Abstract
Case series
Patient: —
Final Diagnosis: PRKAG2 syndrome
Symptoms: Palpitation • dyspnea and fatigue • syncope
Medication: —
Clinical Procedure: Radiofrequency catheter ablation • pacemaker implantion • antiarrhythmic drugs
Specialty: Cardiology
Objective:
Rare disease
Background:
PRKAG2 syndrome diagnosis is already well-defined as Wolff-Parkinson-White syndrome (WPW), ventricular hypertrophy (VH) due to glycogen accumulation, and conduction system disease (CSD). Because of its rarity, there is a lack of literature focused on the treatment. The present study aimed to describe appropriate strategies for the treatment of affected family members with PRKAG2 syndrome with a long follow-up period.
Case Report:
We studied 60 selected individuals from 84 family members (32 males, 53.3%) (mean age 27±16 years). Patients with WPW and/or VH were placed in a group of 18 individuals, in which 11 (61.1%) had VH and WPW, 6 (33.3%) had isolated WPW, and 1 (5.6%) had isolated VH. Palpitations occurred in 16 patients (88.9%), chest pain in 11 (61.1%), dizziness in 13 (72.2%), syncope in 15 (83.3%), and dyspnea in 13 (72%). Sudden cardiac death (SCD) occurred in 2 (11.1%), and 2 patients with cardiac arrest (CA) had asystole and pre-excited atrial flutter-fibrillation (AFL and AF) as the documented mechanism. Transient ischemic attack (TIA) and learning/language disabilities with delayed development were observed. Genetic analysis identified a new missense pathogenic variant (p.K290I) in the PRKAG2 gene. Cardiac histopathology demonstrated the predominance of vacuoles containing glycogen derivative and fibrosis. The treatment was based on hypertension and diabetes mellitus (DM) control, antiarrhythmic drugs (AD), anticoagulation, and radiofrequency catheter ablation (RCA). Six patients (33.3%) underwent pacemaker implantation (PM).
Conclusions:
The present study describes the clinical treatment for a rare cardiac syndrome caused by a PRKAG2 mutation.
MeSH Keywords: Cardiomyopathy, Hypertrophic, Familial; Death, Sudden, Cardiac; Wolff-Parkinson-White Syndrome
Background
The association between Wolff-Parkinson-White syndrome (WPW) and ventricular hypertrophy (VH) has been described as a genetic variant associated with glycogen storage disease (GSD) [1]. Patients may demonstrate frequent tachyarrhythmias leading to sudden cardiac death (SCD), and conduction system disease (CSD). Hence, pacemaker (PM) implantation after the second decade of life is usually required. In addition, ventricular dilatation [2] and even congestive heart failure (CHF) [3] can be concomitant. Due to the rarity of the disease, there is no literature describing treatment. The present study aimed to describe appropriate strategies for the treatment of a large Brazilian family with a novel missense pathogenic variant in the PRKAG2 gene, with a long follow-up period.
Case Reports
Sixty patients from a Brazilian family were retrospectively included from March 2005 to March 2017. Twenty-four individuals were excluded because they did not want to participate, or were not found. All individuals were genetically tested and clinically evaluated every 3 months. These subjects were divided into 2 groups: Group I (G1) consisted of individuals who presented with evidence of pre-excitation or intraventricular conduction abnormalities on surface electrocardiogram (ECG), and/or evidence of VH, as documented by echocardiogram (ECHO), as well as the presence of a new missense variant, who were diagnosed as having PRKAG2 syndrome. group II (G2) consisted of unaffected individuals or individuals who underwent comprehensive clinical assessments and had no positive data.
All study participants underwent blood testing (hemagglutination, ELISA, and immunofluorescence) to detect T. cruzi infection (Chagas’ disease), as well as 12-lead ECG every 3 months, annual transthoracic 3D ECHO, and 24-h Holter monitoring when necessary. Six patients underwent radiofrequency catheter ablation (RCA) and electrophysiological study (EPS) due to refractory arrhythmia from medical treatment. An autopsy was performed on a 22-year-old man who had PRKAG2 syndrome and who developed tachycardiomyopathy before death, due to 2: 1 atrial flutter.
Study protocol
All participants provided written informed consent, and we excluded all individuals that were not found, those with Chagas’ disease, and those who did not consent to participate in the study. This study was approved by the São Rafael Hospital Institutional Review Board (protocol no. 16–12), as well as the Bahiana School of Medicine Institutional Review Board (protocol no. 165.803). All authors have read the manuscript and agreed with the results presented herein.
Electrocardiography
Standard 12-lead ECG was performed using a MicroMed Model 300G ECG Machine (MicroMed, Louisville, KY), and the recordings were examined for ventricular hypertrophy and left ventricular voltage (reported as the maximum S-wave in V1 or V2, plus maximum R-wave in V5 or V6, or the maximum R or S deflection in any lead). Ventricular pre-excitation was diagnosed based on a short PR interval (<120 ms), a widened QRS interval (>110 ms), and the presence of δ delta wave (abnormal initial QRS vector). Conduction system disease was diagnosed if evidence of sinus node dysfunction, such as bradycardia or atrioventricular block, was demonstrated on ECG.
Electrophysiological study (EPS) and RCA
EPS with programmed electrical stimulation and recordings of the 12-lead surface ECG and intracardiac ECG were performed using the EP Tracer System (Swarzer Cardiotek BV, Maastricht, The Netherlands). RCA was performed using IRVINE equipment (model IBI-1500T7, (Biomedical, CA) and irrigated, quadripolar catheters (St. Jude Medical, Saint Paul, MN).
Echocardiography
ECHO was performed using a GE model Vivid Dimension 7 ultrasound machine. A VH diagnosis was made by demonstrating evidence of left ventricular hypertrophy (LVH) (typically asymmetric in distribution, with any diffuse or segmental pattern of left ventricular wall thickening ≥15 mm) [4]. Left ventricular wall thickening was associated with a non-dilated and hyperdynamic chamber in the absence of another cardiac or systemic disease (e.g., hypertension or aortic stenosis) capable of producing the magnitude of hypertrophy evidenced, regardless of whether LV outflow obstruction was present [5,6].
Genetic analysis
Genomic DNA was extracted from all family members, with Chemagic MSM I from whole blood/saliva (Chemagic human blood). DNA integrity was assessed on 0.8% agarose gel. Spectrophotometric measurements were also performed to assess the quality ratios of absorbance, and dsDNA concentration was determined through fluorometry (Qubit, Life Technologies). DNA was fragmented with Bioruptor (Diagenode). Library preparation was performed according to the manufacturer’s instructions (Sure Select XT Custom 0.5–2.9Mb library, Agilent Technologies, Inc). After capture, the indexed library was sequenced in a 6-sample pool cartridge. The sequencing process was developed on a MiSeq System (Illumina) using a 2×150 bp read length. We analyzed the most prevalent 55 genes involved in SCD-related pathologies (ACTC1, ACTN2, ANK2, CACNA1C, CACNB2, CASQ2, CAV3, CRYAB, CSRP3, DES, DMD, DSC2, DSG2, DSP, EMD, FBN1, GLA, GPD1L, HCN4, JPH2, JUP, KCNE1, KCNE2, KCNH2, KCNJ2, KCNQ1, LAMP2, LDB3, LMNA, MYBPC3, MYH6, MYH7, MYL2, MYL3, MYOZ2, PDLIM3, PKP2, PLN, PRKAG2, RYR2, SCN4B, SCN5A, SGCA, SGCB, SGCD, TAZ, TCAP, TGFB3, TGFBR2, TNNC1, TNNI3, TNNT2, TPM1, TTN, and VCL), as previously reported by our group [7]. The genomic coordinates were designed using the tool e Array (Agilent Technologies, Inc.), including all isoforms described by the UCSC. The final size was 432 512 kbp of the encoding regions and UTR boundaries. The coordinates of the sequence data were based on NCBI build 37 (UCSC hg19).
In terms of bioinformatics, the processed raw reads were trimmed and mapped with GEM III, and the output was joined and sorted. Only the uniquely and properly mapped read pairs were selected. Then, the variant call over the cleaned BAM file was performed with SAM tools v.1.2, together with an ad hoc method developed to generate the raw VCF files. Genetic variations were consulted in the Human Gene Mutation Database – HGMD (http://www.biobase-international.com/product/hgmd). The possible pathogenicity of the alteration was consulted in silico using the Condel database (CONsensusDELeteriousness score of missense SNVs (http://bg.upf.edu/condel/analysis), PROVEAN (Protein Variation Effect Analyzer, provean.jcvi.org), and Mutation Taster (www.mutationtaster.org).
Allelic frequency was consulted to identify the common genetic variations in healthy populations in the Exome Variant Server (EVS, http://evs.gs.washington.edu/EVS/), the Exome Aggregation Consortium (ExAC Browser, exac.broadinstitute.org), the dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/) and the 1000 genomes genetic variants database (http://browser.1000genomes.org/). In addition, alignment between different species was performed using the UNIPROT database (http://www.uniprot.org/) to identify conservation of the region that included the genetic variation. Finally, CNV analysis was performed using NGS data, and it focused on capturing significant differences between the expected normalized coverage and obtained normalized coverage for a given sample in the region of interest. Several samples were analyzed to corroborate similar levels of coverage between samples. Non-common (Minor Allele Frequency [MAF] <1%) genetic variants were confirmed using the Sanger method. Molecular screening of the novel variation was performed in 312 control subjects (624 alleles) who were unrelated to any patient. Familial congregation of rare genetic variants was also performed using Sanger technology.
For Sanger sequencing, genomic DNA was extracted from saliva from all subjects using commercial Oragene kits (Cleveland, OH). Polymerase chain reaction products were purified using a commercial reagent (ExoSAP-IT, USB Corporation, Cleveland, OH), and were directly sequenced from both directions using an ABI PRISM 3130XL Automatic DNA Sequencer (Applied Biosystems, USA), with posterior SeqScape Software v2.5 (Life Technologies) analysis to compare the obtained results with the reference sequence from Hg19 (PRKAG2 -CCDS5928-).
Histopathology
From the autopsy, a macroscopic study of the whole-heart weight and ventricular wall thickness measurements was performed. The anatomical assessment was performed by an expert forensic pathologist and confirmed by another independent pathologist. Heart regions were methodically examined. Representative full-thickness myocardium sections (anterior, posterior, and lateral) of both ventricles and atriums were fixed in formalin and included in paraffin blocks (formalin-fixed, paraffin-embedded [FFPE]). For each section (paraffin block), parallel series measuring 10 μm thick were obtained with a microtome (Leica Microtome RM2235), mounted on Super Frost slides (Super frost Plus, Thermo). In both ventricles and atriums, random slices of each section were labeled with hematoxylineosin stain (standard protocol, SIGMA). After washing, the slides were dehydrated in alcohol, cleared in xylene, and cover-slipped with DPX (44581, Fluka). A minimum of 3 labeled slices were studied through a Nikon Eclipse 50i microscope interfaced to a DS-2Mv camera and a HP4600 PC. Pictures of representative areas were taken using NIS-Elements BR3.0 software (Nikon). For electron microscopy, the ventricular heart samples had originally been placed into 3% buffered glutaraldehyde and placed in resin, and 1-μm sections were cut and processed by transmission electron microscopy (Hitachi H-7650, Tokyo, Japan).
Statistical analysis
Descriptive statistics are expressed as the means ±SD of patient characteristics using continuous variables and frequencies, while categorical variables are expressed as percentages. The Fisher exact test or χ2 test were used to evaluate the associations between 2 categorical variables, and 2-sample t tests were used to compare continuous variables between groups. Dependent variables were sudden death (SD), cardiac arrest (CA), transient ischemic attack/stroke (TIA/S), and pacemaker implant (PM). Combined outcomes (CO) were, upon the existence of 1 or more of the following outcomes, SD, CA, TIA/S, or PM. Independent variables were clinical, electrocardiographic, and echocardiographic.
All analyses were performed using SPSS 14.0 for Windows (SPSS Inc., Chicago, IL). The authors had unhindered access to all data and take full responsibility for its integrity.
Clinical findings
From March 2005 to March 2017, 60 individuals from a 4-generation family cohort were included in the present study. All included individuals were Brazilians of African descent, pre-dominantly male (53.3%), with a mean age of 27.4 years (±16.2 years; range, 2 months to 62 years) and tested negative for T. cruzi infection (Chagas’ disease) using hemagglutination, ELISA, and immunofluorescence testing. Of the total of 60 patients studied, 18 (30%) were placed in G1: 11 (18.3%) of these patients had WPW associated with VH and DSC, 6 (10%) had WPW isolated, and 1 (1.6%) had VH isolated. Table 1 describes the demographic characteristic of this study population.
Table 1.
Demographic characteristics of the study population.
| (N=60) | |
|---|---|
| Age (Years) | 27.4±16.2 |
| Color: non White | 60 (100) |
| Gender: Male | 37 (53) |
| WPW+ Ventricular hypertrophy | 11 (18.3) |
| Isolated WPW | 6 (10.0) |
| Isolated ventricular hypertrophy | 1 (1.6) |
| Palpitation | 17 (28.3) |
| Chest pain | 13 (21.7) |
| Pre-syncope | 13 (21.7) |
| Syncope | 14 (23.3) |
| Dyspnea | 14 (23.3) |
| Dizziness | 14 (23.3) |
| Myalgia | 2 (3.3) |
| High blood pressure | 12 (20.0) |
| Diabetes Mellitus | 3 (5.0) |
| Smoking | 4 (4.6) |
| Total Atrioventricular Block | 2 (2.3) |
| Bradycardia | 17 (28.3) |
| Pauses | 6 (10.0) |
| Short PR interval and delta wave | 3 (5.0) |
| Supraventricular Tachycardia | 14 (23.3) |
| Ventricular Tachycardia | 1 (1.7) |
| Right Bundle Brunch Block | 9 (15.0) |
| Left Bundle Brunch Block | 7 (11.5) |
| Atrial fibrillation | 8 (13.3) |
| Atrial flutter | 8 (13.3) |
| Atrial tachycardia | 7 (11.7) |
| Left atrium | 33.2±8.5 |
| Posterior wall | 10.0±8.5 |
| Interventricular septum | 11.0±7.6 |
| Left ventricular ejection fraction | 72.0±8.8 |
WPW – Wolff-Parkinson-White syndrome. Measurements are presented as averages, SD and percentages.
Symptomatic onset was observed in early childhood and adolescence. The most common clinical symptoms in the G1 population were palpitations in 16 patients (88.8%), chest pain in 11 (61.1%), syncope in 15 (83.3%), and dizziness in 13 (72.2%). Four individuals from G1 had syncope and convulsions of unknown cause in their early years of life.
Individuals from G1 had more cardiovascular risk factors than those in G2, such as hypertension observed in 8 (44%) patients from G1 and 4 (9.5%) in G2 (p=0,013). Diabetes mellitus (DM) was only present in G1 (3 (17%) (p=0.014)). Smoking was present in 4 (22%) in G1 and 1 (2.4%) in G2 (p=0.025). Among the 18 affected individuals (G1), 4 females (22.2%) experienced transient cerebral ischemic stroke (TIA) in relation to atrial arrhythmias. WPW, VH, AF, dyslipidemia, DM, and high blood pressure (HBP) were detected in all 4 affected females.
Spontaneous abortions, learning disabilities, retarded growth, autism, and delayed language development
Three women from G1 with WPW and VH reported having at least 1 newborn premature death and experienced spontaneous abortion (22.2%). Three children from G1 died prematurely at 3 to 6 months of age. The following additional incidental observations were made:
Case 1 (G1)
Male, 27 years old, 150 cm, 47 kg. This patient had a history of syncope since infancy and underwent treatment for epilepsy in childhood, without success. Although he walks without difficulty, he has dyslalia, cannot read or write, and has difficulty understanding questions. ECG showed severe sinus bradycardia and right bundle branch block (RBBB) morphology; ECHO showed VH, with normal left ventricular ejection fraction (LVEF) of 60%, posterior wall (PW) 16 mm, and intraventricular septum (IVS) 17 mm. At the age of 26 years, he underwent dual-chamber pacemaker (DDDR) implantation (Entovis DR-T, Medtronic, MN).
Case 2 (G1)
Male, 12 years old, 137 cm, 30 kg. This patient also cannot read or write. He answers questions with great difficulty because he is unable to understand what is asked. ECG showed sinus rhythm, short PR interval, and delta wave until he was 11 years old. At age 12, his ECG presented a pattern of wide QRS complexes with left bundle branch block (LBBB), delta wave, and PR interval. While watching a football game, he experienced an episode of syncope that provoked intense emotion. ECHO showed mild aortic insufficiency and normal LVEF=62%, and the Holter demonstrated periods of asystole (23 seconds). He underwent dual-chamber pacemaker implantation.
Case 3 (G2)
Female, 5 years old, 103 cm, 14 kg. This patient is deaf and mute, is unable to understand or respond to questions, and does not make any eye contact. A diagnosis of autism was confirmed by 2 neurologists. Normal ECG and ECHO results showed (LVEF: 83%) PW 0.5 cm, IVS: 0.5 cm, LA 1.94 cm. No syncope or tachyarrhythmia has been observed to date.
Echocardiography
Echocardiography from all G2 individuals was normal. The predominant pattern observed in G1 consisted of generalized and diffuse LVH, good contractility, and mitral valve insufficiency. The mean left posterior wall ventricular thickness measured 13.13 mm, and the mean interventricular septum thickness averaged 14.4 mm. Hypertrophy, with a predominance of apical and medial portions, was also observed later in life. In some affected family members, mitral regurgitation (n=9, 50%) was observed in the second and third decades of life. Non-obstructive VH predominated in 12 of 18 individuals from G1, and 1 (1.6%) female (G1) had RV and LV outflow tract obstruction.
Electrocardiography
All individuals in G2 had normal ECG. The surviving G1 individuals demonstrated a high incidence (n=15, 83.3%) of bifid T wave and alternant polarity in the same derivation, as well as pacemaker migration, with signs of volume overload of the left atrium and ventricle.
A predominance of short PR intervals, delta wave, wide QRS complexes, and RBBB morphology (n=10, 55.5%) was found in G1, while left bundle branch block (LBBB) was observed in 5 (27.7%) patients from G1 (Figures 1, 2).
Figure 1.
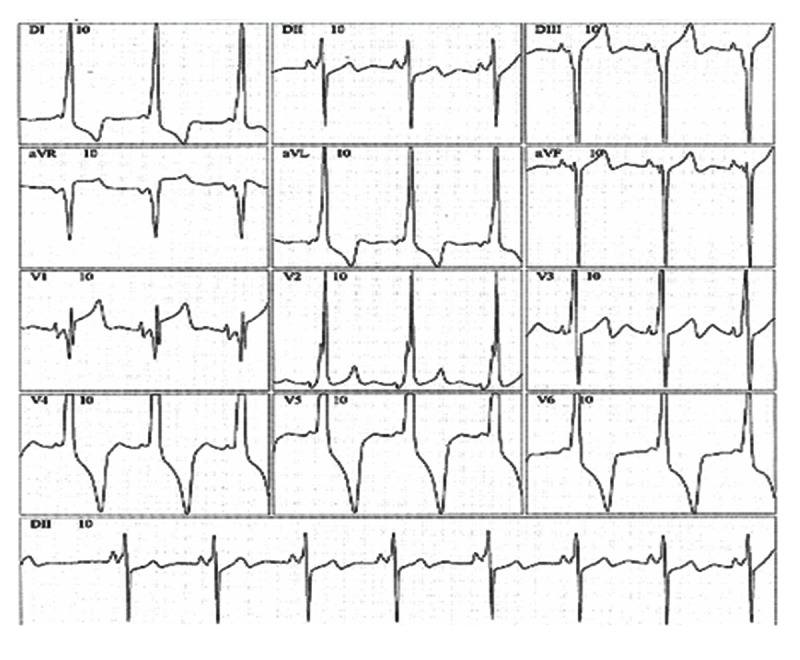
Electrocardiogram with short PR interval, delta wave, and right bundle branch morphology.
Figure 2.
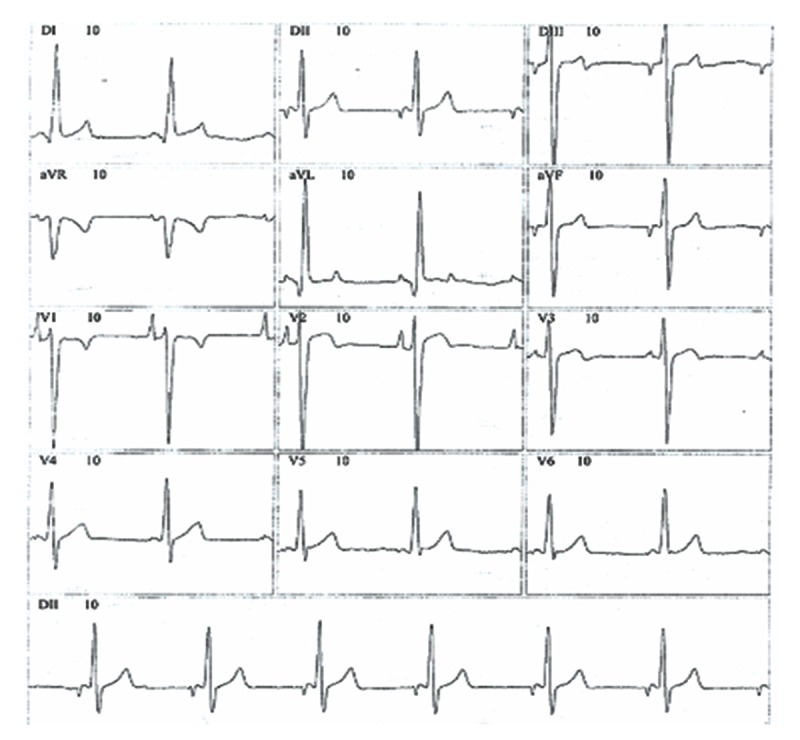
Electrocardiogram with short PR interval, delta wave, and left bundle branch morphology.
Four children from G1 had an isolated short PR interval and delta wave during childhood, and after adolescence they developed widened QRS complexes, bundle brunch morphology, tachyarrhythmias, palpitations, and syncope. Two children have had syncope since their first year of life, and 1 had a PM implanted at age 8 years.
Arrhythmias
Arrhythmias were only detected in G1 individuals, and the most common was supraventricular tachycardia (SVT) in 14 (78%), atrial fibrillation (AF) in 8 (44.4%), atrial flutter (AFL) in 8 (44.4%), and atrial tachycardia (AT) in 7 (38.9%). Spontaneous non-sustained ventricular tachycardia (VT) occurred in 1 patient (5.6%). A statistically significant association was observed between the occurrence of TIA and AFL (p=0.002), hypertension (p=0.023), smoking (p=0.005), and AF (p= 0.06).
Outcomes
In the G1 patients, we found SCD in 2 (11.1%), recovered CA in 2 (11.1%), TIA in 4 (22.2%), and PM implants in 6 (33.3%), while those findings were null in G2 (p=0.002).
Sudden cardiac death (SCD) and documented aborted cardiac arrest (CA)
Two patients had SCD, one (female) at age 37 years, and another (male) at 27 years of age. Both patients were previously known to have suffered from WPW, and each complained of palpitations.
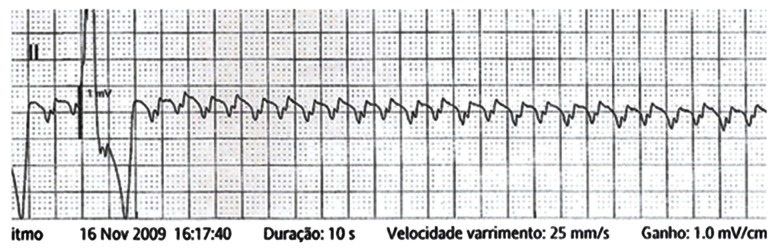
Three episodes of CA were documented in 2 patients from G1. Both individuals had WPW in association with VH, and they had experienced AF with anterograde conduction through the accessory pathway (AP): a 48-year-old female was resuscitated during PM implantation, and a 22-year-old male had 2 documented episodes of CA. At age 16, the male experienced syncope during exercise. Upon examination, AF with anterograde conduction through the AP was detected, and a ventricular rate of 250–300 bpm required resuscitation maneuvers. At age 20 years, common AFL with 1: 1 AV conduction was detected in conjunction with a ventricular rate of 300 bpm. He was treated with amiodarone, but progressive asystole followed. Figure 3 shows transitional PM implantation was required and subsequently removed after 24 h. At age 22 years, he died due to 2: 1 AFL and tachycardiomyopathy, confirmed by autopsy.
Figure 3.
Mechanism of a documented cardiac arrest in a 20-year-old male patient. Atrial flutter with total atrioventricular block.
Anatomopathologic study
During the autopsy, some remarkable alterations were found restricted to the heart. Regardless of the absence of epicardial injuries, there was an evident cardiomegaly (heart weight 786 gr, approximately 2.5 times the normal weight (60 Kg) for this patient), primarily due to biventricular hypertrophy. Random spots of mild endocardial thickening were observed. There were no coronary abnormalities.
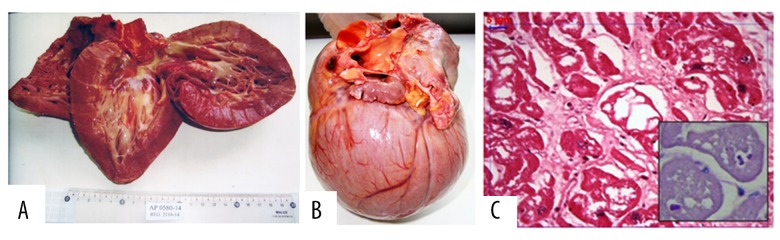
The myocardium showed diffuse hypertrophy, but the arrangement of bundles and muscle cells was regular (differing from the derangement commonly found in hypertrophic cardiomyopathy). Figure 4A and 4B show other irregularities found in fibrotic regions. The increased size of the myocytes was due to severe cytoplasmic vacuolization, in which remaining PAS-positive material could be detected, and this was important to determine the diagnosis of this cardiomyopathy. Despite the morphological damage caused by the formalin fixation, granular deposits compatible with glycogen were identified in lysosomal vesicles or free in the cytoplasm, as well as destroyed organelles that remained within autophagic vacuoles, atrophy and fragmentation of myofibrils, and tumescent mitochondria (Figure 4C).
Figure 4.
(A) Macroscopic image of a heart of a 22-year-old male patient who died suddenly. He suffered from WPW and VH. He was 160 cm tall and weighed 60 Kg. (B) The heart weighed 786 g, with obvious predominance of biventricular hypertrophy and severe symmetrical cardiomegaly, particularly in both ventricles. There was non-specific endocardial thickening. (C) An electron microscopy image of glycogen storage in the myocardial fibers of a mutation carrier patient (c.869A>T, p.K290I). The LV myocardium shows severe vacuolization of the myocardial fiber cytoplasm in an interstitial fibrosis area (HE; 400×). The remains of PAS-positive deposits inside cytoplasmic vesicles are shown in the bottom right quadrant. The scale bar is 5 μm.
Genetic analysis
Genetic sequencing from all patients in G2 revealed individuals with normal results, while a novel missense genetic variant of unknown significance (GVUS) was detected in the PRKAG2 gene (c.869A>T, p.K290I) in all affected individuals from G1. Despite the novel GVUS, alignment between species and in silico databases predict that p.K209I_PRKAG2 will be deleterious. In addition, this novel GVUS has not been identified in any global population databases (Figure 5).
Figure 5.
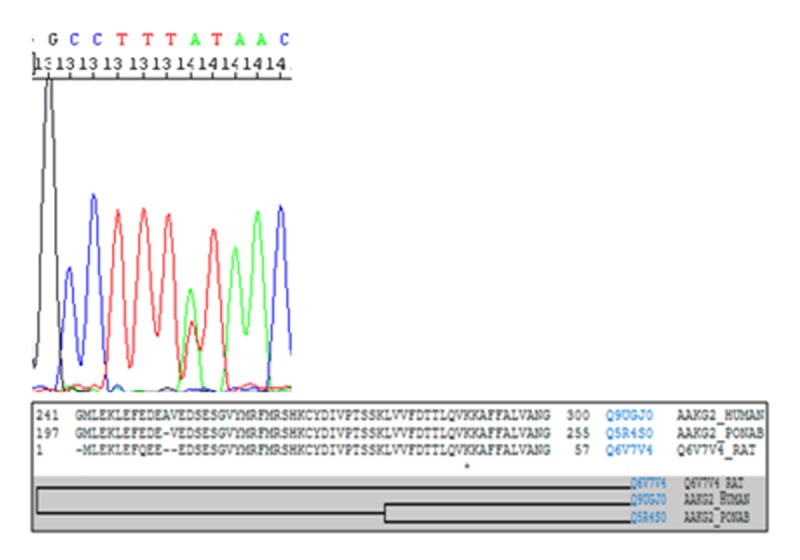
Multiple-sequence alignment and taxonomy. Asterisk indicates the position of conserved amino acids between species due to the genetic variant p.K290I.
Treatment strategies
The goal of treatment was to relieve symptoms and prevent SCD, and it varied depending on severity of symptoms. A low-sodium and low-fat diet, as well as smoking cessation, were recommended to all individuals. Three affected women had DM and were treated with hypoglycemic therapy, especially metformin, with good control of glucose. No DM was observed in G2. GI had 8 (44%) patients with HBP, which was higher than in G2, which had 4 (9.5%) (p=0.013), and all 8 were treated with angiotensin-converting enzyme (ACE) inhibitors, calcium channel blockers (diltiazem), diuretics, and beta-blockers for patients who had already undergone radiofrequency catheterization (RCA). Unlike sotalol, amiodarone was well tolerated with a good rhythm control in all patients from G1.
Anticoagulation
According to the literature, AF, AFL, HBP, and DM, as well as smoking, are controllable risk factors for TIA. Four (22.2%) individuals from G1 had TIA in association with AF, HBP, and DM. We consider PRKAG2 cardiomyopathy itself as an indication for anticoagulation. Based on these findings, all affected patients were advised to undergo anticoagulant therapy, to quit smoking, and to undergo treatment for any incidences of high blood pressure (HBP) and DM. Ten (55.5%) of them responded quite well to treatment with amiodarone for supraventricular tachycardia and atrial tachyarrhythmias.
Pacemaker implantation (PM)
Six individuals from G1 (33.3%) underwent PM implantation due to CSD: 5 received a dual-chamber PM and 1 received an ICD. Five individuals had sinus bradycardia, and 1 experienced total AV block. During the implantation of an atrial lead, a 50-year-old woman suffered aborted cardiac arrest (CA) due to AF with anterograde conduction through the AP and ventricular fibrillation. This patient was successfully resuscitated without neurologic sequelae and received a dual-chamber PM after recovering the sinus rhythm.
The clinical benefit of artificial stimulation was quite evident in all patients and altered the normal course of this disease in terms of symptoms and hospital internments, and they developed fewer atrial tachyarrhythmias.
Implantable cardioverter defibrillators (ICD)
In the present study, the main criteria for ICD implantation were the presence of spontaneous sustained ventricular tachycardia (VT) with obstructive ventricular hypertrophy, syncope, or hemodynamic compromise, and life expectancy >1 year. To date, only 1 ICD was implanted, in a woman with WPW, obstructive LV, conduction system disease, sustained VT, pre-excited AF, and syncope. She had a dual-chamber PM with a low battery and it was performed as an upgrade from a dual-chamber ICD. This patient also had a successful RCA of a left lateral AP, and there was a residual Mahaim-type fiber. She had an inappropriate shock due to atrial tachycardia. After that, she has had no other shocks in 12 years of follow-up.
An attempt was made to avoid PM implantation in children, especially ICDs, due to a high percentage of complications such as infection and inappropriate shock. Recently, a dualchamber PM (DDD) was implanted in an 11-year-old male patient with syncope and periods of asystole (22 seconds), who is now asymptomatic.
Documented cardiac arrest (CA)
One male from G1 had 2 CA at age 16 years due to AF with anterograde conduction though the AP. We performed RCA in the left lateral AP and he also had a Mahaim-type AP. At 20 years of age, he had 1: 1 AFL and, after intravenous amiodarone administration, he had asystole with successful cardiac resuscitation and a dual-chamber PM implantation (Figure 3).
He died last year, at 22 years of age, due to incessant 2: 1 AFL due to tachycardiomyopathy before the programmed RCA. The mother of this patient also had CA at 55 years of age due to pre-excited AF followed by ventricular fibrillation.
We did not have any transplanted patients. There were 3 patients in G1 with heart failure, all due to tachycardiomyopathy that developed after 20 years of age. Two of these patients are under control with drugs and RCA and 1 man died before receiving RCA.
Prevention of sudden cardiac death (SCD)
Non-sustained VT has been associated with a substantial increase in SCD risk in young patients with HCM [8,9]. The present study found that CA was associated with asystole and anterograde conduction through the AP during AF in 22.2% of the affected individuals from G1. Note that VT seemed to have no influence on SCD risk in the affected individuals because CA was observed in only 1 patient (1.7%) with obstructive VH. For the prevention of SCD, we recommended that all patients from G1 avoid playing competitive sports. All symptomatic patients with WPW were treated with RCA. This procedure has been efficient for the supraventricular tachycardia control. At 2–3 years following this procedure, we observed that these patients developed atrial tachyarrhythmia. The oldest affected patient under study, already 61 years of age, is under clinical control, although she has obstructive VH.
Radiofrequency catheter ablation
Another beneficial treatment was RCA recommended as an alternative to avoid SCD. The underlying mechanism behind 3 documented aborted CA were AF with WPW and also total atrioventricular block (AVB). Therefore, we considered RCA, antiarrhythmic drugs, and PM implantation to be more appropriate for the treatment.
Six EPS were performed, with the diagnosis of 8 AP. Two patients had 2 AP, with Mahaim fiber type and 1 more AP (left postero-lateral and right postero-lateral). The AP locations that were successfully treated with RCA with were: (1) right postero-lateral, (1) left postero-lateral, (1) left lateral, (1) right lateral, (1) right antero-septal, and (1) right postero-septal. It was used irrigated catheters in all cases for this success. The use of Mahaim fibers (nodo-fascicular and ventriculo-fascicular) was unsuccessful due to the VH and difficult access.
Pregnancy and PRKAG2 syndrome
A 25-year-old patient, who had WPW and VH, underwent a successful RCA before pregnancy, with total control of supraventricular tachycardia (SVT) during her first gestation and HBP was detected after 20 weeks of pregnancy. She was treated with alfa metildopa (500 mg 12/12h) to control elevated blood pressure and the delivery was performed with cesarean procedure when the baby was already mature, after 36 weeks, without any complications. The male newborn was healthy, with good Apgar score (8) and without the cardiac disease. This boy was included in G2 because his ECG and ECHO were normal.
Forceps delivery was needed in 5 (27.7%) patients from G1 and no in patients from G2. All of them arrived at the hospital in advanced stage of delivery.
Discussion
PRKAG2 cardiac syndrome [2,10] is a rare entity that may include WPW syndrome, VH, conduction system disease, and an increased risk of SCD. Few data were found about therapeutic strategies for this rare and multifaceted pathology. Our study reports on 18 affected individuals in a family of 60 members, with a long period of follow-up and a good experience regarding treatment and complications.
PRKAG2 syndrome has an effect on muscle cell glucose metabolism and insulin-resistant diabetes mellitus, which was described by Gollob [2]. In the present study, we describe a wide spectrum of pathological conditions such as diabetes mellitus, hypertension, congestive heart failure, neurologic disorders, and malignant arrhythmias related to this syndrome.
Sternick et al. have previously described a pathogenic variation in PRKAG2 that was responsible for familial occurrences of RBBB, sinus bradycardia, and short PR intervals [11]. In the present cohort, a predominance of RBBB (n=10, 55.5%) was detected, but LBBB (n=5, 27.7%) was also found in association with a high incidence of stroke, PM implantations, and SCD. Six EPS studies demonstrated Mahaim-type fibers, but also conventional AP. It was difficult to perform the RCA due to the VH; therefore, irrigated catheters were used.
The mechanism of documented CA was total AV block and pre-excited atrial tachyarrhythmias. Therefore, the treatment was based on antiarrhythmic drugs (amiodarone and sotalol), anticoagulation, RCA, and dual-chamber PM.
The children presented with short PR intervals, delta waves, RBBB and LBBB morphologies, and narrow QRS complexes. At approximately age 10 years, they were observed to develop QRS enlargement in association with palpitation due to SVT or syncope, with sinus bradycardia more frequent. One 6-year-old girl was treated with a successful RCA and an 11-year-old boy had a dual-chamber PM implanted, with syncope control. These procedures were beneficial and improved the normal course of this disease.
Maron et al. described substantial LV remodeling with the spontaneous appearance of LV hypertrophy in patients with VH [12]. This finding is also considered to be a common echocardiographic finding in individuals with PRKAG2 cardiac syndrome in later adolescent years (approx. 17–18 years of age) [12]. The present study confirmed this finding in most individuals from G1 (n=15, 83.3%). The autopsy of a 22-year-old patient showed large vacuolar hypertrophy with fibrosis, which differs from sarcomeric hypertrophy, which has the “morphological registered mark”, cellular disorganization, and myofibrillar in large areas of the heart wall. Other forms of hypertrophy, secondary to hypertension, or storage diseases may even exhibit similar clutter, but they are restricted to small isolated points that are insignificant in the total muscle mass.
In the present study, the arrangement of bundles and muscle cells was regular, differing from the derangement commonly found in hypertrophic cardiomyopathy; in addition, severe vacuolization of the myocardial is compatible with glycogen storage disease. It was more difficult to perform the RCA due to the septal and lateral VH in all patients from G1.
In 2008, Tan et al. reported that the p.R302Q variation in PRKAG2 caused pre-excitation and was associated with the presence of Mahaim fibers, supporting the notion that PRKAG2 variants are involved in the development of the cardiac CSD. The present study found typical phenotypic manifestations, given the close proximity of K290I to the classical mutation R302Q. In addition, the literature also correlates PRKAG2 cardiac syndrome and CSD with progressive fibrosis [12–14]. In the present study, 6 individuals from G1 required PM implantation due to sinus bradycardia and total AV block, either of which could have resulted from myocardial fibrosis. Fibrosis may also be included as a pathophysiology responsible for high pacing threshold after 3 years of PM implantation and the natural elimination of AP without RCA.
Four affected patients in G1 had other signs, such as delayed development, learning and language disabilities, early postnatal death, and spontaneous abortions. No ocular changes (retinopathy and/or changes in the retinal pigment epithelium) or complaints related to muscular dystrophy were observed [15,16]. It is also possible to hypothesize that the miscarriages and premature fetal deaths reported by affected females could be due to a severe form of cardiac disease. Regrettably, autopsies were not performed on the fetuses to corroborate this suspicion, and few studies reporting this association have been published to date. One 5-year-old girl has the diagnosis of autism. These data could be found by chance; therefore, these findings must be confirmed to have an association with this cardiac disease.
Accordingly, genetic analysis identified a novel GVUS in PRKAG2 as the potential cause of this new clinical entity. All clinically affected individuals (G1) were observed to carry the novel GVUS, while none of the non-affected (G2) individuals carried this genetic variant. In silico analysis using global population databases predicted the GVUS to be deleterious. In addition, although preliminary genetic screening corroborated the potential malignancy of this GVUS, the diagnosis of a novel variation identified in PRKAG2 was established based on clinical and ECHO aspects. Anatomopathologic study proved that the presence of glycogen storage in vacuoles was responsible for significant VH.
Several drugs can be helpful in treatment of PRKAG2 syndrome, including ACE inhibitors and angiotensin II receptor blockers (Abs), because they may improve reverse remodeling and reduce rates of SCD, as in other types of VH.
Avoidance of playing competitive sports was recommended in patients because many supraventricular arrhythmias were observed during exercise.
The authors consider PRKAG2 cardiomyopathy itself as pathogenic and an indication for anticoagulation. During pregnancy, metabolic control, blood pressure levels control, and surgical intervention (cesarean) as early as possible help avoid complications for the mother and fetus. Amiodarone was the antiarrhythmic drug best tolerated and with better control of tachyarrhythmias.
Similar to Danon’s disease, [16] there were some cases of mental retardation, cardiomegaly, but without hapatomegaly or proximal myopathy, with the need for specialized follow-up with neurologists and speech therapists.
Ventricular Tachycardia is one of the leading causes of SCD in HCM [17]. In PRKAG2 syndrome the observed CA mechanism was asystole and pre-excited AF and not VT, for this reason, this study suggests the treatment based on implanting PM and RCA.
Invasive electrophysiological testing with voltage mapping can be used to identify glycogen replacement and to guide catheter ablation of AP.
One current matter of debate is the role of ablation in patients with asymptomatic WPW pattern [17]. The literature provides little information about ICD therapy for this syndrome, and there is a potential risk of infection and inappropriate shock in children. Cardiac transplant is another option, but it was not indicated in our cohort.
Conclusions
The present study describes the main treatment strategies for a novel genetic variant in PRKAG2 responsible for a glycogen storage disease, with multiple AP and VH, and conduction system disease, with a poor prognosis. The documented mechanisms of CA were pre-excited AF and asystole.
Acknowledgments
This work would not have been possible without the invaluable contributions of physicians Dr. Mario de Seixas Rocha and Dr. Gervásio de Campos. We thank Andris K. Walter for providing English revision and consulting. The CIBERCV is an initiative of the ISCIII, Spanish Ministry of Economy and Competitiveness.
Footnotes
Statements and declarations regarding conflicts of interest
All participants provided written informed consent. This study was approved by the São Rafael Hospital Institutional Review Board (protocol no.16–12), as well as the Bahiana School of Medicine Institutional Review Board (protocol no. 165.803). All authors have read the manuscript and agreed with the results presented herein.
References:
- 1.Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58(25):e212–60. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Gollob MH. Glycogen storage disease as a unifying mechanism of disease in the PRKAG2 cardiac syndrome. Biochem Soc Trans. 2003;31(Pt 1):228–31. doi: 10.1042/bst0310228. [DOI] [PubMed] [Google Scholar]
- 3.Siu BL, Niimura H, Osborne JA, et al. Familial dilated cardiomyopathy locus maps to chromosome 2q31. Circulation. 1999;99(8):1022–26. doi: 10.1161/01.cir.99.8.1022. [DOI] [PubMed] [Google Scholar]
- 4.Arad M, Benson DW, Perez-Atayde AR, et al. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109(3):357–62. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: Morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol. 1995;26(7):1699–708. doi: 10.1016/0735-1097(95)00390-8. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92(4):785–89. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 7.Campuzano O, Sanchez-Molero O, Allegue C, et al. Post-mortem genetic analysis in juvenile cases of sudden cardiac death. Forensic Sci Int. 2014;245C:30–37. doi: 10.1016/j.forsciint.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Danon MJ, Oh SJ, DiMauro S, et al. Lysosomal glycogen storage disease with normal acid maltase. Neurology. 1981;31(1):51–57. doi: 10.1212/wnl.31.1.51. [DOI] [PubMed] [Google Scholar]
- 9.Monserrat L, Elliott PM, Gimeno JR, et al. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: An independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42(5):873–79. doi: 10.1016/s0735-1097(03)00827-1. [DOI] [PubMed] [Google Scholar]
- 10.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31(19):2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 11.Sternick EB, Oliva A, Gerken LM, et al. Clinical, electrocardiographic, and electrophysiologic characteristics of patients with a fasciculoventricular pathway: The role of PRKAG2 mutation. Heart Rhythm. 2011;8(1):58–64. doi: 10.1016/j.hrthm.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 12.Maron BJ, Spirito P, Wesley Y, Arce J. Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. New Engl J Med. 1986;315(10):610–14. doi: 10.1056/NEJM198609043151003. [DOI] [PubMed] [Google Scholar]
- 13.Hagege AA, Dubourg O, Desnos M, et al. Familial hypertrophic cardiomyopathy. Cardiac ultrasonic abnormalities in genetically affected subjects without echocardiographic evidence of left ventricular hypertrophy. Eur Heart J. 1998;19(3):490–99. doi: 10.1053/euhj.1997.0735. [DOI] [PubMed] [Google Scholar]
- 14.Tan HL, van der Wal AC, Campian ME, et al. Nodoventricular accessory pathways in PRKAG2-dependent familial preexcitation syndrome reveal a disorder in cardiac development. Circ Arrhythm Electrophysiol. 2008;1(4):276–81. doi: 10.1161/CIRCEP.108.782862. [DOI] [PubMed] [Google Scholar]
- 15.Arad M, Moskowitz IP, Patel VV, et al. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107(22):2850–56. doi: 10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]
- 16.Maron BJ, Roberts WC, Arad M, et al. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA. 2009;301(12):1253–59. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarsam S, Sidiqi I, Shah D, Zughaib M. Concomitant Wolff-Parkinson-White and atrioventricular nodal reentrant tachycardia: Which pathway to ablate? Am J Case Rep. 2015;16:872–75. doi: 10.12659/AJCR.894647. [DOI] [PMC free article] [PubMed] [Google Scholar]