Abstract
Background:
To evaluate safety, pharmacokinetics, and maximum tolerated dose of roniciclib in patients with advanced malignancies, with dose expansion to evaluate clinical benefit at the recommended phase II dose (RP2D).
Methods:
Two phase I dose-escalation studies evaluated two roniciclib dosing schedules: 3 days on/4 days off or 4 weeks on/2 weeks off. The expansion phase included patients with small-cell lung cancer (SCLC), ovarian cancer, or tumour mutations involving the CDK signalling pathway.
Results:
Ten patients were evaluable in the 4 weeks on/2 weeks off schedule (terminated following limited tolerability) and 47 in the 3 days on/4 days off schedule dose-escalation cohorts. On the 3 days on/4 days off schedule, RP2D was 5 mg twice daily in solid tumours (n=40); undetermined in lymphoid malignancies (n=7). Common roniciclib-related adverse events included nausea (76.6%), fatigue (65.8%), diarrhoea (63.1%), and vomiting (57.7%). Roniciclib demonstrated rapid absorption and dose-proportional increase in exposure. One partial response (1.0%) was observed. In RP2D expansion cohorts, the disease control rate (DCR) was 40.9% for patients with ovarian cancer (n=25), 17.4% for patients with SCLC (n=33), and 33.3% for patients with CDK-related tumour mutations (n=6).
Conclusions:
Roniciclib demonstrated an acceptable safety profile and moderate DCR in 3 days on/4 days off schedule.
Keywords: roniciclib, CDK inhibitor, solid tumours
Cyclin-dependent kinases (CDKs) are key regulators of cell division and other fundamental cellular functions including gene transcription (Nurse et al, 1998). Through overexpression or amplification of cell-cycle activators (e.g., D- or E-type cyclins, CDK4, CDK6) and by inactivation of cell-cycle inhibitors, CDKs contribute to deregulation of the cell cycle in tumour cells (Lapenna and Giordano, 2009; Asghar et al, 2015). CDKs are also involved in regulating basal transcription: for example, CDK7 and CDK9 are involved in the phosphorylation of RNA polymerase II, contributing to initiation and elongation of transcription, respectively (Spangler et al, 2001; Asghar et al, 2015). Polymorphisms of CDK7 and overexpression of CDK9 have been associated with a wide variety of cancer tissues (Peyressatre et al, 2015). The vital role of CDKs in regulating cell proliferation and transcription suggests that the inhibition of CDKs may be a potential therapeutic target in cancer. Selective inhibition of CDKs 4 and 6 has proven to be active in oestrogen receptor-positive breast cancer when used in combination with letrozole, but is limited to malignancies with an intact retinoblastoma gene (Witkiewicz and Knudsen, 2014). A broad-spectrum CDK inhibition profile may be dependent on factors such as tumour type, CDK expression levels, and mutational profile.
Roniciclib (BAY 1000394; Bayer AG, Leverkusen, Germany) is an oral, small-molecule pan-CDK inhibitor with low nanomolar activity against cell-cycle CDKs 1, 2, 4, and 6 and transcriptional CDKs 7 and 9. Roniciclib demonstrated favourable preclinical pharmacokinetic (PK) parameters, with a low clearance and an intermediate half-life across species following intravenous administration and a high volume of distribution, suggesting extensive tissue distribution (Siemeister et al, 2012). After oral application, roniciclib was readily absorbed and showed an intermediate bioavailability of ∼50%. Roniciclib was shown to have broad-spectrum anti-proliferative activity in a large panel of human cancer cell lines of various tumours of diverse genetic backgrounds, and strongly inhibited tumour growth in a dose-dependent manner in xenograft mouse models, including models refractory to standard-of-care drugs (Siemeister et al, 2012). Roniciclib also demonstrated an additive efficacy in combination with cisplatin and etoposide without worsening tolerability of treatment in small-cell lung cancer (SCLC) xenograft models (Siemeister et al, 2012).
A human starting dose of 0.01 mg kg−1 (0.37 mg m−2) was recommended based on preclinical data, including target organ toxicities in the gastrointestinal tract, reproductive organs, and lymphohaematopoietic system; similar in vivo efficacy responses upon various dosing schedules were also observed (Siemeister et al, 2012). Pharmacokinetic and pharmacodynamic data indicated that once-daily dosing of roniciclib achieved a coverage of the cellular anti-proliferative IC50 for 1 day, suggesting nearly complete target inhibition for twice-daily (BID) dosing with a time frame sufficient to cause profound anti-proliferative and cell death-inducing effects (Siemeister et al, 2012).
Here we report two parallel first-in-human phase I studies that evaluated the safety, tolerability, PK, and maximum tolerated dose (MTD) of roniciclib given in a 3 days on/4 days off schedule (NCT01188252) or a 4 weeks on/2 weeks off schedule (NCT01335256) in patients with advanced malignancies. An expansion phase with the recommended phase II dose (RP2D) then assessed the clinical benefit of roniciclib 3 days on/4 days off in patients with SCLC or ovarian cancer, or with solid tumours bearing a distinct tumour mutation in the CDK signalling pathway.
Materials and methods
Both studies were approved by independent ethics committees and institutional review boards for each study site, and were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. All patients provided written, informed consent before participation.
Study designs
These multicentre, open-label, non-randomised, phase I dose-escalation studies consisted of two dosing schedules: 4 weeks on/2 weeks off (42-day cycle) or 3 days on/4 days off (21-day cycle). The primary objective of both studies was to determine the safety and MTD of roniciclib in patients with advanced malignancies. The secondary objectives included assessment of PK, biomarkers, and tumour response profiles.
In the 4 weeks on/2 weeks off schedule, the dose-escalation phase of the study was planned for ∼30 patients with advanced, histologically or cytologically confirmed solid tumours, with up to 25 additional patients planned for an expansion phase at the MTD.
In the 3 days on/4 days off schedule, the study involved nine dose-escalation cohorts of patients with solid tumours and two dose-escalation cohorts of patients with tumours of lymphoid tissues (cohorts L1 and L2). Expansion cohorts at the MTD were planned in patients with SCLC (25 patients), ovarian cancer (25 patients), and tumour mutations involving CDK signalling such as amplification of cyclin D or E or loss of p15 or p16 (six patients). As part of the SCLC cohort, the effect of a high-calorie, high-fat meal on roniciclib PK was investigated.
Treatment
In both studies, increasing doses of roniciclib were administered, starting with a dose of 0.3 mg in a polyethylene glycol-based liquid service formulation, then changing to a tablet formulation at a dose of 5 mg. A bridging cohort compared the bioavailability of the two formulations (in the 3 days on/4 days off schedule only). Details of the dose-escalation schedule are provided in the Supplementary Materials. A dose-limiting toxicity (DLT) was defined by the occurrence of any of the following attributed to roniciclib during cycle 1 of a dose level: absolute neutrophil count <0.5 × 109 l−1 for ⩾7 days; febrile neutropenia with absolute neutrophil count <0.5 × 109 l−1 and fever ⩾38.5 °C; platelets <25 × 109 l−1; any grade 3–5 non-haematological toxicity without a clear alternative explanation; or any grade 4 vomiting.
Roniciclib was administered BID either on a 4 weeks on/2 weeks off schedule of a 42-day cycle or on a 3 days on/4 days off schedule of a 21-day cycle. For PK assessment, a single dose of roniciclib was given on cycle 1, day 1 in both studies and then BID dosing on day 3 for the 4 weeks on/2 weeks off schedule and day 2 for the 3 days on/4 days off schedule. The formulation bridging and food-effect cohorts (3 days on/4 days off schedule only) also had a single dose on cycle 1, day −3. The effect of a high-fat meal on the PK of roniciclib was assessed at the RP2D after administration of roniciclib tablets immediately following consumption of a high-fat, high-calorie meal on cycle 1, day −3.
Dose escalation would not continue in either study if a DLT occurred in more than one of six patients, or in two patients within one cohort. The dose one level below the toxic dose determined the MTD. Patients continued roniciclib therapy until tumour progression, unacceptable toxicity, consent withdrawal, or withdrawal from the study at the discretion of the investigator.
Inclusion criteria
Patients were eligible if they were aged 18 years or older and had histologically or cytologically confirmed solid tumours, or tumours of lymphoid tissue refractory to or not amenable to standard therapy. Patients with solid tumours had to have at least one measurable or evaluable tumour lesion according to Response Evaluation Criteria in Solid Tumors version 1.1. Patients with solid tumours bearing a distinct tumour mutation in the CDK signalling pathway, such as amplification of cyclin D or E or loss of p15 or p16, were enrolled in an additional cohort. Patients had to have a life expectancy of 12 weeks or more and an Eastern Cooperative Oncology Group performance status of 0 or 1. Progressive disease at baseline was not a requirement for study participation. Further inclusion and exclusion criteria are provided in the Supplementary Materials.
Assessments
Safety evaluations included adverse events (AEs), vital signs, laboratory tests, and 12-lead electrocardiograms at screening, at planned times during each cycle, during follow-up, and up to 30 days after the last dose. Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. All patients receiving at least one dose of roniciclib were included in the safety analysis set.
Pharmacokinetic assessments of single-dose and multiple-dose roniciclib were planned in the 4 weeks on/2 weeks off schedule and the 3 days on/4 days off schedule in all patients in the dose-escalation phase, and in at least six patients in each tumour type in the expansion cohorts. The schedule for plasma collection is provided in the Supplementary Materials. Pharmacokinetic data were analysed using non-compartmental methods to estimate maximum drug concentration (Cmax), time to maximum drug concentration (tmax), area under the curve (AUC), AUC from 0 to 12 h after administration (AUC(0–12)), AUC from 0 to 24 h after administration (AUC(0–24)), and half-life of roniciclib and its metabolite, M-1, if possible.
Pharmacodynamic biomarker assessment was performed in the 3 days on/4 days off schedule in the expansion cohorts. The level of expression of proliferating cell nuclear antigen (PCNA) changes during the cell cycle and is associated with cell proliferation or transformation (Stoimenov and Helleday, 2009). Whole blood was collected in PAXgene Blood RNA Tubes (PreAnalytiX GmbH, Hombrechtikon, Switzerland). The schedule for sample collection is provided in the Supplementary Materials. RNA was isolated following the manufacturer’s instructions. Proliferating cell nuclear antigen expression as determined by TaqMan real-time polymerase chain reaction (TaqMan PCNA gene expression assay Hs00427214_g1; Thermo Fisher Scientific Inc., Waltham, MA, USA) was normalised to control genes (GAPDH Hs9999905_m1, SELL Hs01046459_m1, and IGSF6 Hs00175526-m1). All gene expression assays were purchased from Life Technologies (Carlsbad, CA, USA). Proliferating cell nuclear antigen data were pre-processed using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Further details of sample collection in the formulation bridging and food-effect cohorts and for analysis of circulating tumour cells are provided in the Supplementary Materials.
Investigators assessed response using Response Evaluation Criteria in Solid Tumors version 1.1 in patients with solid tumours, and using the pertinent guidelines in patients with tumours of lymphoid tissue. Stable disease was defined as neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, taking as reference the smallest sum diameters at first assessment (after cycle 2). Assessments were made every two cycles in the 3 days on/4 days off schedule and at the end of each cycle for the 4 weeks on/2 weeks off schedule. Response was evaluated in all patients who received study treatment, including patients with missing evaluations or who were not assessed.
Statistical analyses
Descriptive statistics were used to summarise demographic data and other baseline characteristics, AEs, safety parameters, and tumour assessment data. The relative bioavailability of the liquid and tablet formulations and the food effect on single-dose roniciclib were compared based on roniciclib PK parameters, primarily on AUC(0–24) and Cmax, on cycle 1, day −3 and cycle 1, day 1.
Results
Baseline patient demographics and disease characteristics
Fifteen patients with solid malignant tumours who progressed after standard therapy were enrolled in the 4 weeks on/2 weeks off schedule, 10 of whom received treatment and were evaluable for safety and tumour response. The median age at screening was 50.5 years (range, 42–76), and 40% of patients were female. Cancer diagnosis is reported in Supplementary Table 1. Prior systemic therapies were reported in 100% of patients (Table 1). Four patients were assigned to the first dosing cohort (0.3 mg BID) and six patients to the second dosing cohort (0.5 mg BID).
Table 1. Baseline patient demographics and disease characteristics.
| 4 weeks on/2 weeks off schedule |
3 days on/4 days off schedule |
||||||
|---|---|---|---|---|---|---|---|
| Dose-escalation cohorts |
Dose-escalation cohorts |
Dose-expansion cohorts |
Total | ||||
| Solid tumour (n=10) | Solid tumour (n=40) | Lymphoid tissue tumour (n=7) | SCLC 5 mg BID or 5/10 mg BID (n=33) | Ovarian cancer 5 mg BID or 5/10 mg BID (n=25) | Tumour mutation 5 mg BID (n=6) | (N=111) | |
| Median age, years (range) | 50.5 (42–76) | 58.0 (26–73) | 63.0 (36–76) | 60.7 (46–80) | 59.0 (28–75) | 54.5 (43–65) | 59.0 (26–80) |
| Females, n (%) | 4 (40.0) | 16 (40.0) | 1 (14.3) | 15 (45.5) | 25 (100) | 4 (66.7) | 61 (55.0) |
| ECOG performance statusa, n (%) | |||||||
| 0 | 1 (10.0) | 24 (60.0) | 3 (42.9) | 9 (27.3) | 9 (36.0) | 2 (33.3) | 47 (42.3) |
| 1 | 8 (80.0) | 12 (30.0) | 3 (42.9) | 23 (69.7) | 14 (56.0) | 4 (66.7) | 56 (50.5) |
| 2 | – | – | 1 (14.3) | – | – | – | 1 (0.9) |
| Prior systemic therapy (neoadjuvant, adjuvant, palliative, and/or curative), n (%) | 10 (100) | 40 (100) | 7 (100) | 33 (100) | 25 (100) | 6 (100) | 111 (100) |
| Prior radiotherapy, n (%) | 6 (60.0)b | 18 (45.0) | 3 (42.9) | 26 (78.8) | 2 (8.0) | 4 (66.7) | 53 (47.7) |
| Prior local therapy, n (%) | 1 (10.0)c | 1 (2.5) | 1 (14.3) | – | 2 (8.0) | 1 (16.7) | 5 (4.5) |
Abbreviations: BID=twice daily; ECOG=Eastern Cooperative Oncology Group; SCLC=small-cell lung cancer.
Data are missing for seven patients in the 3 days on/4 days off study and one patient in the 4 weeks on/2 weeks off study.
Data are missing for four patients.
Data are missing for nine patients.
A total of 148 patients were enrolled in the 3 days on/4 days off schedule, with 111 receiving treatment and evaluable for safety and tumour response. Overall, 40 patients were enrolled in the solid-tumour dose-escalation cohorts.
In the 3 days on/4 days off schedule, 55.0% of patients were female and median age was 59 years (range, 26–80; Table 1). Approximately half of patients (50.5% 56 patients) had more than three prior systemic anti-cancer therapies (range, 1–11). Patients with SCLC received 1–5 prior lines of treatment (median of two), and patients with ovarian cancer received 3–11 prior lines of treatment (median of five). Colon cancer (nine patients) and mesothelioma (five patients) were the most frequently reported cancer diagnoses in the solid-tumour dose-escalation cohorts (see Supplementary Table 1 for cancer diagnoses).
Dose escalation and RP2D
One patient experienced a DLT in the 0.5 mg BID cohort (hyponatraemia), and several other patients experienced various treatment-emergent AEs (TEAEs) affecting the dose-escalation scheme. Therefore, the study with roniciclib administered in the 4 weeks on/2 weeks off schedule was terminated due to limited tolerability (see additional details below).
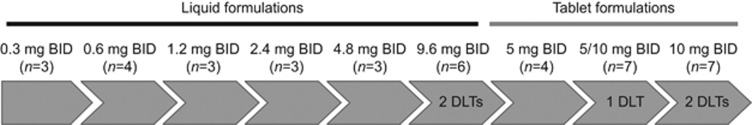
Enrolment and dose escalation in the 3 days on/4 days off schedule using the polyethylene glycol-based liquid service formulation proceeded according to protocol until cohort 6 (9.6 mg BID, formulation bridging cohort), where one patient experienced a DLT (hyponatraemia) leading to expansion of the cohort to six patients. Subsequent dose levels used the 5 mg tablet formulation at 5 mg BID (cohort 7, n=4), 5/10 mg BID (cohort 8, n=7; 5 mg in the morning, 10 mg in the evening), and 10 mg BID (cohort 9, n=7). The 5/10 mg BID dose was initially defined as the MTD for the expansion cohorts. However, after the enrolment of two patients with SCLC and three with ovarian cancer at this dose, the RP2D was changed to 5 mg BID based on safety observations (see below). An additional 59 patients with solid tumours were enrolled in the dose-expansion cohorts with the RP2D (SCLC, n=31; ovarian cancer, n=22; and CDK-related tumour mutation, n=6).
Seven patients were enrolled in the lymphoid malignancy dose-escalation cohorts, 5 mg once daily (n=3) and 5 mg BID (n=4) (see Supplementary Table 1 for cancer diagnoses). Recruitment was stopped before the third dose level because the recruitment rate did not suggest a proper completion of the study within a reasonable time frame.
Exposure and safety
Continuous BID dosing in the 4 weeks on/2 weeks off schedule was not well tolerated. The mean treatment duration was 5.5 weeks (range, 4–10) in the 0.3 mg BID cohort and 7.2 weeks (range, 1–22) in the 0.5 mg BID cohort, with most patients on the study treatment for 4 or more weeks (three out of four patients in the 0.3 mg BID cohort; four out of six patients in the 0.5 mg BID cohort). One patient in the 0.5 mg BID cohort experienced a DLT of grade 3 hyponatraemia, which resolved 3 days after treatment interruption.
The most commonly reported TEAEs were nausea, fatigue, fever, dyspepsia, constipation, vomiting, pain, increased aspartate aminotransferase, and hot flashes (Supplementary Table 2). No grade 4 or 5 TEAEs were reported. One patient permanently discontinued roniciclib because of treatment-emergent lower-extremity oedema (grade 3). Due to the DLT and other drug-related TEAEs affecting the dose-escalation scheme, it was apparent that the study would not be completed within the specified time frame. Therefore, the sponsor chose to terminate the study after the enrolment of the first 10 patients. Ongoing patients were allowed to continue treatment. An MTD was not determined for continuous BID dosing in a 4 weeks on/2 weeks off schedule.
In the 3 days on/4 days off schedule in solid tumours, patients received a median of two cycles (range, 1–12), and median treatment duration was 41 days (range, 1–252), with the majority of patients (73.9%) continuing treatment for less than 50 days.
Dose-limiting toxicities occurred in five patients in the solid-tumour dose-escalation cohorts (Figure 1). Two out of six patients in the 9.6 mg BID cohort, using the polyethylene glycol-based liquid service formulation, reported DLTs (hyponatraemia and oral mucositis), of which mucositis was considered to be related to the polyethylene glycol content of the liquid service formulation. The next cohort started with a decreased dose of 5 mg BID using the tablet formulation, and no DLTs were reported. At 5/10 mg BID, there was one reported DLT (peripheral ischaemia caused by arterial thrombosis in a patient with adrenocortical carcinoma with involvement of the aorta). In the 10 mg BID cohort, two out of six patients reported DLTs (anorexia and elevated troponin). Elevated troponin was considered a thromboembolic event and occurred in a patient with thyroid cancer and a history of aneurysm of the abdominal aorta, cardiac infarction, and ischaemic heart disease. The MTD was determined to be 5/10 mg BID. After the occurrence of three additional thromboembolic events during the 5/10 mg expansion phase (arterial embolism in a patient with ovarian cancer, pulmonary embolism in a patient with SCLC, and intracardial thrombosis in a patient with ovarian cancer), the MTD was reassessed and considered to be 5 mg BID. In the 5 mg BID expansion cohorts, the thromboembolic event rate was 7.9%.
Figure 1.
Patient disposition during dose escalation on the 3 days on/4 days off schedule.
No DLT was reported in the lymphoid malignancy dose-escalation cohorts at doses up to 5 mg BID.
At least one dose modification (reduction or interruption) was reported in 70 patients (63.1%) across all cohorts: 23 patients (20.7%) had dose reductions and 66 patients (59.5%) had dose interruptions or delays, with 44 patients having a single episode. Roniciclib treatment was permanently discontinued in the majority of patients because of disease progression (69 patients; 62.2%), while an additional 12 patients (10.8%) experienced an AE associated with clinical disease progression and discontinued permanently.
The most commonly reported TEAEs of any grade across all cohorts of patients in the 3 days on/4 days off schedule included nausea (80.2%), fatigue (72.1%), diarrhoea (64.9%), and vomiting (61.3% Supplementary Table 3). Nausea was managed by concomitant anti-emetic medications.
Overall, 66 patients (59.5%) experienced an AE of grade 3 (42.3%), 4 (5.4%), or 5 (11.7%). Roniciclib-related TEAEs of any grade were reported in 106 patients (95.5%), with nausea (76.6%), fatigue (65.8%), diarrhoea (63.1%), and vomiting (57.7%) occurring most frequently (Supplementary Table 4). The most common grade 3 roniciclib-related TEAEs were fatigue (7.2%), nausea (5.4%), hypotension (3.6%), and anorexia (3.6%); only two roniciclib-related grade 4 TEAEs were reported (thromboembolic event and peripheral ischaemia; Table 2). Relevant treatment-emergent changes in coagulation parameters, including factor VIII, D-dimers, and von Willebrand factor antigen, were not observed.
Table 2. Summary of treatment-related grade 3 or 4 adverse events occurring in ⩾3 patients (grade 3) or ⩾1 patients (grade 4) overall in the 3 days on/4 days off schedule.
| Adverse event, n (%) | Grade | Cohort 1 0.3 mg BID (n=3) | Cohort 2 0.6 mg BID (n=4) | Cohort 3 1.2 mg BID (n=3) | Cohort 4 2.4 mg BID (n=3) | Cohort 5 4.8 mg BID (n=3) | Cohort 6 9.6 mg BID bridging (n=6) | Cohort 7 5 mg BID (n=4) | Cohort 8 5/10 mg BID (n=7) | Cohort 9 10 mg BID (n=7) | Cohort L1 5 mg QD (n=3) | Cohort L2 5 mg BID (n=4) | SCLC cohort 5/10 mg BID (n=2) | SCLC cohort 5 mg BID (n=23) | Ovarian cohort 5/10 mg BID (n=3) | Ovarian cohort 5 mg BID (n=22) | Tumour mutation cohort 5 mg BID (n=6) | SCLC food- effect cohort 5 mg BID (n=8) | Total (N=111) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue | 3 | – | – | – | – | – | 1 (16.7) | 1 (25.0) | – | – | – | – | – | 1 (4.3) | – | 3 (13.6) | 2 (33.3) | – | 8 (7.2) |
| Nausea | 3 | – | – | – | – | – | 1 (16.7) | – | – | – | – | – | – | 2 (8.7) | – | 1 (4.5) | 1 (16.7) | 1 (12.5) | 6 (5.4) |
| Hypotension | 3 | – | – | – | – | – | – | – | – | 1 (14.3) | – | – | – | 2 (8.7) | – | 1 (4.5) | – | – | 4 (3.6) |
| Anorexia | 3 | – | – | – | 1 (33.3) | – | – | – | – | 1 (14.3) | – | – | – | – | – | – | 1 (16.7) | 1 (12.5) | 4 (3.6) |
| Hyponatraemia | 3 | – | – | – | – | – | 1 (16.7) | – | – | – | – | – | – | 1 (4.3) | – | – | – | 1 (12.5) | 3 (2.7) |
| Hypophosphatemia | 3 | – | – | – | – | – | – | – | – | – | – | 1 (25.0) | – | 1 (4.3) | – | 1 (4.5) | – | – | 3 (2.7) |
| Vomiting | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 (9.1) | – | 1 (12.5) | 3 (2.7) |
| Thromboembolic event | 3 | – | – | – | – | – | – | – | – | – | – | – | – | 1 (4.3) | 2 (66.7) | – | – | – | 3 (2.7) |
| Thromboembolic event | 4 | – | – | – | – | – | – | – | – | – | – | – | 1 (50.0) | – | – | – | – | – | 1 (0.9) |
| Peripheral ischaemia | 4 | – | – | – | – | – | – | – | 1 (14.3) | – | – | – | – | – | – | – | – | – | 1 (0.9) |
Abbreviations: BID=twice daily; QD=once daily; SCLC=small-cell lung cancer.
All adverse events were defined according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Serious TEAEs were reported in 46 patients (41.4%), with the most common being venous and arterial thromboembolic events (eight patients (7.2%), including a case of peripheral ischaemia caused by an arterial thrombosis), dyspnoea (five patients (4.5%)), other general disorders (five patients (4.5%); hemiparesis, device breakage, duodenal obstruction, intestinal obstruction, and general deterioration), ileus (three patients (2.7%)), and unspecified death (three patients (2.7%)). Eleven patients had roniciclib-related serious AEs, with thromboembolic events and peripheral ischaemia being the most frequent (five patients (4.5%)). Eleven patients died between the first day of treatment and 30 days post treatment, primarily because of progressive disease or AEs associated with progressive disease. Reported grade 5 events included death not specified (three patients (2.7%)), other general disorders (two patients (1.8%)), dyspnoea (two patients (1.8%)), and catheter-related infection, encephalopathy, pleural effusion, and other infection in one patient each (0.9%). None of these deaths was attributed to the study drug.
Abnormal electrocardiogram patterns were uncommon across both studies.
Pharmacokinetic evaluation
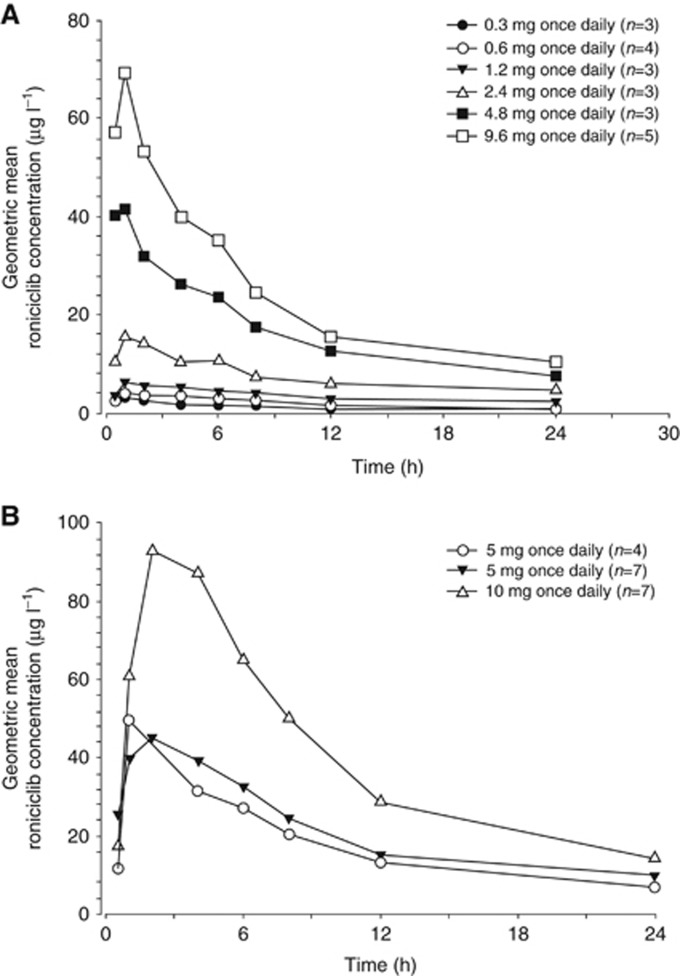
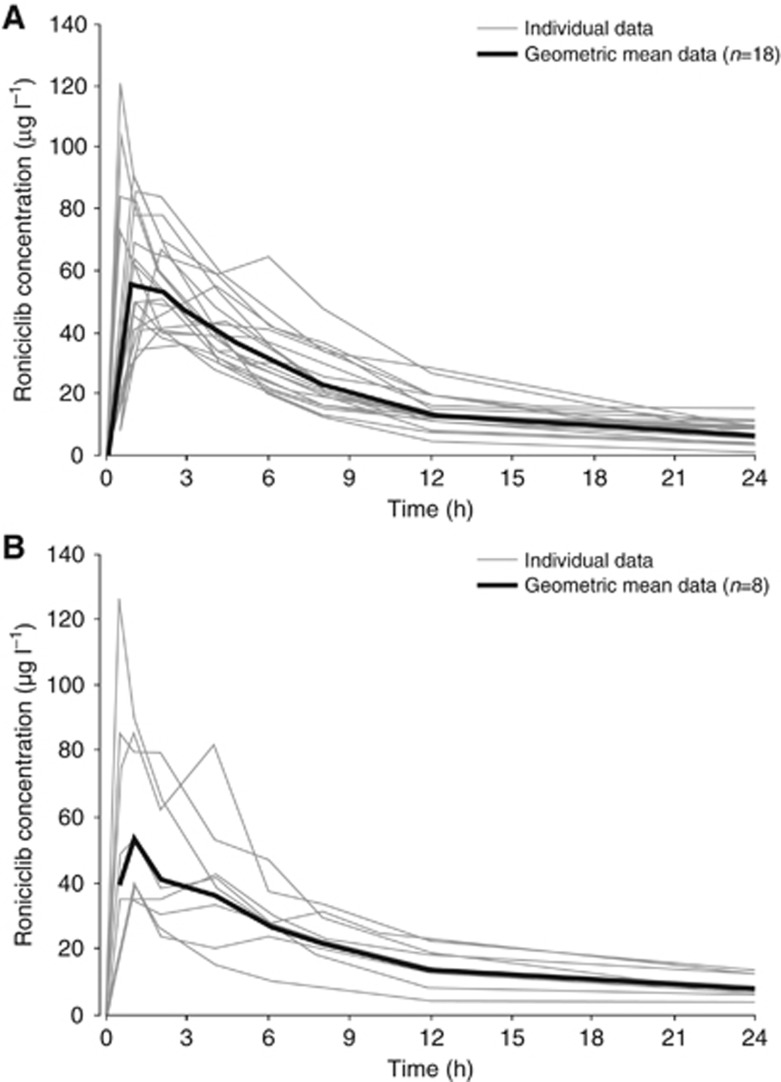
In the 3 days on/4 days off schedule, 39 patients were evaluable for PK analysis in the dose-escalation cohorts, and 31 patients were evaluable in the 5 mg BID dose-expansion cohorts. After oral administration, roniciclib was rapidly absorbed with both the solution and tablet formulations, with a median tmax of ∼1 h (Figure 1). Single-dose roniciclib plasma concentration–time profiles were similar for both dose-escalation cohorts (Figure 2) and dose-expansion cohorts (Figure 3).
Figure 2.
Geometric mean single-dose plasma roniciclib concentration–time profiles on day 1. Dose escalation cohorts 1–6 are shown in (A) and dose escalation cohorts 7–9 are shown in (B).
Figure 3.
Individual and geometric mean single-dose plasma roniciclib concentration–time profiles on day 1. SCLC expansion cohort is shown in (A) and ovarian cancer expansion cohort (5 mg BID and 5/10 mg BID) is shown in (B).
Geometric mean roniciclib PK parameters are presented in Supplementary Table 5. Single-dose and multiple-dose geometric mean PK data indicate that roniciclib exhibits a generally dose-proportional increase in exposure in the dose range studied (0.3–10 mg) with low to moderate inter-patient variability. At the RP2D (5 mg BID), geometric mean multiple-dose roniciclib Cmax and AUC(0–12) values of 98.6 μg l−1 and 662 μg × h l−1, respectively, were estimated on cycle 1, day 10 in the expansion cohorts. On average, roniciclib half-life was estimated to be 8.5 h in the expansion cohorts, which resulted in an approximately two-fold accumulation after BID dosing.
After administration of roniciclib 10 mg as tablets and 9.6 mg as solution formulation, geometric mean roniciclib AUC (from time 0 to the last data point) values were 815 μg × h l−1 and 575 μg × h l−1, respectively, supporting the transition from solution to tablet formulation. After the administration of 5 mg roniciclib tablets immediately following a high-fat, high-calorie meal and under fasting conditions for seven patients, geometric mean roniciclib AUC(0–24) values were 318 μg × h l−1 and 511 μg × h l−1, respectively, indicating a 38% decrease in exposure with food. After administration under fed and fasting conditions, geometric mean roniciclib Cmax values were 29.7 μg l−1 and 71.5 μg l−1, respectively, with a delay in reaching maximum plasma concentrations under fed conditions.
Roniciclib PK parameters were generally comparable in patients enrolled in the 3 days on/4 days off and 4 weeks on/2 weeks off schedules. In both studies, exposure to metabolite M-1 was less than 10% of exposure to roniciclib (data not shown).
Biomarkers
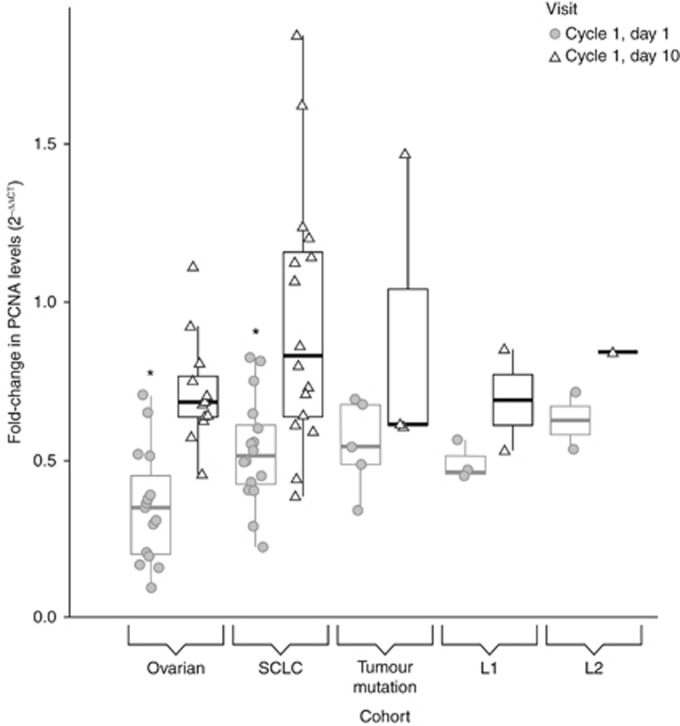
This exploratory analysis aimed to demonstrate the biological activity of roniciclib and to support the RP2D finding. The change in mRNA levels of PCNA in whole blood as a surrogate tissue was determined in response to roniciclib treatment. Roniciclib significantly inhibited expression of PCNA (fold-change from baseline) in peripheral whole blood 6 h post dose on cycle 1, day 1 in ovarian cancer and SCLC patients (Figure 4).
Figure 4.
Changes of PCNA expression levels in whole blood after roniciclib treatment. The boxplot shows the fold-change of PCNA RNA expression on day 1 (circle) and day 10 (triangle) of cycle 1 compared with baseline values for each cohort. Significant changes to baseline values are depicted by asterisks. Cycle 1, day 1 values for both the ovarian cancer and SCLC cohorts are significantly different from baseline PCNA expression. The thick black line in the box indicates the median value, the upper hinge indicates the 75% quantile, and the lower hinge indicates the 25% quantile. Upper and lower whiskers describe the largest or smallest observed values within 1.5-fold of the interquantile range.
PCNA RNA expression levels were evaluated in the whole blood of 59 patients during the expansion phase of the study. The real-time polymerase chain reaction data taken 6 h post dose on cycle 1, day 1 and cycle 1, day 10 were normalised to the pre-treatment expression levels of the respective patient. In the expansion cohorts, 27 patients with ovarian cancer and 32 with SCLC were analysed. For both indications, a decrease in PCNA RNA levels was detected on cycle 1, day 1 that was significantly different from the baseline values (ovarian cancer cohort, ΔΔCT=1.720, P<0.0001; SCLC cohort, ΔΔCT=1.006, P<0.0001). The inhibition of expression was less pronounced on day 10 and did not reach significance (ovarian cancer cohort, ΔΔCT=0.521, P=0.1618; SCLC cohort, ΔΔCT=0.224, P=0.9663; Supplementary Table 6).
Not enough patients were evaluated in the other cohorts to make an assessment.
Although roniciclib treatment caused a pharmacodynamic effect on cycle 1, day 1, no difference between fold-change in PCNA mRNA levels and best response (stable disease or progressive disease) was observed in the 31 samples evaluated from 15 patients with ovarian cancer and 16 patients with SCLC (data not shown). Progressive disease biomarker data from the patient who achieved a partial response in the 3 days on/4 days off schedule were not available; therefore, it was not possible to determine whether a pharmacodynamic effect was observed in blood samples from clinical responders.
Tumour response
No patients in the 4 weeks on/2 weeks off schedule achieved a complete or partial response; stable disease was achieved in five patients, giving a disease control rate (DCR) of 50% (95% CI, 18.71–81.29%). Duration of disease control in the five patients with stable disease ranged from 38 to 153 days. The patient experiencing stable disease for 153 days had otherwise refractory melanoma.
Of the 104 patients in the 3 days on/4 days off schedule with solid tumours, none achieved a complete response, one (1.0%) achieved a partial response, and 33 (31.7%) achieved stable disease, giving a response rate of 1.0% (95% CI, 0.0–5.2%) and a DCR of 32.7% (95% CI, 23.8–42.6%) according to Response Evaluation Criteria in Solid Tumors. Forty patients (38.5%) had radiological progressive disease and 26 (25.0%) were not assessed due to clinical progression or termination due to AEs. The patient with the partial response had ovarian cancer and was treated in cohort 8 (5/10 mg BID). In the 5 mg BID dose-expansion cohorts, the DCR was higher for patients with ovarian cancer (40.9% 95% CI, 20.7–63.7% n=22) than for patients with SCLC (17.4% 95% CI, 5.0–38.8% n=23). The DCR for patients with solid tumours bearing a CDK-related tumour mutation involving CDK signalling was 33.3% (95% CI, 4.3–77.7% n=6).
Of the seven patients in the lymphoid malignancy dose-escalation cohorts, none achieved a complete or partial response and three achieved stable disease, giving a DCR of 42.9% (95% CI, 9.9–81.6%); one patient was not assessed.
Discussion
The aim of the two phase I studies was to assess the safety and tolerability of the pan-CDK inhibitor roniciclib in patients with advanced malignancies. Two different schedules were investigated to determine the optimal dosing strategy.
In contrast to selective CDK4 and 6 inhibitors, pan-CDK inhibitors do not depend on the presence of an intact retinoblastoma gene to have anti-tumour activity (Siemeister et al, 2012). This also allows for their use in indications with frequent loss-of-function mutations. Pan-CDK inhibitors have been studied intensively over the past decade, and although there is a strong mechanistic rationale and promising preclinical data, compounds in development have not yet met expectations in clinical trials. Dinaciclib, an inhibitor of CDKs 1, 2, 5, and 9, has shown encouraging clinical activity as monotherapy and in combinations in phase I trials in advanced malignancies and leukaemia (Nemunaitis et al, 2013; Fabre et al, 2014); however, phase II studies in non-SCLC and advanced breast cancer were disappointing (Mita et al, 2014; Stephenson et al, 2014).
One reason for the failure of pan-CDK inhibitors is their harmful effect on cell-cycle progression of healthy tissues, mainly cells in the bone marrow and the gastrointestinal linings (Pevarello et al, 2010). Since pan-CDK inhibitors act on many phases of the cell cycle, sufficient exposure in the tumour tissue must be reached over its entire duration. It is therefore critical to identify the optimal dose and schedule to allow recovery of healthy tissue while delivering strong anti-proliferative activity. Roniciclib, due to its high solubility and good bioavailability in mice (Siemeister et al, 2012), was predicted to provide adequate exposure in the clinical setting.
In line with preclinical data demonstrating that prolonged exposure of roniciclib was needed to achieve strong anti-proliferative activity (Siemeister et al, 2012) and the predicted intermediate half-life, BID dosing was chosen for clinical trials.
The dosing schedule was not expected to impact on efficacy; however, a potential impact on the safety and tolerability was anticipated. Early toxicity after 7 days of treatment and subsequent patient dropout on the 4 weeks on/2 weeks off schedule suggested that continuous dosing for 4 weeks was not sustainable, so this schedule was terminated. In contrast, the intermittent dosing of roniciclib in the 3 days on/4 days off schedule allowed dose escalation and therefore a higher exposure, resulting in the MTD of 5/10 mg BID. The intermittent dosing schedule is also supported by efficacy data generated in animal studies, as dose-dependent efficacy was observed upon various dosing schedules ranging from once daily to cyclic intermittent dosing (Siemeister et al, 2012). As three additional cases of thromboembolic events (including one arterial event) occurred in the 5/10 mg BID dose-expansion cohorts, 5 mg BID was defined as the RP2D. In the 5 mg BID cohorts, the thromboembolic event rate was 7.9%, which is in the expected range in this patient population and time frame (Mandala et al, 2012; Khorana et al, 2013).
Overall, nausea, fatigue, diarrhoea, and vomiting were the most frequently reported roniciclib-related TEAEs of any grade, and patients received anti-emetic therapy as needed. The most frequently reported grade 3 roniciclib-related TEAEs were fatigue, nausea, anorexia, and hypotension, while only two drug-related grade 4 TEAEs were reported. In general, TEAEs were as anticipated given the mechanism of action of roniciclib and heavily pretreated patients. TEAEs, specifically gastrointestinal effects, were similar to what has been reported in clinical trials of other CDK inhibitors (Cicenas et al, 2014; Aleem and Arceci, 2015) and are likely to be related to the effects on rapidly cycling cells of the gastrointestinal lining (Kumar et al, 2015). Notably, no marked effect on neutrophils was observed. This may be because of the intermittent dosing schedule, allowing sufficient time for recovery, or because of an exposure below the threshold for cell-cycle inhibition in progenitor cells or induction of apoptosis in circulating neutrophils. Overall, with intermittent dosing, the safety profile was acceptable for use in cancer patients.
In the lymphoid malignancy cohort, the MTD was not specifically determined. No DLTs were reported in the two dose-escalation cohorts, and recruitment was stopped early because of a poor recruitment rate.
Roniciclib exhibited favourable PK properties with rapid and reliable absorption, dose-proportional increase in exposure in the dose range studied, and a half-life that supports BID administration. Detailed analysis of PK data derived in different species and in humans indicates that exposure with monotherapy in patients is ∼50% below simulated efficacious exposure in mice using monotherapy (unpublished data). However, that same exposure is in the range of efficacious exposure in mice treated with roniciclib in combination with chemotherapy (unpublished data). Because of lower exposure after consumption of a high-fat meal, roniciclib should be dosed 1 h before, or 2 h after, a meal.
In the 3 days on/4 days off schedule, the solid-tumour cohorts achieved a DCR of 32.7%. The ovarian cancer and SCLC expansion cohorts had DCRs of 40.9% and 17.4%, respectively. In the CDK-related tumour mutation cohort, there was a DCR of 33.3%. In the lymphoid malignancy cohorts, there was a DCR of 42.9%. Although no complete or partial responses were achieved in the expansion cohorts, these DCRs suggest that a potential benefit with roniciclib treatment cannot be excluded in this heavily pretreated patient population. Similar DCRs have been seen in phase I trials of other CDK inhibitors. In phase I trials in advanced malignancies, the CDK1, 2, 5, and 9 inhibitor dinaciclib demonstrated a DCR of 20.8% (Nemunaitis et al, 2013) and the CDK4 and 6 inhibitor palbociclib demonstrated a DCR of 27% (Flaherty et al, 2012). A phase II study of dinaciclib in previously treated patients with SCLC demonstrated no objective responses following treatment (Stephenson et al, 2014).
Proliferating cell nuclear antigen is expressed and can be determined in peripheral blood cells. Inhibition of PCNA has been proposed as a pharmacodynamic marker of cell proliferation. In this study, roniciclib significantly inhibited expression of PCNA in peripheral whole blood 6 h post dose on cycle 1, day 1 in patients with ovarian cancer and SCLC. This indicates biologically relevant exposure of roniciclib at the RP2D. Inhibition of PCNA expression was less pronounced on day 10.
In summary, data from two phase I trials of roniciclib, a pan-CDK inhibitor, demonstrate an acceptable safety profile and a moderate DCR in monotherapy using a 3 days on/4 days off schedule in doses up to 5 mg BID in patients with solid malignant tumours. Inhibition of PCNA was observed in peripheral blood cells, although the observed exposure is slightly lower than predicted efficacious exposure. Roniciclib combined with chemotherapy is being explored as first-line therapy in patients with advanced SCLC (NCT01573338 and NCT02161419).
Acknowledgments
Medical writing assistance was provided by Laura Badtke, PhD (Complete HealthVizion, Chicago, IL, USA) and Louise Picken, PhD (Complete HealthVizion, Macclesfield, UK), based on detailed discussion and feedback from all authors. The authors thank Ulf Buetehorn, PhD, for PK sample analysis and Susanne Reschke for PK calculations. This study was supported by Bayer AG.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
RG has received honoraria from Celgene, Roche, Baxalta, and Pfizer and has performed a consulting role for GlaxoSmithKline, Celgene, Roche, Bayer, Genentech, Clovis, Helsinn Healthcare, Astellas, and ARAID Pharmaceuticals. FB has received personal fees from AstraZeneca, Bristol-Myers Squibb, Eli Lilly Oncology, F Hoffmann-La Roche, Novartis, Merck, MSD, Pierre Fabre, and Pfizer. LG has: received grants from Roche; received personal fees from Roche, Eli Lilly, Bristol-Myers Squibb, Boehringer Ingelheim, and AstraZeneca; and received non-financial support from Roche, Eli Lilly, Boehringer Ingelheim, and AstraZeneca. MP has participated in advisory boards for Eli Lilly and Bristol-Myers Squibb. GZ has: received research funding from Roche; received travel, accommodation, and other expenses from Roche, Pfizer, AstraZeneca, and Bristol-Myers Squibb; performed a consulting or advisory role for Eli Lilly, Pfizer, and Boehringer Ingelheim; acted as a principal investigator in clinical trials for Roche, Bristol-Myers Squibb, Pfizer, and Boehringer Ingelheim; and received personal fees from Roche, Eli Lilly, Bristol-Myers Squibb, Pfizer, AstraZeneca, and Boehringer Ingelheim. GJW has received non-financial support from NantWorks and has received personal fees from Merck, Novartis, Viomics, Paradigm, Amgen, Pfizer, Celgene, Pharmatech, Blend Therapeutics, and Medscape. J-CS has participated in advisory boards for Bayer. AOW, DH, HN, MK, and MO are employees of Bayer AG. PR is an employee of Bayer HealthCare Pharmaceuticals, Inc. JG-O, GKD, BS, IR-C, DS, and RB have no conflicts of interest to declare.
Supplementary Material
References
- Aleem E, Arceci RJ (2015) Targeting cell cycle regulators in hematologic malignancies. Front Cell Dev Biol 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar U, Witkiewicz AK, Turner NC, Knudsen ES (2015) The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14: 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicenas J, Kalyan K, Sorokinas A, Jatulyte A, Valiunas D, Kaupinis A, Valius M (2014) Highlights of the latest advances in research on CDK inhibitors. Cancers 6: 2224–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre C, Gobbi M, Ezzili C, Zoubir M, Sablin MP, Small K, Im E, Shinwari N, Zhang D, Zhou H, Le Tourneau C (2014) Clinical study of the novel cyclin-dependent kinase inhibitor dinaciclib in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia patients. Cancer Chemother Pharmacol 74: 1057–1064. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, LoRusso PM, DeMichele A, Abramson VG, Courtney R, Randolph SS, Shaik MN, Wilner KD, O’Dwyer PJ, Schwartz GK (2012) Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 18: 568–576. [DOI] [PubMed] [Google Scholar]
- Khorana AA, Dalal M, Lin J, Connolly GC (2013) Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 119: 648–655. [DOI] [PubMed] [Google Scholar]
- Kumar SK, LaPlant B, Chng WJ, Zonder J, Callander N, Fonseca R, Fruth B, Roy V, Erlichman C, Stewart AK for the Mayo Phase 2 Consortium (2015) Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 125: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapenna S, Giordano A (2009) Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov 8: 547–566. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Mandala M, Clerici M, Corradino I, Vitalini C, Colombini S, Torri V, De Pascale A, Marsoni S (2012) Incidence, risk factors and clinical implications of venous thromboembolism in cancer patients treated within the context of phase I studies: the ‘SENDO experience’. Ann Oncol 23: 1416–1421. [DOI] [PubMed] [Google Scholar]
- Mita MM, Joy AA, Mita A, Sankhala K, Jou YM, Zhang D, Statkevich P, Zhu Y, Yao SL, Small K, Bannerji R, Shapiro CL (2014) Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer 14: 169–176. [DOI] [PubMed] [Google Scholar]
- Nemunaitis JJ, Small KA, Kirschmeier P, Zhang D, Zhu Y, Jou YM, Statkevich P, Yao SL, Bannerji R (2013) A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med 11: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P, Masui Y, Hartwell L (1998) Understanding the cell cycle. Nat Med 4: 1103–1106. [DOI] [PubMed] [Google Scholar]
- Pevarello P, Bischoff JR, Mercurio C (2010) Targeting cyclin-dependent kinases with small molecule inhibitors. In: Checkpoint Controls and Targets in Cancer Therapy, Siddik ZH (ed), pp 235–244. Humana Press: New York.
- Peyressatre M, Prével C, Pellerano M, Morris MC (2015) Targeting cyclin-dependent kinases in human cancers: from small molecules to peptide inhibitors. Cancers 7: 179–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemeister G, Lücking U, Wengner AM, Lienau P, Steinke W, Schatz C, Mumberg D, Ziegelbauer K (2012) BAY 1000394, a novel cyclin-dependent kinase inhibitor, with potent antitumor activity in mono- and in combination treatment upon oral application. Mol Cancer Ther 11: 2265–2273. [DOI] [PubMed] [Google Scholar]
- Spangler L, Wang X, Conaway JW, Conaway RC, Dvir A (2001) TFIIH action in transcription initiation and promoter escape requires distinct regions of downstream promoter DNA. Proc Natl Acad Sci USA 98: 5544–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson JJ, Nemunaitis J, Joy AA, Martin JC, Jou YM, Zhang D, Statkevich P, Yao SL, Zhu Y, Zhou H, Small K, Bannerji R, Edelman MJ (2014) Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer 83: 219–223. [DOI] [PubMed] [Google Scholar]
- Stoimenov I, Helleday T (2009) PCNA on the crossroad of cancer. Biochem Soc Trans 37: 605–613. [DOI] [PubMed] [Google Scholar]
- Witkiewicz AK, Knudsen ES (2014) Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res 16: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.