Abstract
Background:
The aim of the present study is to evaluate the impact of metastases-directed stereotactic body radiotherapy in two groups of oligometastatic prostate cancer (PC) patients: oligorecurrent PC and oligoprogressive castration-resistant PC (oligo-CRPC).
Methods:
Inclusion criteria of the present multicentre retrospective analysis were: (1) oligorecurrent PC, defined as the presence of 1–3 lesions (bone or nodes) detected with choline positron emission tomography or CT plus bone scan following biochemical recurrence; (2) oligo-CRPC, defined as metastases (bone or nodes) detected after a prostatic-specific antigen rise during androgen deprivation therapy (ADT). Primary end points were: distant progression-free survival (DPFS) and ADT-free survival in oligorecurrent PC patients; DPFS and second-line systemic treatment-free survival in oligo-CRPC patients.
Results:
About 100 patients with oligorecurrent PC (139 lesions) and 41 with oligo-CRPC (70 lesions), treated between March 2010 and April 2016, were analysed. After a median follow-up of 20.4 months, in the oligorecurrent group 1- and 2-year DPFS were 64.4 and 43%. The rate of LC was 92.8% at 2 years. At a median follow-up of 23.4 months, in the oligo-CRPC group 1- and 2-year DPFS were 43.2 and 21.6%. Limitations include the retrospective design.
Conclusions:
Stereotactic body radiotherapy seems to be a useful treatment both for oligorecurrent and oligo-CRPC.
Keywords: oligometastases, prostate cancer, SBRT, SABR, radiotherapy, androgen deprivation therapy
The treatment of patients with metastatic prostate cancer (PC) is rapidly evolving. Androgen deprivation therapy (ADT) with analogues or antagonist of Lh-Rh remains the standard of care; in case of metastatic castrate-resistant PC (mCRPC), Abiraterone, Enzalutamide or Docetaxel could further prolong biochemical control and overall survival (OS) (Tannock et al, 2004; Beer et al, 2014; Ryan et al, 2015). Moreover, Docetaxel with ADT can be now considered as effective treatment in patients with metastatic (high volume) hormone-sensitive PC (Cornford et al, 2017). Concerning the effectiveness of ADT in metastatic PC, it is recognised that its clinical benefit is generally limited to 2–3 years from the beginning of the ADT administration (Harris et al, 2009). Due to this concern, clinicians are frequently motivated to delay the ADT starting using local approaches in patients with limited tumour burden. In fact, it is known that an oligometastatic phase exists; it is generally characterised by a less advanced state of metastatic disease, amenable to local therapy (Hellman and Weichselbaum, 1995). This assumption is particularly true in case of oligometastatic PC due to its potential long-term control and slow rate of progression in several cases (Alongi et al, 2012). In the context of oligometastatic PC patients, two main conditions could be identified: (1) oligorecurrent PC defined as the appearance of metastases following biochemical recurrence in ADT naive patients, showing an endocrine responsiveness; (2) ‘oligoprogressive’ PC defined as a metastatic progression after a prostatic-specific antigen (PSA) rise during ADT, in which PC cells became castration-resistant. In this last condition, the prognosis is worse compared to the oligorecurrent phase.
In the setting of oligometastatic PC, the adoption of oligometastases-directed stereotactic body radiation therapy (SBRT) could potentially achieve local disease control in selected patients with negligible toxicity (Casamassima et al, 2011; Ahmed et al, 2013; Jereczek-Fossa et al, 2012; Muldermans et al, 2016; Ost et al, 2016a, 2016b). In several cases, the site of progression after SBRT for oligometastatic PC recurrences is close to previous treatment volume. This pattern of failure has been also considered amenable to a new course of SBRT rather than other therapeutic approaches (Alongi et al, 2012). Thus, in this context, SBRT could influence the management of ADT administration by two potential ways: (1) delaying the administration of palliative endocrine therapies in case of oligorecurrent PC; (2) postponing the subsequent systemic schedules in case of oligoprogressive castration-resistant PC (oligo-CRPC).
On the basis of these assumptions, here we report the results of a multicentre study evaluating the feasibility and efficacy of SBRT approach for oligorecurrent PC and oligo-CRPC.
Materials and methods
The present study is a multicentre retrospective analysis including 9 centres. For each patient, a consent was obtained prior to inclusion in this study. Inclusion criteria of the present analysis were as follows: (1) histologically proven diagnosis of PC; (2) oligorecurrent PC, defined as the presence of 1–3 lesions (bone or nodes) detected with choline positron emission tomography (PET) or CT plus bone scan following biochemical recurrence; (3) oligo-CRPC, defined as metastases (bone or nodes) detected with choline PET or CT plus bone scan after a PSA rise during ADT; (4) patients treated with SBRT with a dose of at least 5 Gy per fraction to a biological effective dose (BED) of at least 80 Gy using an α/β of 3 Gy.
Exclusion criteria were adjuvant or neo-adjuvant ADT for more than 1 year in oligorecurrent PC and patients treated with SBRT after second-line treatment (Abiraterone, Enzalutamide or Docetaxel).
Primary treatment of PC consisted of radical prostatectomy, RT or a combination of both, at the discretion of the referring Center and according to PC risk categories (Mottet et al, 2017).
SBRT procedures
All patients underwent a CT-based treatment planning with 3 mm slice thickness in supine position. Gross tumour volume, equal to clinical target volume (CTV), was delineated using all the available morphological and metabolic imaging information. A planning target volume (PTV) was created around CTV using isotropic 3 mm margins. Organs at risk were delineated, depending on the location of target volume.
The radiation-adopted schedules followed the local treatment policy of each centre. In order to equalise the different schedules, a BED was calculated using α/β=3 Gy.
Intensity-modulated radiotherapy with static beams or dynamic arcs or 3DCRT techniques were used based on centre internal protocols and technology availability (Supplementary Table 1). At each fraction, patients’ set up and target accuracy was always verified by using cone beam CT. In case of synchronous lesions, all lesions were treated simultaneously. After SBRT, patients were followed every 3–4 months with clinical evaluation, PSA and the same imaging initially used to detect the oligometastases.
End points and statistical analysis
Distant progression-free survival (DPFS, defined as the time between the first day of SBRT to the detection of clinical disease outside the PTV after further biochemical progression) and ADT-free survival (ADT-FS, defined as the time between the first day of SBRT to the start of palliative ADT) were the primary end points for oligorecurrent PC. For oligo-CRPC, the primary end points were DPFS and second-line systemic treatment-free survival (STFS, defined as the time between the first day of SBRT to the start of systemic therapies such as Abiraterone, Enzalutamide or Docetaxel). For both groups of patients, secondary end points were local control (in-field control), OS and toxicity (using CTCAE 4.0 scale). Univariate analysis was performed in order to assess factors influencing outcome in both categories. Survival analysis was realised by using the Kaplan–Meier method and log rank test (a P-value <0.05 was considered statistically significant). Variables analysed in the univariate analysis were as follows: Gleason Score, D’Amico risk group, number and site of metastasis, PSA pre-SBRT, BED, time to castrate resistant (in oligo-CRPC group only); moreover, for the oligorecurrent PC group, time to metastasis, PSA-DT, use of concurrent ADT and prophylactic nodal irradiation were also considered. The following variables were dichotomized at the median value: PSA pre-SBRT, PSA-DT, BED, time to metastasis and time to castrate resistance the median value as cutoff. Variables exhibiting a P-value <0.15 were entered manually in Cox proportional hazard model in a forward stepwise fashion. Statistics was performed using SPSS Software (v. 20.0, Armonk, NY, USA).
Results
Patients
From March 2010 to April 2016, 141 patients for a total of 209 lesions met the inclusion criteria of the current analysis. Among these, 100 patients were treated for oligorecurrent PC and 41 patients underwent SBRT for oligoprogressive mCRPC. The two groups were analysed separately according to their different characteristics and clinical implications.
Oligorecurrent prostate cancer
About 100 patients, with a total of 139 lesions, had an oligorecurrent hormone-sensitive PC. At initial diagnosis, median PSA was 9.8 ng ml−1 (mean 13.9 ng ml−1). According to D’Amico risk groups, 5 patients (5%) were defined as low-risk, 21 patients (21%) as intermediate group, 43 patients (43%) as high-risk group and 31 patients (31%) as very-high-risk group. The primary treatment was radical prostatectomy alone in 24 patients (24%), radical RT in 16 patients (16%), brachytherapy in 2 patients (2%), prostatectomy with adjuvant RT in 35 patients (35%), prostatectomy with salvage RT in 23 patients (23%).
At the time of SBRT, the median age of patients was 67 years (range, 49–81). The median PSA pre-SBRT was 2.4 ng ml−1 (range 0.33–36 ng ml−1). Metastases were detected by means of PET choline in 96 patients (96%) and CT plus bone scan in the remaining cases (4%). Metastases-directed SBRT was delivered for the first course in 1 lesion in 87 patients (87%), 2 lesions in 9 patients (9%) and 3 lesions in 4 patients (4%). Concomitant ADT was given to 24% of patients (median ADT 11 months, range 6–12). In Table 1, the patients’ characteristics are detailed. In Figure 1 the distribution of the treated sites is shown.
Table 1. Baseline characteristics of oligorecurrent prostate cancer.
| Clinical characteristics: oligorecurrent prosate cancer | Value |
|---|---|
| Number of patients | 100 |
| Age, median (range) | 67 (49–81) |
| PSA (ng ml−1) at diagnosis, median | 9.8 |
| D’Amico risk group (D'Amico et al, 1998) at diagnosis | |
| Low | 5 (5%) |
| Intermediate | 21 (21%) |
| High | 43 (43%) |
| Very high | 31 (31%) |
| Adjuvant ADT at first treatment | |
| Yes | 62 (62%) |
| No | 38 (38%) |
| Treatments at diagnosis | |
| RP | 24 (24%) |
| RT | 16 (16%) |
| BRT | 2 (2%) |
| RP+adjuvant RT | 35 (35%) |
| RP+salvage RT | 23 (23%) |
| PSA (ng ml−1) at oligorecurrence (pre-SBRT), median | 2.4 |
| PSA-DT (ng ml−1) at oligorecurrence (pre-SBRT), median (mean) | 6.4 (8.1) |
| Restaging | |
| Choline PET | 96 (96%) |
| CT/bone scan | 4 (4%) |
| Site of lesion | |
| Lymph node | 117 (84.1%) |
| Bone | 22 (15.8%) |
| Number of lesions treated (for the first SBRT course) | 117 |
| 1 lesion | 87 (87%) |
| 2 lesions | 9 (9%) |
| 3 lesions | 4 (4%) |
| Time to metastasis (from primary treatment), median (mean) | 43.9 (60.3) |
| BED (α/β=3 Gy) of SBRT, median (range) | 116 (80–216.6) Gy |
| Prophylactic pelvic RT with SBRT | |
| Yes | 7 (7%) |
| No | 93 (93%) |
| Concomitant ADT with SBRT | |
| Yes | 24 (24%) |
| No | 76 (76%) |
| Median time (months) of concomitant ADT with SBRT (range) | 11 (4–12) |
| PSA (ng ml−1) post SBRT, median (mean) | 0.81 (2.07) |
Abbreviations: ADT=androgen deprivation therapy; BED=biologically effective dose; BRT=brachytherapy; CT=computed tomography; DT=doubling time; PET=positron emission tomography; PSA=prostatic-specific antigen; RP=radical prostatectomy; RT=radiation therapy; SBRT=stereotactic body radiation therapy.
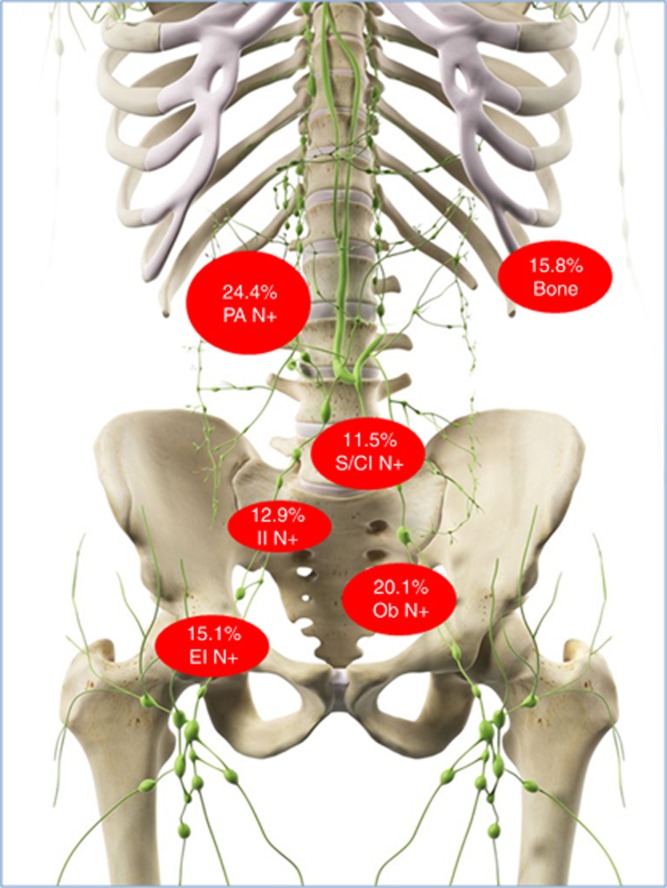
Figure 1.
Sites of oligorecurrences underwent to stereotactic body radiotherapy in the current study. Sites of oligorecurrence: obturator lymph nodes (Ob N)=28 (20.1%); internal iliac lymph nodes (II N)=18 (12.9%); external iliac lymph nodes (EI N)=21 (15.1%); presacral/common iliac lymph nodes (S/CI N)=16 (11.5%); paraortic lymph nodes (PA N)=34 (24.4%); bone=22 (15.8%). A full colour version of this figure is available at the British Journal of Cancer journal online.
At the time of the analysis, the median follow-up from the end of SBRT was 20.4 months (range 3–72 months). The median DPFS was 17.7 months. One-year, 2-year and 3-year DPFS were: 64.4%, 43% and 26.6%, respectively. Fifteen out of 100 patients, after further oligorecurrent disease detected after biochemical progression, underwent a second course of SBRT (for a total of 1 lesion in 11 patients, 2 lesions in 1 patient and 3 lesions in 3 patients): this resulted in a median ADT-FS of 20.9 months (with 1-year, 2-year and 3-year ADT-FS of: 67.4%, 47.3% and 31%, respectively). At 2 years, LC rate (in-field control) was 92.8%, the OS rate was 98.1%. No toxicity superior or equal to G3 was reported. Overall, gastrointestinal toxicity was found in four patients (acute G1) and genitourinary toxicity in three patients (one G1 and two G2 acute and late).
In Table 2, the results of the univariate analyses concerning DPFS and ADT-FS are detailed. At univariate analysis, low PSA-DT (<6.4 months) was shown to be related with a worse DPFS (P=0.06) and a worse ADT-FS (P=0.04). In detail, for patients with a PSA doubling time inferior to 6.4 months, 1-year and 2-year DPFS were equal to 39.3 and 12.8%, as compared to a 1-year and 2-year DPFS equal to 64.5 and 45.6% in case of PSA doubling time superior to 6.4 months. Considering the ADT-FS, for PSA doubling time inferior to 6.4 months at 1- and 2-year ADT-FS were 42.9 and 21.4%. Conversely, for PSA doubling time superior to 6.4 months, 1-year and 2-year ADT-FS were 71.5 and 51.2%. No multivariate analysis was performed as no other variables resulted statistically significant at univariate analysis.
Table 2. Oligorecurrent prostate cancer: results of the univariate analyses concerning DPFS and ADT-FS.
| Median DPFS (months) | 1-year DPFS | 2-year DPFS | 3-year DPFS | Median ADT-FS | 1-year ADT-FS | 2-year ADT-FS | 3-year ADT-FS | |
|---|---|---|---|---|---|---|---|---|
| Whole population (100) | 17.7 | 64.4% | 43% | 26.6% | 20.9 | 67.4% | 47.3% | 31% |
| Initial Gleason Score | P=0.824 | P=0.956 | ||||||
| ⩽7 | 19 | 68.1% | 40.2% | 17.3% | 30.7 | 71.2% | 51.4% | 18.4% |
| >7 | 15 | 58.8% | 41.8% | 13.2% | 19.7 | 67.7% | 51.4% | 18.4% |
| D'Amico risk group (D'Amico et al, 1998) | P=0.868 | P=0.967 | ||||||
| Low/intermediate | 18 | 53.8% | 29.6% | 20.1% | 19 | 63.1% | 34.5% | 23% |
| High/very high | 13.2 | 57.2% | 40.6% | 18.9% | 19 | 65% | 47.2% | 19.2% |
| PSA-DT | P=0.06 | P=0.04 | ||||||
| <6.4 months | 9 | 39.3% | 12.8% | n.r. | 9.9 | 42.9% | 21.4% | n.r. |
| ⩾6.4 months | 20.6 | 64.5% | 45.6% | 21.7% | 25.2 | 71.5% | 51.2% | 33.5% |
| PSA pre-SBRT | P=0.28 | P=0.195 | ||||||
| <2.38 ng ml−1 | 21.1 | 62.9% | 44.1% | 29.3% | 30.7 | 69.4% | 54.9% | 38.4% |
| ⩾2.38 ng ml−1 | 16.5 | 60% | 35.2% | 18.1% | 19.5 | 64.4% | 38.9% | 18.4% |
| Time to metastasis | P=0.178 | P=0.236 | ||||||
| <43.9 months | 16.3 | 66.4% | 41.1% | 29.6% | 20.9 | 76.4% | 49.9% | 36.1% |
| ⩾43.9 months | 12.9 | 54.3% | 37% | 17.5% | 19.1 | 59.6% | 44.4% | 22.2% |
| Lymph node vs bone | P=0.356 | P=0.605 | ||||||
| Lymph node | 21.4 | 63.2% | 39.5% | 17.3% | 23.1 | 69.6% | 48.3% | 30.6% |
| Bone | 11 | 20.2% | n.r. | n.r. | 10.7 | 29.6% | n.r. | n.r. |
| Number of metastases treated | P=0.16 | P=0.56 | ||||||
| One | 13.7 | 59% | 37.1% | 21% | 19.7 | 67.1% | 44.6% | 33% |
| More than one | 33 | 68.9% | 38.4% | 23.1% | 33.3 | 69.7% | 39.6% | 25.9% |
| BED | P=0.51 | P=0.597 | ||||||
| <116 Gy | 13.5 | 57.4% | 43.6% | 15.9% | 21 | 71.1% | 44.2% | 40.8% |
| ⩾116 Gy | 20.2 | 61.2% | 44.1% | 32.4% | 26.5 | 61.9% | 53.1% | 45.5% |
| Prophylactic pelvic RT | P=0.19 | P=0.47 | ||||||
| No | 15.7 | 57% | 37% | 15.8% | 19.7 | 65.7% | 45.7% | 29.4% |
| Yes | 28 | 100% | 67.1% | 33.5% | 26.5 | 100% | 70.1% | 35.9% |
| Concomitant ADT (to SBRT) | P=0.43 | P=0.35 | ||||||
| No | 13.3 | 49.2% | 36.9% | 20.6% | 19.1 | 56.3% | 41.1% | 21.7% |
| Yes | 23.5 | 94% | 44.9% | 9.9% | 25.2 | 98.4% | 45.5% | 37.9% |
Abbreviations: ADT=androgen deprivation therapy; ADT-FS=androgen deprivation therapy-free survival; BED=biologically effective dose; DPFS=distant progression-free survival; DT=doubling time; n.r.=not reached; PSA=prostatic-specific antigen; RP=radical prostatectomy; SBRT=stereotactic body radiation therapy.
Oligoprogressive castration-resistant PC
From March 2010 to April 2016, 41 patients were treated for a total of 70 lesions. In Table 3, the patients’ characteristics are detailed. After a median follow-up of 24 months, 1- and 2-year DPFS were 43.2% and 21.6%, respectively. The median DPFS was 11 months.
Table 3. Baseline characteristics of oligoprogressive prostate cancer.
| Clinical characteristics: oligoprogressive prostate cancer | Value |
|---|---|
| Number of patients | 41 |
| Age, median (range) | 65 (50–81) |
| PSA (ng ml−1) at diagnosis, median | 15 |
| D’Amico risk group (D'Amico et al, 1998) at diagnosis | |
| Low | 0 (0%) |
| Intermediate | 6 (14.6%) |
| High | 16 (39%) |
| Very high | 19 (46.4%) |
| Treatments at diagnosis | |
| RP | 7 (17%) |
| RT | 10 (24.4%) |
| BRT | 1 (2.4%) |
| RP+adjuvant RT | 7 (17%) |
| RP+salvage RT | 11 (26.8%) |
| ADT only | 5 (12.2%) |
| PSA (ng ml−1) at oligoprogression (pre-SBRT), median | 4 |
| Time to castrate resistant (time from start of ADT to the onset of clinical metastases months, median (mean)) | 37 (50.3) |
| Restaging | |
| Choline PET | 38 (93.7%) |
| CT/Scintigraphy | 3 (6.3%) |
| Site of lesion | |
| Lymph node | 49 (70%) |
| Bone | 21 (30%) |
| Number of lesions treated (for the first SBRT course) | 54 |
| 1 lesion | 30 (73.2%) |
| 2 lesions | 9 (22%) |
| 3 lesions | 2 (4.8%) |
| BED (α/β=3 Gy) of SBRT, median (range) | 116 (90–173.33) Gy |
| PSA (ng ml−1) post SBRT, median (mean) | 0.81 (2.07) |
Abbreviations: ADT=androgen deprivation therapy; BED=biologically effective dose; BRT=brachytherapy; CT=computed tomography; PET=positron emission tomography; PSA=prostatic-specific antigen; RP=radical prostatectomy; RT=radiation therapy; SBRT=stereotactic body radiation therapy.
Ten patients underwent a second course of SBRT (for a total of one lesion in six patients, two lesions in two patients, three lesions in two patients) for the appearance of new metastases: this results in a median STFS of 22 months (with 1-, 2-, 3-year STFS of 74.8%, 41.3% and 29.5%, respectively) (Supplementary Data). At the time of the analysis, 20 patients had started systemic treatment (10 patients with Abiraterone or Enzalutamide and 10 patients with Docetaxel). For this subgroup of patients, no significant correlations among all the variables were found at univariate analysis. No adverse urinary-related events were registered, only one case of G1 gastrointestinal late toxicity was observed. Overall survival rate at 2 years was 93.3% and LC rate at 2 years was 90.2%. In Table 4, the results of the univariate analyses concerning DPFS and STFS are detailed.
Table 4. Oligoprogressive castration-resistant prostate cancer: results of the univariate analyses concerning DPFS and second-line STFS.
| Median DPFS (months) | 1-year DPFS | 2-year DPFS | 3-year DPFS | Median STFS | 1-year STFS | 2-year STFS | 3-year STFS | |
|---|---|---|---|---|---|---|---|---|
| Whole population (41 pts) | 11.2 | 43.2% | 21.6% | 11.9% | 22 | 74.8% | 41.3% | 29.5% |
| Initial Gleason Score | P=0.611 | P=0.382 | ||||||
| ⩽7 | 15 | 54.4% | 27.3% | n.r. | 28 | 83.3% | 52.9% | 17.6% |
| >7 | 7 | 34.3% | 11.4% | n.r. | 14 | 57.1% | 28.6% | n.r. |
| D'Amico risk group (D'Amico et al, 1998) | P=0.15 | P=0.096 | ||||||
| Intermediate/high | 24 | 72% | 43.3% | 34.7% | 28 | 85.6% | 64.2% | 42.8% |
| Very high | 7 | 30.7% | 12.3% | 6.1% | 19 | 71.1% | 35.5% | 26.7% |
| PSA pre-SBRT | P=0.644 | P=0.643 | ||||||
| <4 ng ml−1 | 11 | 41.2% | 12% | 12% | 22 | 74.2% | 33.4% | 22.3% |
| ⩾4 ng ml−1 | 11 | 43.9% | 31.4% | 16.7% | 23 | 64.4% | 38.9% | 18.4% |
| Time to castrate resistant | P=0.941 | P=0.39 | ||||||
| <37 months | 14 | 50.5% | 14.4% | 14.4% | 22 | 63.5% | 42.3% | 21.2% |
| ⩾37 months | 11 | 37.2% | 26.6% | n.r. | 19 | 83.3% | 38.2% | n.r. |
| Lymph node vs bone | P=0.791 | P=0.34 | ||||||
| Lymph | 11 | 42.1% | 21.1% | 16.8% | 22 | 80.7% | 43.5% | 34.8% |
| Bone | 6 | 45.7% | 22.9% | 0% | 19 | 75% | 47.7% | 35.8% |
| Number of metastases | P=0.43 | P=0.40 | ||||||
| One | 11 | 44% | 24% | 15% | 24 | 74.6% | 47% | 33.6% |
| More than one | 11 | 42.9% | 14.3% | 14.3% | 22 | 77.1% | 19.3% | n.r. |
| BED | P=0.721 | P=0.238 | ||||||
| <116 Gy | 8 | 31.5% | 21.0% | n.r. | 23 | 90.9% | 45.5% | 40.8% |
| ⩾116 Gy | 11 | 49.4% | 22.4% | 18% | 19 | 67.5% | 38.6% | 23.1% |
Abbreviations: BED=biologically effective dose; DPFS=distant progression-free survival; FS=free survival; n.r.=not reached; PSA=prostatic-specific antigen; SBRT=stereotactic body radiation therapy; STFS=systemic treatment-free survival.
Discussion
In metastatic PC, ADT represents the standard of care. Unfortunately, after an initial response to endocrine therapies, metastatic PC patients develop a castration-resistant condition characterised by PSA rising, appearance of new metastases and/or dimensional progression of previous metastases (Cheng et al, 2012). Recently, SBRT has been strategically considered as a potentially effective and well-tolerated therapeutic tool in the treatment sequence for oligometastatic PC (Alongi et al, 2012). In this phase of the disease, oligometastases-directed SBRT could allow an optimal local control and a significant delay of further systemic treatments.
In a multi-institutional series (Ost et al, 2016a), reporting the safety and efficacy of SBRT for patients diagnosed with ⩽3 metachronous nodal recurrences treated with SBRT, ADT start was delayed by more than 3 years (44 months, range 17–70 months). The authors emphasise the possibility of delaying ADT treatment and, therefore, the onset of endocrine resistance situation. Moreover, the utility of SBRT to ameliorate patients’ quality of life by deferring ADT omission is reported. In fact, it is well-recognised that ADT administration could be a source of complications, especially in terms of cardiovascular adverse events and metabolic syndrome (Efstathiou et al, 2008). In the present study population, median ADT-FS was equal to 20.9 months and median DPFS to 17.7 months. Although the dose delivered was similar to Ost et al (2016 a, 2016 b) experience (i.e., a RT dose of at least 5 Gy per fraction to a BED of at least 80 Gy to all metastatic sites), our results were inferior comparing to their experience. This observation is probably related to several reasons: unlike them, in our population, patients with bone lesions are present and the percentage of adjuvant ADT is smaller (they report a 57% of patients treated with concomitant ADT); furthermore, we report a greater number of lumbar-aortic nodal metastases (M1a). However, in the current population of study, BED prescription dose was satisfactory regardless of the RT techniques.
Looking at the oligo-CRPC patients of our analysis (to our knowledge, we report the largest study on the role of SBRT for this setting of patients), the median DPFS was 11 months, with 1-year and 2-year DPFS of 43.2 and 21.6%. A point of strength of the current results is that during this time interval of 11 months, it was not necessary to start palliative systemic therapy such as chemotherapy or second-line hormonal therapy. Moreover, the 2-year in-field control was 90.2% and OS rate was 93.3%, in the absence of toxicity superior or equal to G3, confirming the high efficacy and the optimal safety profile of SBRT in this more aggressive setting.
We are aware of the limitations of a multi-institutional retrospective study as well of the limited follow-up period of our series; definitive conclusions regarding the impact of SBRT on survival outcomes could not, therefore, be drawn. However, these preliminary findings appear satisfactory and focus on the importance of this multimodal treatment strategy in oligometastatic PC. The identification of this category of patients remains crucial before to offer SBRT. In fact, while for oligometastatic state several definitions are available in the literature, concerning oligorecurrent and oligoprogressive phase, especially for PC patients, a large consensus lacks allowing yet challenging the proper selection of SBRT in this setting.
The recent introduction of functional imaging modalities, such as choline PET, here utilised in 96% of cases, allows to detect the oligometastatic phase earlier when compared to conventional imaging (CT or bone scan) (Hodolic, 2011). The reliability of choline PET for PC has been investigated in several disease phases with different aims: (i) to change treatment intent, from radical to palliative, (ii) to change RT volumes and doses, (iii) to facilitate integration with other therapies (De Bari et al, 2014).
Finally, patient selection is essential in order to optimise clinical results. Predictive factors could help clinicians to stratify patients for receiving the most appropriate and effective treatment. Some clinical features and disease characteristics may still be of value in selecting patients for different treatments. Several parameters were found to be related to long-term outcomes after oligometastases-directed SBRT, such as higher pre-SBRT PSA levels or the number of treated metastases (Muldermans et al, 2016). Similarly to the study by Decaestecker et al (2014), in the current experience, a short PSA-DT (here estimated as inferior to 6.4 months) is related with a worse DPFS and ADT-FS. Certainly, this variable may contribute to assess the decision-making process for SBRT in the oligorecurrent castration-resistant PC setting.
Conclusion
In the present study, SBRT for oligorecurrent PC turned out to be a safe and useful treatment allowing to delay palliative ADT for almost 2 years: the beginning of the systemic second-line therapies can be postponed by SBRT for almost 2 years in case of oligo-CRPC also.
Acknowledgments
Ethical declaration
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Specific and detailed consent was obtained for each patients before the treatment.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
The authors declare no conflict of interest.
Supplementary Material
References
- Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR (2013) Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol 2: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M (2012) Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 17(8): 1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B (2014) Enzalutamide in metastatic prostate cancer before chemotherapy; PREVAIL Investigators. N Engl J Med 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamassima F, Masi L, Menichelli C, Bonucci I, Casamassima E, Lazzeri M, Gulisano M, Aterini S (2011) Efficacy of eradicative radiotherapy for limited nodal metastases detected with choline PET scan in prostate cancer patients. Tumori 97(1): 49–55. [DOI] [PubMed] [Google Scholar]
- Cheng HH, Lin DW, Yu EY (2012) Advanced clinical states in prostate cancer. Urol Clin North Am 39: 561–571. [DOI] [PubMed] [Google Scholar]
- Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, van der Poel HG, van der Kwast TH, Rouvière O, Wiegel T, Mottet N (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 71(4): 630–642. [DOI] [PubMed] [Google Scholar]
- D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280(11): 969–974. [DOI] [PubMed] [Google Scholar]
- De Bari B, Alongi F, Lestrade L, Giammarile F (2014) Choline-PET in prostate cancer management: the point of view of the radiation oncologist. Crit Rev Oncol Hematol 91(4): 234–247. [DOI] [PubMed] [Google Scholar]
- Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, De Vos F, Huysse W, Hautekiet A, Maes G, Ost P (2014) Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 9: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, Smith MR (2008) Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92-02. Eur Urol 54(4): 816–823. [DOI] [PubMed] [Google Scholar]
- Harris WT, Mostaghel EA, Nelson PS, Montgomery B (2009) Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimized androgen depletion. Nat Clin Pract Urol 6(2): 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13(1): 8–10. [DOI] [PubMed] [Google Scholar]
- Hodolic M (2011) Role of (18)F-choline PET/CT in evaluation of patients with prostate carcinoma. Radiol Oncol 45(1): 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jereczek-Fossa BA, Beltramo G, Fariselli L, Fodor C, Santoro L, Vavassori A, Zerini D, Gherardi F, Ascione C, Bossi-Zanetti I, Mauro R, Bregantin A, Bianchi LC, De Cobelli O, Orecchia R (2012) Robotic image guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys 82(2): 889–897. [DOI] [PubMed] [Google Scholar]
- Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Matveev VB, Moldovan PC, van den Bergh RC, Van den Broeck T, van der Poel HG, van der Kwast TH, Rouvière O, Schoots IG, Wiegel T, Cornford P (2017) EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71(4): 618–629. [DOI] [PubMed] [Google Scholar]
- Muldermans JL, Romak LB, Kwon ED, Park SS, Olivier KR (2016) Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys 95: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, Henderson D, Casamassima F, Orecchia R, Surgo A, Brown L, Tree A, Miralbell R, De Meerleer G (2016. b) Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol 69(1): 9–12. [DOI] [PubMed] [Google Scholar]
- Ost P, Jereczek-Fossa BA, Van As N, Zilli T, Tree A, Henderson D, Orecchia R, Casamassima F, Surgo A, Miralbell R, De Meerleer G (2016. a) Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences. Clin Oncol (R Coll Radiol) 28(9): e115–e120. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, Carles J, Flaig TW, Taplin ME, Higano CS, de Souza P, de Bono JS, Griffin TW, De Porre P, Yu MK, Park YC, Li J, Kheoh T, Naini V, Molina A, Rathkopf DE COU-AA-302 Investigators (2015) Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16(2): 152–160. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA TAX 327 Investigators (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351(15): 1502–1512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.