Abstract
Purpose
Children with cataract and their families face intensive medical and surgical management, with numerous hospital attendances, topical medications, and surgical procedures, as well as uncertainty about the child’s future visual ability, education, and independence. Little is known about the impact on functional visual ability, vision-, and health-related quality of life (VR-, HR-QoL).
Patients and methods
Seventy two children aged 2–16 years (mean 8.45, SD 4.1) treated for developmental or secondary cataract and their parents/carers completed three validated instruments measuring functional visual ability, VR-, and HR-QoL: the Cardiff Visual Ability Questionnaire for Children (CVAQC), Impact of Vision Impairment for Children (IVI-C), and PedsQL V 4.0.
Results
All scores are markedly reduced: median (interquartile range (IQR)) CVAQC score −1.42 (−2.28 to −0.03), mean (SD) IVI-C score 65.67 (16.91), median (IQR) PedsQL family impact score 75 (56.94–88.19), parent report 71.74 (51.98–88.5), self-report 76.09 (61.96–89.13). Psychosocial PedsQL subscores are lower than physical subscores. Parent-completed tools (PedsQL family and parent report) state greater impact on HR-QoL than tools completed by children/young people, particularly in teenagers. Older children/young people have higher functional visual ability scores than younger children.
Conclusions
Cataract has a marked a long-term impact on functional visual ability and quality of life of children and young people, with HR-QoL affected to degrees reported in children with severe congenital cardiac defects or liver transplants.
Introduction
With an incidence of 1–3 per 10 000 live births, congenital/infantile cataract is a rare, but sight-threatening condition.1 Congenital/infantile cataract poses significant management challenges. The risk of developing glaucoma is high, with 31% of children either having glaucoma or being monitored as ‘glaucoma suspect’ at 5 years after cataract surgery,2 and an overall annual incidence of postoperative glaucoma of 5.25 per 100 cataract operations.3, 4 Visual outcomes are often disappointing. Younger age at surgery is associated with better visual outcomes.5, 6 Unilateral cases have a worse visual prognosis in the affected eye than bilateral: at 6 years after surgery, a median visual acuity 0.48 in bilateral and 1.0 logMAR (affected eye) in unilateral cases has been reported.3 Amblyopia and its management with hours of occlusion or blurring of the better seeing eye may affect the child’s motor co-ordination, functioning, and emotional state.7, 8
Children with cataract require frequent hospital attendances, which means that children miss school and parents lose time from work. If glaucoma develops, additional treatment in the form of eye drops (95%)2 or glaucoma surgery (up to 40% at 5 years of follow-up)2 is required, carrying an additional burden of anxiety for the child and the family. Yet little is known about the impact of cataract and its management on children and their families.
Studies using parental proxy VR-QoL tools such as the Children’s Visual Function Questionnaire found that family impact and treatment difficulty scores are worse in families of children with unilateral compared with those with bilateral cataracts, and in those treated with an aphakic contact lens compared with those treated with an intraocular lens implant.9 Bilateral congenital/infantile cataract is associated with reduced VR-QoL scores compared with healthy controls.10 A study in China using a specifically developed self-report tool showed a correlation of QoL scores with visual acuity, surgery, and density of cataract.11, 12
Over recent years, self-report instruments have been developed to measure health-related quality of life (HR-QoL) in children with various conditions, and vision-related quality of life (VR-QoL) and functional visual ability (FVA) in children with sight impairment. For example, the Impact of Vision Impairment for Children (IVI-C) instrument13 can be used to assess the impact of visual impairment on VR-QoL, and the Cardiff Visual Ability Questionnaire for Children (CVAQC)14 and the Functional vision questionnaire for children and young people with visual impairment,15 to assess the impact on activities of daily living (FVA).
While none of these has been used specifically in children with cataract, previous studies in this population have applied a generic HR-QoL tool for children and families, the PedsQL Inventory (www.pedsql.org).16, 17 The PedsQL covers different domains (physical and psychosocial—emotional, social, school) and contains age-specific versions as well as separate questionnaires for parents/carers, one as a report about their child, and another to evaluate the impact of the child’s condition on the family. Using the PedsQL, it has been reported that 6 years after treatment for congenital/infantile cataract, parents and children report lower HR-QoL scores than normative controls.18
However, none of the previous studies included children/young people with secondary cataract, and none offers data on long-term impact of cataract treatment in early childhood.
The main objective of the present work was to explore FVA, VR-QoL, and HR-QoL in children treated or monitored for developmental and secondary cataract and their parents/carers, using CVAQC, IVI-C, and PedsQL. We included young people up to the age of 16 years to explore long-term impact of childhood cataract (ChC) and its management.
Materials and methods
This work is part of a larger cross-sectional observational study, approved by the National Research Ethics Committee South Central—Oxford A (14/SC/1052). Between 25 June 2014 and 03 June 2015 we enrolled children and young people aged 2–16 years who attended clinics at Moorfields Eye Hospital, London, UK. In the present study, we included children with congenital/infantile and cataract secondary to uveitis. At enrolment, exclusion criteria were: visually not significant cataract, inability to communicate in English, surgical intervention (incisional or laser) within 1 month of date of completing questionnaires (before or after). We enrolled consecutive patients, noting reasons for not wanting to take part. We gave parents/carers and children age-appropriate information material about the study and addressed any questions. Parents/carers gave written consent, and children could sign an assent form.
We recorded age at study participation, gender, and ethnic background. From the medical notes, we recorded ocular and systemic diagnoses, age at diagnosis of the eye condition and best corrected visual acuity (BCVA) with both eyes open in logMAR on the day of study participation. Where visual acuity was recorded as ‘counting fingers’, we noted a BCVA of 1.3 logMAR, for ‘hand movements only’ we noted 1.6 logMAR, for ‘perception of light’ 2.7 logMAR, and for ‘no perception of light’ or ‘ocular prosthesis/artificial eye’, 3 logMAR.19 We also recorded details of previous and current treatment, such as number of previous surgical interventions (sum of interventions right and left eye, including incisional surgery, laser treatment, removal of sutures, injections, excluding examinations under anaesthesia), number of general anaesthetics including examinations under anaesthesia and number of current topical medications (sum of eye drop applications per day right and left eye).
Main outcome measures
FVA was assessed with the CVAQC.14 The 25 questions with answers selected on a four-point scale were completed by children from the age of 5 and covered areas such as education, near and distance vision, getting around, social interaction, entertainment, and sports. We transformed the raw CVAQC scores into logarithmic scores.14 The resulting scores range from −3.00 (normal visual ability) to +2.80 (severe visual impairment).
To evaluate VR-QoL, children aged 8 years or older completed the IVI-C questionnaire regarding mobility, interaction, school, and emotional state. Due to delays in agreements and permissions we started using the IVI-C13 5 weeks after the start of the study (01 August 2014). We scored responses as recommended by the developers, allocating values between 0 and 4 to the responses from ‘never’ to ‘always’. We did not allocate a score when the response ‘no, for other reasons’ was selected. As the tool comprises 24 items, the resulting raw scores range from 0 to 96, with 96 indicating normal VR-QoL.
To assess HR-QoL age-specific versions of the PedsQL Inventory were completed by children between the ages of 5–16 years and their parents/carers. The questionnaire for children aged 8–12 years consists of 23 questions in the domains of ‘my health and activities’, ‘my feelings’, ‘how I get along with others’, and ‘about school’ answers are given on a 5-point Likert scale from 0 (never a problem) to 4 (always a problem). The set for young children (5–7) and teenagers aged 13–18 years comprises of 23 questions about physical, emotional, social, and school functioning; answers are on a 5-point Likert scale. We asked parents to complete two questionnaires, the PedsQL Parental report about the child/young person, and the PedsQL Family report, which measures the impact of a child’s condition on the family. The parental report on: children aged 5 years and older; children aged 8–12 years, and that for teenagers aged 13–18 years consisted of 23 questions with answers on a 5-point Likert scale. The parental report on children aged 2–4 years consisted of 21 questions. The family impact questionnaire includes ‘physical’, ‘emotional’, ‘social’, ‘cognitive’ functioning of the parent, ‘communication’, ‘worry’, ‘daily activities’, and ‘family relationships’. This questionnaire contains 36 questions with answers on a 5-point Likert scale. Parents and children from the age of 5 can self-administer the questionnaire (PedsQL administration guidelines). We calculated the PedsQL scores as detailed in the scoring instructions. If items were left blank, we adjusted the denominator, using the number of completed items instead of the number of total items. It is recommended to remove questionnaires from the analysis if 50% or more of the items have been left blank; this did not occur in our sample. In summary, self, parent and family overall total, physical, and psychosocial HR-QoL scores are generated with PedsQL scores ranging from 0 to 100, 100 indicating normal HR-QoL.
All questionnaires were self-administered and completed on the same day, during a regular clinic appointment. When children needed help completing the questionnaires, they were assisted by a member of the research team or play leaders, but not by family members.
Statistics
We aimed for an overall sample size of between 50 and 100 children with cataract, as recommended for Bland–Altman limits of agreement analysis. Demographic and clinical data, and the CVAQC, IVI-C, and PedsQL scores were transferred to a dedicated database in Microsoft Office Excel (Microsoft, Redmond, WA, USA) by a member of the research team. Calculation of scores and data transfer were double-checked by a second member of the team.
Analysis was carried out in Microsoft Office Excel (Microsoft), SPSS v23 (IBM, Armonk, New York, NY, USA), and Stata (V14, StataCorp LLC, College Station, TX, USA). Where data were missing, data sets were excluded from the relevant analyses. We applied descriptive statistics throughout, reporting means and SDs or median and interquartile range (IQR) as appropriate.
Where data were missing for individual items in the PedsQL and IVI-C, we adjusted the denominator accordingly. The conversion from raw to logarithmic CVAQC scores takes into account missing data.14
We assessed relationships between age and quality of life scores using Spearman rank correlation and assessed whether differences observed between the age groups were statistically significant using the Rank Sum test or independent t-test. Agreement between adult and child PedsQL scores was assessed using Bland–Altman techniques.
Statistical significance was set at the 5% level and all tests conducted were two-tailed.
Results
Enrolment
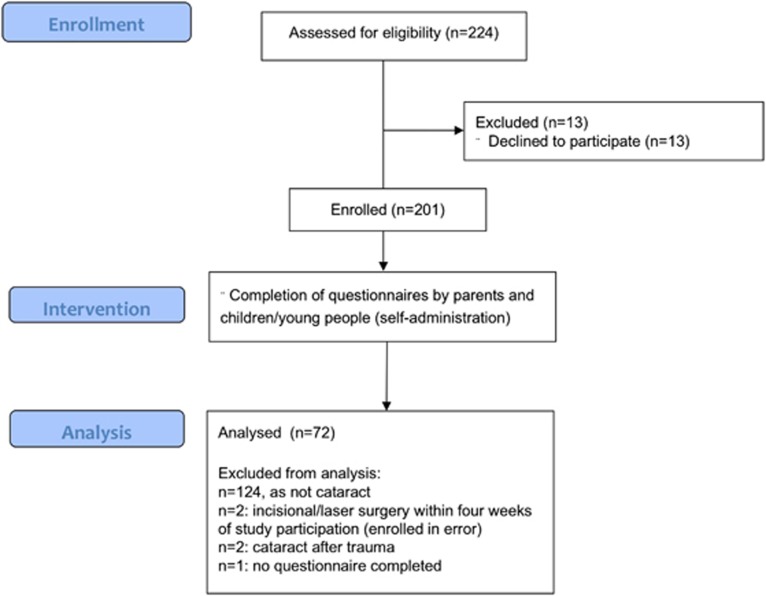
We screened ~3800 sets of medical notes to identify children eligible to take part in the wider study. We approached 214 children and their families; 13 declined taking part, all because of a perceived lack of time to complete the questionnaires. We enrolled 201 children and young people (Figure 1). As this report focuses on ChC, we excluded 124 data sets of children who did not have cataract or a history of cataract surgery. We removed two data sets as children had undergone incisional surgery or laser treatment within 4 weeks of study participation and had been enrolled in error. In addition, we removed two data sets of children with traumatic cataract, which was not included in our inclusion criteria, and one data set, as neither parents nor child completed the questionnaires after having given consent. The statistical analysis was carried out on the remaining 72 data sets (Figure 1).
Figure 1.
Enrolment, intervention, and analysis flowchart (modified from CONSORT, www.consort-statement.org).
Missing data
The proportion of missing data was low. No data were missing for age, gender, diagnoses, laterality, BCVA, and number of daily eye drops. Ethnicity was unknown in eight participants (11.11%). Age at diagnosis of the eye condition could not be determined exactly in three children (4.17%). Two children had previous surgical interventions at other centres, and information about previous number of operations and general anaesthetics was incomplete (2.78%). For all questionnaires administered, response and completion rates were high. The response rate for the PedsQL family report was 95.83%, for the parent report 97.22%, for the self-report 90.74% (Supplementary Material). The proportions of fully completed questionnaires were 88.41%, 90.00%, and 97.96%, respectively. CVAQC and IVI-C response rates were 90.74% and 77.78%, respectively. CVAQC and IVI-C scores both contain a ‘for other reasons’ category; selection of this category is taken into account during calculation of the scores.
Participants
The mean (SD) age of participants was 8.45 (SD 4.10) years. Eighteen children were 2–4 years old, 16 were 5–7 years old, 25 were 8–12 years old, and 13 were 13–16 years old (Table 1). Thirty-eight participants (52.78%) were female. Sixty-eight percent of participants were White, 15.28% Asian or Asian British, 4.17% Black or Black British, 1.39% other; ethnicity was unknown in 11.11%.
Table 1. Demographic and clinical characteristics, associated conditions and lens status in study participants.
| Age 2–4 years | Age 5–7 years | Age 8–12 years | Age 13–16 years | All age groups | |
|---|---|---|---|---|---|
| n=18 | n=16 | n=25 | n=13 | n=72 | |
| Age at study participation | |||||
| Mean (SD) | 3.66 (0.91) | 6.26 (0.85) | 9.88 (1.33) | 15.05 (1.14) | 8.45 (4.10) |
| Age at diagnosis | |||||
| Mean (SD) | 0.23 (0.54) | 1.07 (1.79) | 1.52 (3.17) | 2.36 (4.52) | 1.25 (2.84) |
| BCVA | |||||
| Median (IQR) | 0.30 (0.1–0.62) | 0.2 (0–0.43) | 0.1 (0–0.28) | 0.1 (0–0.6) | 0.18 (0.02–0.43) |
| Number of daily eye drops | |||||
| Median (IQR) | 0 (0–2) | 0 (0–0) | 0 (0–3) | 8 (0–10) | 0 (0–2.25) |
| Number of eye interventions | |||||
| Median (IQR) | 2 (2–4) | 2 (2–5) | 3.5 (2–5) | 4 (2–8) | 3 (2–5) |
| Number of general anaesthetics | |||||
| Median (IQR) | 2 (2–5) | 2.5 (2–4) | 3.5 (2–6) | 5 (3–8) | 3 (2–5.75) |
| Associations | n | % | |||
| Congenital/infantile cataract or lensectomy only | 27 | 37.5 | |||
| Cataract/lensectomy complicated by secondary glaucoma | 31 | 43.06 | |||
| Cataract and glaucoma secondary to uveitis | 2 | 2.78 | |||
| Cataract and neonatal or aniridic glaucoma (no lensectomy) | 2 | 2.78 | |||
| Cataract and microphthalmia, lensectomy, glaucoma | 1 | 1.39 | |||
| Cataract and microphthalmia | 8 | 11.11 | |||
| Lens subluxation / Marfan syndrome and glaucoma | 1 | 1.39 | |||
| Lens status | |||||
| One eye prosthesis, other aphakic | 1 | 1.39 | |||
| Aphakia both eyes | 28 | 38.89 | |||
| One eye aphakic, other no surgery | 16 | 22.22 | |||
| Lens implants both eyes | 7 | 9.72 | |||
| One eye lens implant, other eye no surgery | 13 | 18.06 | |||
| No surgery to either eye | 7 | 9.72 | |||
Clinical details
Sixty-nine participants (95.83%) had congenital/infantile cataract, of these, 31 (43.06%) had developed glaucoma after cataract surgery. Eight also had microphthalmia; another had microphthalmia, cataract surgery, and secondary glaucoma. Two had cataract associated with either aniridia or primary congenital glaucoma. In total, 37 children and young people (51.39%) had glaucoma.
Lens status
Over a third of participants were aphakic in both eyes (n=28, 38.89% Table 1). Sixteen (22.22%) had aphakia in one eye, while the other eye had not undergone surgery. In one case, one eye was aphakic, the other replaced by an ocular prosthesis. Seven participants (9.72%) had lens implants in both eyes, and another seven had not undergone surgery.
The condition was bilateral in 43 cases (59.72%), and the mean age (SD) at diagnosis was 1.25 years (SD 2.84) (Table 1). Median BCVA in the better seeing eye or with both eyes open was 0.18 logMAR (IQR 0.02–0.43). Children applied a median of 0 (0–2.25) eye drops each day, with a maximum of 16 daily drops. The median number of eye operations (incisional or laser) performed on both eyes combined was 3 (2–5, maximum 35), and the median number of general anaesthetics including examinations under anaesthesia the children/young people had undergone was 3 (2–5.75, maximum 26).
Functional visual ability
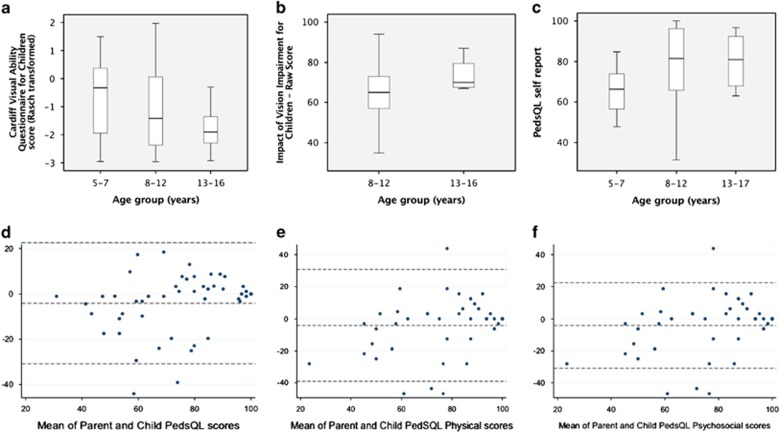
Forty-nine of 54 children and young people aged 5–16 years completed the CVAQC (90.74%). The median score was −1.42 (IQR −2.28 to −0.03), indicating moderate impairment of FVA (Table 2). Scores were better in older children than in the younger age group: −1.91 (−2.30 to −1.36) in 13–16 year olds, −1.42 (−2.37 to 0.06) in 8–12 year olds, and −0.36 (−1.94 to 0.37) in 5–7 year olds (−3.00 indicating normal visual ability; Figure 2a). There was evidence of an association between age and CVAQC score (Spearman’s rho correlation coefficient SRCC −0.291, P=0.04).
Table 2. Scores for functional visual ability (FVA), vision- and health-related quality of life (VR-QoL, HR-QoL) reported by parents/carers and children.
| Age 2–4 years | Age 5–7 years | Age 8–12 years | Age 13–16 years | All age groups | |
|---|---|---|---|---|---|
| CVAQC | |||||
| Median (IQR) | −0.36 (−1.94 to 0.37) | −1.42 (−2.37 to 0.06) | −1.91 (−2.30 to−1.36) | −1.42 (−2.28 to −0.03) | |
| IVI-C | |||||
| Mean (SD) | 63.82 (17.96) | 73.5 (9.26) | 65.67 (16.91) | ||
| PedsQL family report | |||||
| Median (IQR) | 65.28 (51.39 to 76.47) | 78.17 (63.89 to 86.28) | 78.13 (57.09 to 92.01) | 61.79 (50.87 to 87.41) | 75 (56.94 to 88.19) |
| PedsQL parent report | |||||
| Median (IQR) | 72.12 (55.95 to 87.5) | 65.22 (49.46 to 82.88) | 80.43 (59.27 to 95.11) | 61.41 (55.16 to 78.80) | 71.74 (51.98 to 88.5) |
| PedsQL self-report | |||||
| Median (IQR) | 66.30 (57.07 to 73.91) | 81.52 (65.76 to 96.2) | 80.98 (68.75 to 91.85) | 76.09 (61.96 to 89.13) | |
| PedsQL family report physical subscore | |||||
| Median (IQR) | 66.67 (50 to 100) | 79.17 (61.46 to 100) | 90.00 (68.75 to 100) | 58.33 (48.96 to 81.25) | 79.17 (58.33 to 100) |
| PedsQL family report psychosocial subscore | |||||
| Median (IQR) | 65.83 (51.67 to 79.46) | 77.08 (64.79 to 83.75) | 78.33 (54.17 to 90.26) | 65.06 (51.88 to 85.63) | 73.33 (54.79 to 85.63) |
| PedsQL parent report physical subscore | |||||
| Median (IQR) | 79.69 (53.13 to 89.84) | 73.44 (51.56 to 94.53) | 87.50 (61.61 to 93.75) | 59.38 (49.22 to 95.31) | 81.25 (50 to 93.75) |
| PedsQL parent report psychosocial subscore | |||||
| Median (IQR) | 67.31 (54.33 to 86.54) | 62.50 (52.08 to 75.42) | 73.33 (51.55 to 90.83) | 64.17 (52.92 to 72.5) | 68.33 (53.33 to 85.71) |
| PedsQL self-report physical subscore | |||||
| Median (IQR) | 71.88 (56.25 to 85.94) | 84.38 (66.41 to 99.22) | 87.50 (67.97 to 95.31) | 82.81 (64.84 to 94.53) | |
| PedsQL self-report psychosocial subscore | |||||
| Median (IQR) | 61.67 (53.33 to 72.50) | 79.17 (62.50 to 91.67) | 79.17 (70 to 91.67) | 73.33 (60 to 85.95) | |
Possible CVAQC scores (FVA) extend from −3.00 (normal visual ability) to +2.80 (severe visual impairment). Possibe IVI-C scores are between 0 and 96 (96=normal VR-QoL); participants in this study reported markedly reduced VR-QoL. Possible PedsQL scores range from 0 to 100 (100=normal HR-QoL); in this study, scores were severely reduced in all versions and subscales of the instrument (parent report, family report, self-report, physical and psychosocial subscores).
Figure 2.
Top: Box plots of median and interquartile range (IQR) Cardiff Visual Ability for Children (a), Impact of Vision Impairment for Children (b), and PedsQL self-report scores (c) of children with cataract. There is a trend towards less impairment with increasing age (decreasing scores on CVAQC, increasing scores on IVI-C and PedsQL), but with large overlap of the IQR. Bottom: Bland–Altman plots of agreement between parent and child PedsQL scores; x axis: mean of parent and child scores; y axis: difference of parent and child scores. Parents report lower overall scores than their children; the lower the scores, the larger the disagreement (d). The plot for physical and psychosocial PedsQL subscores shows no systematic bias (e), but parents tended to report lower scores than their children on the psychosocial scale (f).
Vision-related quality of life
Twenty-one of 27 eligible children and young people aged 8–16 years completed the IVI-C (77.78%). The mean score was 65.67 (SD 16.91). The mean score in the older age group (73.5, SD 9.26, in 13–16 year olds) was higher than that in the younger age group (63.82, SD 17.96, in 8–12 year olds), with 96 indicating normal VR-QoL (Figure 2b). The observed difference was not statistically significant in these data, however there were just four subjects in the older age group and thus power to detect such a difference was limited.
Health-related quality of life
The median of the overall self-report completed by children/young people (n=49/54, 90.74%) was 76.09 (61.96–89.13; Figure 2c), the median of the parent report about the child (n=70, 97.22%) was 71.74 (51.98–88.19), and the median score of the family report completed by 69/72 parents/carers (response rate 95.93%, Supplementary Material) was 75 (56.94–88.19, Table 2 with 100 indicating normal HR-QoL. Figure 2d shows a Bland–Altman plot assessing agreement between parent and child PedsQL scores. There is evidence of decreasing dispersion—for individuals with lower scores, there is larger disagreement between child and adult score than for individuals with higher scores. The average disagreement (ie, estimated bias) between adult and child score was −4.06 (−7.94, −0.18), indicating significant evidence that parents scores are lower than their child’s.
The overall self-report scores were slightly higher in the older age groups than the younger ones: median (IQR) 66.30 (57.07–73.91) in 5–7 year olds, 81.52 (65.76–96.2) in 8–12 year olds, and 80.98 (68.75–91.85) in 13–16 year olds (Table 2) and the Family impact PedsQL scores tended to be higher in parents of children aged 5–12 years than in very young children and teenagers (Table 2).
The median ‘physical wellbeing’ PedsQL subscores were 82.81 (64.84–94.53) in the self-report, 81.25 (50–93.75) in the parent report, and 79.17 (58.33–100) in the family report. The median ‘psychosocial wellbeing’ subscores were 73.33 (60–85.95) in the self-report, 68.33 (53.33–85.71) in the parent report, and 73.33 (54.79–85.63) in the family report. Psychosocial scores appeared more affected than physical scores. Figures 2e and f are Bland–Altman plots for physical and psychosocial scores. There was no evidence of systematic bias for physical scores, but parents tended to give lower scores than their children on the psychosocial score with an estimated difference of −4.19 (−8.12, −0.26).
Associations
Our study was not powered to detect statistically significant associations. However, older age at study participation tended to be associated with better CVAQC and PedsQL self-report scores. Bilaterality of cataract tended to be associated with worse PedsQL self-report, and parent scores and poorer BCVA tended to be associated with worse a lower CVAQC score, IVI-C score, PedsQL self-report, parent and family scores.
Discussion
Key results
The principal aim of this study was to explore the effects of ChC on FVA, VR-QoL, and general HR-QoL as reported by children/young people and their parents/carers. Due to its inclusive design, involving children of a broad age range and their parents, this study delivers new insights into the impact of ChC on families.
ChC is not only associated with sight impairment, but is often complicated by a need for multiple additional operations and anaesthetics and the need for daily eye drops, most commonly for secondary glaucoma. The effect on FVA, VR-QoL, and HR-QoL is profound. Children themselves report reduced levels of FVA and VR-QoL; this is most pronounced in younger children. HR-QoL, reported by the young person themselves and by parents on behalf of their child, is significantly reduced, as is the HR-QoL experienced by the family. Children and young people of all age groups report a greater impact on psychosocial than physical well-being.
Limitations
Our study design carries a number of potential sources of bias. First, enrolling children attending a single site may induce selection bias. We attempted to reduced further bias by approaching consecutive patients; only a small proportion (n=13/224, 5.8%) declined to take part. However, families may have stopped attending clinics due to dissatisfaction with the services, or unwillingness or inability to comply with intense treatment regimes. On the other hand, families of children with good visual outcome may equally stop attending and be lost to follow-up. We have no data to estimate the proportion of families who stop attending, but from clinical experience consider the overwhelming majority of parents to be eager to provide the best possible healthcare for their child. Lack of a control group of healthy children may be considered a limitation. However, CVAQC and IVI-C were specifically developed for children with sight impairment; this would lead to a ceiling effect if used in healthy children, and comparison with healthy children would be misleading. For the PedsQL, normative data are available from large numbers of healthy children, and a control group is not required. We limited inclusion to families able to communicate in English, which may induce selection bias. However, during enrolment we did not encounter any family who could not communicate in English.
It would be interesting to explore a possible association between the number of surgical interventions and QoL. Previous studies have used the number of surgical procedures as a proxy of painful episodes the child had encountered as part of their eye treatment.20 Similarly, the number of general anaesthetics (including EUAs, as these are often arranged on the understanding that should findings indicate a need for additional surgery, this will be carried out under the same anaesthetic) has been used as a proxy for episodes of emotional upset and anxiety.20 However, our study was not powered to explore associations between QoL and putative risks factors for a reduction in QoL. As we were mindful of the need to avoid multiple significance tests and the potential for misinterpretation of non-statistically significant findings, we focused our analysis on the main objectives of the study.
While logMAR visual acuity is a well-established measure of visual function, it is not always possible to use logMAR methods in children with sight impairment, and ‘hand movements’ or ‘counting fingers’ at a specified testing distance are still occasionally used. Complete blindness, ‘no perception of light’, or ‘artificial eye/ocular prosthesis’ can also not be expressed in logMAR. In order to allow a quantitative analysis, we followed the approach of using logMAR values of 1.3–3 in these cases.19 This may have led to an under- or overestimate of logMAR acuity in some cases. The heterogeneity of our study population, including children with secondary cataract and secondary glaucoma, may be considered problematic, but it allowed us to gather the views of older children and young people, which no previous study had explored. Another strength of our study is that children/young people completed the questionnaires by themselves, or were supported by play specialists if necessary. This eliminated parental perceptions influencing the children’s answers, though it may not have fully eliminated an adult’s bias from children’s answers.
Interpretation
The reduction in HR-QoL in children with cataract we report here is comparable to levels reported by children with severe congenital heart defects or liver transplants.21, 22 psychosocial subscores are reduced to levels comparable to children undergoing treastment for acute lymphoblastic leukaemia.23 A previous study exploring HR-QoL in children who had undergone surgery for congenital cataract and their parents also reported reduced levels;18 the scores we observed in the equivalent age group are even lower. In addition, the present study extends the finding of reduced HR-QoL to children who have not undergone surgery and children with secondary cataract.
A novel finding is that family and parental HR-QoL scores are higher in children aged 5–12 compared with younger children and teenagers. The cause for this is unclear; possibly child and family initially adjust, but in teenagers expectations and frustrations about education and transition to independence may increase. Alternatively, as better QoL is associated with better BCVA, the observed improvement in vision over time may explain increased levels in HR-QoL.
Two of the tools we used, the CVAQC and the IVI-C, have not previously been used in children with cataract, so direct comparison with other studies is not possible.
Generalisability
Within the limits of the study design, that is, selection bias which may have led to inclusion of a higher proportion of more treatment-adherent families and the limitation of enrolling participants at a single site in one highly developed country, our findings can be generalised to other children with cataract who receive care in similar settings.
Conclusions
While treatment for cataract in adults is a highly successful sight-restoring procedure, cataract in children can have a dramatic effect on the life of affected children/young people and their families. More research is needed to evaluate the impact of multiple interventions and life-long hospital follow-up. Families and young people may benefit from support to address psychosocial problems and difficulties with children’s activities of daily living.

Acknowledgments
We thank Miss Anneka Tailor for supporting data collection and entry, and Miss Konstantina Prapa for facilitating enrolment of participants into the study. We thank all children, young people, parents, and carers who took part in this study. The study was not supported by specific funding. AHDN and VT are employed by the National Institute for Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital and UCL Institute of Ophthalmology, and as such the work was supported by the NIHR.
Author contributions
AD-N and MP developed the study protocol. VT enrolled participants, collected data and entered data onto the electronic database which AD-N had developed. AD-N and CB conducted data analysis. All authors reviewed and discussed, and interpreted the data acquired. AD-N drafted the manuscript, which was then critically reviewed and modified by all authors.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
The authors declare no conflict of interest.
Supplementary Material
References
- Foster A, Gilbert C. Cataract in children. Acta Paediatr 2003; 92(12): 1376–1378. [DOI] [PubMed] [Google Scholar]
- Freedman SF, Lynn MJ, Beck AD, Bothun ED, Orge FH, Lambert SR et al. Glaucoma-related adverse events in the first 5 years after unilateral cataract removal in the infant aphakia treatment study. JAMA Ophthalmol 2015; 133(8): 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak M, Rahi JS, , British Congenital Cataract Interest G. Incidence of and factors associated with glaucoma after surgery for congenital cataract: findings from the British Congenital Cataract Study. Ophthalmology 2008; 115(6): 1013–1018 e1012. [DOI] [PubMed] [Google Scholar]
- Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR, , Infant Aphakia Treatment Study Group. Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study. Am J Ophthalmol 2014; 158(5): 892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann EE, Lynn MJ, Lambert SR, , Infant Aphakia Treatment Study Group. Baseline characteristics of the infant aphakia treatment study population: predicting recognition acuity at 4.5 years of age. Invest Ophthalmol Vis Sci 2014; 56(1): 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann EE, Stout AU, Lynn MJ, Yen KG, Kruger SJ, Lambert SR et al. Stereopsis results at 4.5 years of age in the infant aphakia treatment study. Am J Ophthalmol 2015; 159(1): 64–70 e61-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci 2008; 49(2): 594–603. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B. Effect of amblyopia on self-esteem in children. Optom Vis Sci 2008; 85(11): 1074–1081. [DOI] [PubMed] [Google Scholar]
- Birch EE, Cheng CS, Felius J. Validity and reliability of the Children's Visual Function Questionnaire (CVFQ). J AAPOS 2007; 11(5): 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes M, Salomão S, Berezovsky A, Tartarella M. Assessing vision-related quality of life in children with bilateral congenital cataracts. Arq Bras Oftalmol 2009; 72(4): 467–480. [DOI] [PubMed] [Google Scholar]
- Chen W, Ye H, Deng D. Development and evaluation of the scale of quality of life for children with bilateral congenital cataract. Zhonghua Yan Ke Za Zhi 2007; 43(3): 239–244. [PubMed] [Google Scholar]
- Ye H, Chen W, Deng D, Lin Z, Yang W. Quality of life assessment in children with congenital bilateral cataract. Zhonghua Yan Ke Za Zhi 2007; 43(11): 996–999. [PubMed] [Google Scholar]
- Cochrane GM, Marella M, Keeffe JE, Lamoureux EL. The impact of vision impairment for children (IVI_C): validation of a vision-specific pediatric quality-of-life questionnaire using Rasch analysis. Invest Ophthalmol Vis Sci 2011; 52(3): 1632–1640. [DOI] [PubMed] [Google Scholar]
- Khadka J, Ryan B, Margrain TH, Court H, Woodhouse JM. Development of the 25-item Cardiff Visual Ability Questionnaire for Children (CVAQC). Br J Ophthalmol 2010; 94(6): 730–735. [DOI] [PubMed] [Google Scholar]
- Tadic V, Cooper A, Cumberland P, Lewando-Hundt G, Rahi JS. Vision-related quality of life G. Development of the functional vision questionnaire for children and young people with visual impairment: the FVQ_CYP. Ophthalmology 2013; 120(12): 2725–2732. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001; 39(8): 800–812. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQL 4.0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med 2002; 25(2): 175–193. [DOI] [PubMed] [Google Scholar]
- Chak M, Rahi J, , British Congenital Cataract Interest Group. The health-related quality of life of children with congenital cataract: findings of the British Congenital Cataract Study. Br J Ophthalmol 2007; 91(7): 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities ‘hand motion’ and ‘counting fingers’ can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci 2006; 47(3): 1236–1240. [DOI] [PubMed] [Google Scholar]
- Freedman B, Jones S, Lin A, Stinnett S, Muir K. Vision-related quality of life in children with glaucoma. J AAPOS 2014; 18(1): 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RL, Day T, Wade A, Bull C, Wren C, Dezateux C et al. Patient-reported quality of life outcomes for children with serious congenital heart defects. Arch Dis Child 2014; 99(5): 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbers CA, Neighbors K, Martz K, Bucuvalas JC, Webb T, Varni JW et al. Health-related quality of life in pediatric liver transplant recipients compared with other chronic disease groups. Pediatr Transplant 2011; 15(3): 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L, Yanofsky R, Klaassen RJ, Dix D, Pritchard S, Winick N et al. Quality of life during active treatment for pediatric acute lymphoblastic leukemia. Int J Cancer 2011; 128(5): 1213–1220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.