2004. Am J Clin Nutr 2009; 89: 372–381. [DOI] [PubMed] [Google Scholar]
- Tang Z, Li M, Zhang X, Hou W. Dietary flavonoid intake and the risk of stroke: a dose-response meta-analysis of prospective cohort studies. BMJ Open 2016; 6: e008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr 2012; 95: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursu J, Nurmi T, Tuomainen TP, Ruusunen A, Salonen JT, Voutilainen S. The intake of flavonoids and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr 2007; 98: 814–818. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Takahashi R. Flavonoids and asthma. Nutrients 2013; 5: 2128–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geybels MS, Verhage BA, Arts IC, van Schooten FJ, Goldbohm RA, van den Brandt PA. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am J Epidemiol 2013; 177: 1388–1398. [DOI] [PubMed] [Google Scholar]
- Kocic B, Kitic D, Brankovic S. Dietary flavonoid intake and colorectal cancer risk: evidence from human population studies. J Buon 2013; 18: 34–43. [PubMed] [Google Scholar]
- Woo HD, Kim J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J Gastroenterol 2013; 19: 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora-Ros R, Fedirko V, Trichopoulou A, Gonzalez CA, Bamia C, Trepo E et al. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int J Cancer 2013; 133: 2429–2443. [DOI] [PubMed] [Google Scholar]
- Zamora-Ros R, Ferrari P, Gonzalez CA, Tjonneland A, Olsen A, Bredsdorff L et al. Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Res Treat 2013; 139: 163–176. [DOI] [PubMed] [Google Scholar]
- Hui C, Qi X, Qianyong Z, Xiaoli P, Jundong Z, Mantian M. Flavonoids, flavonoid subclasses and breast cancer risk: a meta-analysis of epidemiologic studies. PLoS One 2013; 8: e54318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard DR, Ross R, Janssen I. Coffee, tea and their additives: association with BMI and waist circumference. Obes Facts 2010; 3: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes LA, Arts IC, Ambergen T, Brants HA, Dagnelie PC, Goldbohm RA et al. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: a longitudinal analysis from the Netherlands Cohort Study. Am J Clin Nutr 2008; 88: 1341–1352. [DOI] [PubMed] [Google Scholar]
- Assini JM, Mulvihill EE, Huff MW. Citrus flavonoids and lipid metabolism. Curr Opin Lipidol 2013; 24: 34–40. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture Agricultural Research Service. USDA Database for the Flavonoid Content of Selected Foods, Release 3.0. 2011. Available from: Nutrient Data Laboratory Home Page https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03.pdf.
- Bhagwat S, Haytowitz DB, Wasswa-Kintu S. USDA’s Expanded Flavonoid Database for the assessment of Dietary Intakes, Release 1.1. U.S. Department of Agriculture, Agricultural Research Service. Nutrient Data Laboratory Home Page 2015. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-special-interest-databases-on-flavonoids/.
- Baik I, Lee M, Jun NR, Lee JY, Shin C. A healthy dietary pattern consisting of a variety of food choices is inversely associated with the development of metabolic syndrome. Nutr Res Pract 2013; 7: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernarelli JA, Lambert JD. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur J Nutr 2013; 52: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoia ML, Rimm EB, Mukamal KJ, Hu FB, Willett WC, Cassidy A. Dietary flavonoid intake and weight maintenance: three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016; 352: i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy A, Rogers G, Peterson JJ, Dwyer JT, Lin H, Jacques PF. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am J Clin Nutr 2015; 102: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]