Abstract
Case series
Patient: Male, 23 • Female, 20
Final Diagnosis: Ataxia telnagiectasia
Symptoms: Gagging • coughing • hoarseness • articulatory inaccuracy
Medication: —
Clinical Procedure: Oral motor and swallowing assessment
Specialty: Neurology
Objective:
Rare disease
Background:
The body of literature on oral motor and swallowing disorders in patients with ataxia telangiectasia (AT) is limited.
Case Report:
The purpose of this study was to characterize oral motor and swallowing disorders in two siblings with AT, based on oral motor and swallowing assessments. Specific procedures were applied for oral motor and swallowing assessments and both patients underwent videofluoroscopy (VFS). Case 1 presented vocal instability, change in postural control during feeding; food retention in oral cavity; slower oral transit time; and multiple swallowing (signs for solid and liquid). Case 2 presented parted lips at rest and reduced muscle strength; reduced strength and mobility of the tongue; vocal weakness and instability; reduced speech precision and intelligibility; decreased intonation pattern; food retention in oral cavity during feeding; slower oral transit time; multiple swallowing (signs for solid and liquid); poor bolus ejection; incoordination and difficulty in controlling the sips of water taken from the cup; altered cervical auscultation after swallowing and respiratory distress (liquid and puree). For both patients VFS results revealed laryngeal penetration for liquid.
Conclusions:
Although the literature describes the occurrence of dysarthria and swallowing disorders in patients with AT, little attention has been given to describing which oral motor deficits are responsible for these disorders. Early identification of swallowing alterations and rehabilitation could decrease the risk of aspiration pneumonia. Future studies are necessary in order to investigate the deterioration process of swallowing in AT and the influence of rehabilitation in maintaining functional health.
MeSH Keywords: Ataxia Telangiectasia, Deglutition Disorders, Outcome and Process Assessment (Health Care), Speech Disorders, Voice Disorders
Background
Ataxia telangiectasia (AT) is a progressive, rare, and monogenic neurodegenerative disease with a pattern of autosomal recessive inheritance [1–3]. The prevalence of this disease is estimated to be between 1 in 40,000 and 1 in 300,000 live births, and the life expectancy for people with AT is 25 years [3,4]. The main causes of death are respiratory failure, lung disease, complications arising from cancer and from chemotherapy treatment, and neural deterioration [1–3].
AT is difficult to diagnose before the patient reaches the age of two years, unless the disease is already present in the family. Although early diagnosis can play a major role in family planning, and can also help avoid years of costly diagnostic testing, the clinical diagnoses becomes most apparent after the age of 10 years, when symptoms become fully expressed [5]. AT is characterized by ataxia, immunodeficiency, sinopulmonary infection, premature aging, nutritional impairment, dysarthria, and oculomotor abnormalities [3,6,7]. Another characteristic that may be present in AT is the appearance of conjunctival telangiectasia. Typically, the first signs of this syndrome appear when the child begins to walk [3,6,7].
The literature also describes impaired swallowing in patients with AT and the potential association between dysphagia and pulmonary disease in these patients. Symptoms often include coughing and choking during meals, incoordination between the breathing and swallowing events, absence of the cough reflex, and difficulty in maintaining weight or loss of weight [5,7].
Several studies of the more than 30 known types of ataxia have indicated that aspiration pneumonia is the main cause of death, which can be directly associated with oropharyngeal dysphagia [7–9]. It is, therefore, imperative to identify risk factors for possible swallowing disorders in this population. The earlier the detection of dysphagia, the earlier an adequate treatment can be selected. This not only shortens the time-frame for reestablishment of the patient’s overall health status, but also reduces the overall rehabilitation costs, including the performance of unnecessary procedures [10].
Although the literature describes the occurrence of dysarthria and swallowing disorders in patients with AT, little attention has been given to the description of oral motor deficits responsible for these disorders. Oral motor rehabilitation can contribute to the development of clinical protocols and to the establishment of therapeutic guidelines to reduce the risk of pneumonia and to promote safe eating in these patients.
The purpose of this study was to characterize oral motor and swallowing disorders in AT, based on the clinical assessment of oral motor performance and swallowing.
Case Report
The study was approved by the Institutional Review Board to ensure ethical conduct of research studies with human participants (CAPPesq HCFMUSP 240/10). Written informed consent was obtained from all participants.
We report the case of two Caucasian siblings affected by AT. These patients were referred by the medical team of the Division of Oral Motor Disorders (Hospital das Clínicas, School of Medicine, University of São Paulo, Brazil) for assessment and possible treatment. Regarding family history, the parents were not consanguineous, had no diagnosed chronic disease, and had five children: one who was stillborn, three who were affected by AT (two males and one female, one of the males having died), and one daughter who was not affected by AT. In order to characterize oral motor performance and swallowing, we conducted the following assessments: oral-motor assessment, clinical swallowing assessment, and videofluoroscopy (VFS).
Oral-motor clinical assessment
For the assessment of oral motor function, six items were scored using a binary (normal/abnormal) approach [11]. The items scored included lip closure, tongue movement, palatal elevation, gag reflex, voice quality, and speech motor control. Lip, tongue, and soft palate function were scored by assessing the symmetry, strength, and agility of each isolated movement. Gag reflex, elicited through the use of a standard method (mouth mirror touching the pillars of fauces, dorsum of the tongue, and into the vallecula), was rated as abnormal if the reflex was absent or reduced (stimulation needs to be repeated for several times in order to obtain an adequate response). Voice quality was assessed by auditory-perceptual analysis using the GRBASI Scale [12]. Speech motor control was evaluated based on articulatory precision as well as on agility in spontaneous speech and repetition of the syllables /pa/, /ta/, /ka/.
Nonverbal apraxia was evaluated based on the performance of the following movements: protruding the tongue, blowing, biting lower lip, puckering lips, coughing, puffing out cheeks, blowing a kiss, alternating between puckering lips and smiling, and clicking the tongue [13]. Instructions were given orally and supplemented with articulatory cues if patients were unable to perform the movements in response to verbal commands alone. Performance on each item was scored on a categorical scale of four points: 1) demonstrates imprecision or incomplete movement; 2) presents effort to perform movement and several trials are needed to reach final movement; 3) presents full movement, but movement is slow and irregular; and 4) presents no effort, precision and full movement.
Swallowing clinical assessment
The clinical evaluation of swallowing included the application of a clinical protocol [14], followed by the classification of the swallowing functional level according to the American Speech-Language-Hearing Association National Outcome Measurement System (ASHA-NOMS) [15]. The clinical protocol included the offer of water and puree/solid volumes and was divided in two sections: the water swallow test and the puree/solid swallow test. Test results were marked as either pass or fail for each one of the observed items. The patients were assessed during the swallow of water offered on a conventional cup, fruit puree offered on a spoon, and half a piece of bread. The tests were repeated, if necessary, up to three times to confirm results.
The ASHA-NOMS swallowing level scale [15] is a multidimensional tool designed to measure both the supervision level required and the diet level by assigning a single number, between 1 and 7. 1 – Individual is not able to swallow safely by mouth. All nutrition and hydration is received through non-oral means (e.g., nasogastric tube). 2 – Individual is not able to swallow safely by mouth for nutrition and hydration but may take some consistency with consistent maximal cues in therapy only. Alternative method of feeding is required. 3 – Alternative method of feeding required as individual takes less than 50% of nutrition and hydration by mouth, and/or swallowing is safe with consistent use of moderate cues to use compensatory strategies and/or requires maximum diet restriction. 4 – Swallowing is safe but usually requires moderate cues to use compensatory strategies, and/or individual has moderate diet restriction and/or still requires tube feeding and/or oral supplements. 5 – Swallow is safe with minimal diet restriction and/or occasionally requires minimal cueing to use compensatory strategies. May occasionally self-cue. All nutrition and hydration needs are met by mouth at mealtime. 6 – Swallowing is safe and individual eats and drinks independently and may rarely require minimal cueing. Usually self-cues when difficulty occurs. May need to avoid specific food items (e.g., popcorn and nuts), or requires additional time (due to dysphagia). 7 – Individual’s ability to eat independently is not limited by swallow function. Swallowing would be safe and efficient for all consistencies. Compensatory strategies are used effectively when needed.
Videofluoroscopic assessment of swallowing
VFS assessments were performed in a lateral plane by a VFS trained radiographer and a trained speech and language therapists. Liquid barium (Opti-bar) with 100% w/v was used. The protocol adopted for the swallowing assessment involved the ingestion of food with different consistencies and is routinely used at our hospital to investigate swallowing characteristics, especially the presence of aspiration.
Swallowing was analyzed by reviewing the digitalized images of each swallow. Penetration/aspiration was determined by using an 8-point multidimensional perceptual scale [16]. Scores were attributed as follows: 1 – material does not enter the airway; 2 – material enters the airway, remains above the vocal folds, and is ejected from the airway; 3 – material enters the airway, remains above the vocal folds, and is not ejected from the airway; 4 – material enters the airway, touches the vocal folds, and is ejected from the airway; 5 – material enters the airway, touches the vocal folds, and is not ejected from the airway; 6 – material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway; 7 – material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort; 8 – material enters the airway, passes below the vocal folds, and no effort is made to eject it.
Clinical case 1
A 23-year-old man was referred to the Division of Oral Motor Disorders due to gastroesophageal reflux disease (GERD), frequent choking during meal times, and hoarseness. The patient presented visible telangiectasia on the sclerae of both eyes. The patient was diagnosed with AT at the age of seven years and referred for evaluation after having stopped walking at the age of 12 years. The patient also had been referred after having presented with five episodes of pneumonia.
Previous medical history revealed immunoglobulin G deficiency types 2 and 3 (i.e., IgG2 and IgG3), growth deficit, and hypothyroidism. At the time of the oral motor and swallowing assessment, the patient was making use of medication for GERD. Medical records indicated: bilateral pleural thickening with volumetric reduction of the upper lung lobes, especially to the left and minimal bronchiectasis in the right upper lung lobe (computed tomography (CT) of the lungs); diffuse esophageal ectasia, hepatic calcifications and signs of liver fat deposition; mild erosive distal esophagitis (esophagoduodenoscopy); thyroid echogenicity and echo texture within normal limits (ultrasound); polysomnography with normal results. No other examination results were available in the patient’s medical files.
The results obtained in the oral motor clinical assessment, swallowing, and VFS assessment are present in Table 1 and Figure 1.
Table 1.
Clinical features of oral motor and swallowing assessment (Case 1).
| Oral-motor clinical assessment | |
|---|---|
| Lip closure | Normal |
| Tongue movement | Normal |
| Palatal elevation | Normal |
| Gag reflex | Normal |
| Voice quality (GRBAS-I) | Abnormal – (G3R0B0A0S0-I3) – vocal instability |
| Speech-motor control (speech intelligibility, prosody and articulatory precision) | Normal |
| Nonverbal apraxia | |
|---|---|
| Protruding the tongue | 4 |
| Blowing | 4 |
| Biting lower lip | 4 |
| Puckering lips | 4 |
| Coughing | 3 |
| Puffing out cheeks | 4 |
| Blowing a kiss | 4 |
| Alternating between puckering lips and smiling | 3 |
| Clicking the tongue | 3 |
| Swallowing clinical assessment | |
|---|---|
| Tested for all food consistencies |
|
| Swallowing functional Level | ASHA NOMS Level 6 |
| Videofluoroscopic assessment of swallowing | |
|---|---|
| Presence of penetration/aspiration (Figure 1) | Score 4 |
Figure 1.
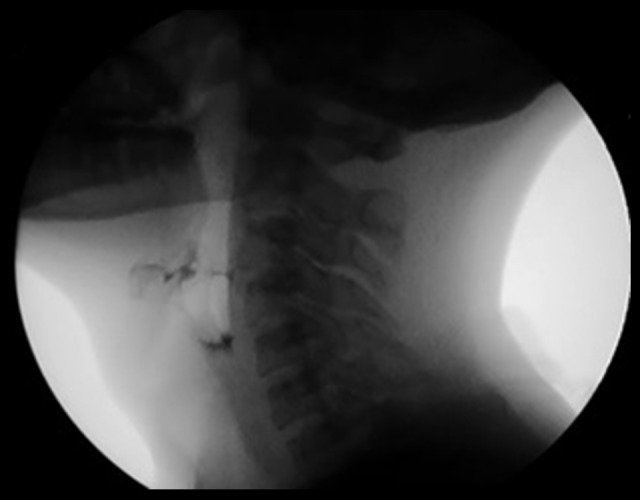
Liquid swallowing image from videofluoroscopy, Clinical Case 1. The occurrence of liquid penetration into the supraglottic region during swallowing in Clinical Case 1 is presented.
Clinical case 2
A 20-year-old woman was referred to the Division of Oral Motor Disorders due to GERD and frequent choking during meal times. The patient presented visible telangiectasia on the sclerae of both eyes. The patient was diagnosed with AT at the age of three years and was referred after having stopped walking at the age of 10 years. The patient had a gastrostomy at the age of eight, but at the time of our assessment the patient was also being fed orally. The patient also had been referred having presented with twelve episodes of pneumonia.
Previous medical history revealed immunoglobulin G deficiency type 2 (i.e., IgG2), growth deficit, and hypothyroidism. At the time of oral motor and swallowing assessment, the patient was making use of medication for: GERD, hypothyroidism (levothyroxine); pneumonia (antibiotics); vertigo (flunarizine); and immunoglobulin deficiency (human gamma-globulin). Medical records indicated: bilateral pleural thickening with volumetric reduction of the upper lung lobes, diffuse minimal bronchiectasis, and minimal bilateral pleural effusion and pneumonia (CT of the lungs); mild erosive distal esophagitis (esophagoduodenoscopy); heterogeneous echogenicity of the thyroid (ultrasound); and bilateral and symmetric volumetric reduction of the cerebellum (CT). No other examination results were available in the patient’s medical files.
The results obtained in the oral motor clinical assessment, swallowing, and VFS assessment are shown in Table 2 and Figure 2.
Table 2.
Clinical features of oral motor and swallowing assessment (Case 2).
| Oral-motor clinical assessment | |
|---|---|
| Lip closure | Abnormal – parted lips at rest and reduced muscle strength |
| Tongue movement | Abnormal – reduced strength and mobility |
| Palatal elevation | Normal |
| Gag reflex | Normal |
| Voice quality (GRBAS-I) | Abnormal – (G1R0B0A1S0-I1) – vocal instability |
| Speech-motor control (speech intelligibility, prosody and articulatory precision) | Abnormal – reduced speech precision and intelligibility, decreased intonation pattern, irregular DDK |
| Nonverbal apraxia | |
|---|---|
| Protruding the tongue | 3 |
| Blowing | 3 |
| Biting lower lip | 3 |
| Puckering lips | 2 |
| Coughing | 2 |
| Puffing out cheeks | 1 |
| Blowing a kiss | 3 |
| Alternating between puckering lips and smiling | 2 |
| Clicking the tongue | 3 |
| Swallowing clinical assessment | |
|---|---|
| Tested for all food consistencies |
|
| Swallowing functional Level | ASHA NOMS Level 5 |
| Videofluoroscopic assessment of swallowing | |
|---|---|
| Presence of penetration/aspiration (Figure 2) | Score 4 |
Figure 2.

Liquid swallowing image from videofluoroscopy, Clinical Case 2. The occurrence of liquid penetration into the supraglottic region during swallowing in Clinical Case 2 is presented.
Discussion
The early identification of risk factors for oropharyngeal dysphagia in patients with AT can help prevent undesired outcomes, such as malnutrition, dehydration, and aspiration pneumonia, and possibly avoid additional costs due to prolonged hospitalizations and decrease morbidity associated with dysphagia [17,18]. In our case study, the VFS indicated that both patients presented signs of bolus entering the airway system, with food reaching the vocal folds. This scenario, associated with oral-motor alterations and especially the reduction of muscle strength and mobility of tongue, has been reported to increase the risk for bronchial aspiration [3].
Ataxia is a symptom of cerebellar change and is usually the first diagnostic hallmark of AT, and is usually identified in the first years of life [1,3,19]. Symptoms include changes in gait, fine motor skills, speech, and feeding [1,3]. All of these characteristics were observed during our assessments of both patient cases and Case 2 presented bilateral and symmetric volumetric reduction of the cerebellum (via CT); which has also been reported in a recent study [19]. In addition, incoordination between the palatopharyngeal and laryngeal movements may cause vocal instability, as was observed in both of our patients. Difficulties regarding articulatory precision, speech intelligibility, and prosody were also observed in Case 2. Currently, there are no studies that have investigate speech characteristics in patients with AT. For this reason, we believe that our findings may contribute to the management of dysphonia and dysarthria these patients.
Fine motor skill deficits can also be observed in cases of AT and may have an impact in feeding, as it is difficult to hold utensils to bring food to the mouth [1]. During the swallowing assessment, Case 2 presented difficulty in controlling sips of water taken from the glass due to hand movement coordination deficits. This difficulty can become a risk factor in patients with oropharyngeal dysphagia, considering that the speed of bolus flow from the mouth into the pharynx may be sufficiently fast that it does not provide enough time for the airway closure before the bolus arrives at the larynx and airway [20].
In most cases of AT, immunodeficiency remains stable throughout the patient’s life. Laryngotracheal aspiration-related problems, however, tend to increase alongside neural deterioration [5]. Lower respiratory tract infections also seem to progress over time and constitute an important risk factor, along with recurrent aspiration, especially in older patients [5]. Both patients in our study presented medical history of immunodeficiency and pneumonia and Case 2 was under treatment for pneumonia at the time of our assessment. Although the patients in our study did not present signs of aspiration during VFS, images indicated bolus penetration in the supraglottic region. It has been noted in the literature that the presence of food in the airway system is an important risk factor for pneumonia [21]. Over time, the presence of immunodeficiency and the progression of motor disorders itself tends to worsen the overall pulmonary condition [7]. Monitoring swallowing, therefore, is of utmost importance, and guidelines for treating neurologic dysphagia must be followed to promote safe swallowing and enable greater protection of the lower airway system.
Pulmonary insufficiency has been identified as a major cause of death in AT. According to Davis [5], there are three main ways to preserve a patient’s pulmonary function: reduce aspirations, increase mucociliary clearance, and reduce lung inflammation. Gastrostomy seems to be an important resource used in many patients with AT [6], in addition to reducing the risk of aspiration; it helps to improve the nutritional status of these patients. In our study, Case 2 made use of a gastrostomy tube as a means of improving nutrition (i.e., extreme low weight).
Conclusions
Even though dysphagia is part of the symptoms presented by patients with AT, the body of literature on oral motor and swallowing disorder in these patients is limited, and there are currently no standardized quality indicators for swallowing rehabilitation programs designed for this type of patient. Little emphasis is given to the early identification of swallowing alterations; attention is only given when the patient is already severely compromised. Treatment of AT involves lifestyle modifications. Early identification of swallowing disorders enables early oral rehabilitation (i.e., compensatory strategies, diet modification), which in turn could decrease the risk of aspiration pneumonia. Finally, future studies are necessary to investigate the deterioration of the process of swallowing in this population, and to identify the most effective rehabilitation processes that can maintain healthy function for a longer period of time.
Footnotes
Conflict of interests
The authors declare no conflict of interests.
References:
- 1.Chakor RT, Bharote H. Inherited ataxia with slow saccades. J Postgrad Med. 2012;58(4):318–24. doi: 10.4103/0022-3859.105471. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhary MW, Al-Baradie RS. Ataxia-telangiectasia: Future prospects. Appl Clin Genet. 2014;10(7):159–67. doi: 10.2147/TACG.S35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothblum-Oviatt C, Wright J, Lefton-Greif MA, et al. Ataxia telangiectasia: A review. Orphanet J Rare Dis. 2016;11(1):159. doi: 10.1186/s13023-016-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swift M, Morrell D, Cromartie E, et al. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 1986;39(5):573–83. [PMC free article] [PubMed] [Google Scholar]
- 5.Davies EG. Update on the management of the immunodeficiency in ataxia-telangiectasia. Expert Rev Clin Immunol. 2009;5(5):565–75. doi: 10.1586/eci.09.35. [DOI] [PubMed] [Google Scholar]
- 6.Lefton-Greif MA, Crawford TO, McGrath-Morrow S, et al. Safety and caregiver satisfaction with gastrostomy in patients with Ataxia Telangiectasia. Orphanet J Rare Dis. 2011;6:23. doi: 10.1186/1750-1172-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefton-Greif MA, Crawford TO, Winkelstein JA, et al. Oropharyngeal dysphagia and aspiration in patients with ataxia-telangiectasia. J Pediatr. 2000;136(2):225–31. doi: 10.1016/s0022-3476(00)70106-5. [DOI] [PubMed] [Google Scholar]
- 8.Isono C, Hirano M, Sakamoto H, et al. Differences in dysphagia between spinocerebellar ataxia type 3 and type 6. Dysphagia. 2013;28(3):413–18. doi: 10.1007/s00455-013-9450-4. [DOI] [PubMed] [Google Scholar]
- 9.Rüb U, Brunt ER, Petrasch-Parwez E, et al. Degeneration of ingestive-related brainstem nuclei in spinocerebellar ataxia type 2, 3, 6 and 7. Neuropathol Appl Neurobiol. 2006;32:635–49. doi: 10.1111/j.1365-2990.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 10.Phillips SA, Ross PD, Chalmers K, MacDougall G. Can we improve dysphagia referrals? J Laryngol Otol. 2007;121(6):584–87. doi: 10.1017/S0022215106002064. [DOI] [PubMed] [Google Scholar]
- 11.Mangilli LD, Sassi FC, Santos SS, Andrade CRF. Oral sensorimotor function for feeding in patients with tetanus. Acta Tropica. 2009;111(3):316–20. doi: 10.1016/j.actatropica.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Hirano M. Clinical examination of voice. New York: Springer Verlag; 1981. [Google Scholar]
- 13.Presotto M, Olchik MR, Shuh AFS, Rieder CRM. Assessment of nonverbal and verbal apraxia in patients with Parkinson’s disease. Parkinsons Dis. 2015;2015:840327. doi: 10.1155/2015/840327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padovani AR, Medeiros GC, Andrade CRF. Protocolo fonoaudiológico de introdução e transição da alimentação por via oral (PITA). In: Andrade CRF, Limongi SCO, editors. Disfagia: prática baseada em evidências. São Paulo: Sarvier; 2012. pp. 74–85. [in Portuguese] [Google Scholar]
- 15.American Speech-Language-Hearing Association . National Outcome Measurement System (NOMS): Adult Speech-Language Pathology User’s guide. Rockville (MD): National Center for Evidence-Based Practice in Communication Disorders; 2003. [Google Scholar]
- 16.Rosenbek JC, Robbins JA, Roechker EB, et al. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 17.Chichero JA, Heaton S, Bassett L. Triaging dysphagia: Nurse screening for dysphagia in an acute hospital. J Clin Nurs. 2009;18(11):1649–59. doi: 10.1111/j.1365-2702.2009.02797.x. [DOI] [PubMed] [Google Scholar]
- 18.Lefton-Greif MA, Perlman AL, He X, et al. Assessment of impaired coordination between respiration and deglutition in children and young adults with ataxia telangiectasia. Dev Med Child Neurol. 2016;58(10):1069–75. doi: 10.1111/dmcn.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navratil M, Duranovic V, Nogalo B, et al. Ataxia-Telangiectasia presenting as cerebral palsy and recurrent wheezing: A case report. Am J Case Rep. 2015;16:631–36. doi: 10.12659/AJCR.893995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steele CM, Alsanei WA, Ayanikalath S, et al. A systematic review. Dysphagia. 2015;30(1):2–26. doi: 10.1007/s00455-014-9578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman R, Vilardell N, Clavé P, Speyer R. Effect of bolus viscosity on the safety and efficacy of swallowing and the kinematics of the swallow response in patients with oropharyngeal dysphagia: white paper by the European Society for Swallowing Disorders (ESSD) Dysphagia. 2016;31:232–49. doi: 10.1007/s00455-016-9696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


