Abstract
Background:
Pre-existing non-cancer conditions may complicate and delay colorectal cancer diagnosis.
Method:
Incident cases (aged ⩾40 years, 2007–2009) with colorectal cancer were identified in the Clinical Practice Research Datalink, UK. Diagnostic interval was defined as time from first symptomatic presentation of colorectal cancer to diagnosis. Comorbid conditions were classified as ‘competing demands’ (unrelated to colorectal cancer) or ‘alternative explanations’ (sharing symptoms with colorectal cancer). The association between diagnostic interval (log-transformed) and age, gender, consultation rate and number of comorbid conditions was investigated using linear regressions, reported using geometric means.
Results:
Out of the 4512 patients included, 72.9% had ⩾1 competing demand and 31.3% had ⩾1 alternative explanation. In the regression model, the numbers of both types of comorbid conditions were independently associated with longer diagnostic interval: a single competing demand delayed diagnosis by 10 days, and four or more by 32 days; and a single alternative explanation by 9 days. For individual conditions, the longest delay was observed for inflammatory bowel disease (26 days; 95% CI 14–39).
Conclusions:
The burden and nature of comorbidity is associated with delayed diagnosis in colorectal cancer, particularly in patients aged ⩾80 years. Effective clinical strategies are needed for shortening diagnostic interval in patients with comorbidity.
Keywords: diagnostic interval, delay, colorectal cancer, multimorbidity, comorbidity, inflammatory bowel disease
Cancer survival in England is lower than the European average, particularly for patients aged 65 years or older and in the first year after diagnosis (Coleman et al, 2011). This discrepancy is attributed in part to late diagnosis, which is generally thought to contribute to advanced stage at diagnosis, and thus to the poorer survival observed (Hamilton et al, 2016). Shortening the diagnostic interval (i.e., the time between presenting with a symptom of cancer and ultimate diagnosis; Weller et al, 2012) was made a specific government public policy priority in Improving Outcomes: A Strategy for Cancer (Government, 2011).
Diagnostic intervals decreased between 2001–2002 and 2007–2008 for colorectal and five other cancer sites, and longer diagnostic intervals were associated with increasing age, being female and presenting with symptoms that did not qualify for referral under national guidelines in place in 2005 (National Institute for Health and Clinical Excellence, 2005; Neal et al, 2014; Din et al, 2015). Consultation rates generally increase before cancer is diagnosed (Lyratzopoulos et al, 2012, 2013, 2015; Shephard et al, 2012, 2013, 2015a, 2015b, c; Stapley et al, 2012, 2013). These excess consultations may represent ‘missed’ opportunities for expediting the diagnosis. The 2010 National Cancer Patient Experience Survey reported that the probability of three or more pre-referral consultations was greater in young compared with old patients, in women vs men and in those from ethnic minority groups compared with whites (Lyratzopoulos et al, 2012), supporting this assertion. Alternatively, the excess consultations may simply reflect an increase in the medical complexity of patients with symptomatic cancer.
Risk factors for cancer—including increasing age, social deprivation and other lifestyle factors such as smoking and obesity (Parkin et al, 2011; National Cancer Intelligence Network, 2014; Office for National Statistics, 2016)—are all also associated with multimorbidity, that is, the existence of two or more concurrent diagnoses (Valderas et al, 2009, 2015; Violán et al, 2014). A cohort study of 99 997 adults in England using The Clinical Practice Research Datalink (http://www.cprd.com/intro.asp) (CPRD) reported that 58 115 (58%) patients had multimorbidity, and accounted for 78% of all consultations made between 1 April 2005 and 31 March 2008 (Salisbury et al, 2011). Pre-existing conditions could create competing demands for clinical care (Jaen et al, 1994; Nutting et al, 2001; Ricci-Cabello et al, 2015b), with a higher burden of care making recognition of symptoms and signs less likely. Symptoms identified as risk markers for cancer are also features of other, more common, diseases. These conditions provide reasonable alternative explanations for the early features of cancer, encouraging the patient or clinician to misattribute them to an existing condition rather than a new one, such as cancer (Lyratzopoulos et al, 2015). This problem may be more severe the higher the number of conditions present, and multimorbidity may therefore obscure the diagnosis of cancer, lengthening the diagnostic interval.
There is relatively little research exploring associations between multimorbidity and cancer diagnosis. A significant event audit was conducted in 92 general practices in the North of England Cancer Network for the diagnosis of lung cancer (Mitchell et al, 2013). Few patients had the headline symptom of haemoptysis, whereas pre-existing chest disease was common. This comorbidity often offered a plausible alternative explanation for the symptoms, such as infection, with no further diagnostic possibilities considered initially. This delayed investigation of some of those with cancer. A prospective cohort study of patients referred for suspicion of colorectal cancer in England investigated the symptoms, clinical factors and socio-demographic factors associated with time to diagnosis of colorectal cancer, other abdominal cancer or non-cancer diagnoses. The study suggested that patients and healthcare providers may normalise persistent rectal bleeding, and that the presence of gastrointestinal comorbidities delays diagnosis, probably through misattribution of symptoms to pre-existing conditions. The same study also found that patients with self-reported anxiety or depression experienced a longer time to diagnosis (cancer or otherwise), indicating altered perception of the seriousness of physical symptoms in the presence of a mental health condition (Walter et al, 2016). No study has investigated the impact of increasing burden from morbidity on diagnostic interval.
We explored the associations between diagnostic interval, demographics and number of conditions in a large cohort of patients with colorectal cancer, and hypothesised that greater morbidity would increase diagnostic interval. Furthermore, we investigated the relative effects of conditions that are unrelated to colorectal cancer, but could compete for clinical attention, and of conditions providing alternative explanations for key diagnostic features of cancer.
Materials and methods
Data set
The Clinical Practice Research Datalink (http://www.cprd.com/intro.asp) (CPRD) is the largest database of electronic, anonymised longitudinal medical records from primary care in the world (http://www.cprd.com/intro.asp, accessed 14 Feb 2017). At the time the data for this study were recorded, the database (then known as the General Practice Research Database, GPRD) contained over 5.4 million active patient records, drawn from over 670 primary care practices within the UK, including all consultations and diagnoses. Our patient inclusion criteria were:
A record of a primary diagnosis of incident colorectal cancer between 1st January 2007 and 31st December 2009. Cases were excluded where additional code(s) before the cancer code indicated palliative care or oncology treatment (including bowel surgery, chemotherapy or radiotherapy). In these instances, it was unclear when the diagnosis was made.
At least 1 full year of CPRD records preceding the cancer diagnosis, with at least one consultation in that time (patients who did not consult provided no opportunity for the GP to be involved in their cancer diagnosis).
Age at diagnosis was 40 years or older. Younger patients were excluded owing to the rarity of colorectal cancer diagnoses in this age group, in keeping with similar primary care studies (Hamilton, 2009; Neal et al, 2014; Din et al, 2015).
Symptom codes
Eleven features of colorectal cancer were selected based on previous studies in primary care (Hamilton et al, 2005, 2008, 2009; Hippisley-Cox and Coupland, 2012). Specific symptoms were rectal bleeding, diarrhoea, constipation, change in bowel habit, rectal mass, abdominal pain, and abdominal mass; nonspecific features were weight loss, appetite loss, fatigue and anaemia (determined from a blood test result).
Diagnostic interval
The date of diagnosis was taken as that of the first entry of a code for colorectal cancer (Hamilton, 2009; Neal et al, 2014). We defined a patient’s diagnostic interval as the length of time (in days) between the first presentation of a symptom coded in their medical record and their date of diagnosis (Weller et al, 2012). We restricted analyses to symptoms occurring in the year preceding the patient’s cancer diagnosis. It is possible for patients to experience symptoms more than a year before diagnosis (Corner et al, 2005). It is difficult, however, to establish whether these early symptoms are a result of the cancer, or of benign or incidental conditions, such as those also identified in the present study. In the CAPER studies, no symptom present more than a year before diagnosis was reliably more common in cases of colorectal cancer than in controls (Hamilton, 2009). Diagnostic intervals were therefore restricted to a maximum of 365 days to minimise the risk of misattributing a symptom as the index symptom of cancer. Patients with no identifiable symptom codes were excluded as a diagnostic interval could not be calculated for them, following the methodology of previous studies (Hamilton, 2009; Neal et al, 2014; Din et al, 2015).
Multimorbidity
To explore the effects of the two mechanisms by which multimorbidity is hypothesised to lengthen diagnostic interval, we collated two lists of conditions; designated ‘competing demands’ and ‘alternative explanations’. While both may result in diagnostic delay of cancer, their mechanisms of action would be essentially different. The first list would place additional demands on patient care, and would thereby limit the ability of GPs to focus on key symptoms, while the latter may falsely reassure GPs that key symptoms could be reasonably attributed to pre-existing conditions, rather than to a new one.
For the ‘competing demand’ set of conditions, we selected 12 important chronic conditions unrelated to colorectal cancer, 11 of which are components of the Quality and Outcomes Framework (QOF); the pay-for-performance scheme in the UK. QOF conditions are well defined, and recording is likely to be reliable and comprehensive, being linked to practice payments. The 11 conditions were coronary heart disease, heart failure, hypertension, asthma, chronic obstructive pulmonary disease (COPD), dementia, depression, chronic kidney disease (CKD), epilepsy, osteoporosis and rheumatoid arthritis. Anxiety was also included as a competing demand, as previous work has linked anxiety to increased diagnostic intervals (Robertson et al, 2004; Walter et al, 2016). These conditions are defined by Read Codes (Herrett et al, 2010) specified by the QOF business rules, which were obtained from the code-list repository ClinicalCodes.org (https://clinicalcodes.rss.mhs.man.ac.uk/), verified by a GP (JMV), and then applied to the CPRD data to identify patients with each chronic condition. Some Read Codes for anxiety or depression included the other condition (e.g., E200300 ‘anxiety with depression’), and initial modelling treating these conditions separately revealed multicollinearity between the two. We therefore combined anxiety and depression into one condition-group.
We identified conditions or therapies that may provide alternative explanations for each of the following specific features of colorectal cancer: abdominal pain, rectal bleeding, irregular bowel movement (diarrhoea and/or constipation) and anaemia. Two experienced GPs and a researcher with expertise in colorectal cancer sequentially considered for each key symptom/sign the following plausible alternative explanations: frequent conditions that are part of the differential diagnosis of those symptoms/signs in Primary Care, and secondary effects of medications frequently used in the primary care. A long list of candidate conditions was iteratively reviewed until the final set of comorbid conditions was agreed by consensus. The six conditions/therapies selected were endometriosis (abdominal pain), diverticulosis or diverticulitis (rectal bleeding, irregular bowel movement and abdominal pain), haemorrhoids (rectal bleeding), irritable bowel syndrome (IBS; irregular bowel movement and abdominal pain), warfarin therapy (anaemia) and codeine therapy (irregular bowel movement). Read Codes for each of these conditions were identified by a clinical member of the research team (JMV) using a code library provided by CPRD. These Read Codes were then used to identify patients with each condition. Patients’ therapy records were inspected to ascertain if they were prescribed warfarin or codeine.
We identified patients with inflammatory bowel disease (IBD), which is both an alternative explanation for symptoms (irregular bowel movement, abdominal pain and rectal bleeding) and a risk factor for colorectal cancer. Finally, we also identified patients with cancers other than colorectal, using code-lists and procedures described elsewhere (Neal et al, 2014).
Patients were only categorised as having a comorbidity if a code relating to the diagnosis was entered before their first cancer symptom. For each patient, we calculated their number of ‘competing demand’ conditions, their number of ‘alternative explanation’ conditions, and their total number of conditions. Both IBD and non-colorectal cancer were included in the count of total number of conditions.
Patient characteristics
We grouped patients into 5-year age bands, and combined groups that contained <10% of the sample to facilitate subgroup analyses. We also calculated each patient’s mean yearly consultation rate, averaging their number of consultations over the 1–3 years before their colorectal cancer diagnosis, as data availability permitted (each patient had at least 1 year of the data preceding diagnosis).
Data analysis
The relationships between key variables were explored graphically. The diagnostic interval had a highly skewed distribution, and therefore descriptive analyses report the median diagnostic interval and the associated interquartile range. For all regression analyses, diagnostic intervals were log transformed (0.1 was added to each patient’s diagnostic interval prior to the transformation in order to retain patients with values of 0 days), and consequently are reported as geometric means with 95% confidence intervals. Unadjusted univariate linear regressions investigated the separate effect of the predictor variables of age, gender, consultation rate, the three condition counts, presence of IBD and presence of non-colorectal cancer on diagnostic interval. A regression model then included predictors significant in the univariate analyses (P<0.10), unless there were indications of multicollinearity. A final model compared the effect on diagnostic interval of each condition separately, controlling for age and gender in order to identify any specific condition which may in itself pose a particular challenge in the diagnosis of cancer. All analyses were conducted in Stata\SE version 14.
Results
The CPRD supplied the records of 6287 patients with colorectal cancer. Out of these, 454 (7%) were excluded as they had a treatment code, a palliative care code or a code indicating metastatic spread before their first cancer code, casting doubt on the date of diagnosis. Of the remaining 5833 cases, a further 1321 (23%) patients with no recorded features of colorectal cancer in the year preceding diagnosis were excluded, as no diagnostic interval could be calculated. The remaining 4512 patients are summarised in Table 1, showing that the total number of comorbid conditions increased sharply with age and that mean yearly consultation rates were higher for participants with a greater number of conditions. 1127/4512 (25.0%) had at least one ‘competing demand’ condition in addition to at least one ‘alternative explanation’ condition.
Table 1. Sample demography and morbidity.
| n (%) | Median diagnostic interval (IQR) | Mean no. of conditions (s.d.) | Mean yearly consultation rate (s.d.) | |
|---|---|---|---|---|
| Full sample | 4512 (100.0) | 84 (39, 192) | 2.0 (1.6) | 7.7 (6.4) |
| Men | 2436 (54.0) | 79 (37, 176) | 1.8 (1.5) | 7.5 (6.0) |
| Women | 2076 (46.0) | 89 (40, 210) | 2.1 (1.7) | 8.1 (6.7) |
| Age group (years) | ||||
| 40–59 | 619 (13.7) | 71 (35, 147) | 1.1 (1.2) | 5.3 (4.8) |
| 60–64 | 510 (11.3) | 65 (29, 130) | 1.5 (1.3) | 6.5 (6.1) |
| 65–69 | 562 (12.5) | 75.5 (38, 148) | 1.8 (1.5) | 7.3 (5.6) |
| 70–74 | 683 (15.1) | 78 (38, 178) | 2.0 (1.6) | 7.8 (6.1) |
| 75–79 | 821 (18.2) | 80 (38, 194) | 2.2 (1.6) | 8.5 (6.2) |
| 80–84 | 685 (15.2) | 109 (50, 234) | 2.5 (1.8) | 9.2 (7.2) |
| 85+ | 632 (14.0) | 123.5 (50, 263) | 2.5 (1.7) | 8.8 (7.3) |
| Number of 'competing demand' conditions | ||||
| None | 1221 (27.1) | 64 (32, 143) | — | 4.6 (4.0) |
| One | 1378 (30.5) | 80.5 (39, 176) | — | 7.5 (5.4) |
| Two | 990 (21.9) | 89.5 (42, 206) | — | 8.8 (6.0) |
| Three | 564 (12.5) | 113 (49, 241.5) | — | 10.3 (7.3) |
| Four or more | 359 (8.0) | 136 (59, 258) | — | 12.7 (9.2) |
| Number of 'alternative explanation' conditions | ||||
| None | 3098 (68.7) | 77 (37, 175) | — | 7.0 (5.9) |
| One | 1125 (24.9) | 101 (42, 217) | — | 8.7 (6.5) |
| Two or more | 289 (6.4) | 107 (50, 245) | — | 11.9 (8.3) |
Abbreviation: IQR=interquartile range.
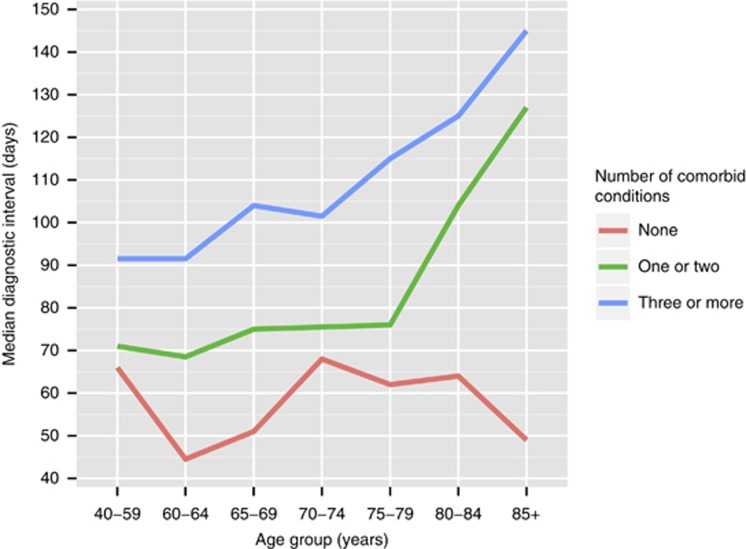
Figure 1 shows that diagnostic interval increased non-linearly with greater morbidity, with no clear trend up to 75–79 years of age, but with a sizeable increase in diagnostic interval thereafter. This graph appears to show a clear interaction effect between age and morbidity, such that the increase in diagnostic interval after 80 years of age only occurs in the groups reporting at least one comorbid condition, with no clear trend between age and diagnostic interval for those without comorbidities.
Figure 1.
Median diagnostic intervals across age groups by total number of conditions.
All variables explored as potential predictors in the unadjusted univariate analyses were significantly associated with diagnostic interval (Supplementary Table 1). There was strong multicollinearity between the count of total number of conditions and the number of ‘competing demand’ conditions (as expected): only the counts of ‘competing demand’ and ‘alternative explanation’ conditions entered the main model. The strong relationship between consultation rate and morbidity (Table 1) gave rise to multicollinearity with the count of ‘competing demand’ conditions, so consultation rate was excluded.
With the remaining predictors included (Table 2), gender was no longer significantly associated with diagnostic interval. A single ‘competing demand’ condition delayed diagnosis by 10 days, and a single ‘alternative explanation condition’ delayed diagnosis by 9 days. Patients with four or more ‘competing demand’ conditions had intervals over one month longer than those without comorbid conditions. Inflammatory bowel disease, which is both an alternative explanation of symptoms and a risk factor for colorectal cancer (Lukas, 2010; Kim and Chang, 2014), increased the diagnostic interval by 26 days.
Table 2. Regression model estimating associations between diagnostic interval and number of ‘competing demand’ and/or ‘alternative explanation’ conditions.
| Coeff. (95% CI) | P-value | Exponentiated coeff. (95% CI) | Diagnostic interval change in days (95% CI)a | |
|---|---|---|---|---|
| Female gender | 0.02 (−0.05, 0.09) | 0.544 | 1.02 (0.95, 1.09) | 2 (−3, 7) |
| Age group (years)b | 0.07 (0.05, 0.09) | <0.001 | ||
| 40–59 (reference) | 0.00 | — | 1.00 | |
| 60–64 | −0.13 (−0.27, 0.02) | 0.083 | 0.88 (0.76, 1.02) | −9 (−18, 1) |
| 65–69 | 0.05 (−0.09, 0.18) | 0.505 | 1.05 (0.91, 1.20) | 4 (−7, 15) |
| 70–74 | 0.06 (−0.08, 0.19) | 0.395 | 1.06 (0.93, 1.21) | 5 (−6, 16) |
| 75–79 | 0.10 (−0.03, 0.23) | 0.132 | 1.11 (0.97, 1.26) | 8 (−2, 20) |
| 80–84 | 0.32 (0.19, 0.45) | <0.001 | 1.38 (1.21, 1.57) | 29 (16, 44) |
| 85+ | 0.34 (0.21, 0.48) | <0.001 | 1.41 (1.23, 1.62) | 31 (17, 47) |
| No. of 'competing demand' conditionsb | 0.09 (0.06, 0.12) | <0.001 | ||
| None (reference) | 0.00 | — | 1.00 | |
| One | 0.12 (0.03, 0.21) | 0.009 | 1.13 (1.03, 1.24) | 10 (2, 18) |
| Two | 0.20 (0.10, 0.30) | <0.001 | 1.22 (1.11, 1.34) | 17 (8, 26) |
| Three | 0.30 (0.18, 0.41) | <0.001 | 1.34 (1.20, 1.51) | 26 (15, 39) |
| Four or more | 0.35 (0.20, 0.49) | <0.001 | 1.41 (1.22, 1.63) | 32 (17, 49) |
| No. of 'alternative explanation' conditionsb | 0.09 (0.03, 0.14) | 0.003 | ||
| None (reference) | 0.00 | — | 1.00 | |
| One | 0.12 (0.04, 0.19) | 0.003 | 1.12 (1.04, 1.21) | 9 (3, 16) |
| Two or more | 0.13 (−0.01, 0.27) | 0.061 | 1.14 (0.99, 1.32) | 11 (0, 24) |
| Inflammatory bowel disease | 0.29 (0.17, 0.41) | <0.001 | 1.34 (1.18, 1.51) | 26 (14, 39) |
| Non-colorectal cancer | 0.11 (−0.05, 0.28) | 0.173 | 1.12 (0.95, 1.32) | 9 (−4, 24) |
Abbreviation: CI=confidence interval.
Calculated using the geometric mean (as used by the log-tranformed regression model) of 76.71.
Sensitivity analyses entered these covariates as ordinal variables to assess their global effects, which are reported above the effects of their separate levels.
A further model (Table 3) tested the interaction on diagnostic interval between age and morbidity (Figure 1). This model supports there being no clear trend in diagnostic interval across age for patients with no comorbidities, in contrast to increasing diagnostic intervals across age, especially for those aged ⩾80 years, for those with at least one comorbidity. As a sensitivity analysis, we stratified our key findings by two age categories (40–74 years, 75+ years). No notable differences were found between these models and that presented in Table 2.
Table 3. Interaction between age group and presence of comorbidity on diagnostic interval.
| Coeff. (95% CI) | P-value | Exponentiated coeff. (95% CI) | Diagnostic interval change in days (95% CI)a | |
|---|---|---|---|---|
|
Age group by comorbidity | ||||
| 40–59, no comorbidity (reference) | 0.00 | — | 1.00 | — |
| 40–59, ⩾1 comorbidity | 0.04 (−0.15, 0.23) | 0.655 | 1.04 (0.86, 1.26) | 3 (−11, 20) |
| 60–79, no comorbidity | −0.17 (−0.34, 0.00) | 0.053 | 0.84 (0.71, 1.00) | −12 (−22, 0) |
| 60–79, ⩾1 comorbidity | 0.20 (0.06, 0.35) | 0.006 | 1.22 (1.06, 1.41) | 17 (5, 32) |
| 80+, no comorbidity | 0.08 (−0.14, 0.30) | 0.466 | 1.08 (0.87, 1.35) | 6 (−10, 27) |
| 80+, ⩾1 comorbidity | 0.53 (0.39, 0.68) | 0.000 | 1.71 (1.47, 1.98) | 54 (36, 75) |
Abbreviation: CI=confidence interval.
The model controlled for gender. 40–59 year olds with no comorbidities were the reference group.
Calculated using the geometric mean (as used by the log-tranformed regression model) of 76.71.
Out of the 20 studied conditions and therapies, four were significantly associated with longer diagnostic intervals (Table 4). These were inflammatory bowel disease, coronary heart disease, diverticulosis or diverticulitis and anxiety/depression.
Table 4. Regression model estimating associations between diagnostic interval and individual conditions.
| Coeff. (95% CI) | P-value | Exponentiated coeff. (95% CI) | Diagnostic interval change in days (95% CI)a | |
|---|---|---|---|---|
|
'Competing demand' conditions | ||||
| Anxiety/depression | 0.11 (0.03, 0.20) | 0.007 | 1.12 (1.03, 1.22) | 9 (3, 17) |
| Asthma | 0.06 (−0.05, 0.16) | 0.284 | 1.06 (0.95, 1.18) | 5 (−4, 13) |
| Chronic kidney disease | 0.04 (−0.06, 0.13) | 0.450 | 1.04 (0.94, 1.14) | 3 (−4, 11) |
| COPD | 0.12 (−0.01, 0.25) | 0.075 | 1.13 (0.99, 1.28) | 10 (−1, 22) |
| Coronary heart disease | 0.18 (0.09, 0.27) | <0.001 | 1.20 (1.09, 1.31) | 15 (7, 24) |
| Dementia | −0.05 (−0.34, 0.24) | 0.722 | 0.95 (0.71, 1.27) | −4 (−22, 20) |
| Diabetes mellitus | 0.16 (−0.07, 0.39) | 0.164 | 1.18 (0.94, 1.48) | 14 (−5, 37) |
| Epilepsy | 0.04 (−0.07, 0.14) | 0.510 | 1.04 (0.93, 1.15) | 3 (−5, 12) |
| Heart failure | 0.11 (−0.03, 0.26) | 0.132 | 1.12 (0.97, 1.29) | 9 (−3, 23) |
| Hypertension | 0.04 (−0.03, 0.11) | 0.254 | 1.04 (0.97, 1.11) | 3 (−2, 9) |
| Osteoporosis | 0.00 (−0.16, 0.17) | 0.986 | 1.00 (0.85, 1.18) | 0 (−12, 14) |
| Rheumatoid arthritis | 0.23 (−0.02, 0.48) | 0.069 | 1.26 (0.98, 1.62) | 20 (−1, 48) |
|
'Aternative explanation' conditions | ||||
| Codeine therapy | 0.06 (−0.09, 0.22) | 0.423 | 1.07 (0.91, 1.24) | 5 (−7, 19) |
| Diverticulosis/diverticulitis | 0.17 (0.03, 0.30) | 0.015 | 1.18 (1.03, 1.35) | 14 (3, 27) |
| Endometriosis | −0.31 (−0.9, 0.28) | 0.299 | 0.73 (0.41, 1.32) | −21 (−46, 24) |
| Haemorrhoids | 0.02 (−0.07, 0.12) | 0.632 | 1.02 (0.93, 1.13) | 2 (−5, 10) |
| Irritable bowel syndrome | 0.11 (−0.02, 0.25) | 0.103 | 1.12 (0.98, 1.29) | 9 (−2, 22) |
| Warfarin therapy | 0.06 (−0.09, 0.22) | 0.409 | 1.07 (0.92, 1.24) | 5 (−6, 19) |
| Inflammatory bowel disease | 0.29 (0.16, 0.41) | <0.001 | 1.33 (1.18, 1.51) | 26 (14, 39) |
| Non-colorectal cancer | 0.11 (−0.05, 0.28) | 0.178 | 1.12 (0.95, 1.32) | 9 (−4, 25) |
Abbreviations: CI=confidence interval; COPD=chronic obstructive pulmonary disease.
Calculated using the geometric mean (as used by the log-tranformed regression model) of 76.71. This model controlled for age and gender. Conditions in bold were significantly associated with diagnostic interval.
Discussion
The current study is one of the first to investigate the specific impact of the burden and nature of comorbid conditions on time to diagnosis of colorectal cancer. There was a clear association between increasing comorbidity and longer time to diagnosis in colorectal cancer, with the increase ranging from 9 to 32 days, and seen particularly in those aged 80 years or greater. This finding was observed for both genders, and little difference in effects was seen between conditions we considered to be unrelated to a colorectal cancer diagnosis (the ‘competing demand’ conditions) and those giving a plausible diagnostic alternative to colorectal cancer.
The observed increases in time to diagnosis reported in the present study are clinically significant. They correspond to an increase of 13% for people with a single ‘competing demand’ condition, and of 12% for people with a single ‘alternative explanation’ condition. For those with four or more ‘competing demand’ conditions, the increase amounts to 41%, and for the single condition of IBD the increase is 34%. In addition, it is important to note that these effects are independent and that a quarter of patients had both types of conditions.
Strengths and limitations
The main strength of this study is its primary care setting, which is the commonest starting point for cancer diagnosis. Features of possible cancer were collected before the diagnosis was established, eliminating recall bias. The study was large and generalisable, as CPRD data is representative of patients across the UK. A further key strength of this study is the modelling of both the burden and the nature of comorbidity. Our categorisation of comorbidities into ‘competing demand’ conditions and alternative diagnoses was based on clinical plausibility. In oo maximise reliability of coding, all the comorbid conditions in the category were part of the AQOF. These illnesses would generally impose a greater burden of care than conditions not included in the incentive scheme. Other clinicians may have generated different conditions and categories. We acknowledge that this is a novel approach to the classification of comorbidity and as such we cannot rely on or compare our methods with a gold standard methodology for the identification of alternative explanations. Further research in the area would be needed. Also, it may be too simplistic to assume from the very similar increases in diagnostic interval for the two main groups that all comorbidities have the same effect on diagnostic interval: it remains possible they act through different mechanisms, but with similar sized effects.
The main disadvantage of using electronic records as our data source is that we were reliant upon the quality of the doctors’ recording for symptoms. Cancer recording is very good in the CPRD (Dregan et al, 2012; Boggon et al, 2013). Linkage to the cancer registry was not available, and would have allowed us to check the date of diagnosis, though again discrepancies between the CPRD and cancer registry on this point are minor (Tate et al, 2009). Patient records have been widely used in similar cancer studies before, including the CPRD data. Symptoms may be unvoiced by the patient, not recorded by the GP, or documented solely in the text, such that the records are inaccessible to researchers: the latter appears to happen less where a symptom is known to be strongly associated with cancer (Price et al, 2016). Laboratory data and prescribing data are of very high quality, so the study is unlikely to have overlooked patients with anaemia, or those who have been prescribed warfarin or codeine. We only studied symptoms previously reported to be associated with colorectal cancer, including most of those in contemporaneous and current NICE guidances (NICE, 2005, 2015). Nearly a quarter of eligible patients had no apparent feature of colorectal cancer in the year before diagnosis: although some of this will represent under-recording, though some will reflect the quarter of patients who present as an emergency, often bypassing primary care (McPhail et al, 2013).
Comparison with previous studies
Our findings are supported by a prospective cohort study of patients referred for suspicion of colorectal cancer in two regions in England. The study reported that anxiety, depression and gastrointestinal comorbidities were associated with longer times to diagnosis of colorectal cancer, other abdominal cancers or non-cancer conditions (Walter et al, 2016). A pre-existing diagnosis of dementia is reported to be associated with under-diagnosis or post-mortem diagnosis of colon cancer, but the diagnostic interval was not reported (Gupta and Lamont, 2004). A small qualitative study reported weak evidence that early presentation with a possible symptom of colorectal cancer may, perversely, have contributed to diagnostic delay because the symptom was normalised by the patient and/or the GP. This may be because a symptom was falsely attributed to a benign condition, for example rectal bleeding initially attributed to existing haemorrhoids, or the patient was investigated but there were negative findings on colonoscopy (Bain et al, 2002). A cohort study in Scotland reported that the odds of delayed diagnosis were doubled by a past history of depression or anxiety. The authors ascribed this to the misattribution of nonspecific abdominal symptoms to benign gastrointestinal conditions, which are relatively common in patients with depression or anxiety (Robertson et al, 2004).
The relationship between diagnostic interval and age is inconsistent athecross cancers, though overall diagnostic intervals increase with age by an estimated 7.8 days (95% CI 6.4–9.1) per decade of age (Din et al, 2015). Survival from cancer worsens with age (Quaresma et al, 2015). The increase in multimorbidity with age has been well-characterised (Salisbury et al, 2011; Barnett et al, 2012; Violán et al, 2014). We hypothesised that some of the worse survival in older age groups could relate to diagnostic delay caused in turn by comorbidity, and our results support this, with the increase in diagnostic intervals considerably greater than the 5.4 days (95% CI 2.4–8.5) decrease seen in the first 5 years of 2-week clinics for urgent investigation of suspected cancer (Neal et al, 2014).
Implications for practice and research
The crucial point is whether these modest, but real, delays in diagnosis matter in terms of reducing survival (or delaying treatment of symptoms). For some patients, the delay will be immaterial; for others, tumour progression or a complication may occur. It is clear from analysis of several international cohorts (Torring et al, 2011; Torring et al, 2012) that survival worsens with diagnostic delay. The co morbid patients in this study are already disadvantaged by their comorbidity; additional disadvantage—even if modest—is unhelpful.
The impact of the burden and nature comorbidity on cancer diagnosis has received little attention so far. Our observations merit replication using alternative data sources, using expanded lists of comorbid conditions and exploring the potential impact on different cancer sites. These studies should include theoretically sound models that account for the possible different mechanisms that may operate simultaneously. They include the competing demands placed by both chronic and acute conditions, the presence of known risk factors, or the potential misattribution of signs and symptoms to existing conditions, among others (Valderas, 2015; Ricci-Cabello et al, 2015a). Pending replication of our observations, effective interventions for minimising diagnostic delays that focus on the burden and nature of comorbid conditions would be needed, especially in patients aged over 80 years. This may necessitate creation of evidence-based guidelines for the review of patients with a possible cancer symptom who are not offered investigation (the so-called ‘safety-netting’), which incorporate specific recommendations for comorbid patients.
Conclusions
This is one of the first studies to investigate the impact of the burden and nature of existing comorbid conditions on time to diagnosis of cancer. An increased time to diagnosis in colorectal cancer, ranging from 9 to 32 days, was associated with conditions that give a plausible diagnostic alternative, or that are unrelated to colorectal cancer, yet place competing demands at the time of diagnosis. Effective clinical strategies are needed for shortening the diagnostic interval in the presence of comorbidity, which should be particularly targeted at patients aged 80 years or older.
Acknowledgments
FUNDING: There was no direct funding for this work. JMV was supported by a National Institute of Health Research (NIHR) Clinician Scientist Award for the study of the management of patients with multimorbidity in primary care. The study was approved by the CPRD’s Independent Scientific Advisory Committee (reference number 09_0111). Read code lists for the conditions studied are available from the authors on request.
Author contributions
LM, SP, JMV and WH designed the study. SP and LM prepared the data set, and LM conducted the analyses. All authors interpreted the results, and were involved in drafting and revising the manuscript. JMV will act as guarantor.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Bain NS, Campbell NC, Ritchie LD, Cassidy J (2002) Striking the right balance in colorectal cancer care—a qualitative study of rural and urban patients. Fam Pract 19(4): 369–374. [DOI] [PubMed] [Google Scholar]
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B (2012) Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380(9836): 37–43. [DOI] [PubMed] [Google Scholar]
- Boggon R, van Staa TP, Chapman M, Gallagher AM, Hammad TA, Richards MA (2013) Cancer recording and mortality in the General Practice Research Database and linked cancer registries. Pharmacoepidemiol Drug Saf 22(2): 168–175. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, McGahan CE, Turner D, Marrett L, Gjerstorff ML, Johannesen TB, Adolfsson J, Lambe M, Lawrence G, Meechan D, Morris EJ, Middleton R, Steward J, Richards MA (2011) Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 377(9760): 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner J, Hopkinson J, Fitzsimmons D, Barclay S, Muers M (2005) Is late diagnosis of lung cancer inevitable? Interview study of patients' recollections of symptoms before diagnosis. Thorax 60(4): 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din NU, Ukoumunne OC, Rubin G, Hamilton W, Carter B, Stapley S, Neal RD (2015) Age and gender variations in cancer diagnostic intervals in 15 cancers: analysis of data from the UK Clinical Practice Research Datalink. PloS One 10(5): e0127717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dregan A, Moller H, Murray-Thomas T, Gulliford MC (2012) Validity of cancer diagnosis in a primary care database compared with linked cancer registrations in England. Population-based cohort study. Cancer Epidemiol 36(5): 425–429. [DOI] [PubMed] [Google Scholar]
- Government HM (2011) Improving Outcomes: A Strategy for Cancer. The Department of Health: London, UK. [Google Scholar]
- Gupta SK, Lamont EB (2004) Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc 52(10): 1681–1687. [DOI] [PubMed] [Google Scholar]
- Hamilton W (2009) The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer 101(Suppl 2): S80–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng KK, Marshall T (2008) The importance of anaemia in diagnosing colorectal cancer: a case-control study using electronic primary care records. Br J Cancer 98(2): 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Lancashire R, Sharp D, Peters TJ, Cheng K, Marshall T (2009) The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Round A, Sharp D, Peters TJ (2005) Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer 93(4): 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Walter FM, Rubin G, Neal RD (2016) Improving early diagnosis of symptomatic cancer. Nat Rev Clin Oncol 13(12): 740–479. [DOI] [PubMed] [Google Scholar]
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ (2010) Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 69(1): 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C (2012) Identifying patients with suspected colorectal cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 62(594): e29–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaen CR, Stange KC, Nutting PA (1994) Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract 38(2): 166–171. [PubMed] [Google Scholar]
- Kim ER, Chang DK (2014) Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol 20(29): 9872–9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M (2010) Inflammatory bowel disease as a risk factor for colorectal cancer. Dig Dis 28(4-5): 619–624. [DOI] [PubMed] [Google Scholar]
- Lyratzopoulos G, Abel GA, McPhail S, Neal RD, Rubin GP (2013) Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer 108(3): 686–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP, Abel GA (2012) Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 13(4): 353–365. [DOI] [PubMed] [Google Scholar]
- Lyratzopoulos G, Vedsted P, Singh H (2015) Understanding missed opportunities for more timely diagnosis of cancer in symptomatic patients after presentation. Br J Cancer 112(Suppl 1): S84–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail S, Elliss-Brookes L, Shelton J, Ives A, Greenslade M, Vernon S, Morris EJA, Richards M (2013) Emergency presentation of cancer and short-term mortality. Br J Cancer 109(8): 2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell ED, Rubin G, Macleod U (2013) Understanding diagnosis of lung cancer in primary care: qualitative synthesis of significant event audit reports. Br J Gen Pract 63(606): e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Intelligence Network (2014) Cancer by Deprivation in England. Incidence, 1996-2010. Mortality 1997–2011. Public Health England: London, UK. [Google Scholar]
- National Institute for Health and Clinical Excellence (2005) Referral Guidelines for Suspected Cancer. NICE: London, UK. [Google Scholar]
- Neal RD, Din NU, Hamilton W, Ukoumunne OC, Carter B, Stapley S, Rubin G (2014) Comparison of cancer diagnostic intervals before and after implementation of NICE guidelines: analysis of data from the UK General Practice Research Database. Br J Cancer 110(3): 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE (2005) Referral Guidelines for Suspected Cancer. NICE: London, UK. [Google Scholar]
- NICE (2015) Suspected Cancer: Recognition and Referral [NG12]. NICE. [PubMed] [Google Scholar]
- Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L (2001) Competing demands in the office visit: what influences mammography recommendations? J Am Board Fam Pract 14(5): 352–361. [PubMed] [Google Scholar]
- Office for National Statistics (2016) Cancer Registration Statistics. England 2014. Office for National Statistics: Newport. [Google Scholar]
- Parkin DM, Boyd L, Walker LC (2011) 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 105(Suppl): S77–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SJ, Stapley SA, Shephard E, Barraclough K, Hamilton WT (2016) Is omission of free text records a possible source of data loss and bias in Clinical Practice Research Datalink studies? A case-control study. BMJ Open 6(5): e011664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaresma M, Coleman MP, Rachet B (2015) 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: a population-based study. Lancet 385(9974): 1206–1218. [DOI] [PubMed] [Google Scholar]
- Ricci-Cabello I, Stevens S, Kontopantelis E, Dalton ARH, Griffiths RI, Campbell JL, Doran T, Valderas JM (2015. a) Impact of the prevalence of concordant and discordant conditions on the quality of diabetes care in family practices in England. Ann Fam Med 13(6): 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Cabello I, Violán C, Foguet-Boreu Q, Mounce LTA, Valderas JM (2015. b) Impact of multi-morbidity on quality of healthcare and its implications for health policy, research and clinical practice. A scoping review. Eur J Gen Pract 21(3): 192–202. [DOI] [PubMed] [Google Scholar]
- Robertson R, Campbell NC, Smith S, Donnan PT, Sullivan F, Duffy R, Ritchie LD, Millar D, Cassidy J, Munro A (2004) Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer 90(8): 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA (2011) Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 61(582): e12–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E, Neal R, Rose P, Walter F, Hamilton WT (2013) Clinical features of kidney cancer in primary care: a case-control study using primary care records. Br J Gen Pract 63(609): e250–e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E, Stapley S, Neal RD, Rose P, Walter FM, Hamilton W (2012) Clinical features of bladder cancer in primary care. Br J Gen Pract 62: e598–e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard EA, Neal RD, Rose P, Walter FM, Litt EJ, Hamilton WT (2015. a) Quantifying the risk of multiple myeloma from symptoms reported in primary care patients: a large case-control study using electronic records. Br J Gen Pract 65(631): e106–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard EA, Neal RD, Rose PW, Walter FM, Hamilton WT (2015. b) Quantifying the risk of Hodgkin lymphoma in symptomatic primary care patients aged >/=40 years: a case-control study using electronic records. Br J Gen Pract 65(634): e289–e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard EA, Neal RD, Rose PW, Walter FM, Hamilton WT (2015. c) Quantifying the risk of non-Hodgkin lymphoma in symptomatic primary care patients aged >/=40 years: a large case-control study using electronic records. Br J Gen Pract 65(634): e281–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W (2012) The risk of pancreatic cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer 106(12): 1940–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W (2013) The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer 108(1): 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate AR, Martin AG, Murray-Thomas T, Anderson SR, Cassell JA (2009) Determining the date of diagnosis—is it a simple matter? The impact of different approaches to dating diagnosis on estimates of delayed care for ovarian cancer in UK primary care. BMC Med Res Methodol 9: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torring ML, Frydenberg M, Hamilton W, Hansen RP, Lautrup MD, Vedsted P (2012) Diagnostic interval and mortality in colorectal cancer: U-shaped association demonstrated for three different datasets. J Clin Epidemiol 65(6): 669–678. [DOI] [PubMed] [Google Scholar]
- Torring ML, Frydenberg M, Hansen RP, Olesen F, Hamilton W, Vedsted P (2011) Time to diagnosis and mortality in colorectal cancer: a cohort study in primary care. Br J Cancer 104(6): 934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderas JM (2015) Multimorbidity, not a health condition or complexity by another name. Eur J Gen Pract 21(4): 213–214. [DOI] [PubMed] [Google Scholar]
- Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M (2009) Defining comorbidity: implications for understanding health and health services. Ann Fam Med 7(4): 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violán C, Foguet-Boreu Q, Flores-Mateo G, Salisbury C, Blom J, Freitag M, Glynn L, Muth C, Valderas JM (2014) Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PloS One 9(7): e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter FM, Emery JD, Mendonca S, Hall N, Morris HC, Mills K, Dobson C, Bankhead C, Johnson M, Abel GA, Rutter MD, Hamilton W, Rubin GP (2016) Symptoms and patient factors associated with longer time to diagnosis for colorectal cancer: results from a prospective cohort study. Br J Cancer 115(5): 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, Campbell C, Andersen RS, Hamilton W, Olesen F, Rose P, Nafees S, van Rijswijk E, Hiom S, Muth C, Beyer M, Neal RD (2012) The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 106(7): 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.