Abstract
Purpose
The aim of this case series is to raise awareness of the emerging issue of serious retinal damage caused by the prolonged use of hydroxychloroquine (HCQ) and the importance of adequate and appropriate monitoring of visual function during treatment.
Patient and methods
This is a small retrospective case series of 3 patients on long-term HCQ who developed serious symptomatic retinal toxicity confirmed on imaging and functional testing.
Results
All 3 patients were treated with HCQ for over 15 years; two for rheumatoid arthritis (RA), and the third for systemic lupus erythematosus (SLE). All 3 patients had macular involvement varying in severity confirmed with characteristic features on imaging and functional testing (Optical Coherence Tomography (OCT), Autofluorescence (AF) and Humphrey 10-2 visual fields).
Conclusion
HCQ is widely used to treat autoimmune conditions with a proven survival benefit in patients with SLE. However, long-term use can be associated with irreversible retinal toxicity. These cases highlight that HCQ, like chloroquine, can also cause visual loss in susceptible individuals. Early detection of presymptomatic retinal changes by the introduction of appropriate screening and monitoring is mandatory to limit the extent of irreversible visual loss due to HCQ retinal toxicity.
Introduction
HCQ is a disease-modifying drug used in the treatment of various rheumatological and dermatological conditions. In spite of its improved safety profile compared with its predecessor, chloroquine, it is now becoming clearer that retinal toxicity is a side effect of prolonged use and is emerging as a significant cause of visual loss for susceptible patients on this therapy.
Retinopathy caused by antimalarials, first described in 1963,1 is usually asymptomatic, and by the time symptoms of photosensitivity and patchy visual loss develop, retinal changes are already present. The introduction of more sophisticated imaging has enabled the identification of pre-symptomatic retinal changes indicative of toxicity.2, 3
Case 1
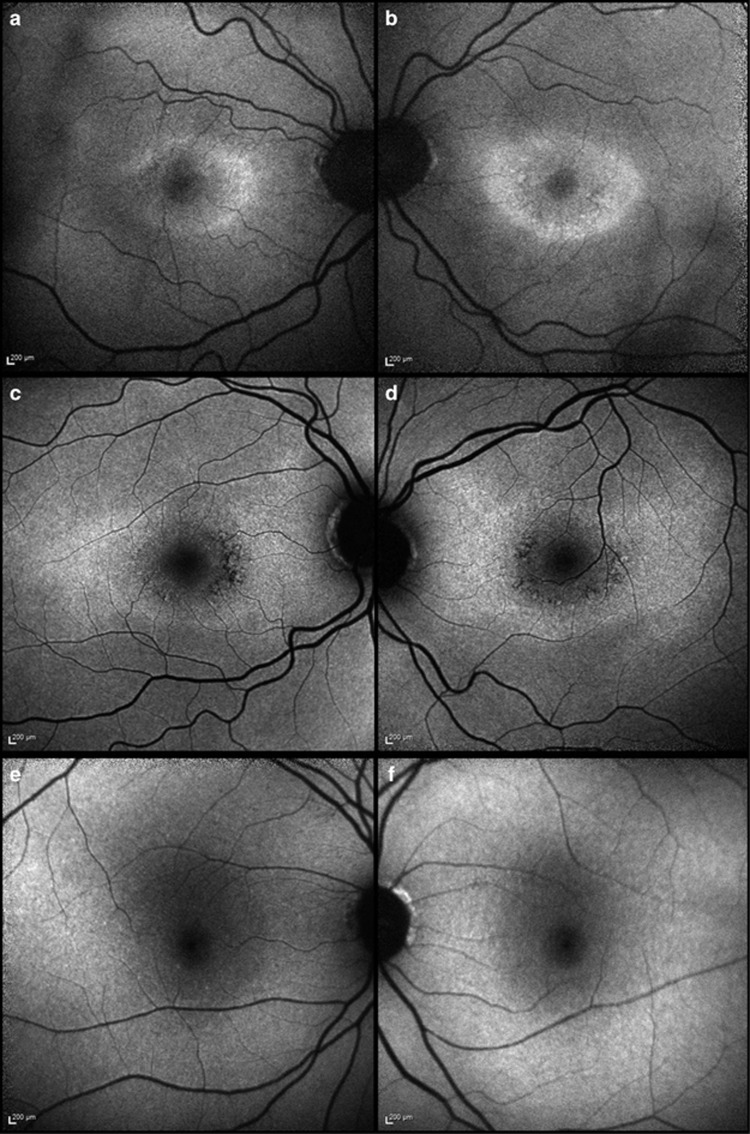
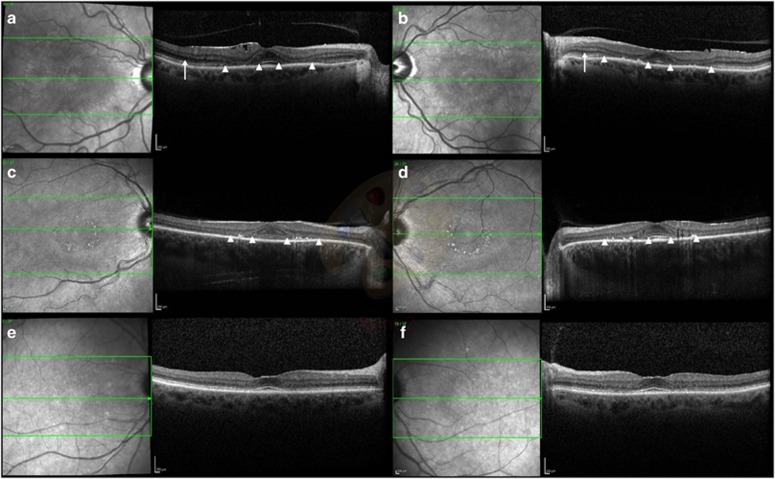
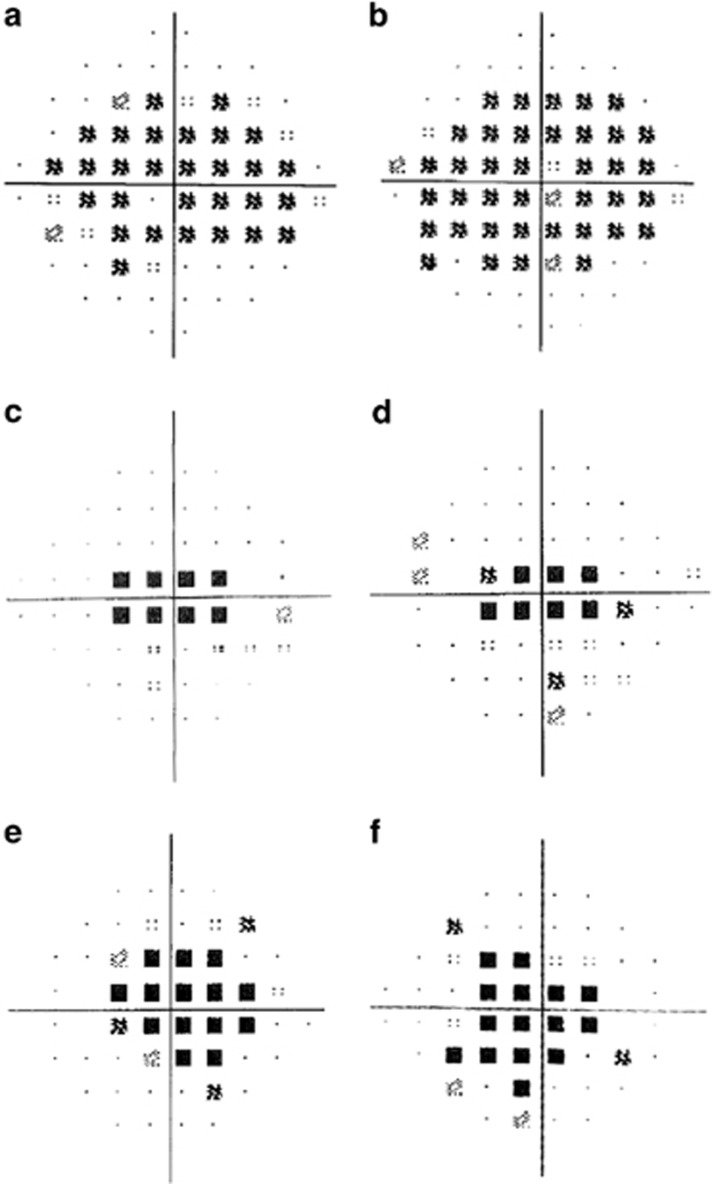
A 63-year-old caucasian female presented with glare and decreased vision for 9 months having taken HCQ 400 mg per day for RA for 16 years (cumulative dose of 2336 g; 4.93 mg/kg per day). Her medications included omeprazole, methotrexate, and folic acid. Her renal function was 84 ml/min/1.73 m2. Visual acuities were 6/6 right and 6/7.4 left. Fundoscopy and imaging revealed subtle disturbances of the macular retinal pigment epithelium, increased AF signal on Scanning Laser Ophthalmoscopy (SLO), with bilateral parafoveal thinning on Spectral Domain OCT imaging (Figures 1a and b, Figures 2a and b).4 Ring scotomata were identified on visual field testing (Figures 3a and b); electrophysiology demonstrated normal rod and cone responses, but abnormal macular function. Repeat testing 6 months later revealed stable visual acuities and no deterioration on electrophysiological testing.
Figure 1.
Autofluorescence (AF) imaging in HCQ retinopathy. (a, b) Case 1 demonstrates a significant bilateral increase in parafoveal AF signal. (c, d) Case 2 demonstrates a more widespread increase in AF signal with patchy decreasing signal parafoveally indicative of atrophic change bilaterally. (e, f) Case 3 demonstrates very minimal changes on AF.
Figure 2.
The OCT features of HCQ retinopathy in severe and mild retinopathy. (a, b) Case 1 demonstrates parafoveal thinning of outer retinal segments in both eyes with disruption of the ellipsoid zone and photoreceptor inner and outer segments (between white arrowheads). Epiretinal membrane formation is noted, more pronounced in the right eye. Posterior vitreous separation is seen bilaterally. The external limiting membrane is not visualised in the parafovea but is seen more peripherally (arrow). (c, d) Case 2 demonstrates bilateral parafoveal thinning of the ellipsoid zone and photoreceptor inner and outer segments (between white arrowheads) with corresponding retinal pigment epithelium changes on scanning laser ophthalmoscopy. The external limiting membrane is not visualised. (e, f) Case 3 demonstrates fine retinal pigment epithelium changes. The external limiting membrane is preserved.
Figure 3.
Visual field exams in HCQ retinopathy. All presented plots are those from Humphrey automated perimetry of the central field (10–2). (a, b) Case 1 demonstrates characteristic ring scotomas. (c, d) Case 2 demonstrates central scotomas. (e, f) Case 3 demonstrates central visual field defects.
Case 2
A 44-year-old caucasian female noted decreased vision and central scotomas 6 months prior to presentation, having taken a daily dose of HCQ 400 mg for RA for 15 years (cumulative dose 2910 g; 5.6 mg/kg per day). Her medications included leflunomide, methotrexate, omeprazole, loratadine, folic acid. Her renal function was 84 ml/min/1.73 m2. Visual acuities were 6/6 in both eyes. Fundoscopy and imaging revealed bilateral Bull's eye maculopathy with typical AF and OCT findings (Figures 1c and d, Figures 2c and d). Visual field testing showed central sensitivity losses with decreased macular function on the multifocal ERG. (Figures 3c and d).
Case 3
A 74-year-old caucasian female with SLE presented with a 4 month history of bilateral central scotomata in the last 4 months, having taken 400 mg of HCQ per day for 16 years, followed by 200 mg per day for 6 months (total dose of 2372 g; 4.93 mg/kg per day (on 400 mg per d)). Her medications included losartan, atorvastatin, furosemide, fenofibrate, colchicine, and probenecid. She had not previously taken tamoxifen. She had chronic kidney disease (stage 3) with a 4-year history of deteriorating renal function: her eGFR at the time of diagnosis was 28 ml/min/1.73 m2. Visual acuities were 6/6 in both eyes. Imaging and functional testing confirmed a central maculopathy (Figures 1e and f, Figures 2e and f, Figures 3e and f).
Discussion
HCQ is a widely used drug found to be of significant benefit in rheumatological and dermatological conditions, with demonstrated survival benefits for SLE.5 However, like Chloroquine, where a similar bull’s eye maculopathy develops, HCQ has now also been shown to be associated with significant retinal toxicity in susceptible individuals. While older studies have estimated HCQ toxicity to be infrequent at ~0.5–1% of those taking HCQ for greater than 5 years,6 recent epidemiological data indicate that retinal toxicity occurs in greater than 10% of patients, who have taken HCQ for over 10 years, and 20–50% of patients taking HCQ for greater than 20 years.7
The 2009 Royal College of Ophthalmologists Guidelines on HCQ screening advise referral to an Ophthalmologist only if the patient has baseline visual impairment, eye disease confirmed by an optometrist, or if the patient notices visual symptoms.8 This was based on the premise that the introduction of HCQ was a safe option and was not thought to be associated with the same toxicity as chloroquine. Patients were previously instructed to self-monitor with an Amsler grid, but regular screening has not been common practice in the UK.
The American Academy of Ophthalmology (AAO) 2016 guidelines recommend baseline ophthalmologic examination including fundoscopy with further testing if abnormalities are present at baseline, as well as annual automated central visual field testing (Humphrey 10-2), SD-OCT, and AF after 5 years of exposure or sooner.3 Amsler grid monitoring was not included in the recommendations because it was regarded as subjective and unreliable.
It is now clear that visual acuities, 10–2 visual fields, and imaging with OCT and FAF and if indicated, multifocal ERG, should be used to look for pre-symptomatic retinal toxicity.3, 7 Some patients’ retinopathy progresses despite stopping treatment; involvement of the external limiting membrane on OCT carries negative prognostic value, suggestive of irreversible photoreceptor damage.9 We review the specific retinal imaging and functional testing features in more detail in the accompanying review.10 Asian patients demonstrate a pericentral pattern of disease, and wider field imaging and visual field testing (30–2) may be required in these patients.11, 12
Rheumatologists, Ophthalmologists and General practitioners who are involved in the care of these patients need to be aware of the need for initial screening and regular monitoring of patients. These cases highlight the importance of recognising and managing this emerging problem. Instituting a standardised screening protocol would enable cessation of therapy as early as possible, thus reducing the risk and extent of retinal toxicity. In the accompanying review10, we present a proposal for screening and monitoring, which includes practical suggestions for imaging and functional testing.
Footnotes
The authors declare no conflict of interest.
References
- Grupper C, Bregeat P, Juge P. [SEvere retinopathy during treatment of lupus erythematosus by synthetic antimalarials (2 cases)]. Bull Soc Fr Dermatol Syphiligr 1963; 70: 824–832. [PubMed] [Google Scholar]
- Nika M, Blachley TS, Edwards P, Lee PP, Stein JD. Regular examinations for toxic maculopathy in long-term chloroquine or hydroxychloroquine users. JAMA Ophthalmol 2014; 132: 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 2016; 123: 1386–1394. [DOI] [PubMed] [Google Scholar]
- Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the 'flying saucer' sign). Clin Ophthalmol 2010; 4: 1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007; 66: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology 2003; 110: 1321–1326. [DOI] [PubMed] [Google Scholar]
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 2014; 132: 1453–1460. [DOI] [PubMed] [Google Scholar]
- The Royal College of Ophthalmologists. Hydroxychloroquine and OcularToxicity Recommendations on Screening, The Royal College of Ophthalmologists, 2009. Available at: https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2009-SCI-010-Ocular-Toxicity.pdf (accessed on 16 September 2016).
- Mititelu M, Wong BJ, Brenner M, Bryar PJ, Jampol LM, Fawzi AA. Progression of hydroxychloroquine toxic effects after drug therapy cessation: new evidence from multimodal imaging. JAMA Ophthalmol 2013; 131: 1187–1197. [DOI] [PubMed] [Google Scholar]
- Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye 2017. (in press); doi:10.1038/eye.2016.298. [DOI] [PMC free article] [PubMed]
- Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology 2015; 122: 110–116. [DOI] [PubMed] [Google Scholar]
- Lee DH, Melles RB, Joe SG, Lee JY, Kim JG, Lee CK et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology 2015; 122: 1252–1256. [DOI] [PubMed] [Google Scholar]