Abstract
Purpose
To investigate 1-year visual and anatomic outcomes of intravitreal aflibercept for neovascular age-related macular degeneration (nAMD) given at a fixed 8-weekly interval.
Methods
Retrospective, single-practice data analysis from an electronic medical record system of 255 eyes (223 patients) with treatment-naïve nAMD receiving 8-weekly aflibercept.
Results
Mean logarithm of the minimum angle of resolution best-corrected visual acuity (BCVA) improved from 0.66 at baseline to 0.50 at month 11 (P<0.0001). Mean central retinal thickness (CRT) decreased from 311 μm at baseline to 211 μm at month 11 (P<0.0001). Our mean VA gain of eight ETDRS letters was comparable to the VIEW 1 and VIEW 2 Trials’ results at the end of year 1. After loading at month 5, mean BCVA was 0.48 (P<0.0001), and mean CRT was 235 μm. At month 5, 143 eyes (56%) were inactive defined by the absence of macular haemorrhage and intraretinal fluid (IRF) and subretinal fluid (SRF) on optical coherence tomography, and 112 eyes (44%) remained active. At month 11, 136 eyes (53%) were inactive, and 119 eyes (47%) remained active. At month 11, 77% of inactive eyes after loading remained inactive, and 77% of the active eyes after loading remained active. At month 11, mean BCVA of the inactive group was 0.51, and mean BCVA of the active group was 0.48 (P=0.54).
Conclusions
Aflibercept administered by fixed dosing over 1 year improved VA and macular morphology in treatment-naïve eyes. Active lesions at month 11 do not have worse VA outcomes compared with inactive lesions. The macular status after loading is a reliable indicator of disease activity at the end of year 1.
Introduction
Central vision loss caused by age-related macular degeneration (ARMD) is the leading cause of blindness among the elderly in developed countries. Approximately 10–15% of all cases are neovascular AMD.1 In the United Kingdom, there were an estimated 263 000 cases of wet AMD in 2007–2009, with an annual incidence of 39 700 new cases.1 Neovascular AMD is characterised by choroidal neovascularisation that is driven by a complex process that involves a signal protein called vascular endothelial growth factor A (VEGF-A).2 Anti-VEGF agents such as aflibercept have become the mainstay of treatment for neovascular AMD. Aflibercept has gained FDA approval for the treatment of neovascular AMD in 2011 based on a comparison with ranibizumab in the VIEW 1 and VIEW 2 trials.3, 4 Aflibercept administered at 8-weekly intervals over the first year, following a loading dose of 3 monthly injections, delivered the same VA results as administering monthly ranibizumab.3 An 8-weekly fixed regimen requires fewer monitoring visits and helps solve capacity issues compared with a Pro Re Nata or treat-and-extend regimen. However, there is need to evaluate whether similar results to clinical trials can be achieved in routine clinical practice. The aim of this study was to investigate the visual and anatomic outcomes of aflibercept given at a fixed 8-weekly interval dosing in our practice at Southampton Eye Unit, University Hospital Southampton NHS foundation trust.
Materials and methods
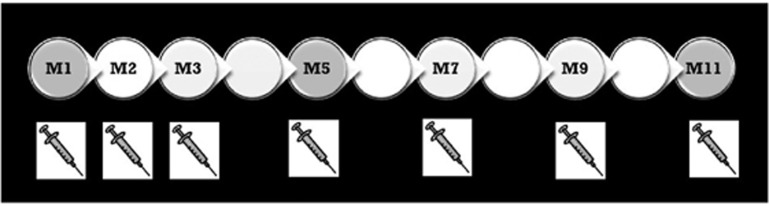
We retrospectively reviewed the clinical records of 255 eyes of 223 patients using an electronic medical record system at Southampton Eye Unit. Patients were treatment naïve diagnosed with neovascular age-related macular degeneration (nAMD) who received aflibercept over 1 year per the VIEW 1 and VIEW 2 Clinical Trials’ protocol; 3 consecutive monthly aflibercept intravitreal injections (Q4W) followed by continuous bimonthly fixed-interval dosing injections for the rest of year 1 (Q8W). At baseline visit, before the first injection, patients had their best-corrected visual acuity (BCVA) evaluated using their own prescription. In addition to that, spectral domain optical coherence tomography (SD-OCT) and in a good proportion of cases fundus fluorescein angiography or indocyanine green angiography were carried out. Missing observations were not imputed. Last observation carried forward was not used in these analyses. Further dilated fundus exams and SD-OCT scans were performed at month 5 (after loading dose) and at month 11 (before their last injection). In total, patients had one baseline visit and two follow-ups during year 1. A total of seven intravitreal injections were performed (Figure 1).
Figure 1.
A total of seven intravitreal injections during year 1. SD-OCT scans are performed only three times during the whole year; at baseline visit, at month 5 (after loading) and at month 11 (end of year 1). Grey circles represent the follow-up visits where an SD-OCT scan is performed.
VAs were carried out on each visit (follow-ups with SD-OCT scan included, and in injection-only clinics) using the logarithm of the minimum angle of resolution (LogMar) BCVA. Patients were consented before receiving their first intravitreal treatment.
The primary outcome measure was mean change in LogMar BCVA at year 1. Secondary outcomes included SD-OCT findings at month 5 (after loading) and at the end of year 1 (month 11), in addition to the proportion of patients with active/inactive disease despite the Q8W dosing regimen. LogMar BCVA and central retinal thickness (CRT) obtained from SD-OCT scans were analysed. At month 5 (after loading; before IVI no. 4) and at month 11 (before IVI no. 7; last IVI of year 1), maculae were classified into dry (inactive disease) and wet (active disease): inactive disease was defined by the absence of intraretinal (IRF) or subretinal fluid (SRF) on macular OCT scan, whereas active disease was defined by the presence of macular haemorrhage, SRF, IRF, and intraretinal cysts (IRCs) on SD-OCT scan. A paired t-test was used to compare means of BCVA and CRT at last follow-up to postloading and to baseline. The unpaired t-test was used to compare means of BCVA and CRT of the active vs inactive group. A P-value of <0.05 was considered to be statistically significant.
Results
A total of 255 eyes of 223 patients were retrospectively recruited. One hundred and twenty-four right eyes and 131 left eyes were analysed. Seventy-two per cent of the patients were of female gender. Mean age of this cohort of patients was 82 years.
Mean baseline BCVA was 0.66 LogMar (range 0.30–1.2 LogMar). Mean BCVA at month 5 (after loading) was 0.48 (range − 0.02 to 1.22 LogMar). Mean BCVA at month 11 was 0.50 (range 0.0–2.0 LogMar). The mean BCVA improved from 0.66 at baseline to 0.50 at month 11 (P<0.0001). Mean VA gain of eight ETDRS letters in a real-world setting was comparable to VIEW 1 and VIEW 2 Trials’ of 8.4 letters in the integrated analysis of the VIEW 1 and VIEW 2 studies (7.9 letters in VIEW 1 and 8.9 letters in VIEW 2 at end of year 1).4 The VA gain after loading (represented by a mean BCVA of 0.48 LogMar) was maintained throughout the 1-year treatment plan (mean BCVA of 0.50 at M11).
Regardless of their baseline VA (range 0.3–1.2 LogMar), eyes that fulfilled the criteria were retrospectively enrolled. The overall cohort included 255 eyes. Data from 38 eyes (37 patients) were not included as they had not completed a full year 1 of treatment at the time of data extraction. Mean baseline BCVA of this small group was 0.61 LogMar and their mean BCVA at the time of data extraction was 0.53 LogMar. Twenty eyes/38 eyes did not receive further anti-VEGF injections after loading as their disease was considered to be stable/dry at month 5. The overall dropout rate is (38/255) circa 15% in year 1. The overall mean BCVA was not affected by these dropouts. In this cohort of patients, the retention rate in year 2 is circa 94%. Dropouts in year 2 after having completed year 1 of treatment are minimal as patients follow a predefined proactive pathway till the end of year 2, which in the majority of cases meets our inclusion criteria.
The mean CRT decreased from 311 μm at baseline to 235 μm at month 5 (P<0.0001), and continued to decrease down to 211 μm at month 11 (P<0.0001).
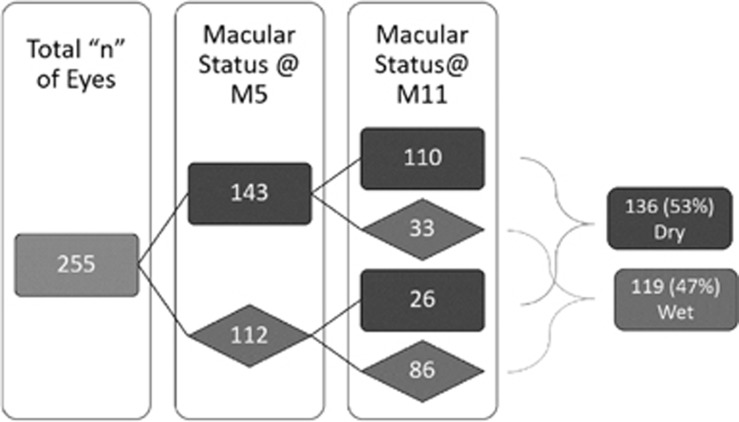
At month 5 (after loading), 143 eyes (56%) were inactive as defined by the absence of IRF or SRF on OCT, whereas 112 eyes (44%) remained active with the presence of SRF, IRF, IRC, or macular haemorrhage. At month 11, 136 eyes (53%) were inactive, whereas 119 eyes (47%) remained active with the presence of SRF, IRF, IRCs, or macular haemorrhage. Seventy-seven per cent (110 eyes) of the inactive eyes after loading remained inactive at month 11. Seventy-seven per cent (86 eyes) of the active eyes after loading remained active at month 11 (Figure 2).
Figure 2.
The proportion of active/inactive disease based on the macular status on SD-OCT scan at months 5 and 11. Fifity-six per cent (143 eyes) of the treated eyes were deemed to be inactive at M5. Forty-four per cent (112 eyes) were deemed to be active at M5. Seventy-seven per cent (110/143 eyes) of the inactive eyes after loading remained inactive at month 11. Seventy-seven per cent (86/112 eyes) of the active eyes after loading remained active at month 11. One hundred and thirty-six eyes (53%) were inactive, whereas 119 eyes (47%) were active at the end of year 1. Dark grey is inactive. Light grey is active.
Mean LogMar BCVA at month 11 for the active and inactive groups was 0.48 and 0.51, respectively (P=0.53). Mean CRT at month 11 for the active and inactive groups was 231 and 191 μm, respectively (P=0.0001). Table 1 defines the differences between the two groups throughout the 1-year treatment plan.
Table 1. Mean BCVA and CRT of the inactive vs active group at different stages during the 1-year treatment plan.
| Characteristic | Group A (dry at M11) | Group B (wet at M11) | P-value | 95% Confidence interval |
|---|---|---|---|---|
| Mean baseline VA LogMar | 0.68 | 0.61 | 0.06 | −0.00 to 0.14 |
| Mean VA at M5 LogMar | 0.53 | 0.46 | 0.05 | −0.00 to 0.14 |
| Mean VA at M11 LogMar | 0.51 | 0.48 | 0.53 | −0.06 to 0.11 |
| Mean baseline CRT | 308 | 314 | 0.71 | −37.47 to 25.60 |
| Mean CRT at M5 | 222 | 249 | 0.005 | −46.98 to −8.15 |
| Mean CRT at M11 | 191 | 231 | 0.0001 | −59.88 to −19.76 |
Abbreviations: BCVA, best-corrected visual acuity; CRT, central retinal thickness; LogMar, logarithm of the minimum angle of resolution. At month 5 (P=0.05) and at month 11 (P=0.53), mean BCVA of the active group was not worse than that of the inactive group. Differences were statistically significant at M5 but not at M11. The difference between the means of CRT of both groups at month 11 was statistically significant.
Discussion
Visual and anatomic outcomes of treating patients diagnosed with nAMD in a clinical trial setting are promising. Nevertheless, replicating those outcomes in a real-world setting has often not been achieved. Reasons include a more heterogenous group of patients than seen in clinical trials and also undertreatment of nAMD due to the challenge of patients, carers and health-care providers following optimum treatment regimens.5 In our practice, lack of capacity results in a lack of appointment slots for follow-up or Pro Re Nata (PRN) intravitreal injections. Therefore, we investigated whether a care pathway that improves capacity could provide as good results as a hypothetical Eye Unit where there were no capacity issues and patients could receive regular monthly follow-ups and immediate anti-VEGF therapy with no delay.
Aflibercept was administered every 8 weeks, after a loading phase of 3 monthly injections (VIEW protocol) as this has been shown to deliver VA results equivalent to administering ranibizumab on a monthly basis over the first year.3 Our cohort of patients received seven intravitreal injections but only three clinic visits during the 1-year treatment plan (Figure 1).
The benefits of this pathway can be detailed mathematically. For N patients, a PRN treatment strategy would result in a baseline visit, and after three intravitreal injections, a monthly follow-up visit with OCT imaging every month. In 12 months, this would be 10 clinic visits. In our pathway, they would receive just three clinic visits (baseline, months 5 and 11). Therefore, the savings in clinic visits is 7N. In our unit, we see circa 1000 patients per year and so we save 7000 clinic visits and 7000 OCT scans which greatly helps our capacity. The average number of ranibizumab injections in a PRN scheme is estimated to be 8. Thus, the number of intravitreal injections saved in a year is N or for a 1000 patients this equates to 1000 injections. There is thus a considerable cost saving to health commissioners with this approach too.
However, it is important to evaluate if such a care pathway delivers good outcomes for patients. In this study, we have shown that VA outcomes in this cohort of patients at end of year 1 were comparable to clinical trial outcomes. The mean BCVA improved from 0.66 at baseline to 0.50 at month 11 (P<0.0001). The mean letter gain was +8 compared favourably with +8.4 in the integrated analysis of the VIEW 1 and VIEW 2 studies, being 7.9 in VIEW 1 and 8.9 in VIEW 2. Baseline VA in the VIEW study was 53.6 ETDRS letters (52 letters in our cohort) and the mean 1-year VA was 62 ETDRS letters (60 letters in our cohort of patients). VA measurement in a real-world clinical setting is based on patient’s habitual correction. This is likely to underestimate the actual changes in vision; however, it may better reflect what vision patients actually experience.6
Mean change in VA is the main primary visual outcome in multiple clinical trials. Secondary outcomes such as the SD-OCT-based monitoring of the fluid status of the macula after loading and at end of year 1 of treatment are as important as visual gains. Treatment of nAMD is OCT based and there is no doubt that anatomic changes after treatment would define many variables such as efficacy and success of the anti-VEGF agent, in addition to its vital role in plotting the road map for year 2 treatment with an anti-VEGF as aflibercept.
At month 11 (before intravitreal injection number 7; the last of year 1), 136 eyes (53%) were deemed inactive as defined by the absence of IRF or SRF on SD-OCT, whereas 119 eyes (47%) remained active with the presence of SRF, IRF, IRCs, or macular haemorrhage (Figure 2).
The two groups (active compared with inactive), defined by retinal fluid status at month 11, were balanced with respect to CRT at baseline. Regarding mean baseline BCVA, there was an ~3 ETDRS letters difference between the two groups (Table 1). For eyes without retinal fluid at month 11 (INACTIVE GROUP), mean change in BCVA from baseline at month 11 was nine letters. The corresponding change for eyes with retinal fluid present at month 11 (ACTIVE GROUP) was seven letters (Table 1).
The OCT-based macular status at month 5 was a reliable indicator of the macular status at month 11. Fifty-six per cent (143 eyes) of the treated eyes were deemed to be dry at M5. Forty-four per cent (112 eyes) were deemed to be wet at M5. Seventy-seven per cent (110 eyes) of the inactive eyes after loading remained inactive at month 11. Seventy-seven per cent (86 eyes) of the active eyes after loading remained active at month 11. It seems that the macular status after loading indicates which treatment plan should be adopted in year 2.
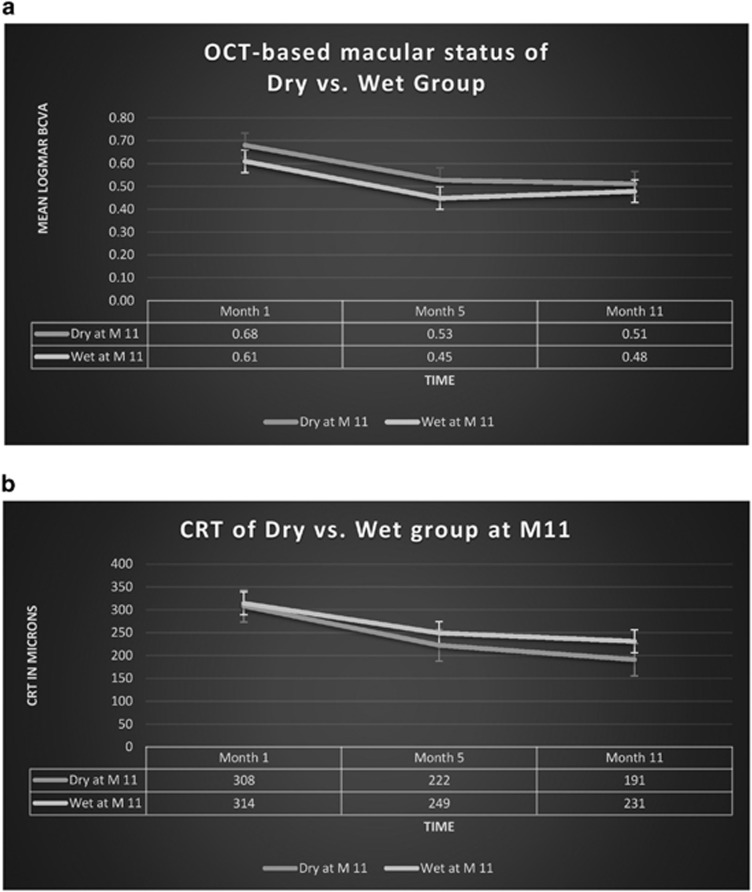
As it can be observed in Figure 3a, the best VA outcome of the inactive group was observed at month 11. After switching into the Q8W fixed-dosing, the inactive group continued to gain vision until the end of year 1. On the contrary, the active group had the best VA after loading (month 5) as it experienced a small decline of VA after switching from the monthly dosing into the Q8W dosing. Despite that, CRT continued to get better and to drop down with more injections given after the loading dose in both groups (Figure 3b).
Figure 3.
(a) Change of VA over time of both the inactive and active groups (based on OCT status at month 11). SEM bars can be observed at the three different time points (months 1, 5, and 11). (b) Change of CRT over time of both the inactive and active groups (based on OCT status at month 11). SEM bars can be observed at the three different time points (months 1, 5, and 11).
In the integrated analysis of the VIEW 1 and VIEW 2 RCTs, the proportion maintaining vision (losing <15 ETDRS letters) was 95%, whereas the proportion gaining ≥15 ETDRS letters was 31%. In our study, the proportion maintaining vision (losing <15 ETDRS letters) was 94%, whereas the proportion gaining ≥15 ETDRS letters was 24%.
In the inactive group, the (n) and proportions of the 136 eyes with follow-up at 1 year gaining 5, (6–10) or 15 letters were (25) 18%, (21) 15%, and (38) 28%, respectively, and the proportion maintaining vision (losing <15 ETDRS letters) was 94%. In the active group, the (n) and proportions of the 119 eyes with follow-up at 1 year gaining 5, (6–10) or 15 letters were (10) 8%, (27) 23%, and (24) 20%, respectively, and the proportion maintaining vision (losing <15 ETDRS letters) was 94%.
A treatment protocol in which the maximum VA gain is achieved with the minimum of clinic visits would not only alleviate capacity issues but also offer a cost-effective option. We have run an economic analysis which demonstrated that our Year 1 VIEW protocol (with three clinic visits in 1 year of treatment) using aflibercept is the most cost-effective option compared with treat-and-extend, PRN, and monthly dosing using ranibizumab (Table 2).
Table 2. Economic analysis of aflibercept per VIEW at our department vs ranibizumab in three different treatment regimens.
| Dosing protocol | Year 1 VIEW | Treat-and-extend | PRN | Monthly |
|---|---|---|---|---|
| Drug | Aflibercept | Ranibizumab | Ranibizumab | Ranibizumab |
| Cost of drug per intravitreal injection (£) | 816.00 | 742.00 | 742.00 | 742.00 |
| Mean number of doses per year | 7 | 10.1 | 8.6 | 12 |
| Mean number of outpatient visits with OCT, VA and IVI (£245) | 3 | 10.1 | 8.6 | 12 |
| Mean number of outpatient visits with OCT and VA (£127) | 0 | 0 | 3.4 | 0 |
| Mean number of outpatient visits with VA (£118) | 4 | 0 | 0 | 0 |
| Total mean cost of treatment (£) | 6919.00 | 9968.70 | 8920.00 | 11 844.00 |
Abbreviations: IVI, intravitreal injection; OCT, optical coherence tomography; PRN, Pro Re Nata; VA, visual acuity.
Despite the net price per aflibercept injection being higher than that for ranibizumab injections, our protocol demands fewer injections and fewer clinic visits than published trials using alternative dosing strategies. We have included the mean number of doses and follow-up visits as reported by these landmark trials. Our economic analysis is summarised in Table 2. The tariffs were obtained from the clinical coding department in our trust. We compared our data using the Year 1 VIEW protocol with data from Wycoff et al.’s RCT for treat-and-extend dosing,7 PrONTO study data for PRN dosing,8 and ANCHOR study data for monthly dosing.9 The net price for aflibercept 40 mg/ml solution for intravitreal injection is £816.00 per 0.1 ml vial.10 The net price for ranibizumab 10 mg/ml is £742.00 per 0.23 ml vial.11 However, the NHS has negotiated confidential discounted prices for both aflibercept and ranibizumab where the price of each drug to the NHS are very similar. Therefore, the price savings of our protocol may be even greater than reported here. Limitations of our economic analysis are that different eye units may have different numbers of outpatient visits with or without OCT scans, thereby altering the total cost per patient for treat-and-extend and PRN dosing. However, our analysis demonstrates that our Year 1 VIEW protocol achieves a mean saving of £4925 against the predetermined monthly protocol.
Conclusions
Intravitreal aflibercept administered by fixed dosing over 1 year improved both VA and macular morphology in a large number of treatment-naïve eyes with nAMD. Our results demonstrate that for treatment-naïve patients with nAMD, treated in a real-world clinical setting, implementing a PROACTIVE fixed-dosing bimonthly regimen of aflibercept, results in a mean change in VA comparable to that achieved in clinical trials, irrespective of retinal fluid status at a single late time point.
The macular status after loading is a reliable indicator of disease activity at the end of year 1. Our results demonstrate that it is possible to safely implement a reduced visit care pathway and that this results in excellent clinical outcomes. Widespread adoption of this protocol could markedly help the United Kingdom Hospital Eye Service to cope with the considerable capacity issues they face implementing anti-VEGF therapies for neovascular AMD. Such adoption would result in improved capacity and allow more patients to be treated in a timely manner in addition to the fact that it might be much more cost effective. This, in turn, would improve visual outcomes for patients affected by this devastating disease. Relying on an early time point (in this case OCT status after loading) might help in planning the individual care pathway for patients in year 2 of their treatment with an anti-VEGF such as aflibercept (ongoing fixed dosing or a treat-and-extend protocol), thus allowing a better service delivery in AMD Units across the United Kingdom.
Footnotes
HA has received travel support to educational meetings from Bayer Pharmaceuticals. SK has received travel support to educational meetings from Bayer Pharmaceuticals. SRR has received travel support to educational meetings from Bayer Pharmaceuticals. AL has received travel support to educational meetings from Bayer Pharmaceuticals.
References
- Owen C, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 2012; 96(5): 752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge JS, Ioffe E, Cao J, Papadopoulos N, Thurston G, Wiegand SJ et al Clincal development of VEGF trap biology of VEGF and its receptors. In: Figg WD, Folkman J (eds). Angiogenesis. Springer: New York, NY, USA, 2008, pp 415–420. [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik J-F, Kaiser PK, Nguyen QD et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012; 119(12): 2537–2548. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, Brown DM, Chong V, Nguyen QD et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety six-week results of the VIEW studies. Ophthalmology 2014; 121(1): 193–201. [DOI] [PubMed] [Google Scholar]
- Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014; 121: 1092–1101. [DOI] [PubMed] [Google Scholar]
- Talks JS, Lotery AJ, Ghanchi F, Sivaprasad S, Johnston RL, Patel N et al United Kingdom Aflibercept Users Group. First-year visual acuity outcomes of providing aflibercept according to the VIEW study protocol for age-related macular degeneration. Ophthalmology 2016; 123(2): 337–343. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015; 122(12): 2514–2522. [DOI] [PubMed] [Google Scholar]
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W et al. Variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009; 148(1): 43–58. [DOI] [PubMed] [Google Scholar]
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR Study. Ophthalmology 2009; 116(1): 57–65. [DOI] [PubMed] [Google Scholar]
- NICE, aflibercept price. Available at: https://www.evidence.nhs.uk/formulary/bnf/current/8-malignant-disease-and-immunosuppression/81-cytotoxic-drugs/815-other-antineoplastic-drugs/aflibercept/aflibercept#Search?q=afliberceptprice. 6 February 2017.
- NICE, Lucentis® (Novartis). Available at: https://www.evidence.nhs.uk/formulary/bnf/current/11-eye/118-miscellaneous-ophthalmic-preparations/1182-ocular-diagnostic-and-peri-operative-preparations-and-photodynamic-treatment/subfoveal-choroidal-neovascularisation/ranibizumab/lucentis. 6 February 2017.