Abstract
Many G protein-coupled receptors (GPCRs) are reported to be involved in the pathogenesis of multiple sclerosis (MS), and ~40% of all identified GPCRs rely on the Gαq/11 G protein family to stimulate inositol lipid signaling. However, the function of Gα subunits in MS pathogenesis is still unknown. In this study, we attempted to determine the role of Gαq in the pathogenesis of experimental autoimmune encephalomyelitis (EAE), a well-known mouse model of MS. We discovered that compared with wild-type mice, Gαq-knockout mice exhibited less severe EAE symptoms, with lower clinical scores, reduced leukocyte infiltration and less extensive demyelination. Moreover, a significantly lower percentage of Th17 cells, one of the key players in MS pathogenesis, was observed in Gαq-knockout EAE mice. Studies in vitro demonstrated that deficiency of Gαq in CD4+ T cells directly impaired Th17 differentiation. In addition, deficiency of Gαq significantly impaired DC-derived IL-6 production, thus inhibiting Th17 differentiation and the Gαq-PLCβ-PKC and Gαq-MAPKs signaling pathways involved in the reduced IL-6 production by DCs. In summary, our data highlighted the critical role of Gαq in regulating Th17 differentiation and MS pathogenesis.
Keywords: Dendritic cells, EAE, Gαq, IL-6, Th17 differentiation, MS
Introduction
Multiple sclerosis (MS) is a common autoimmune inflammatory disease of the central nervous system (CNS) characterized by immune-mediated demyelination, axonal loss and tissue destruction. The animal model, experimental autoimmune encephalomyelitis (EAE), has many pathological and histological similarities with MS. The pathogenesis of MS is still unclear. Many environmental risk factors have been reported to be involved in MS pathogenesis, such as viral infection, sunlight exposure and cigarette smoking.1, 2 Recently, many studies have shown that both Th1 and Th17 cells play a role in MS.3 T cell polarization, leukocyte migration and infiltration into the CNS are very important steps involved in EAE pathogenesis.4 However, how various environmental stimuli participate in MS and EAE pathogenesis is unclear. G protein-coupled receptors (GPCRs) are known to mediate most of the physiological responses to environmental stimuli, suggesting that GPCRs may play an important role in MS pathogenesis by mediating signal transduction.
GPCRs, which are important drug targets, mediate a number of biological processes by transmitting signals across the cell membrane.5 The binding of agonist and receptor leads to the activation of the G protein Gα subunit and the Gβγ dimer, which can regulate a set of independent effectors.6 Gα subunits are divided into five different families: Gαq, Gαs, Gαi, Gαv and Gα12, according to sequence similarity.7, 8 The Gαq/11 family has four family members; Gαq and Gα11 are ubiquitously expressed, Gα14 is expressed in the kidney, liver and lung, and Gα15/16 is only found in hematopoietic cells.9 Many cellular proteins have been reported to interact with Gαq, such as phospholipase C β (PLCβ), Akt and MAPK members.10
Many GPCRs have been reported to participate in MS or EAE pathogenesis.11 In MS patients, β-adrenergic receptors have been reported to be increased in PBMCs and correlated with disease activity;12 a significant decrease in kappa opioid receptors has been found in the spinal cords of MS patients and are thought to be related to neuropathic pain.13 CVT-6883, an A2BAR-specific antagonist, alleviates EAE pathogenesis via reducing IL-6 secretion from dendritic cells (DCs);14 CXCR3 signaling reduces the severity of EAE by controlling the distribution of effector and regulatory T cells in the CNS.15 However, the function of G proteins in MS or EAE is still not clear. It has been estimated that almost 40% of all identified GPCRs rely on the Gαq/11 family to stimulate signaling. In this study, we found that Gαq-KO mice showed less severe clinical symptoms of EAE and reduced CNS infiltration of inflammatory cells. Further research demonstrated that Gαq-KO mice showed reduced Th17 differentiation by inhibiting IL-6 production by DCs, which was mediated by Gαq-PLCβ and MAPK signaling. Our results revealed a critical role of Gαq in regulating EAE pathogenesis by influencing Th17 differentiation and IL-6 production in DCs.
Materials and methods
Mice
C57BL/6 mice were purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Gαq-KO mice were derived from the C57BL/6 strain, and genotype identification was based on PCR.16 All mice were maintained at the Tongji University animal care facility in pathogen-free conditions. The experiments were carried out in accordance with the Tongji University Animal Care Committee guidelines.
EAE induction
Female mice (8–10 weeks of age) were immunized with MOG35-55 (200 μg) in CFA, which contained heat-killed Mycobacterium tuberculosis (5 mg/ml). Pertussis toxin (200 ng/mouse) was IP injected on day 0 and day 2. Clinical appearance was assessed daily and scored as follows: 0, normal; 1, paralyzed tail; 2, mildly paralyzed hind legs; 3, totally paralyzed hind legs; 3.5, paraplegia with mildly paralyzed forelimbs; 4, paraplegia with paralyzed forelimbs; 4.5, moribund and 5, death.
Histopathological and immunohistochemical analysis
For the histological analysis, mice were anesthetized with chloral hydrate and then perfused with phosphate buffer saline (PBS), followed by 4% paraformaldehyde (pH 7.4). Lumbar spinal cord samples were collected and fixed in 4% paraformaldehyde ⩾12 h. Paraffin-embedded tissue sections (5 μm) were stained with H&E and Luxol fast blue to analyze inflammatory infiltration and demyelination. Image-Pro software was used to calculate the number of infiltrating cells and the percentage of myelin loss in inflammatory foci per section for quantization of inflammation and demyelination levels, respectively. Paraffin-embedded sections of the spinal cords were rehydrated and put in antigen retrieval solution at 95 °C for 20 min before proceeding to immunohistochemistry. Sections were incubated with rabbit polyclonal anti-NFH antibody (N4142, 1:500) and mouse anti-GFAP antibody (MAB360, 1:500) at 4 °C overnight. The secondary antibody was conjugated to Alexa Fluor 546 (Thermo Fisher, A-11010, 1:1000) or 488 (Thermo Fisher, A-11001, 1:1000), and nuclei were stained with DAPI. An Olympus IX51 inverted fluorescence microscope was used for fluorescence detection.
Reverse transcription and quantitative real-time PCR
Total RNA was extracted from mouse tissues (lymph nodes, spleen, cerebrum and lumbar spinal cord) using TRI reagent (Molecular Research Center, Inc.). Reverse transcription was performed with random hexamer primers and murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). Quantitative real-time PCR was assayed in a LightCycler quantitative PCR apparatus with SYBR Green 2 × qPCR Master Mix (Bioneer, Seoul, South Korea). Expression was normalized relative to β-actin and then to the expression of the control. The primer sequences are listed in Supplementary Table 1.
Flow cytometry
Lymphocytes from tissues or CD4+ T cells from in vitro differentiation assays were incubated with PMA (50 ng/ml; Sigma, St Louis, MO, USA), ionomycin (750 ng/ml; Sigma) and brefeldin A (10 μg/ml; Sigma) for 5 h at 37 °C. Surface markers were incubated with relevant antibodies for 30 min at 4 °C in the dark. Then, the cells were subjected to fixation and permeabilization, followed by intracellular cytokine (IL-17 and IFN-γ) staining with relevant antibodies for 30 min at 4 °C in the dark. A Foxp3 staining buffer set (eBioscience, San Diego, CA, USA) was used to detect Treg cells after fixation, permeabilization and staining with antibody for 30 min at 4 °C in the dark. Flow cytometric analysis was carried out with a Guava EasyCyte 8HT system and GuavaSoft software.
ELISA
Serum was collected from the orbital venous blood. To collect culture supernatants, lymphocytes were isolated from the draining lymph nodes, seeded into 96-well plates (2 × 105/well/100 μl and restimulated with MOG35–55(20 μg/ml) for 3 days at 37 °C. The concentration of IL-17A, IFN-γ, TGF-β and IL-6 in the serum and the culture supernatants was measured using ELISA kits (eBioscience).
CD4+ T cell isolation and in vitro differentiation
CD4+ T cells were isolated by magnetic depletion of non CD4+ T cells using a cocktail of biotin-conjugated antibodies from single-cell suspensions of mouse spleen (Invitrogen, Oslo, Norway). Cells were activated with anti-CD28 (2 μg/ml) and anti-CD3 (2 μg/ml). Anti-IL-4 (10 μg/ml) and IL-12 (10 ng/ml) were added for Th1 polarization. Anti-IFN-γ (10 μg/ml), anti-IL-4 (10 μg/ml), IL-6 (30 ng/ml), TGF-β1 (3 ng/ml), IL-1β (10 ng/ml) and TNF-α (10 ng/ml) were added For Th17 polarization. Anti-IFN-γ (10 μg/ml), IL-2 (10 ng/ml) and TGF-β1 (5 ng/ml) were added for Treg polarization. Cells were collected on day 4 for analysis.
DC generation, stimulation, IL-6 measurement and migration assay
Bone marrow progenitors, isolated from the femurs and tibias of mice, were cultured in complete medium supplemented with murine GM-CSF (20 ng/ml) and murine IL-4 (1 ng/ml) for 7 days to generate bone marrow-derived dendritic cells (BMDCs). LPS (1 μg/ml) was added and incubated for 24 h to induce maturation of BMDCs. The supernatants were collected, and IL-6 was measured by ELISA assay. Transwell chambers with 5 μm pore size (Costar 3421, Corning, Kennebunk, ME, USA) was used for the migration assay. A total of 5 × 105 LPS-matured BMDCs were placed in the upper chamber, and CCL19 (100 ng/ml; Peprotech, Rocky Hill, NJ, USA), CCL21 (100 ng/ml; Peprotech), and CXCL12 (200 ng/ml; Peprotech) were placed in the lower chamber at 37 °C. After incubation for 3 h, cells in the lower chamber were collected and stained with CD11c for flow cytometric analysis. The numbers of migrated BMDCs are given as the fold increase over the blank control.
DC-T cell co-culture
A CD4+CD62L+T cell Isolation Kit II (Miltenyi Biotec, Auburn, CA, USA) was used to isolate naive T cells from single-cell suspensions from the spleen; in the first step, non-CD4+ cells were magnetically depleted, and magnetically labeled CD62L+ T cells were positively selected in the second step. In the DC-T cell co-culture assay, naive T cells and BMDCs were cultured at a ratio of 3:1 in complete medium supplemented with anti-CD28 (2 μg/ml), anti-CD3 (2 μg/ml) for 72 h. Anti-IFN-γ (10 μg/ml), TGF-β1 (3 ng/ml), and anti-IL-4 (10 μg/ml) were added for Th17 polarization. Cells were collected on day 4, and the percentage of Th17 cells in the CD4+ gate was detected by flow cytometric analysis.
Statistical analysis
A two-way ANOVA test was used to assess the significance of EAE clinical scores between the two groups throughout the disease course. The Mann–Whitney U-test was used to compare the significance of EAE clinical scores between the two groups on a given day. Student’s t-test was used to assess the other analyses. All data are expressed as the mean±s.e.m. P values<0.05 were considered significant.
Results
G protein Gαq-KO mice develop less severe EAE
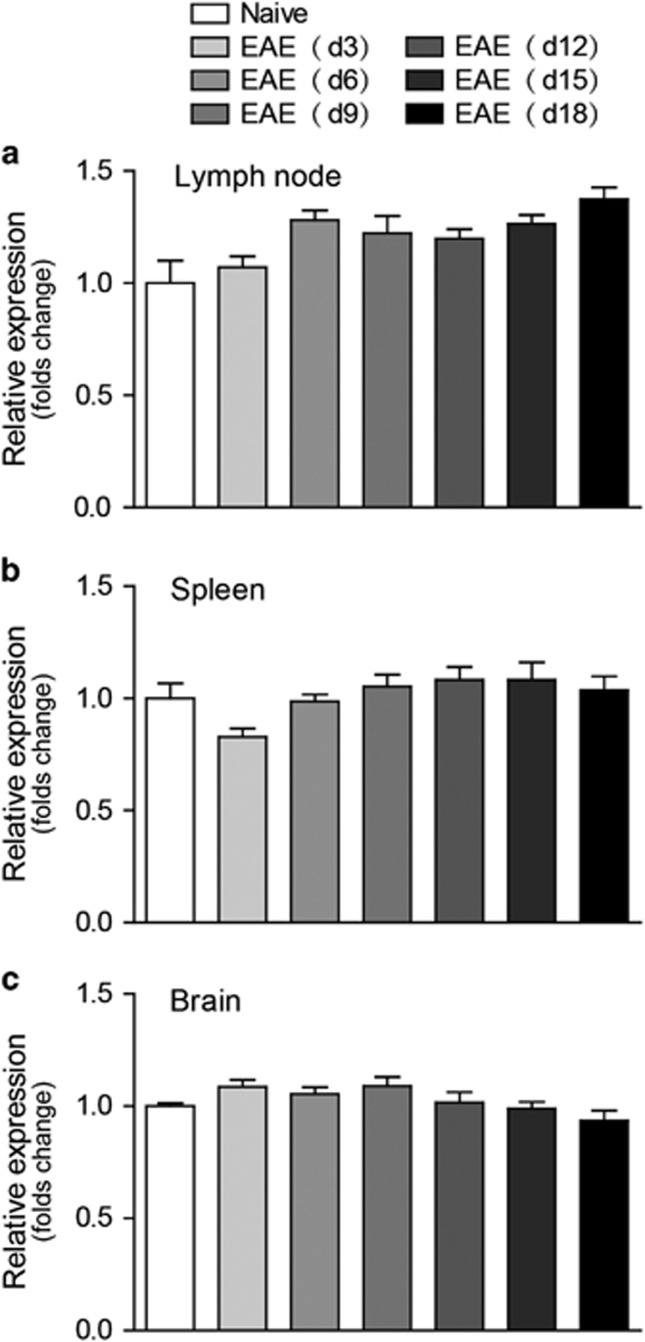
Approximately 40% of GPCRs rely on the Gαq/11 G protein family to perform their functions. In addition, some GPCRs, such as the A2B adenosine receptor, β2-adrenergic receptor and CXCR3, coupled to Gαq/11 protein, were reported to play a role in the pathogenesis of EAE and other autoimmune disease. Thus, we were interested in the role of Gαq in EAE pathogenesis. C57BL/6 mice were induced to develop EAE with MOG35-55, and we assessed the mRNA level of Gαq in different tissues (lymph nodes, spleen and brain) on days 3, 6, 9, 12, 15 and 18 post-immunization (PI). Expression of Gαq was abundant in the detected tissues, and a slight upregulation of Gαq was observed in lymph nodes (Figure 1a), while no significant changes were found in either the spleen or central nervous system during EAE pathogenesis (Figures 1b and c). The stable expression level of Gαq is reasonable, as Gαq plays broad and critical roles in the regulation of physiology.
Figure 1.
Expression profile of Gαq in the peripheral immune tissues and central nervous system during EAE pathogenesis. Total RNA was isolated from the spleen, lymph nodes and brains of naive controls and EAE mice on days 3, 6, 9, 12, 15, and 18 post immunization. qPCR was performed to analyze gene expression. The expression was normalized to relative β-actin and then to the expression of the control. (a–c) Relative expression level of Gαq in lymph nodes (a), spleen (b) and brain (c). The data are expressed as the mean±s.e.m. (n=6).
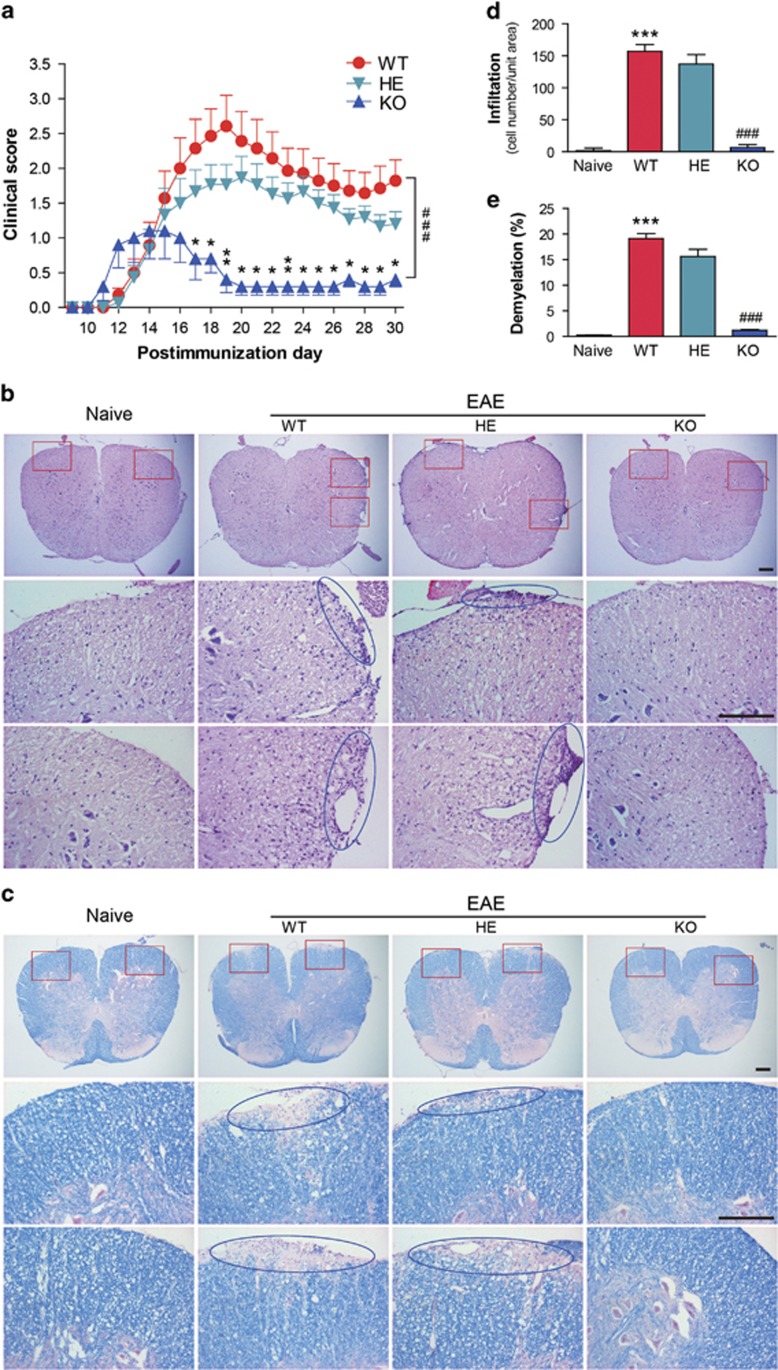
To investigate the function of Gαq during EAE development, Gαq-KO mice (PCR identification, Supplementary Figure 1) were subjected to EAE induction with MOG35-55 immunization, together with the relative heterozygotes and wild-type littermate controls. The clinical appearance of EAE was assessed daily for 30 days. Our results showed that compared with the WT controls, EAE severity was significantly alleviated in the Gαq-KO mice, with low incidence (80%), and a slight relief of disease symptoms was observed in the heterozygous group (Figure 2a, Table 1). Because our previous work showed that the expression of some proinflammatory cytokines, such as IL-17 and IL-6, was uniformly upregulated on day 15 and reduced on day 18 in the CNS (data not shown), the lumbar spinal cords, which had the most leukocyte infiltration and the most severe demyelination, were collected on day 17 to detect leukocyte infiltration and myelin loss in CNS. Based on the quantization of H&E staining with Image-Pro software, we found that compared with WT mice, leukocyte infiltration in the lumbar spinal cord was remarkably decreased in Gαq-KO mice (Figures 2b and d), and less demyelination in the Gαq-KO mice was also shown by Luxol fast blue staining (Figures 2c and e). Fluorescent staining of the nucleus with DAPI in the paraffin sections of spinal cords was also performed to test leukocyte infiltration in the CNS, which confirmed that deficiency of Gαq efficiently prevented leukocyte infiltration of the spinal cord (Supplementary Figures 2A and B). We also assessed the gliosis and neurofilament expression in the inflammatory foci of the spinal cord by immunostaining with antibodies targeting GFAP (gliosis) or NFH (neurofilaments) (Supplementary Figure 2C).17, 18 The increase of GFAP-reactive gliosis, which correlated with EAE progression, was suppressed in Gαq-KO mice. NFH staining showed that the extensive axonal damage in the spinal cord sections of WT mice was absent in Gαq-KO mice. All these results demonstrated that Gαq was functionally involved in EAE pathogenesis.
Figure 2.
Gαq-KO suppresses EAE pathogenesis. (a) Clinical scores of EAE in WT (n=14), heterozygous (n=15) and Gαq-KO (n=5) mice. The data are expressed as the mean±s.e.m. ###P<0.001 versus WT group throughout the disease course (two-way analysis of variance (ANOVA)), *P<0.05 and **P<0.01 versus WT on any given day (Mann–Whitney U-test). (b) Hematoxylin and eosin and (c) Luxol Fast Blue staining of the paraffin sections of the spinal cords isolated from naive and EAE-induced WT, heterozygous, and Gαq-KO mice on day 17 PI, and boxed areas in the top column are enlarged and presented at the bottom; the circular areas show infiltration or demyelination. Scale bars, 200 μm. (d and e) Quantization of CNS infiltrates; the percentages of demyelination presented in (b) and (c) were quantified by Image-Pro. Five mice from each group were killed, and 20 sections from each mouse were analyzed. The data are expressed as the mean±s.e.m. ***P<0.001 versus naive group, ###P<0.001 versus WT-EAE.
Table 1. Development of EAE.
| Group | Incidence | Day of disease onset | Maximum clinical score | Score>3 |
|---|---|---|---|---|
| Wild type | 14 of 14(100%) | 15.0±0.9 | 3.1 (±0.4) | 9 of 14 |
| heterozygote | 14 of 15(93.3%) | 14.2±0.4 | 2.4 (±0.3) | 7 of 15 |
| Gαq-KO | 4 of 5(80%) | 11.5±0.3 | 1.1 (±0.4)* | 0 of 5 |
Development of MOG35–55-induced EAE. Incidence reflects the number of mice that developed EAE relative to the total mice in a group; Day of disease onset is presented in days (mean±s.e.m.); Maximum clinical score is the average of the highest score reached by each mouse in a group (mean±SEM.); Score>3 shows the number of mice achieving an EAE score of greater than 3 relative to the total mice in a group. *P<0.05, versus wild type (Student’s t-test).
Gαq deficiency inhibits in vivo Th17 development
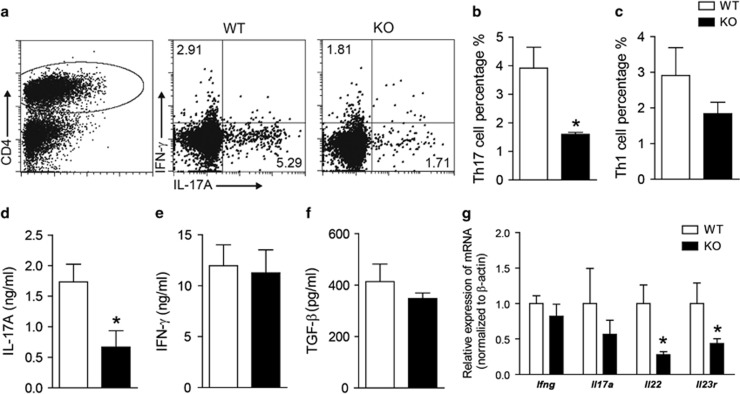
Th1 cells and Th17 cells are thought to be the major pathogenic effector cells in EAE. To reveal the role of Gαq in the development of T cells during EAE pathogenesis, we detected the percentages of Th1 and Th17 in the CD4+ cell population from the lymphocytes of EAE mice. Our previous findings have shown that proinflammatory cytokines such as IL-17A increased on days 6 and 9 and reduced on day 12 in the spleen and lymph nodes (data not shown). Therefore, the EAE mice were killed on day 10. Compared with the WT controls (5.29%), the percentage of the Th17 subset was significantly reduced in the lymph nodes of Gαq-KO mice (1.71%) (Figures 3a and b). Compared with WT mice (2.91%), the percentage of the Th1 subset was also decreased (1.81%) but was not significant (Figures 3a and c). IL-17A is the main effector cytokine of the Th17 subset; thus, we measured IL-17A production from lymph node cells with MOG restimulation in vitro for 72 h. The ELISA results showed that IL-17A was also significantly suppressed by nearly 61% in Gαq-KO EAE mice (Figure 3d). However, IFN-γ secreted by Th1 cells or TGF-β secreted by Tregs was not significantly altered between the Gαq-KO and WT lymphocytes (Figures 3e and f). Th17-associated genes (il22, il17a, il23r), which are typically upregulated in peripheral lymphoid organs during EAE progression, were also detected in the lymph nodes. Our results indicated that the expression of these genes in Gαq-KO mice was lower than in WT mice (Figure 3g). In contrast, compared with WT mice; ifng, a Th1-related gene, showed no significant change (Figure 3g). All these data demonstrated that Th17 development was impaired in Gαq-KO mice during EAE pathogenesis.
Figure 3.
Gαq-KO inhibits in vivo Th17 development. Leukocytes were isolated from the lymph nodes of WT mice and Gαq-KO mice on day 10 PI and analyzed with flow cytometry. (a–c) Th1 and Th17 cells were analyzed by intracellular staining of IFN-γ and IL-17A, respectively, in the CD4+ gate. (d–f) Lymph node leukocytes from EAE-induced WT mice and Gαq-KO mice were re-stimulated in vitro with MOG35–55 (20 μg/ml) for 72 h, and IL-17A (d), IFN-γ (e) and TGF-β (f) in supernatants were analyzed with ELISA. (g) qPCR analysis of Th1- and Th17-related gene expression in lymph nodes. The data are expressed as the mean±s.e.m. (n=3), * P<0.05 versus WT-EAE.
Gαq deficiency inhibits in vitro Th17 differentiation
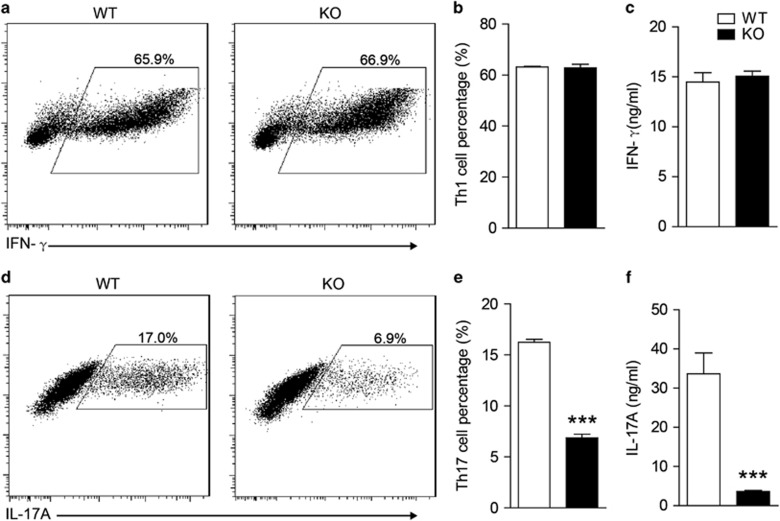
Gαq has been reported to be involved in the pathways that regulate the migration and survival of T cells.16, 19 We further investigated whether Gαq directly influenced the polarization of T cells. Th1, Th17, and Treg cells, three subsets of CD4+ T cells, were induced from CD4+ T cells using different combinations of cytokines and antibodies as previously described.20 As depicted in Figure 4, Gαq deficiency in CD4+ T cells did not affect Th1 differentiation (Figures 4a and b), but a significant impairment of Th17 differentiation, from 17 to 6.9%, was observed (Figures 4d and e). We also found that Gαq deletion induced Treg differentiation from 22.58 to 29.20% (Supplementary Figure 3), which was in accordance with the less severe EAE symptoms, as Tregs play an immunosuppressive role during EAE pathogenesis. Then, we measured IL-17A and IFN-γ levels in the supernatant by ELISA. The results showed that Gαq deletion led to reduction of IL-17A secretion by nearly 89.2% (Figure 4f), but it did not influence IFN-γ production (Figure 4c). All these data demonstrated that a deficiency in Gαq directly impaired Th17 differentiation.
Figure 4.
Gαq-KO inhibits in vitro Th17 differentiation. (a–f) Naive CD4+ T cells from WT and Gαq-KO mice were induced to differentiate into Th1 (a, b) or Th17 (d, e) cells in vitro. Antigen-specific Th1 and Th17 responses were measured by ELISA for IFN-γ and IL-17A production in the supernatant (c, f). The data are expressed as the mean±s.e.m. (n=3), ***P<0.001 versus WT.
Gαq deletion leads to reduction of IL-6 production by DCs
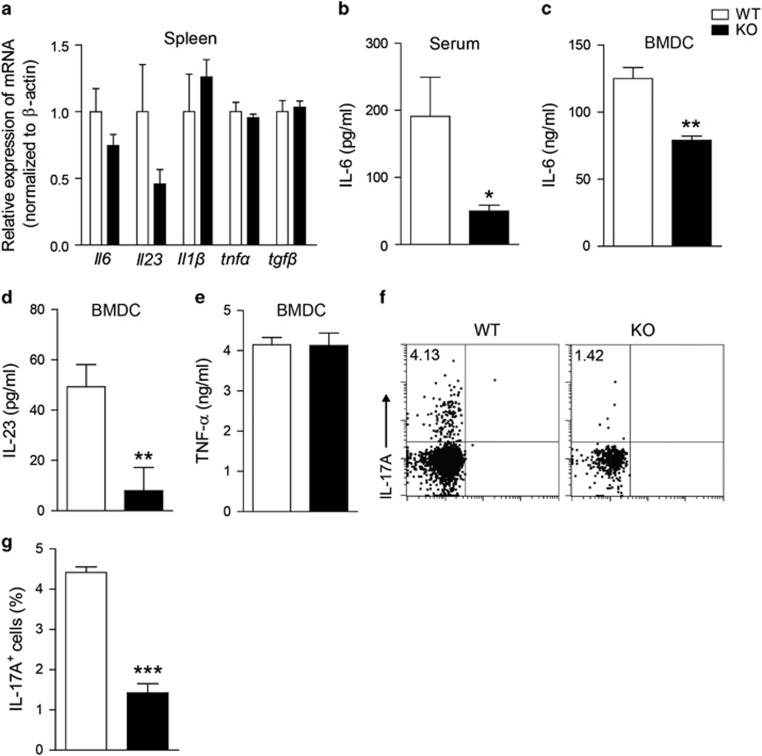
DCs, as the most powerful APCs in the peripheral immune system, can influence the fate of naive CD4+ T cells by producing cytokines, such as IL-1, IL-6 and IL-23.21, 22 IL-6 initiates the development of Th17 and forms a positive feedback loop with IL-17.23 To test whether Gαq could modulate Th17 differentiation indirectly through regulating cytokine production by DCs, we detected changes in IL-6 at the mRNA and protein levels during EAE pathogenesis. The expression of the IL-6 gene in the spleen was decreased in Gαq-KO mice (Figure 5a), and compared with WT controls, the serum concentration of IL-6 was significantly reduced by nearly 74% in Gαq-KO EAE mice on day 10 PI (Figure 5b). These data indicated that Gαq was involved in regulating IL-6 expression during EAE pathogenesis. To identify the role of Gαq in the IL-6 production of DCs, BMDCs induced from bone marrow progenitors, were stimulated with LPS, and the IL-6 production capacity was evaluated. Compared with WT controls, IL-6 production was significantly decreased by nearly 40% in Gαq-KO DCs (Figure 5c). We then detected the secretion of two other cytokines, IL-23 and TNF-α, which also play roles in Th17 development. IL-23 was significantly decreased by nearly 83.7% in Gαq-KO DCs (Figure 5d), but TNF-α production was not affected by Gαq deficiency (Figure 5e). This result suggested that decreased production of IL-23 was another cause of impaired Th17 differentiation in Gαq-KO mice.
Figure 5.
Gαq-KO impairs IL-6 production both in vivo and in vitro. (a) qPCR analysis of il6, il23, il1β, tnfα, and tgfβ mRNA expression in splenocytes from WT or Gαq-KO EAE mice. (b) Serum was collected from WT mice and Gαq-KO EAE mice on day 10 PI, and the IL-6 level was measured. (c-e) BMDCs were induced and further stimulated with LPS (1 μg/ml) for 24 h, and the IL-6 (c), IL-23 (d), and TNF-α (e) in the culture supernatant were measured. (f, g) Th17 differentiation was monitored with FACS analysis in the in vitro DC–T cell co-culture system, the representative FACS (f) and statistics data (g) are shown. The data are expressed as the mean±s.e.m. (n=3), *P<0.05, **P<0.01, ***P<0.001 versus the WT group.
Naive T cells can be induced into Th17 cells in a polarizing condition containing anti-CD3/CD28, IL-6 and TGF-β. Therefore, we co-cultured WT or Gαq-KO DCs with naive WT CD4+ T cells to test the DCs’ capacity for inducing Th17 development. Compared with the WT group, the percentage of Th17 cells in the Gαq-KO group was reduced from 4.13 to 1.42% (Figures 5f and g). The impaired production of IL-6 and IL-23 from Gαq-deficient DCs contributed to the reduced Th17 differentiation.
In addition to cytokine production, the migration ability of DCs is closely related to EAE pathogenesis. Here, we detected the role of Gαq in DC migration with three different chemokines and found that migration of Gαq-KO DCs was also suppressed (Supplementary Figure 4).
Gαq mediates IL-6 production in DCs via Gαq-PLCβ-PKC and Gαq-MAPK signaling
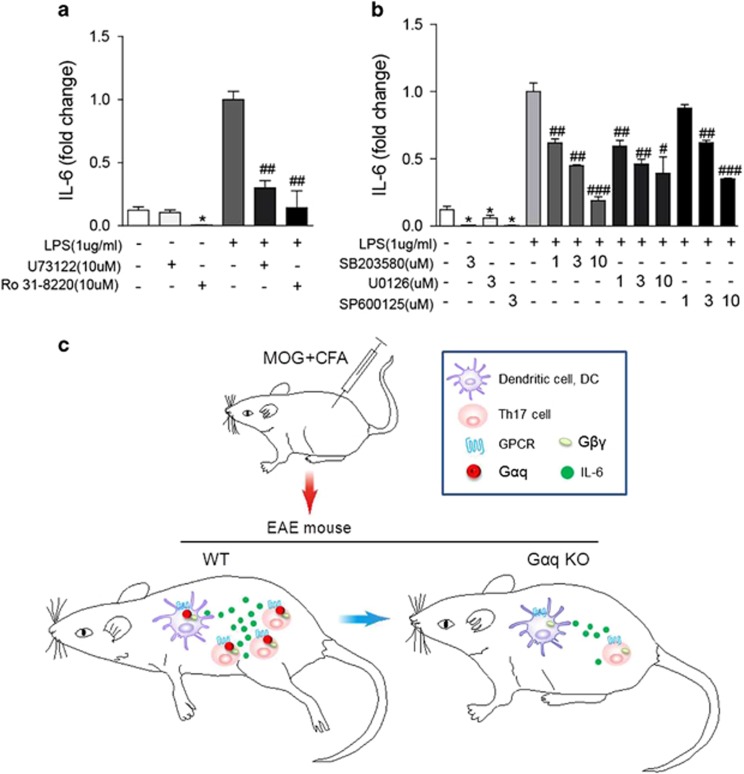
PLCβ is a canonical effector of Gαq. To investigate whether PLCβ was involved in the Gαq-mediated production of IL-6 in DCs, BMDCs were pretreated with inhibitors targeting different signaling pathways before stimulation with LPS or not. Treatment with the PLCβ inhibitor U73122 and the protein kinase C (PKC) inhibitor Ro 31-8220 led to reduced IL-6 production induced by LPS (Figure 6a). It has been reported that Gαq signaling can stimulate MAPKs including ERK1/2, JNK, and p38 via PLC-DAG-PKC, as well as PLC-IP3-Ca2+ signaling mechanisms.24, 25, 26, 27 We used a p38 inhibitor (SB203580), an ERK inhibitor (U0126) and a JNK inhibitor (SP600125) to test which pathway might be involved in IL-6 production by DCs. The results showed that all three MAPK inhibitors reduced IL-6 production by DCs in a dose-dependent manner (Figure 6b). Immature DCs secreted very low amounts of IL-6 into the supernatant. Inhibition by various inhibitors could also be observed (Figures 6a and b). Thus, we concluded that Gαq might regulate IL-6 production in DCs via Gαq-PLCβ-PKC and Gαq-MAPK signaling.
Figure 6.
The downstream pathways involved in Gαq-mediated regulation of IL-6 production in DCs. BMDCs were generated in vitro and stimulated with LPS (1 μg/ml) or not for 24 h in the presence of various pathway inhibitors, and IL-6 production was measured by ELISA. (a-b) The effect of the PLCβ inhibitor U73122 and the PKC inhibitor Ro 31-8220 (a) and the MAPK inhibitors SB203580, U0126 and SP600125 (b) on IL-6 production from BMDCs. (c) The working model that deficiency of Gαq ameliorates EAE through impaired DC/IL-6 production and Th17 differentiation. The data are expressed as the mean±s.e.m. (n=3), *P<0.05 versus DCs treated without LPS and inhibitors; #P<0.05, ##P<0.01, ###P<0.001 versus DCs treated with LPS alone.
Discussion
MS is an autoimmune disease primarily mediated by overactive T cells and is characterized by demyelination in the CNS and problems with strength and muscle control. It is one of the foremost causes of nontraumatic neurologic disability in young adults.28 To date, there is still no curative treatment for multiple sclerosis. The eight disease-modifying drugs approved by the FDA can only reduce disease activity and are accompanied by a variety of side-effects. Interferon therapies, the first and most common modifying agents for MS, have side effects, including injection-site reactions and flu-like symptoms. Glatiramer acetate, the best option in the early stages of MS, is often associated with injection pain, rapid heartbeat and shortness of breath. Most of these approved drugs are given by subcutaneous injections or intramuscular injections. A major step towards mechanistic novelty has been taken through the first oral drug, fingolimod, which targets a GPCR called sphingosine 1-phosphate receptor (S1P1). Despite its convenience and superior efficacy, side effects such as headache, influenza and diarrhea are still a major concern for fingolimod. To develop new therapies with enhanced curative efficacy and fewer side effects, more underlying molecular mechanisms should be discovered.29, 30
Many factors, such as chemokines, neural transmitters, neuropeptides and hormones, are thought to have an effect on MS-associated morbidity, and many receptors for these factors are GPCRs. In addition to the fingolimod receptor, S1P1, an increasing number of GPCRs have been reported to be involved in the pathogenesis of MS/EAE.11 Gαq/11, one of the G protein subfamilies, has recently attracted attention for its immune regulation in autoimmunity. In the murine systemic lupus erythematosus (SLE) model, the mRNA and protein level of Gαq/11 in splenocytes is up-regulated and correlated with disease activity.31 Blocking Gαq/11 in lupus-prone mice effectively reduces the clinical evaluation of disease activity, such as serum IgG levels, anti-DNA antibody levels and proteinuria.32 Gαq overexpression results in mice that are prone to developing progressive cardiac dilation, which is considered an autoimmune disease, with infiltration of inflammatory cells in the heart.33 Acute pancreatitis, resulting from the inflammatory auto-digestion of the pancreas, is associated with high expression of Gαq in pancreatic tissue.34
Many GPCRs, considered therapeutic targets for MS/EAE, are reported to be Gαq-coupled. The receptor cysLT1 is thought to predominantly couple with Gαq, and intracellular-extracellular calcium and Erk activation have been observed through the pathway.35, 36 Our previous work found that Montelukast and Zafirlukast, two oral antagonists of this receptor, could attenuate symptoms of EAE, with reduced infiltration of inflammatory cells in the CNS.20 Prostaglandin E2 has been found to be increased in the CSF of MS patients and reported to facilitate the EAE response. Its receptors (EP1-EP4), revealed as a therapeutic target for MS by targeted lipidomics, are thought to couple with Gαq and mediate inflammatory pain sensitization. In contrast, the neuroprotective effect mediated by PGE2 is dependent on Gαi-coupled signaling.37, 38, 39 The receptor H1R, which has been identified as a susceptibility gene in EAE, has been reported to trigger a regulatory mechanism via a Gαq-PLC-RAC-mediated pathway, and its antagonist, pyrilamine, ameliorates the clinical signs of EAE, with alteration of Th1 cell/Th17 cell activation.40, 41, 42 These findings suggest that blockage of Gαq signaling may have a protective effect in MS/EAE pathogenesis.
Because Gαq is ubiquitously expressed in mammalian cells, the mRNA expression was slightly increased in the peripheral immune tissues and CNS tissues during the EAE pathogenesis. However, deficiency of Gαq significantly alleviated clinical symptoms of EAE. Gαq-KO mice showed suppressed inflammation and neuroprotective effects with remarkably reduced CNS leukocyte infiltration, less demyelination and less axonal damage. These results indicated that Gαq played a prominent role in the immune regulation of EAE pathogenesis.
Our in vivo analysis in an EAE model revealed that blockage of Gαq inhibited development of Th17 cells, with a lower percentage in CD4+ lymphocytes as well as suppressed production of Th17-associated cytokines and receptors, including IL-17A, IL-22 and IL-23r. The in vitro assay further confirmed that the inhibition was specifically of Th17 cells. We hypothesized that Gαq-coupled platelet activating factor receptor (PAFR) antagonists, which have been proven to alleviate EAE, were involved in the inhibition of Th17. Because the Gαq-AC-PKA-Src-STAT3 pathway was impaired in Gαq-KO mice, PAFR failed to induce Th17 differentiation.43, 44 It has been reported that TCR-mediated immune responses are regulated by Gαq through an Lck-dependent pathway. Gαq deficiency in T cells leads to reduced activation of Lck.45 The reduced activity of STAT3 caused by suppressed activation of Lck could be an explanation for the impaired differentiation of Th17 cells in Gαq-KO mice.46 However, the influence of Lck is not observed in Th1 differentiation,47 which is consistent with our results that the Th17 response, but not the Th1 response, was suppressed in Gαq-KO mice. Moreover, the aberrant production of IL-17 can also result from the Gαq-KO-induced inhibition of RhoA, which influences the production of IL-17 and IL-21 but has no effect on Th1 cells.48, 49 Because RhoA also mediates the migration of Th17, its inhibition may influence the percentage of Th17 in lymph nodes from EAE and further contribute to the reduction of leukocyte infiltration in the CNS.50
Th17 differentiation can be influenced by DCs through secretion of Th17 cell-polarizing cytokines.51 One pro-inflammatory cytokine, IL-6, which has been shown to be crucial in the initiation of Th17 development,23 was reduced in both the spleen and serum of Gαq-KO EAE mice. Decreases of IL-6 and IL-23 were further confirmed in BMDCs, indicating that deficiency of Gαq in DCs might suppress Th17 through an impaired cytokine profile. This inhibition of Gαq-KO DCs on Th17 was similar to that of SCH23390-treated DCs. SCH23390 is an antagonist to a Gα-coupled receptor, D1-like-R and exerts protective effects in EAE.52, 53 It is thought that blockage of D1-like-R obstructs intracellular cAMP mediated by Gαs and consequently leads to reduced secretion of IL-6.54 However, our results suggested that Gαq also positively mediated IL-6 production in DCs. PLCβ–PKC, which is the most well-known downstream pathway of Gαq, has been reported to regulate IL-6 in many types of cells.14, 55 In addition, the members of the MAPK family, which are involved in IL-6 production, are also important signaling molecules in the Gαq signaling cascade.56 By using various chemical inhibitors, we found that both the Gαq–PLCβ–PKC and the Gαq–MAPK pathway might be involved in IL-6 production in DCs.
In summary, we demonstrated a critical role of Gαq in regulating EAE pathogenesis by influencing Th17 differentiation and IL-6 production in DCs (Figure 6c). There should be a dual regulatory mechanism involved in Gαq signaling, due to the fact that some agonists of Gαq-coupled receptors, such as alpha-1A-adrenoceptor and gonadotropin-releasing hormone receptors, relieve EAE severity. However, our findings suggested that in EAE/MS, pro-inflammatory signals of Gαq played dominant roles over the anti-inflammatory signals, which supplied important clues for understanding the pathogenesis of MS and provided new insights into strategies for disease therapy.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2014CB541903, 2012CB910404), the National Natural Science Foundation of China (31171348, 31371414), the Shanghai Municipal Education Commission (14zz042), the State Key Laboratory of Drug Research (SIMM1302KF-09) and the Fundamental Research Funds for the Central Universities.
Footnotes
Supplementary Information accompanies this paper on Cellular & Molecular Immunology website (http://www.nature.com/cmi)
Supplementary Material
References
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol 2007; 61: 288–299. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol 2007; 61: 504–513. [DOI] [PubMed] [Google Scholar]
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med 2010; 16: 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol 2005; 23: 683–747. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Gilman AG. G proteins. Trends Biochem Sci 1992; 17: 383–387. [DOI] [PubMed] [Google Scholar]
- Standfuss J, Edwards PC, D'Antona A, Fransen M, Xie G, Oprian DD et al. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature 2011; 471: 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science 1991; 252: 802–808. [DOI] [PubMed] [Google Scholar]
- Oka Y, Saraiva LR, Kwan YY, Korsching SI. The fifth class of Galpha proteins. Proc Natl Acad Sci USA 2009; 106: 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal 2006; 18: 135–150. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shi G. Gq-coupled receptors in autoimmunity. J Immunol Res 2016; 2016: 3969023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du CS, Xie X. G protein-coupled receptors as therapeutic targets for multiple sclerosis. Cell Res 2012; 22: 1108–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukos Y, Leonard JP, Thomaides T, Thompson AJ, Cuzner ML. beta-Adrenergic receptor density and function of peripheral blood mononuclear cells are increased in multiple sclerosis: a regulatory role for cortisol and interleukin-1. Ann Neurol 1992; 31: 657–662. [DOI] [PubMed] [Google Scholar]
- Lynch JL, Alley JF, Wellman L, Beitz AJ. Decreased spinal cord opioid receptor mRNA expression and antinociception in a Theiler's murine encephalomyelitis virus model of multiple sclerosis. Brain Res 2008; 1191: 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Du C, Lv J, Zhao G, Li Z, Wu Z et al. Blocking A2B adenosine receptor alleviates pathogenesis of experimental autoimmune encephalomyelitis via inhibition of IL-6 production and Th17 differentiation. J Immunol 2013; 190: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT et al. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol 2007; 179: 2774–2786. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhang Y, He Y, Li Y, Lund FE, Shi G. The deficiency of Galphaq leads to enhanced T-cell survival. Immunol Cell Biol 2014; 92: 781–790. [DOI] [PubMed] [Google Scholar]
- Mei F, Guo S, He Y, Wang L, Wang H, Niu J et al. Quetiapine, an atypical antipsychotic, is protective against autoimmune-mediated demyelination by inhibiting effector T cell proliferation. PLOS ONE 2012; 7: e42746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JD, Herbin O, de la Hera B, Vidaurre OG, Moy GA, Sun Q et al. Nuclear export inhibitors avert progression in preclinical models of inflammatory demyelination. Nat Neurosci 2015; 18: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L, Stanley P, Willenbrock F, Hogg N. The Galphaq/11 proteins contribute to T lymphocyte migration by promoting turnover of integrin LFA-1 through recycling. PLOS ONE 2012; 7: e38517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Du C, Lv J, Wei W, Cui Y, Xie X. Antiasthmatic drugs targeting the cysteinyl leukotriene receptor 1 alleviate central nervous system inflammatory cell infiltration and pathogenesis of experimental autoimmune encephalomyelitis. J Immunol 2011; 187: 2336–2345. [DOI] [PubMed] [Google Scholar]
- Comabella M, Montalban X, Munz C, Lunemann JD. Targeting dendritic cells to treat multiple sclerosis. Nat Rev Neurol 2010; 6: 499–507. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nature Reviews Immunology 2003; 3: 984–993. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007; 8: 967–974. [DOI] [PubMed] [Google Scholar]
- Wang Y, Falting JM, Mattsson CL, Holmstrom TE, Nedergaard J. In brown adipocytes, adrenergically induced beta(1)-/beta(3)-(Gs)-, alpha(2)-(Gi)- and alpha(1)-(Gq)-signalling to Erk1/2 activation is not mediated via EGF receptor transactivation. Exp Cell Res 2013; 319: 2718–2727. [DOI] [PubMed] [Google Scholar]
- Chan AS, Wong YH. Gbetagamma signaling and Ca2+ mobilization co-operate synergistically in a Sos and Rac-dependent manner in the activation of JNK by Gq-coupled receptors. Cell Signal 2004; 16: 823–836. [DOI] [PubMed] [Google Scholar]
- Sugawara Y, Nishii H, Takahashi T, Yamauchi J, Mizuno N, Tago K et al. The lipid raft proteins flotillins/reggies interact with Galphaq and are involved in Gq-mediated p38 mitogen-activated protein kinase activation through tyrosine kinase. Cell Signal 2007; 19: 1301–1308. [DOI] [PubMed] [Google Scholar]
- Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene 2007; 26: 3122–3142. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 2010; 162: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg MM. Multiple sclerosis review. P T 2012; 37: 175–184. [PMC free article] [PubMed] [Google Scholar]
- Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov Med 2011; 12: 213–228. [PMC free article] [PubMed] [Google Scholar]
- Jacobson JD, Ansari MA, Kinealy M, Muthukrishnan V. Gender-specific exacerbation of murine lupus by gonadotropin-releasing hormone: potential role of G alpha(q/11). Endocrinology 1999; 140: 3429–3437. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Dhar M, Muthukrishnan V, Morton TL, Bakht N, Jacobson JD. Administration of antisense oligonucleotides to Galpha(Q/11) reduces the severity of murine lupus. Biochimie 2003; 85: 627–632. [DOI] [PubMed] [Google Scholar]
- Aminzadeh MA, Tseliou E, Sun B, Cheng K, Malliaras K, Makkar RR et al. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur Heart J 2015; 36: 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia SH, Hu CX, Fang JM, Di Y, Zhao ZL, Liu LR. G[alpha]i2 and G[alpha]q expression change in pancreatic tissues and BN52021 effects in rats with severe acute pancreatitis. Pancreas 2008; 37: 170–175. [DOI] [PubMed] [Google Scholar]
- Peres CM, Aronoff DM, Serezani CH, Flamand N, Faccioli LH, Peters-Golden M. Specific leukotriene receptors couple to distinct G proteins to effect stimulation of alveolar macrophage host defense functions. J Immunol 2007; 179: 5454–5461. [DOI] [PubMed] [Google Scholar]
- Foster HR, Fuerst E, Branchett W, Lee TH, Cousins DJ, Woszczek G. Leukotriene E4 is a full functional agonist for human cysteinyl leukotriene type 1 receptor-dependent gene expression. Sci Rep 2016; 6: 20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y, Matsushita T, Kita Y, Uematsu S, Akira S, Kira J et al. Targeted lipidomics reveals mPGES-1-PGE2 as a therapeutic target for multiple sclerosis. Proc Natl Acad Sci USA 2009; 106: 21807–21812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat 2010; 91: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilak M, Wu L, Wang Q, Haughey N, Conant K St, Hillaire C et al. PGE2 receptors rescue motor neurons in a model of amyotrophic lateral sclerosis. Ann Neurol 2004; 56: 240–248. [DOI] [PubMed] [Google Scholar]
- Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KS, Watanabe T et al. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science 2002; 297: 620–623. [DOI] [PubMed] [Google Scholar]
- Zappia CD, Granja-Galeano G, Fernandez N, Shayo C, Davio C, Fitzsimons CP et al. Effects of histamine H1 receptor signaling on glucocorticoid receptor activity. Role of canonical and non-canonical pathways. Sci Rep 2015; 5: 17476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedotti R, DeVoss JJ, Youssef S, Mitchell D, Wedemeyer J, Madanat R et al. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci USA 2003; 100: 1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo DD, Bazan NG, Hunt JD. Activation of platelet-activating factor receptor-coupled G alpha q leads to stimulation of Src and focal adhesion kinase via two separate pathways in human umbilical vein endothelial cells. J Biol Chem 2004; 279: 3497–3508. [DOI] [PubMed] [Google Scholar]
- Drolet AM, Thivierge M, Turcotte S, Hanna D, Maynard B, Stankova J et al. Platelet-activating factor induces Th17 cell differentiation. Mediators Inflamm 2011; 2011: 913802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai J, Methi T, Andressen KW, Levy FO, Torgersen KM, Vang T et al. The heterotrimeric G-protein alpha-subunit Galphaq regulates TCR-mediated immune responses through an Lck-dependent pathway. Eur J Immunol 2008; 38: 3208–3218. [DOI] [PubMed] [Google Scholar]
- Brisslert M, Bian L, Svensson MN, Santos RF, Jonsson IM, Barsukov I et al. S100A4 regulates the Src-tyrosine kinase dependent differentiation of Th17 cells in rheumatoid arthritis. Biochim Biophys Acta 2014; 1842: 2049–2059. [DOI] [PubMed] [Google Scholar]
- Kemp KL, Levin SD, Stein PL. Lck regulates IL-10 expression in memory-like Th1 cells. Eur J Immunol 2010; 40: 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JQ, Kalim KW, Li Y, Zhang S, Hinge A, Filippi MD et al. RhoA orchestrates glycolysis for TH2 cell differentiation and allergic airway inflammation. J Allergy Clin Immunol 2016; 137: e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest 2010; 120: 3280–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Azreq MA, Kadiri M, Boisvert M, Page N, Tessier PA, Aoudjit F. Discoidin domain receptor 1 promotes Th17 cell migration by activating the RhoA/ROCK/MAPK/ERK signaling pathway. Oncotarget 2016; 7: 44975–44990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev 2008; 19: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochem Biophys Res Commun 2008; 373: 286–291. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Wang HY, Friedman E. Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J Neurochem 2001; 78: 981–990. [DOI] [PubMed] [Google Scholar]
- Nakano K, Yamaoka K, Hanami K, Saito K, Sasaguri Y, Yanagihara N et al. Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol 2011; 186: 3745–3752. [DOI] [PubMed] [Google Scholar]
- Lee CH, Shieh DC, Tzeng CY, Chen CP, Wang SP, Chiu YC et al. Bradykinin-induced IL-6 expression through bradykinin B2 receptor, phospholipase C, protein kinase Cdelta and NF-kappaB pathway in human synovial fibroblasts. Mol Immunol 2008; 45: 3693–3702. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003; 374: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.