Abstract
Background:
There is limited data on the efficacy of anti-programmed death 1 (PD-1) antibodies in patients (pts) with melanoma brain metastasis (BM), particularly those which are symptomatic.
Method:
We retrospectively assessed pts with melanoma BM treated with PD-1 antibodies, nivolumab and pembrolizumab. Clinicopathologic and treatment parameters were collected and outcomes determined for intracranial (IC) response rate (RR) using a modified RECIST criteria, with up to five IC target lesions used to determine IC response, disease control rate (DCR) and progression-free survival (PFS).
Results:
A total of 66 pts were identified with a median follow up of 7.0 months (range 0.8–24.5 months). A total of 68% were male and 45% BRAF V600 mutation positive. At PD-1 antibody commencement, 50% had an elevated LDH; 64% had local therapy to BM prior to commencing anti-PD1, of which 5% had surgical resection, 14% stereotactic radiosurgery (SRS), 18% whole-brain radiotherapy (WBRT), 27% had surgery and radiotherapy. Twenty-one per cent started anti-PD-1 as first line systemic therapy. No pt had prior anti-PD-1 treatment. The IC overall RR was 21 and DCR 56%. Responses occurred in 21% of pts with symptomatic BM. The median OS was 9.9 months (95% CI 6.93–17.74). Pts with symptomatic BM had shorter PFS than those without symptoms (2.7 vs 7.4 months, P=0.035) and numerically shorter OS (5.7 vs 13.0 months, P=0.068). Pts requiring corticosteroids also had a numerically shorter PFS (3.2 vs 7.4 months, P=0.081) and OS (4.8 vs 13.1 months, P=0.039).
Conclusions:
IC responses to anti-PD-1 antibodies occur in pts with BM, including those with symptomatic BM requiring corticosteroids. Prospective trials evaluating anti-PD-1 therapy in pts with BM are underway.
Keywords: metastatic melanoma, brain metastases, anti-PD1 therapy, corticosteroids, pembrolizumab, nivolumab
In total, 40–50% of patients with stage IV melanoma develop brain metastases during the course of their disease (Carlino et al, 2012); and the median overall survival (OS) from diagnosis of brain metastases is approximately 5 months (Raizer et al, 2008). Patients treated with surgery and/or stereotactic or whole-brain radiotherapy (WBRT) may have better outcomes with median OS rates ranging from 8.7 to 10.4 months (Fife et al, 2004) although this may be partially due to a selection bias. Melanoma has long been considered radio-resistant (Dossgg and Memula, 1982); despite this radiotherapy, particularly radiosurgery, remains an important contributor to the treatment of melanoma brain metastasis in the modern era (Choong et al, 2017). Targeted therapy using BRAF/MEK inhibitors and immunotherapy with checkpoint blockade have dramatically changed the therapeutic landscape and the prognosis for patients with metastatic melanoma (Menzies and Long, 2014); but the therapeutic benefit of immune checkpoint inhibitors in patients with brain metastases remains unclear. BRAF inhibitors have demonstrated intracranial (IC) efficacy in patients with brain metastases (Long et al, 2012; McArthur et al, 2017). There is also evidence that the anti-CTLA-4 antibody ipilimumab, can lead to anti-tumour responses in this patient population, although efficacy is limited in those with symptomatic brain metastasis requiring corticosteroids (Margolin et al, 2012; Queirolo et al, 2014). While historically radiotherapy or palliation has been the predominant treatment modality for patients with multiple symptomatic brain metastasis the development of active systemic agents has led to an increasing interest in systemic therapy as the initial treatment for patients with brain metastasis (Carlino et al, 2012).
More recently, immunotherapy with anti-programmed death-1 (PD-1) antibodies has shown impressive and often durable extra-cranial responses in patients with metastatic melanoma (Larkin et al, 2015; Robert et al, 2015a, 2015b). There is, however, only limited evidence of efficacy in melanoma patients with brain metastases treated with these drugs, as these patients were excluded from initial clinical trials.
In this retrospective study we report the clinical efficacy of anti-PD-1 antibodies in a large cohort of melanoma patients with brain metastases. Our study includes patients with symptomatic brain metastases requiring corticosteroids as well as patients with additional poor prognostic factors in whom the benefit of this treatment is unknown.
Materials and methods
We retrospectively assessed the efficacy of the anti-PD-1 antibodies, pembrolizumab and nivolumab, in consecutively treated patients with metastatic melanoma and brain metastases treated between October 2012 and March 2016 across five major melanoma centres in Australia. Patients with measurable brain metastases (measuring ⩾5 mm) who received ⩾1 dose of anti-PD-1 therapy were included in the final analysis. Patients with leptomeningeal disease and those who received prior anti-PD-1 therapy were excluded. Data collected included: baseline demographics, mutational status (BRAF/NRAS) and prognostic variables (American Joint Committee on Cancer (AJCC; 7th edition) stage of disease, serum lactate dehydrogenase (LDH), Eastern Cooperative Oncology Group performance status (ECOG), corticosteroid use); baseline number of IC lesions, sum of dimensions (SoD) and number of extracranial (EC) sites; details of PD-1 inhibitor treatment (type, dosage, number of cycles received); prior systemic treatments and local therapy to BMs; and time to end-point data. End points evaluated were IC response rate (RR), disease control rate (DCR) of IC lesions, IC progression-free survival (PFS) and OS. Consistent with prior studies, including prospective trials examining the efficacy of systemic therapy in melanoma patients with brain metastasis, IC response using CT and/or MRI was determined by a modified Response Evaluation Criteria In Solid Tumors (mRECIST) criteria (Long et al, 2012). Up to five IC target lesions were included, imaging was performed at 6–8 week intervals, with tumour measurements and reporting of scans carried out by radiologists. Target lesions were considered as any brain lesion measuring ⩾5 mm, including previously treated with radiotherapy (SRS and/or WBRT). A IC complete response (CR) was defined as a disappearance of all IC lesions, a IC partial response as a 30% decrease in the sum of the longest diameter (SoD) of all target lesions without the development of any new lesions. When possible IC responses were confirmed by repeating scans at 6–8 week intervals. IC progressive disease (PD) was defined as either an increase of 20% in SoD of target brain lesion that remained consistent or continued to increase on subsequent imaging, development of new brain lesions, or unequivocal increase in size of non-target brain lesions or clinical progression with central nervous system (CNS) symptoms. SD was defined as none of CR, PR or PD. Pseudoprogression or radiation treatment changes were defined as increased contrast enhancement and oedema which resolved or stabilised within 3 months on serial imaging. In cases where the distinction between progressive IC disease and radiation-related changes/pseudoprogression was not clear a neuro-radiologist at participating sites was consulted. PFS was defined as time between date of commencement of therapy to date of progression or death. EC response assessments were made as per the RECIST v1.1. Kaplan–Meier estimates of PFS and OS from commencement of anti-PD1 therapy were calculated separately for patients grouped by CNS symptoms and corticosteroid use and compared using a log-rank test, where P⩽0.05 was considered to be statistically significant. Hazard ratio and its 95% confidence interval were estimated through a univariate Cox proportional hazard model for respectively CNS symptoms and corticosteroid use. The study was approved by individual institution ethics committees and patients either prospectively consented to inclusion or consent was waived as per individual institution ethics committee guidance.
Results
Patient demographics and anti-PD-1 therapy
A total of 66 patients with BM who received either pembrolizumab or nivolumab between October 2012 and March 2016 were identified. Baseline demographic data are detailed in Table 1. The median follow up after commencement of anti-PD-1 therapy was 7.0 months (range 0.8–24.5 months). The median age was 62 years, majority were male (68%) and 42% of patients had BMs at first diagnosis of advanced melanoma. BRAF V600 mutations were detected in 45% of patients. No pt had prior anti-PD-1 treatment. The median interval from diagnosis of BM to commencement of anti-PD1 therapy was 2.3 months (range 0.03–62.7 months). At PD-1 inhibitor commencement 32% of pts had an ECOG of ⩾2, 50% had an elevated LDH level, only 21% of patients were treatment naive and 64% had prior local therapy to BM, indicating a cohort with poor prognostic features.
Table 1. Baseline demographics.
| Demographics | N (%) |
|---|---|
| Median age–years (range) | 62 (19–85) |
| Male | 45 (68) |
| BM at stage IV diagnosis | |
| No | 38 (58) |
| Yes | 28 (42) |
| Mutational status | |
| BRAF V600 mutated | 30 (45) |
| Other | 36 (55) |
| ECOG PS | |
| 0 | 16 (24) |
| 1 | 29 (44) |
| 2 | 19 (29) |
| 3 | 2 (3) |
| LDH | |
| ⩽ULN | 30 (45) |
| ⩾ULN | 33 (50) |
| Unknown | 3 (5) |
| Local therapy to BMs prior to anti-PD-1 | |
| Nil | 24 (36) |
| Surgery | 3 (5) |
| SRS | 9 (14) |
| WBRT | 12 (18) |
| Combination | 18 (27) |
| Type of anti-PD-1 therapy | |
| Nivolumab | 6 (9) |
| Pembrolizumab | 60 (91) |
| Lines of prior systemic treatment | |
| 0 | 14 (21) |
| 1 | 33 (50) |
| 2 | 15 (23) |
| ⩾3 | 4 (6) |
Abbreviations: BM=brain metastases; ECOG PS=Eastern Cooperative Oncology Group performance status; PD1=programmed death-1; SRS=stereotactic radiosurgery, ULN=upper limit of normal; WBRT=whole-brain radiotherapy.
Most patients (90%) had more than one BM at the start of anti-PD-1 therapy (Table 2). Median SoD of IC target lesions was 23.5 mm (range 5–153 mm). Median number of EC sites was 3 (range 0–7). The majority of patients (70%) had asymptomatic BMs at the start of anti-PD-1 therapy. Of the 20 pts with symptomatic BM at treatment commencement, 15 (75%) patients required corticosteroids for symptom control, with doses ranging from dexamethasone (0.5 mg to 12 mg) or equivalent alternative corticosteroid. Baseline tumour characteristics are detailed in Table 2.
Table 2. Baseline tumour characteristics.
| Characteristics | N (%) |
|---|---|
| Total number of IC metastases | |
| 1 | 7 (10) |
| 2–4 | 34 (52) |
| 5–10 | 19 (29) |
| >10 | 6 (9) |
| Median SoD of IC targets (mm; range) | 23.5 (5–153) |
| Median number of EC metastatic sites (range) | 3 (0–7) |
| Symptomatic BM | |
| No | 46 (70) |
| Yes | 20 (30) |
| On steroids for symptomatic BM | |
| No | 51 (77) |
| Yes | 15 (23) |
Abbreviations: BM=brain metastases; EC=extracranial; IC=intracranial; SoD=sum of dimensions.
Of the 42 (64%) of pts who received local treatment for BM prior to commencing anti-PD-1 therapy, three (5%) had surgical resection, nine (14%) had SRS and 12 (18%) had WBRT. In total, 18 (27%) patients had a combination of surgery and radiotherapy. Radiation therapy was administered within 12 weeks of starting anti-PD1 treatment in 19 (29%) patients.
Pembrolizumab was received by 60 (91%) patients at 2 mg kg−1 intravenous dosing every 3 weeks and six (9%) patients received nivolumab at 3 mg kg−1 intravenously every 2 weeks.
Efficacy analyses
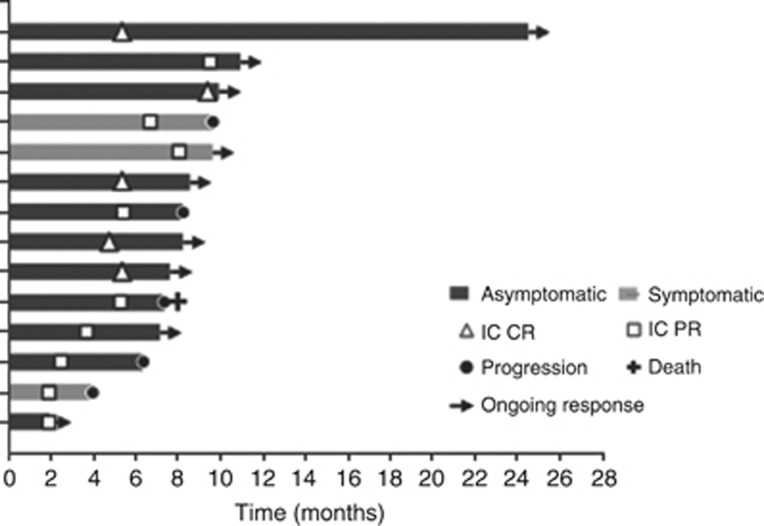
Of the 66 patients, 41 (62%) were evaluated using MRI scans and 25 (38%) using CT scans. Objective IC responses were observed in 14 (21%) patients with 5 (8%) patients achieving a CR (examples in Figure 1). IC disease control (defined as CR, PR and SD) was achieved in 37 (56%) patients and 29 (44%) patients had PD of which 13 (20%) pts experienced clinical progression without confirmatory imaging. Of the pts that achieved an objective response, three (21%) pts had symptomatic BM. Durable responses were seen in most pts that achieved an objective response to anti-PD-1 treatment (Figure 2).
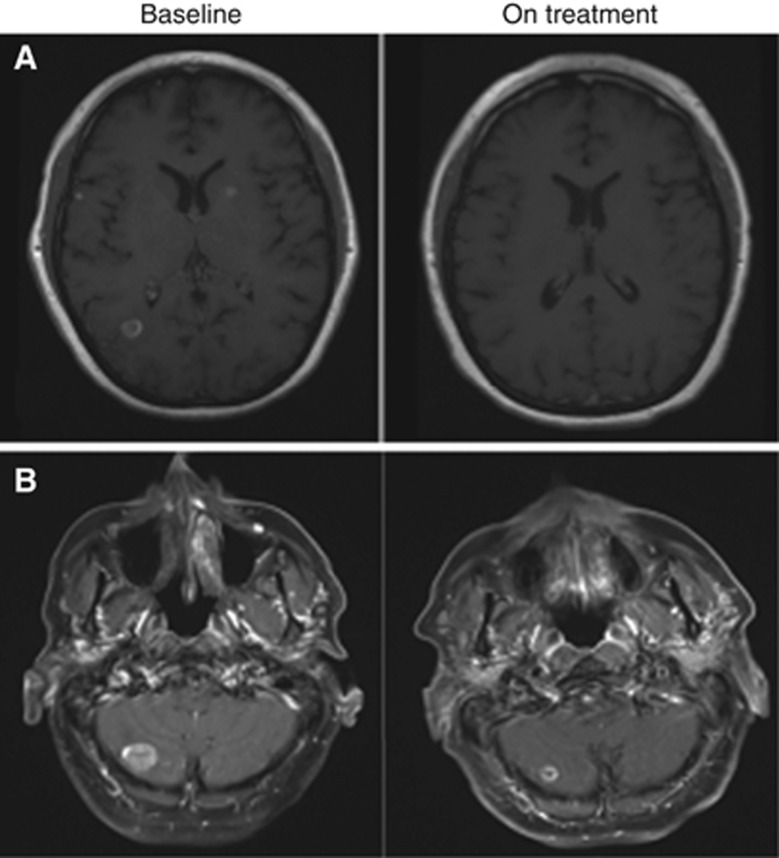
Figure 1.
Radiological examples of intracranial response. Intracranial responses seen with anti-PD-1 therapy. (A) MRI showing a partial response in a patient with symptomatic brain metastases on 4 mg dexamethasone at baseline and 6 months later. (B) MRI showing a partial response seen in a patient on anti-PD-1 therapy with no prior local intracranial therapy at baseline and 2 months later.
Figure 2.
Swimmer’s plot showing durable responses in patients who achieved an objective response to anti-PD1 therapy.
After excluding pts who had no EC sites of disease or had clinical IC progression without restaging imaging, 48 pts were evaluated for EC response, with objective responses seen in 18 (38%) pts. Both IC and EC objective responses (IC and EC ORR) were in nine (14%) pts. In 29 (60%) pts the best IC and EC responses were concordant (i.e., IC and EC were both PR/CR, both SD or both PD).
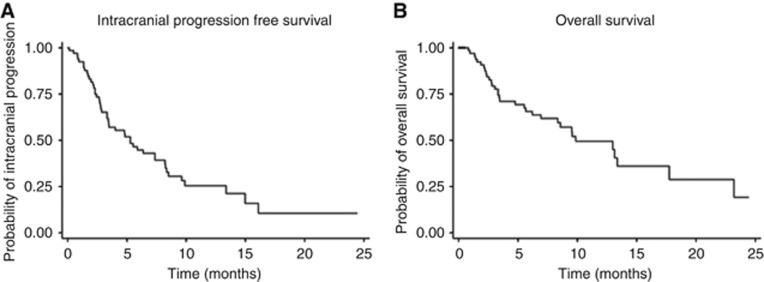
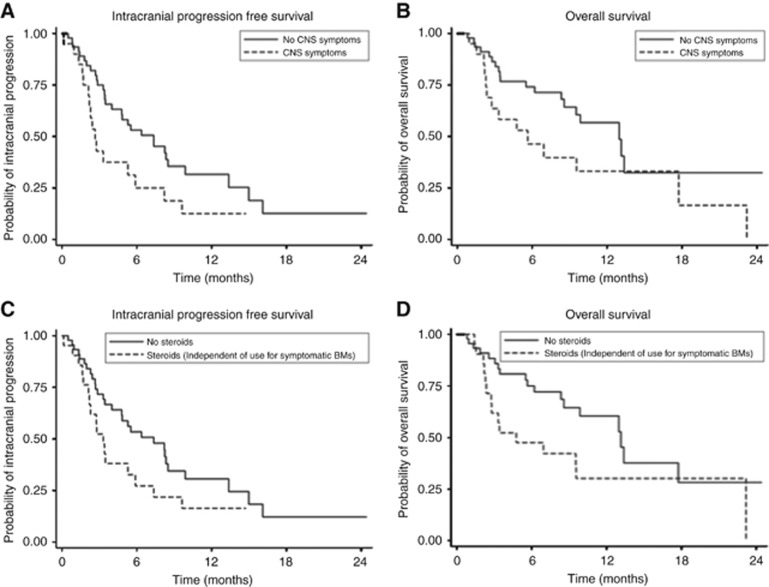
The median IC PFS was 5.3 months (95% CI 3.3–8.2 months) (Figure 3A). The IC PFS was significantly lower in patients with symptomatic BM than pts with asymptomatic metastases (2.7 vs 7.4 months, P=0.035). Patients on corticosteroids had a shorter PFS than those not on corticosteroids (3.2 vs 7.4 months, P=0.081) (Figure 4). The median EC PFS was not reached, and further EC disease assessments were not continued after IC progression or after changes in treatment.
Figure 3.
Intracranial PFS and OS. (A) The overall median intracranial PFS was 5.3 months (95% CI 3.3–8.2 months). (B) The median OS was 9.9 months (95% CI 6.9–17.7).
Figure 4.
Impact of CNS symptoms and steroids on intracranial PFS and OS. (A) The intracranial progression-free survival (PFS) was significantly lower in patients with symptomatic brain metastases than in asymptomatic patients: 2.7 vs 7.4 months (HR 1.95 (95% CI 1.05–3.63), P=0.0348). (B) Patients with symptomatic brain metastases had a shorter median survival than patients with asymptomatic metastases: 5.7 vs 13.0 months (HR 1.91 (95% CI 0.95–3.84), P=0.068). (C) Patients on corticosteroids had a shorter PFS than those not on corticosteroids: 3.2 vs 7.4 months (HR 1.72 (95% CI 0.93–3.17), P=0.081). (D) Patients on corticosteroids had a shorter OS than those not on corticosteroids: 4.8 vs 13.1 months (HR 2.06 (95% CI 1.04–4.11) P=0.039).
The median OS was 9.9 months (95% CI 6.9–17.7 months) (Figure 3B). Patients with symptomatic brain metastases had a shorter median survival than patients with asymptomatic metastases (5.7 vs 13.0 months, P=0.068). Similarly, the median OS of pts on corticosteroids was significantly shorter than those not on corticosteroids, (4.8 vs 13.1 months, P=0.039) (Figure 4).
On univariate analysis, pts receiving anti-PD-1 therapy as first-line treatment compared to subsequent line showed numerical superiority but not statistically significant improvement in IC PFS (8.3 vs 5.3 months, P=0.809) or OS (9.5 vs 13.0 months, P=0.904). Similarly, prior local therapy with SRS or WBRT before commencing anti-PD-1 therapy did not significantly influence IC PFS (5.3 vs 6.4 months, P=0.645) or OS (13.1 vs 8.3 months, P=0.201). Furthermore, receipt of radiotherapy within 12 weeks of commencing anti-PD-1 therapy did not significantly influence IC PFS (5.3 vs 6.4 months, P=0.770) or OS (13.0 vs 8.3 months, P=0.192).
Discussion
This is the first study to report on the efficacy of anti-PD-1 therapy in a large cohort of melanoma patients with symptomatic brain metastases and poor ECOG performance status. We demonstrate that over half of all patients treated with anti-PD-1 therapies experienced IC disease control and 20% had a partial or complete IC response. The IC response rate in our study was lower than EC responses; however, the EC response rate in our study cohort was similar to those reported in trials which demonstrated objective response rates of 21–34% and 31–40% with pembrolizumab (Ribas et al, 2015, 2016; Robert et al, 2015b) and nivolumab (Topalian et al, 2014; Larkin et al, 2015; Weber et al, 2015; Robert et al, 2015a) respectively. This is despite the inclusion of patients with poor prognostic factors many of whom would have been excluded from clinical trials: including those with active brain metastases, pts of poor performance status (ECOG ⩾2), and patients on corticosteroids. Our study demonstrates a similar IC efficacy compared to a recently published prospective trial (Goldberg et al, 2016) which included a small cohort of melanoma pts (n=18) with asymptomatic brain metastases measuring <2 cm that did not require corticosteroids. While a high concordance was seen between IC and EC responses in this study, this was lower than that seen with single-agent dabrafenib (Azer et al, 2014).
Radiotherapy has been shown to have immunomodulatory effects (Formenti and Demaria, 2013; Sridharan and Schoenfeld, 2015) including the upregulation of inflammatory cytokines and PD-L1 and facilitation of T cell infiltration. Preclinical studies have demonstrated enhanced efficacy of anti-PD-1 antibodies when combined with radiotherapy (Dovedi et al, 2014) as well as responses in non-irradiated tumours (Park et al, 2014, 2015). Retrospective studies (Ahmed et al, 2015, 2016; Liniker et al, 2016; Qian et al, 2016) have reported on the feasibility of concurrent and sequential anti-PD-1 therapy with radiotherapy and demonstrated favourable OS and IC disease control compared to historical controls. One of these studies suggested improved disease control when a concurrent schedule was applied and also demonstrated superiority of anti-PD-1 blockade compared to anti-CTLA-4 blockade in this setting (Qian et al, 2016). In keeping with these observations there was a trend to improved OS in our cohort in patients who received radiotherapy prior to or during anti-PD-1 therapy, however, differences in patient-related factors are also likely to have affected survival, and formal randomised studies will be required to determine if the combination of radiotherapy is synergistic with anti-PD1 in the setting of brain metastasis. It is worth noting that these prior studies only included asymptomatic patients with low-volume brain metastases that were suitable for stereotactic radiotherapy and who therefore differed from our patient population.
Ipilimumab and MAPK inhibitors can be effective treatments in patients with melanoma and brain metastases with durable responses seen in a proportion of patients (Long et al, 2012; Margolin et al, 2012; Dummer et al, 2014; Queirolo et al, 2014). Targeted therapy with BRAF inhibitors leads to an overall higher IC response rate than treatment with anti-CTLA-4 blockade mirroring the differences seen with these agents in achieving EC responses. In a prospective clinical trial with ipilimumab in patients with brain metastases, IC disease control was seen in 24% of neurologically asymptomatic patients not requiring steroids while only 10% of symptomatic patients on steroids exhibited disease control. The latter cohort of patients also experienced poorer EC responses (5 vs 27%) and poorer OS (3.4 vs 7 months) (Margolin et al, 2012). In a retrospective analysis of 146 patients with brain metastases who were treated with ipilimumab in an expanded access program (Queirolo et al, 2014), 26 patients had received steroid therapy at baseline and had an overall DCR of 15%, while the remaining 119 patients achieved a global DCR of 29%. In our patient cohort the IC response rate was better than in those reported with ipilimumab, with a similar poorer outcome seen in patients with symptomatic BM on corticosteroids.
The impact of high dose corticosteroids on immunotherapeutic outcome remains unclear with most trials excluding patients on immunosuppressive doses of systemic steroids (>10 mg per day prednisone equivalents), and retrospective analyses reporting conflicting outcomes (Downey et al, 2007; Horvat et al, 2015; Menzies et al, 2017). Furthermore, corticosteroids are frequently administered to neurologically symptomatic patients who usually have a poor ECOG status, a variable shown independently to affect PFS, OS and efficacy of anti-PD-1 therapies (Dudnik et al, 2016). Our study has shown that patients on corticosteroids have a poorer outcome, but despite this anti-PD-1 therapy was able to achieve disease control in a proportion of these patients.
This study has some limitations in being retrospective. First, safety data was not collected in a standardised and reliable manner and the short patient follow up further limited this. Furthermore, imaging modalities to evaluate responses to treatment differed among the institutions involved in the study, preventing reliable comparison of outcomes stratified by number of BM, and potentially confounding the response assessment due to sensitivity and consistency of the imaging modality used. Similarly interpretation and measurement bias may have occurred when reporting responses in the different patient groups such as those who were symptomatic or on steroids, particularly given tumour assessment was performed at clinician discretion.
In conclusion, our study demonstrates that the anti-PD1 antibodies, nivolumab and pembrolizumab, have significant activity in melanoma patients with brain metastases including symptomatic patients requiring corticosteroids. Current ongoing clinical trials will provide further prospective evidence about the IC efficacy of anti-PD1 blockade and include the ABC trial (NCT02374242) which is evaluating the activity of the anti-PD1 antibody, nivolumab, alone and in combination with ipilimumab, the CheckMate 204 trial (NCT02320058), evaluating the efficacy of nivolumab in combination with ipilimumab followed by nivolumab monotherapy (Margolin et al, 2015), and the CA209-322 (NCT02621515) trial, evaluating nivolumab in metastatic melanoma with symptomatic brain metastases. As the treatment paradigm for melanoma patients with brain metastases evolves, choosing the appropriate systemic treatment or combination therapy and the optimal sequencing of local and systemic therapies will be the next challenge faced by oncologists.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
JC has sat on advisory boards for Novartis and GSK; RFK is a consultant advisor for Merck, BMS, Novartis and Amgen; GVL is a consultant advisor to Amgen, Merck MSD, Novartis, Roche, Array and Pierre-Fabre; AMM is a consultant advisor to Merck MSD, Norvatis and Chugai; MSC is a consultant advisor for Merck MSD, BMS, Novartis and Amgen. The remaining authors declare no conflict of interest.
References
- Ahmed K, Abuodeh Y, Echevarria M, Arrington J, Stallworth D, Hogue C, Naghavi A, Kim S, Kim Y, Patel B, Sarangkasiri S, Johnstone PA, Sahebjam S, Khushalani N, Forsyth PA, Harrison LB, Yu M, Etame AB, Caudell JJ (2016) Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol 27(12): 2288–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed K, Stallworth D, Kim Y, Johnstone P, Harrison L, Caudell J, Yu H, Etame A, Weber J, Gibney G (2015) Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 27(3): 434–441. [DOI] [PubMed] [Google Scholar]
- Azer MW, Menzies AM, Haydu LE, Kefford RF, Long GV (2014) Patterns of response and progression in patients with BRAF-mutant melanoma metastatic to the brain who were treated with dabrafenib. Cancer 120: 530–536. [DOI] [PubMed] [Google Scholar]
- Carlino MS, Fogarty GB, Long GV (2012) Treatment of melanoma brain metastases: a new paradigm. Cancer J 18: 208–212. [DOI] [PubMed] [Google Scholar]
- Choong ES, Lo S, Drummond M, Fogarty GB, Menzies AM, Guminski A, Shivalingam B, Clarke K, Long GV, Hong AM (2017) Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer 75: 169–178. [DOI] [PubMed] [Google Scholar]
- Dossgg LL, Memula N (1982) The radioresponsiveness of melanoma. Int J Radiat Oncol Biol Phys 8: 1131–1134. [DOI] [PubMed] [Google Scholar]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, Mckenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, Jones H, Wilkinson RW, Honeychurch J, Illidge TM (2014) Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74: 5458–5468. [DOI] [PubMed] [Google Scholar]
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL (2007) Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 13: 6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudnik E, Moskovitz M, Wollner M, Zer A, Bar J, Agbarya A, Idan T, Shechtman Y, Amna MA, Peled N (2016) 181P: Anti-PD-1 antibodies in non-small cell lung cancer (NSCLC): The real-life setting experience. J Thor Oncol 11: S136. [Google Scholar]
- Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, Veronese L, Hilfiker PR, Felderer L, Rinderknecht JD (2014) Vemurafenib in patients with BRAFV600 mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer 50: 611–621. [DOI] [PubMed] [Google Scholar]
- Fife K, Colman M, Stevens G, Firth I, Moon D, Shannon K, Harman R, Petersen-Schaefer K, Zacest A, Besser M (2004) Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 22: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Formenti SC, Demaria S (2013) Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 105(4): 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, Yu J, Hegde U, Speaker S, Madura M, Ralabate A, Rivera A, Rowen E, Gerrish H, Yao X, Chiang V, Kluger HM (2016) Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17(7): 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat TZ, Adel NG, Dang T-O, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D’angelo SP, Woo KM, Panageas KS, Wolchok JD, Chapman PB (2015) Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 33(28): 3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1): 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liniker E, Menzies A, Kong B, Cooper A, Ramanujam S, Lo S, Kefford R, Fogarty G, Guminski A, Wang T (2016) Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. OncoImmunology 5: e1214788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Algazi A (2012) Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 13: 1087–1095. [DOI] [PubMed] [Google Scholar]
- Margolin K, Ernstoff MS, Hamid O, Lawrence D, Mcdermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13: 459–465. [DOI] [PubMed] [Google Scholar]
- Margolin KA, Tawbi HA-H, Ernstoff MS, Hodi FS, Mcdermott DF, Edwards R, Avila A, Atkins MB (2015) A multi-center phase II open-label study (CheckMate 204) to evaluate safety and efficacy of nivolumab (NIVO) in combination with ipilimumab (IPI) followed by NIVO monotherapy in patients (pts) with melanoma (MEL) metastatic to the brain. ASCO Annual Meeting Proceedings. J Clin Oncol33: abstr TPS9080.
- McArthur G, Maio M, Arance A, Nathan P, Blank C, Avril M, Garbe C, Hauschild A, Schadendorf D, Hamid O, Fluck M, Thebeau M, Schachter J, Kefford R, Chamberlain M, Makrutzki M, Robson S, Gonzalez R, Margolin K (2017) Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol 28(3): 634–641. [DOI] [PubMed] [Google Scholar]
- Menzies A, Johnson D, Ramanujam S, Atkinson V, Wong A, Park J, Mcquade J, Shoushtari A, Tsai K, Eroglu Z, Klein O, Hassel JC, Sosman JA, Guminski A, Sullivan RJ, Ribas A, Carlino MS, Davies MA, Sandhu SK, Long GV (2017) Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28(2): 368–376. [DOI] [PubMed] [Google Scholar]
- Menzies AM, Long GV (2014) Systemic treatment for BRAF-mutant melanoma: where do we go next? Lancet Oncol 15: e371–e381. [DOI] [PubMed] [Google Scholar]
- Park S, Dong H, Zhao W, Grams M, Liu X, Harrington S, Furutani K, Krco C, Olivier K, Markovic S (2014) PD-1 blockade enhances radiation therapy-induced abscopal effect. Int J Radiat Oncol Biol Phys 90: S57–S58. [Google Scholar]
- Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, Mansfield AS, Furutani KM, Olivier KR, Kwon ED (2015) PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res 3: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian JM, Yu JB, Kluger HM, Chiang VL (2016) Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 122(19): 3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queirolo P, Spagnolo F, Ascierto PA, Simeone E, Marchetti P, Scoppola A, Del Vecchio M, Di Guardo L, Maio M, Di Giacomo AM (2014) Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol 118: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizer JJ, Hwu W-J, Panageas KS, Wilton A, Baldwin DE, Bailey E, Von Althann C, Lamb LA, Alvarado G, Bilsky MH (2008) Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol 10: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu W-J, Weber JS (2016) Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315: 1600–1609. [DOI] [PubMed] [Google Scholar]
- Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, Mcneil C, Kalinka-Warzocha E (2015. a) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372: 320–330. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, Mcneil C, Lotem M (2015. b) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- Sridharan V, Schoenfeld JD (2015) Immune effects of targeted radiation therapy for cancer. Discov Med 19: 219–228. [PubMed] [Google Scholar]
- Topalian SL, Sznol M, Mcdermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32: 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, D’angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16: 375–384. [DOI] [PubMed] [Google Scholar]