Abstract
Purpose
This study aimed to evaluate the prevalence and associated factors of involutional blepharoptosis in a nationwide representative sample in Korea.
Methods
Cross-sectional study 20 941 Korean men and women 40 years of age and older who participated in last 2 years (2008 and 2009) of the 4th wave of the Korean National Health and Nutrition Examination Survey (KNHANES) IV and the first 2 years (2010 and 2011) of KNHANES V. Ocular examinations were performed by ophthalmologists trained in procedure and grading methods, and blepharoptosis was defined as a marginal reflex distance <2 mm on either eye.
Results
The prevalence of involutional blepharoptosis in the Korean adult was 13.5% (95% CI: 12.1%, 14.9%). It was increasing along with aging (5.4% among 40’s and 32.8% in people over 70 years old). A statistically significant negative association between levator function and blepharoptosis was found. With right eye, while only 5.4% (95% CI: 4.5%, 6.2%) had blepharoptosis among people whose levator function was excellent (≥12 mm), 71.4% (95% CI: 60.0%, 85.9%) of people whose levator function was poor (≤4 mm) had blepharoptosis. Hypertension, diabetes, higher body mass index (BMI), and lower education had statistically significant association with blepharoptosis adjusting all other confounders.
Conclusions
The distribution and proportional changes of levator function and marginal reflex distance 1 (MRD1) with aging implicate a contributory role of muscular degeneration. Strong association with hypertension, diabetes, BMI, and education level suggests that etiology of involutional blepharoptosis would be multifactorial and further investigation would be necessary to determine precise mechanism and contribution of factors.
Introduction
Age-related blepharoptosis (ptosis) is one of the common conditions encountered in ophthalmology clinics. It can cause superior visual field defect at primary and downward reading gaze and affect quality of life.1
The pathophysiology and characteristics of involutional ptosis was established by many clinical studies.2, 3, 4, 5, 6, 7 In terms of risk factors associated with ptosis, dehiscence of the levator aponeurosis is thought to be the most accountable cause in involutional ptosis.4, 5 However, most of the studies were performed with patients who underwent surgery in tertiary or referred hospitals limiting generalizability of the study results. Few studies reported prevalence and characteristics of blepharoptosis in a large general population.
The Korean Center for Disease Control and Prevention has conducted a series of Korea National Health and Nutrition Examination Surveys (KNHANES) since 1998. As parts of the fourth and fifth KNHANES (2007–2012), ophthalmological interviews and examinations were conducted between 2008 and 2011. In the present study, we examined the prevalence and associated factors of involutional blepharoptosis in a nationwide representative sample in Korea.
Materials and methods
Korea National Health and Nutrition Examination Surveys
The KNHANES is a cross-sectional and nationally representative survey of the health and nutritional status of the civilian, non-institutionalized Korean population using stratified, multistage clustered probability sampling design. Sampling units are defined based on the data of household registries, including geographic area, sex, and age groups. All members of each selected household were asked to participate in the survey, and a participation rate between 2008 and 2011 ranged from 77.8 to 82.8%. The survey consists of a health interview, a nutritional survey, and a health examination survey including ophthalmologic examinations. All examinations and interviews were conducted in a specially equipped mobile examination center. Ocular examinations were performed by ophthalmologists trained in procedure and grading methods. All participants were given information about the study and gave written informed consent. This survey was reviewed and approved by the Institutional Review Board of the South Korea Centers for Disease Control and Prevention. Additional details regarding the study design and methods are provided elsewhere.8, 9
Study population
Participants who completed the ophthalmological examination and aged over 40 years (n=22 832) were included in the study. Participants with a history of previous eyelid or intraocular surgery or with a medical condition that might affect the position of the upper eyelid or motility, including thyroid disease, systemic collagen disease, myopathy or cerebrovascular disease were excluded. After excluding, total 17 286 participants were included in the final analysis.
Definition of blepharoptosis
The marginal reflex distance 1 (MRD1) was defined as the distance from the upper eyelid margin to the corneal light reflex in the primary position excluding the effect of dermatochalasis or brow ptosis. The value of the MRD1 were measured and categorized into 5 subgroups (1) ≤4.0 mm; (2) 3.0–3.9 mm; (3) 2.0–2.9 mm; (4) 1.0–1.9 mm; and (5) <1.0 mm, and blepharoptosis was defined as a marginal reflex distance (MRD1) of <2 mm on either eye.10, 11 The levator function was estimated by measuring the upper eyelid excursion from downgaze to upgaze eliminating the frontalis muscle function for 4 subgroups (1) ≤12 mm; (2) 8–11 mm; (3) 5–7 mm; and (4) <4 mm). An autorefractor (KR8800; Topcon, Tokyo, Japan) was used for measurement of refractive errors, which were converted to spherical equivalents. Myopia was defined as a spherical equivalent of higher than −0.75 diopters (D). Hyperopia was defined as a spherical equivalent of more than +1.0 D. A slit-lamp examination (Haag-Streit model BQ-900; Haag-Streit AG, Koeniz, Switzerland) was performed for determination of diseases in the anterior segment of the eye and measurement of the intraocular pressure.
Other variables
Hypertension was defined as systolic blood pressure of 140 mm Hg or higher, diastolic blood pressure of 90 mm Hg or higher, and/or current use of blood pressure-lowering medication. Diabetes was defined as fasting plasma glucose of 126 mg/dl or higher, and/or current use of oral hypoglycemic agents or insulin. In addition, demographic and socioeconomic information such as age, gender, education, income, and job status was obtained from the health interview. The educational level of participants was classified as follows: (1) less than elementary school graduate; (2) middle school graduate; (3) high school graduate; and (4) more than college graduate. The participants’ occupations were categorized into four groups according to work types and working-environment: (1) white-collar workers—managers, office workers, or professionals; (2) indoor blue-collar workers—service workers, sellers, technicians, mechanics or assemblers; (3) outdoor blue-collar workers—farmers, fishermen, or simple laborers; and (4) unemployed—never been employed or household wives. Household income status was classified into four quartiles based on the household annual income. With a multivariate model, only education was included due to high correlations (>0.4) among education, occupation, and household income.
Statistical analysis
Survey weights were calculated to account for survey year, stage of selection and non-response. Statistical analysis used the survey commands of Stata (release 12.1; StataCorp LP, College Station, TX, USA) to account for survey weights and for the complex sampling design. Descriptive statistics, such as mean, median, proportion, standard error, and 95% confidence interval (CI), were reported when appropriate. Continuous variables were expressed as mean and standard deviations and categorical variables as percentages. Differences in the distribution of continuous and categorical variables by blepharoptosis were evaluated using the t-tests, or the χ2-test, respectively. Multi-variable logistic regression models were used to determine the risk factors for involutional blepharoptosis. Age and sex adjusted prevalence of blepharoptosis by levator function were computed using marginal standardization. The predicted conditional probability of blepharoptosis can be obtained from marginal standardization with confounders fixed at its mean, therefore providing a less biased estimate of disease prevalence for the overall population.12
For the logistic regression assessing the odds ratio (OR) of blepharoptosis by the risk factors, we used progressively adjusted models. The minimally adjusted model was crude. In model 2, we accounted for the demographics, lifestyle and cardiovascular factors of age, gender, hypertension, hyperlipidemia, diabetes, history of CVD, BMI, smoking, drinking status, and education. In model 3, we additionally adjusted for the presence of other eye diseases that may potentially affect the association including myopia, hyperopia, strabismus, pterygium, and cataract.
Results
The overall prevalence of involutional blepharoptosis in the Korean adult population was 13.5% (95% CI: 12.1%, 14.9%) and the prevalence in age of 40–49, 50–59, 60–69, and over 70 years old was 5.4% (95% CI: 4.3%, 6.6%), 11.6% (95% CI: 9.7%, 13.4%), 19.8% (95% CI: 17.4%, 22.1%), and 32.8% (95% CI: 29.4%, 36.1%) respectively (data not shown).
The demographic characteristics in the study are summarized in Table 1. The mean age of study participants was 55.1 years. People with blepharoptosis were more likely to be older (62.9 vs 53.9 years old), higher body mass index (BMI, 24.3 vs 23.9 kg/m2), hypertensive (40.1 vs 23.6%), and diabetic (16.6 vs 8.7%) compared to people without disease. People who had lower education and lower income were more likely to have blepharoptosis: only 9.6% of white-collar workers had blepharoptosis while 20.6% of outdoor blue-collar workers had it. People with blepharoptosis were also more likely to have hyperopia (30.8 vs 17.7%), strabismus (2.2 vs 0.9%), and cataract (62.7 vs 33.78%) compared to people without it.
Table 1. Characteristics of the study populationa.
| Characteristic | Overall N=17286 |
Involutional Blepharoptosis |
P-value | |
|---|---|---|---|---|
| No N=15138 (87.6%) | Yes N=2148 (12.4%) | |||
| Age, years (mean, SE) | 55.1 (0.2) | 53.9 (0.2) | 62.9 (0.4) | <0.001 |
| Sex, female | 51.2 (0.4) | 51.4 (0.5) | 50.1 (1.4) | 0.72 |
| BMI, kg/m2 (mean, SE) | 23.9 (3.4) | 23.9 (3.7) | 24.3 (8.0) | <0.001 |
| Hypertension (Yes) | 25.9 (0.5) | 23.6 (0.5) | 40.1 (1.4) | <0.001 |
| Hyperlipidemia (Yes) | 11.2 (0.3) | 11.0 (0.3) | 12.3 (0.8) | 0.17 |
| Diabetes (Yes) | 9.7 (0.3) | 8.7 (0.3) | 16.6 (1.0) | <0.001 |
| CVD (Yes) | 5.6 (0.2) | 5.2 (0.3) | 8.0 (0.6) | <0.001 |
| Smoking (Yes) | 46.7 (0.5) | 46.5 (0.5) | 48.3 (1.3) | 0.19 |
| Alcohol drinker (Yes) | 83.0 (0.4) | 84.0 (0.4) | 76.3 (1.1) | <0.001 |
| Education | <0.001 | |||
| <Elementary school | 31.5 (0.7) | 28.4 (0.8) | 51.8 (1.7) | |
| Middle school | 15.8 (0.4) | 16.2 (0.5) | 12.9 (0.9) | |
| High school | 31.5 (0.6) | 33.1 (0.7) | 21.4 (1.1) | |
| ≥College | 19.9 (0.8) | 21.4 (0.8) | 12.1 (1.3) | |
| Household incomeb | <0.001 | |||
| Very low | 20.7 (0.6) | 18.9 (0.6) | 32.2 (1.7) | |
| Low | 24.9 (0.6) | 24.8 (0.6) | 25.5 (1.4) | |
| High | 25.8 (0.6) | 26.5 (0.6) | 21.4 (1.2) | |
| Very high | 26.8 (0.8) | 28.2 (0.8) | 18.1 (1.6) | |
| Occupationc | <0.001 | |||
| While-collar | 15.8 (0.5) | 16.8 (0.6) | 9.6 (1.1) | |
| Indoor blue-collar | 26.8 (0.7) | 28.0 (0.7) | 18.8 (1.3) | |
| Outdoor blue-collar | 20.8 (0.9) | 20.8 (0.9) | 20.6 (1.5) | |
| Unemployed | 35.2 (0.7) | 33.1 (0.7) | 49.0 (1.5) | |
| Myopia (yes) | 34.6 (0.7) | 35.7 (0.7) | 27.3 (1.3) | <0.001 |
| Hyperopia (yes) | 19.5 (0.4) | 17.7 (0.4) | 30.8 (1.3) | <0.001 |
| IOP, mmHg (mean, SE) | 14.1 (0.1) | 14.1 (0.5) | 14.0 (0.9) | 0.38 |
| Pterygium (yes) | 8.2 (0.4) | 7.6 (0.4) | 11.8 (0.8) | <0.001 |
| Strabismus (yes) | 1.2 (0.1) | 0.9 (0.1) | 2.2 (0.4) | <0.001 |
| Cataract (yes) | 37.6 (1.0) | 33.8 (1.0) | 62.7 (2.1) | <0.001 |
Values are survey-weighted mean (SE) or percentage (SE) for continuous or categorical variables, respectively. IOP: Intraocular pressure; CVD: History of cardiovascular disease (myocardial infarction or angina).
Household income status was classified into four quartiles based on household annual income.
(1) White-collar workers—managers, office workers, or professionals; (2) indoor blue-collar workers—service workers, sellers, technicians, mechanics or assemblers; (3) outdoor blue-collar workers—farmers, fishermen, or simple laborers; and (4) unemployed—never been employed or household wives.
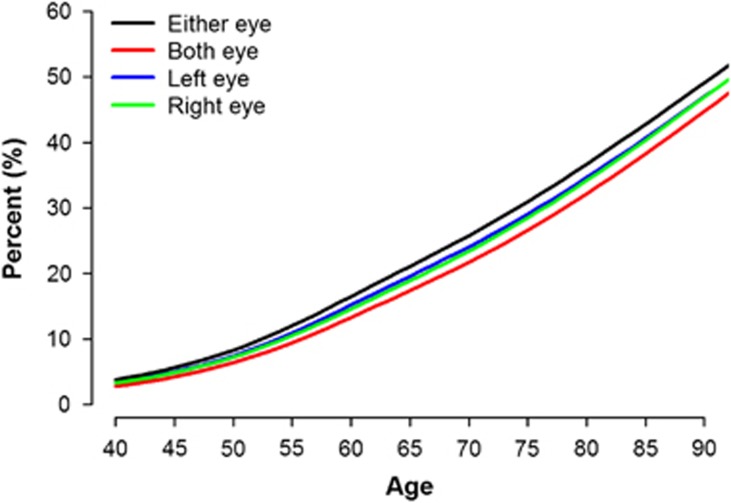
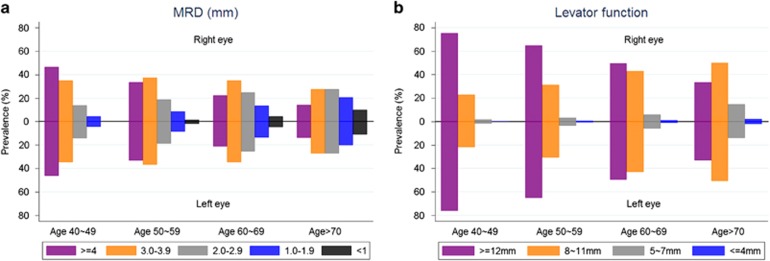
Figure 1 presents increasing prevalence of involutional blepharoptosis along with aging. According to the Figure 2a, with older age group, the proportion of MRD1≥4 mm is decreasing (right eye: 47.6% in 40–49, 34.8% in 50–59, 21.8% in 60–69, and 14.3% in 70 years older) while the proportion of MRD<1 mm substantially increasing (right eye: 0.3% in 40–49, 1.6% in 50–59, 4.4% in 60–69, and 10.7% in 70 years older).
Figure 1.
Prevalence of blepharoptosis along with aging (N=17 286). Each line represents prevalence of blepharoptosis of right, left, either and both eyes among study participants, and the prevalence were presented up to 90 years old.
Figure 2.
(a) Marginal Reflex Distance 1 (MRD) by Age Groups Proportion of MRD≥4, 3.0–3.9, 2.0–2.9, and <1 mm in right and left eye were presented by age group. Each color represents different group categorized by MRD. (b) Levator Function by Age Groups Proportion of levator function≥12, 8–11, 5–7, and ≤4 in right and left eye were presented by age group. Each color represents different levator function group.
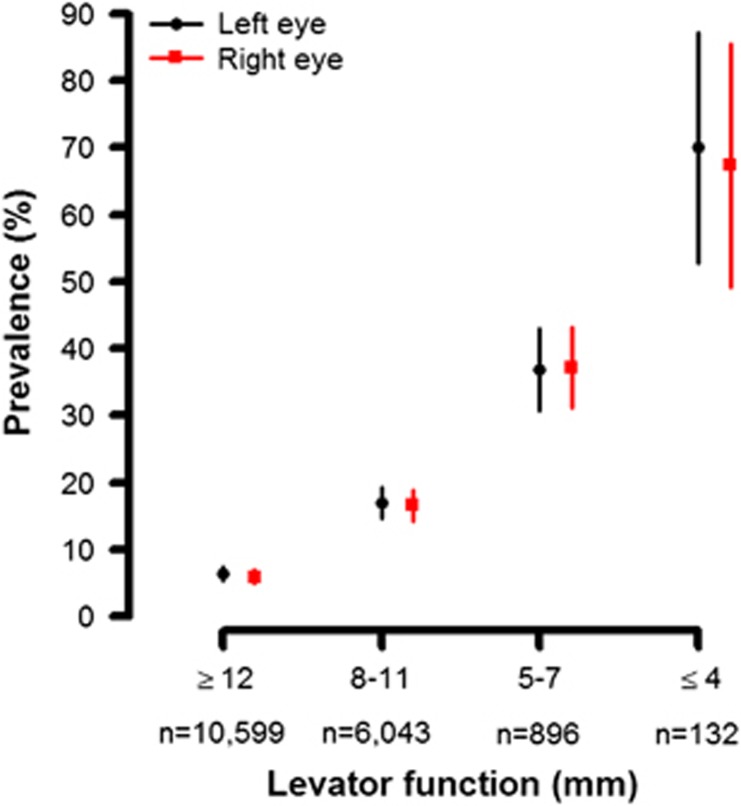
According to Figure 2b, the proportion of people whose levator function was excellent (≥12 mm) decreased as age increased (right eye: 76.9% in 40–49, 66.1% in 50–59, 49.8% in 60–69, and 33.7% in 70 years older) and increased prevalence of people whose levator function<12 was observed. With adjustment of age and gender, a statistically significant negative association between levator function and blepharoptosis was found (Figure 3). While only 5.4% (95% CI: 4.5%, 6.2%) and 5.5% (95% CI: 4.7%, 6.4%) for right and left eye, respectively, had blepharoptosis among people whose levator function was excellent (≥12 mm), 71.4% (95% CI: 60.0%, 85.9%) and 78.9% (95% CI: 64.4%, 93.4%) of people whose levator function was <=4 mm had blepharoptosis with right and left eye.
Figure 3.
Adjusted prevalence of blepharoptosis by levator function. Age and sex adjusted prevalence of blepharoptosis of right and left eye by levator function was presented. Results were computed using marginal standardization and box plots represent 95% confidence interval.
In a multivariate model, the adjusted OR for blepharoptosis with 1 year age increase was 1.05 (95% CI: 1.04, 1.06), and having hypertension (OR: 1.18, 95% CI: 1.01, 1.38) or diabetes (OR: 1.32, 95% CI: 1.09, 1.58), and higher BMI (OR: 1.05, 95% CI: 1.03, 1.07) was associated with increased odds of blepharoptosis. On the other hands, being female and having higher education was inversely associated with the outcome (Table 2). After adjusting all demographic and clinical characteristics, people with strabismus were 2.06 (95% CI: 1.17, 3.62) times more likely to have blepharoptosis and it was statistically significant.
Table 2. Factors associated with involutional blepharoptosis.
|
Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Age, years | 1.07 | 1.06, 1.07 | <0.01 | 1.06 | 1.05, 1.07 | <0.01 | 1.05 | 1.04, 1.06 | <0.01 |
| Female (vs male) | 0.95 | 0.85, 1.07 | 0.40 | 0.79 | 0.65, 0.95 | <0.01 | 0.69 | 0.57, 0.84 | <0.01 |
| Hypertension (Yes) | 2.18 | 1.92, 2.48 | <0.01 | 1.20 | 1.04, 1.40 | 0.02 | 1.18 | 1.01, 1.38 | 0.04 |
| Hyperlipidemia (Yes) | 1.13 | 0.95, 1.35 | 0.17 | 0.91 | 0.75, 1.11 | 0.36 | 0.87 | 0.71, 1.06 | 0.17 |
| Diabetes (Yes) | 2.11 | 1.78, 2.50 | <0.01 | 1.36 | 1.14, 1.62 | <0.01 | 1.32 | 1.09, 1.58 | <0.01 |
| CVD (Yes) | 1.57 | 1.29, 1.90 | <0.01 | 0.85 | 0.68, 1.05 | 0.12 | 0.82 | 0.65, 1.04 | 0.10 |
| BMI, kg/m2 | 1.03 | 1.02, 1.05 | <0.01 | 1.05 | 1.03, 1.07 | <0.01 | 1.05 | 1.03, 1.07 | <0.01 |
| Smoking (Yes) | 1.08 | 0.96, 1.21 | 0.19 | 1.00 | 0.83, 1.21 | 0.97 | 0.97 | 0.80, 1.18 | 0.76 |
| Alcohol drinker (Yes) | 0.61 | 0.53, 0.71 | <0.01 | 0.95 | 0.81, 1.12 | 0.54 | 0.90 | 0.77, 1.06 | 0.24 |
| Education | |||||||||
| Elementary school (ref) | 1.00 | 1.00 | 1.00 | ||||||
| Middle school | 0.44 | 0.36, 0.53 | <0.01 | 0.73 | 0.60, 0.88 | <0.01 | 0.73 | 0.60, 0.89 | <0.01 |
| High school | 0.35 | 0.30, 0.42 | <0.01 | 0.77 | 0.63, 0.94 | <0.01 | 0.78 | 0.64, 0.95 | 0.01 |
| ≥College | 0.31 | 0.24, 0.41 | <0.01 | 0.72 | 0.54, 0.96 | 0.03 | 0.73 | 0.54, 0.98 | <0.01 |
| Myopia (yes) | 0.70 | 0.61, 0.80 | <0.01 | 1.07 | 0.92, 1.25 | 0.37 | |||
| Hyperopia (yes) | 2.18 | 1.90, 2.49 | <0.01 | 1.07 | 0.92, 1.24 | 0.39 | |||
| Strabismus (yes) | 2.27 | 1.49, 3.45 | <0.01 | 2.06 | 1.17, 3.62 | 0.01 | |||
| Pterygium (yes) | 1.61 | 1.34, 1.92 | <0.01 | 0.90 | 0.75, 1.09 | 0.27 | |||
| Cataract (yes) | 3.29 | 2.75, 3.93 | <0.01 | 1.41 | 1.12, 1.78 | <0.01 | |||
Abbreviations: CI, confidence interval; CVD, history of cardiovascular disease (myocardial infarction or angina); IOP, intraocular pressure; OR, odds ratio; ref, reference.
Model 1: Unadjusted
Model 2: Adjusted for age, gender, hypertension, hyperlipidemia, diabetes, history of CVD, BMI, smoking and drinking status, education.
Model 3: Model 2+eye diseases (presence of myopia, hyperopia, strabismus, pterygium, and cataract).
Discussion
The estimated prevalence of involutional blepharoptosis in our study was 13.5%. This is comparable with the results of the previous studies. The British study of 400 random, age-stratified subjects above 50 years of age reported 11.5% of blepharoptosis.13 A hospital-based, cross-sectional study conducted in Nepal14 reported 37.6% of ptosis cases and 10.7% of them were aponeurotic ptosis. The retrospective study in a tertiary care hospital in Singapore reported blepharoptosis was the most commonly encountered conditions occupying 19.5% of surgical cases in an oculoplastic division.15
Considering that our study was conducted with national representative sample, the rate depicts the prevalence of blepharoptosis in general people in Korea. With a MRD1 of 2 mm, 25% of superior visual field defect is known to be disturbed in various studies.16, 17, 18 Cahill et al19 suggested that ptosis and upper eyelid blepharoplasty surgery were found to be functionally beneficial for the patients with MRD1 of 2 mm or less in the primary gaze. To prevent potential injuries due to visual field defect such as motor-vehicle or fall injuries, it would be necessary for educating public about blepharoptosis and have them surgical treatment if the condition requires.
In our study, the prevalence of blepharoptosis was increasing along with age. It was 5.4% among 40’s and it was estimated up to 32.8% in people over 70 years old. This can be explained with aponeurotic or muscular aspects. Aponeurotic dehiscence or disinsertion is a well-known cause of involutional ptosis.20 The increased prevalence of ptosis from structural instability might be accounted for aponeurotic abnormalities by repetitive stretching or minor damages. In terms of muscular aspects, the loss of muscular function with aging would be associated with increased prevalence of ptosis with aging. According to a previous study, cytochrome c oxidase lacking muscle fibers were shown to be increased with aging in the extraocular muscles, and the highest defect density was observed in the levator palpebrae muscle.21 In addition, an extraocular movement study found that maximum duction angle of extraocular movement was decreased with aging.22 A marked decline appeared around the sixth decade with the greatest decrements occurring in supraduction suggesting decreasing ocular muscular function along with aging. Therefore, involutional ptosis might be due to muscular abnormalities with aging changes in muscles.
Classically, involutional blepharoptosis was thought not to be a muscular disease nor associated with a change in levator function, but rather to be primarily abnormalities of the aponeurosis.2, 3, 4, 5, 6, 7 In our study, the proportion of population with severe ptosis is increased with aging, and the proportion of people with excellent levator function also decreased with advancement of age. Moreover, a statistically significant correlation was observed between the degree of eyelid ptosis (MRD1) and levator function. Furthermore, we found much higher prevalence of blepharoptosis among people whose levator function was poor compared to people whose levator function was excellent. This is similar to the results of the retrospective cohort study by Pereira et al,23 which reported significant correlation between MRD1 and levator function with 136 patients with involutional ptosis. Therefore, our findings in large representative population seem to support the hypothesis that abnormality of the levator muscle itself might be a contributing factor in the development of involutional blepharoptosis.
In our study, hypertension, diabetes, and higher BMI had statistically significant association with blepharoptosis adjusting all other confounders. Although age is a strong risk factor of involutional ptosis, these systemic factors seem to play contributory roles in reducing levator muscle function. Specifically, disturbance of microcirculation on the levator muscle might cause fatty degeneration of the muscle and precipitate loss of function resulting in ptosis.24, 25 The study by Shirado26 presented the relationship between dyslipidemia and age-related involutional blepharoptosis in a series of 251 Japanese patients aged 60 years or older. Dyslipidemia was suggested as a possible determinant of the presence of involutional ptosis (Odds ratio: 4.008, P=0.002). However, the study was a hospital-based cross-sectional study and a causal relationship could not be determined. In our study, hyperlipidemia was not associated with blepharoptosis. Instead, other factors were shown to be related with the etiology of involutional blepharoptosis and further prospective studies covering larger patient participants will be required.
In addition, it would be meaningful to investigate localized risk factors of blepharoptosis such as physical or outdoor activities in future studies. In our study, we found involutional ptosis was associated with education level, household income, and occupation. People with higher education (marker of socioeconomic status) level tended to have a lower risk of blepharoptosis compared to people with lower education adjusting age, gender, comorbidity, BMI, smoking, drinking, and eye diseases. People with low socioeconomic status (eg, blue-collar workers) would experience more repetitive mechanical stimuli to the eyelid such as eyelid rubbing or sweat dabbing during occupational physical exertions, which might cause aponeurosis damage resulting in higher prevalence of blepharoptosis compared to people with higher education and white-collar jobs. Moreover, recently Kase et al27 suggested not only aging but also oxidative stress would play a role in the pathologic study of muscle structures in the levator aponeurosis in involutional blepharoptosis. This might reflect possible association between social-behavioral factors and pathologic factors on in involutional blepharoptosis.
Several limitations need to be considered in the interpretation of our results. First, this is a cross-sectional study, which limits the assessment of temporal relationships as well as causal inferences. Second, KNHANES did not include all the information or concurrent conditions that may influence eyelid position such as contact lens usage or traumatic eyelid injury history. Therefore, some participants included in the study may not be of age-related origin. Third, levator function and MRD1 could not be recorded as continuous values; linear regression cannot be performed to assess the correlation. Fourth, in this study, we excluded participants who did not complete the ophthalmological examination of KNAHNES. They would be participants with advanced visual defects that prevent them from completing the tests. As a result, the prevalence of blepharoptosis in our study could be underestimated due to this selection bias. However, the estimated prevalence of involutional blepharoptosis in our study was 13.5% which was comparable with the results of the previous studies. Last, since our study sample used a nationally representative sample of Korean men and women, our findings may not be generalizable to people in other countries or to subjects of other race or ethnicities. In spite of these limitations, this is the first population-based study that measured detailed eyelid parameters and determined the prevalence of blepharoptosis. Furthermore, availability of data on a number of potential risk factors and the large sample size are important strengths of our study that add plausibility to the findings.
In conclusion, the present study provides the first representative data on involutional blepharoptosis among Korean adult population. The distribution and proportional changes of levator function and MRD1 with aging implicate a contributory role of muscular degeneration. Strong association with hypertension, diabetes, BMI, and education level suggests that etiology of involutional blepharoptosis would be multifactorial and further investigation would be necessary to determine precise mechanism and contribution of factors.

Acknowledgments
Author contributions
MK designed and analyzed the study and drafted all versions of the manuscript. KW advised on design, analysis, and reviewed successive drafts of the manuscript. JC, YK, SK, and SY designed the study and reviewed successive drafts of the manuscript. DZ designed some of the study materials, contributed to analysis.
Footnotes
The authors declare no conflict of interest.
References
- Battu VK, Meyer DR, Wobig JL. Improvement in subjective visual function and quality of life outcome measures after blepharoptosis surgery. Am J Ophthalmol 1996; 121: 677–686. [DOI] [PubMed] [Google Scholar]
- Anderson RL, Dixon RS. Aponeurotic ptosis surgery. Arch Ophthalmol 1979; 97: 1123–1128. [DOI] [PubMed] [Google Scholar]
- Berke RN. A simplified blaskovics operation for blepharoptosis: results in 91 operations. Trans Am Ophthalmol Soc 1951; 49: 297–350. [PMC free article] [PubMed] [Google Scholar]
- Frueh BR. The mechanistic classification of ptosis. Ophthalmology 1980; 87: 1019–1021. [DOI] [PubMed] [Google Scholar]
- Jones LT, Quickert MH, Wobig JL. The cure of ptosis by aponeurotic repair. Arch Ophthalmol 1975; 93: 629–634. [DOI] [PubMed] [Google Scholar]
- Paris GL, Quickert MH. Disinsertion of the aponeurosis of the levator palpebrae superioris muscle after cataract extraction. Am J Ophthalmol 1976; 81: 337–340. [DOI] [PubMed] [Google Scholar]
- JA NOculoplastic Surgery: the Requisites in Ophthalmology. Mosby: St Louis, MO, USA, 2001. [Google Scholar]
- Yoon KC, Mun GH, Kim SD, Kim SH, Kim CY, Park KH et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol 2011; 25: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Lee SY, Kwon HS, Lee SH, Jung MH, Han K et al. Gender differences in the association of insulin resistance with metabolic risk factors among Korean adolescents: Korea National Health and Nutrition Examination Survey 2008-2010. Diabetes Res Clin Pract 2013; 99: 54–62. [DOI] [PubMed] [Google Scholar]
- Small RG. Stabilization of eyelid height after aponeurotic ptosis repair. Ophthalmology 1999; 106: 2043–2044. [DOI] [PubMed] [Google Scholar]
- Small RG, Sabates NR, Burrows D. The measurement and definition of ptosis. Ophthal Plast Reconstr Surg 1989; 5: 171–175. [DOI] [PubMed] [Google Scholar]
- Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014; 43: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan GV, Tallis RC, Leatherbarrow B, Forman WM. A community survey of ptosis of the eyelid and pupil size of elderly people. Age Ageing 1995; 24: 21–24. [DOI] [PubMed] [Google Scholar]
- Thapa R, Karmacharya PC, Nepal BP. Etiological pattern of blepharoptosis among patients presenting in teaching hospital. JNMA J Nepal Med Assoc 2006; 45: 218–222. [PubMed] [Google Scholar]
- Tan MC, Young S, Amrith S, Sundar G. Epidemiology of oculoplastic conditions: the Singapore experience. Orbit 2012; 31: 107–113. [DOI] [PubMed] [Google Scholar]
- Hacker HD, Hollsten DA. Investigation of automated perimetry in the evaluation of patients for upper lid blepharoplasty. Ophthal Plast Reconstr Surg 1992; 8: 250–255. [DOI] [PubMed] [Google Scholar]
- Meyer DR, Linberg JV, Powell SR, Odom JV. Quantitating the superior visual field loss associated with ptosis. Arch Ophthalmol 1989; 107: 840–843. [DOI] [PubMed] [Google Scholar]
- Meyer DR, Stern JH, Jarvis JM, Lininger LL. Evaluating the visual field effects of blepharoptosis using automated static perimetry. Ophthalmology 1993; 100: 651–658, discussion 8–9. [DOI] [PubMed] [Google Scholar]
- Cahill KV, Bradley EA, Meyer DR, Custer PL, Holck DE, Marcet MM et al. Functional indications for upper eyelid ptosis and blepharoplasty surgery: a report by the American Academy of Ophthalmology. Ophthalmology 2011; 118: 2510–2517. [DOI] [PubMed] [Google Scholar]
- Wouters RJ, van den Bosch WA, Mulder PG, Lemij HG. Upper eyelid motility in blepharoptosis and in the aging eyelid. Invest Ophthalmol Vis Sci 2001; 42: 620–625. [PubMed] [Google Scholar]
- Muller-Hocker J, Schneiderbanger K, Stefani FH, Kadenbach B. Progressive loss of cytochrome c oxidase in the human extraocular muscles in ageing—a cytochemical-immunohistochemical study. Mutat Res 1992; 275: 115–124. [DOI] [PubMed] [Google Scholar]
- Shechtman D, Shallo-Hoffmann J, Rumsey J, Riordan-Eva P, Hardigan P. Maximum angle of ocular duction during visual fixation as a function of age. Strabismus 2005; 13: 21–26. [DOI] [PubMed] [Google Scholar]
- Pereira LS, Hwang TN, Kersten RC, Ray K, McCulley TJ. Levator superioris muscle function in involutional blepharoptosis. Am J Ophthalmol 2008; 145: 1095–1098. [DOI] [PubMed] [Google Scholar]
- Dortzbach RK, Sutula FC. Involutional blepharoptosis. A histopathological study. Arch Ophthalmol 1980; 98: 2045–2049. [DOI] [PubMed] [Google Scholar]
- Shore JW, McCord CD Jr. Anatomic changes in involutional blepharoptosis. Am J Ophthalmol 1984; 98: 21–27. [DOI] [PubMed] [Google Scholar]
- Shirado M. Dyslipidaemia and age-related involutional blepharoptosis. J Plast Reconstr Aesthet Surg 2012; 65: e146–e150. [DOI] [PubMed] [Google Scholar]
- Kase S, Noda M, Yoshikawa H, Yamamoto T, Ishijima K, Ishida S. Oxidative stress in the levator aponeurosis in Asian involutional blepharoptosis. Ophthal Plast Reconstr Surg 2014; 30: 290–294. [DOI] [PubMed] [Google Scholar]