Abstract
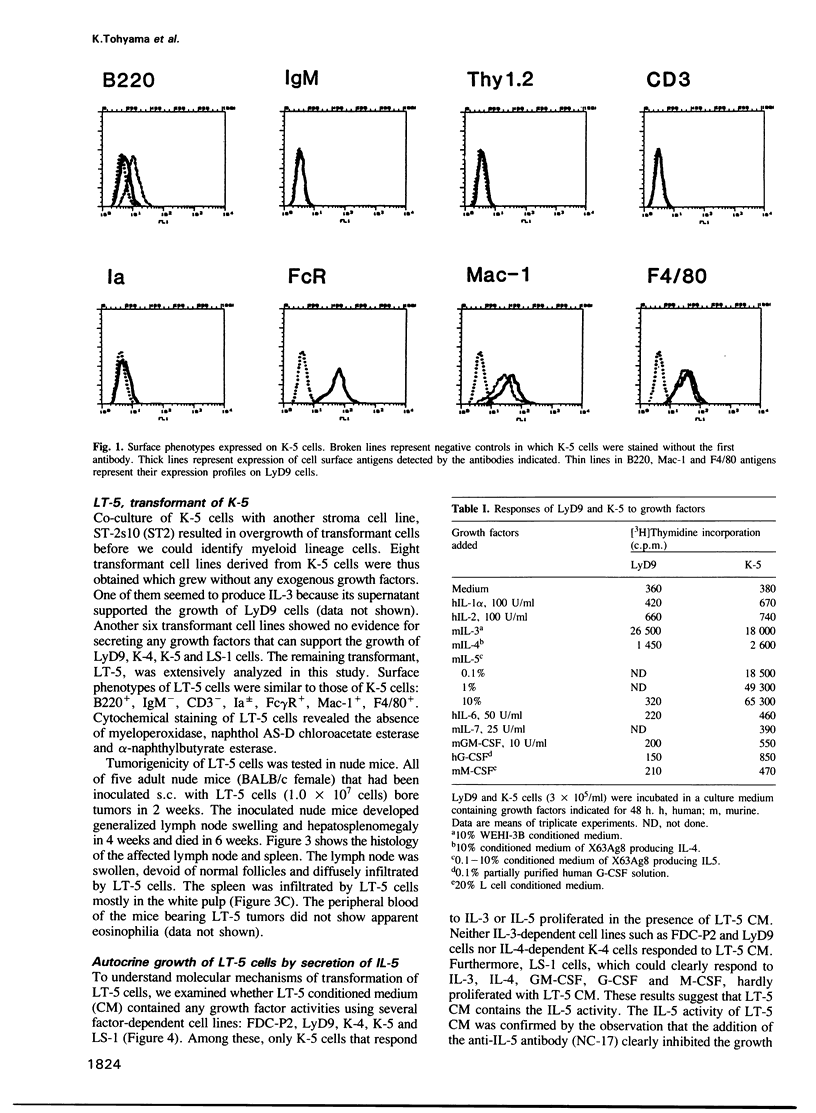
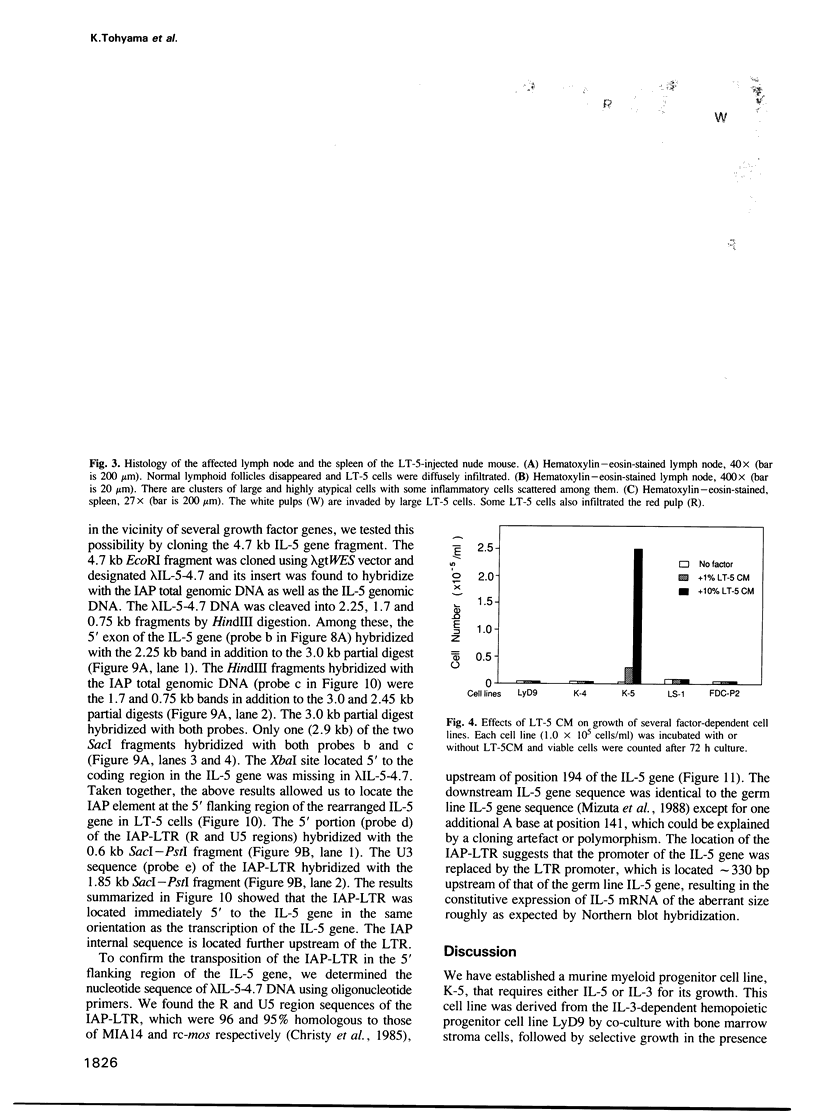
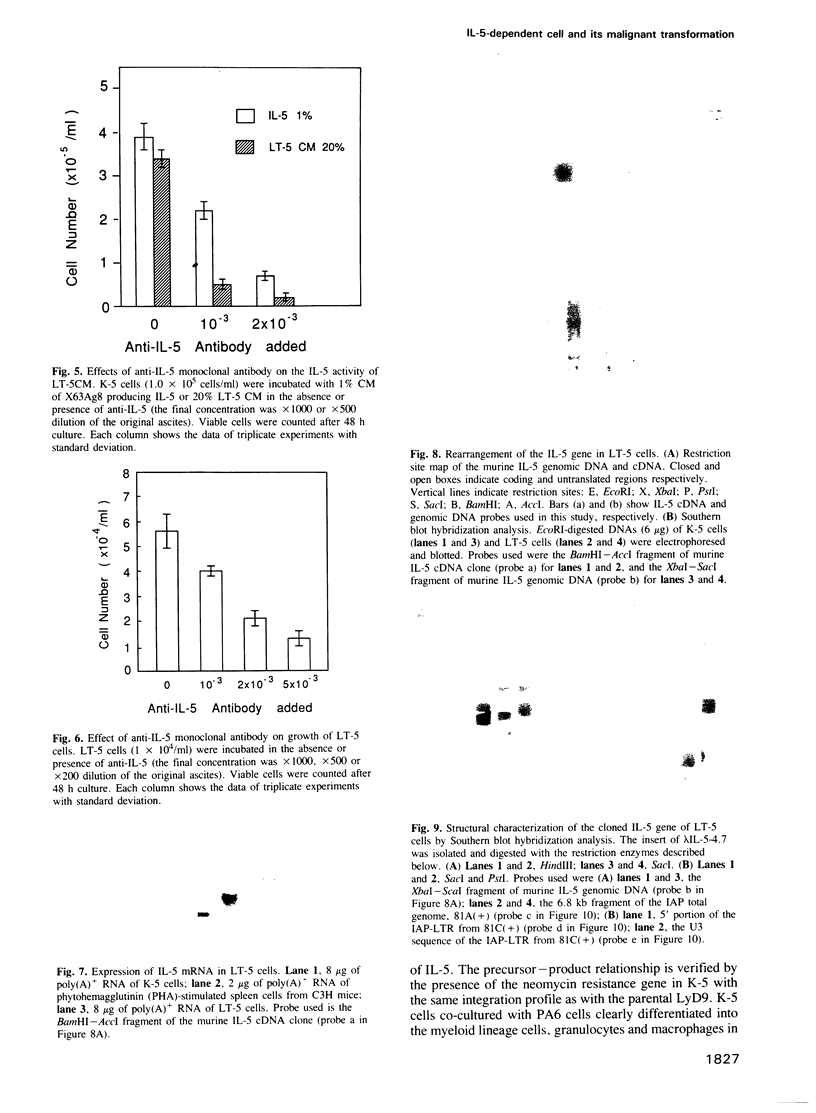
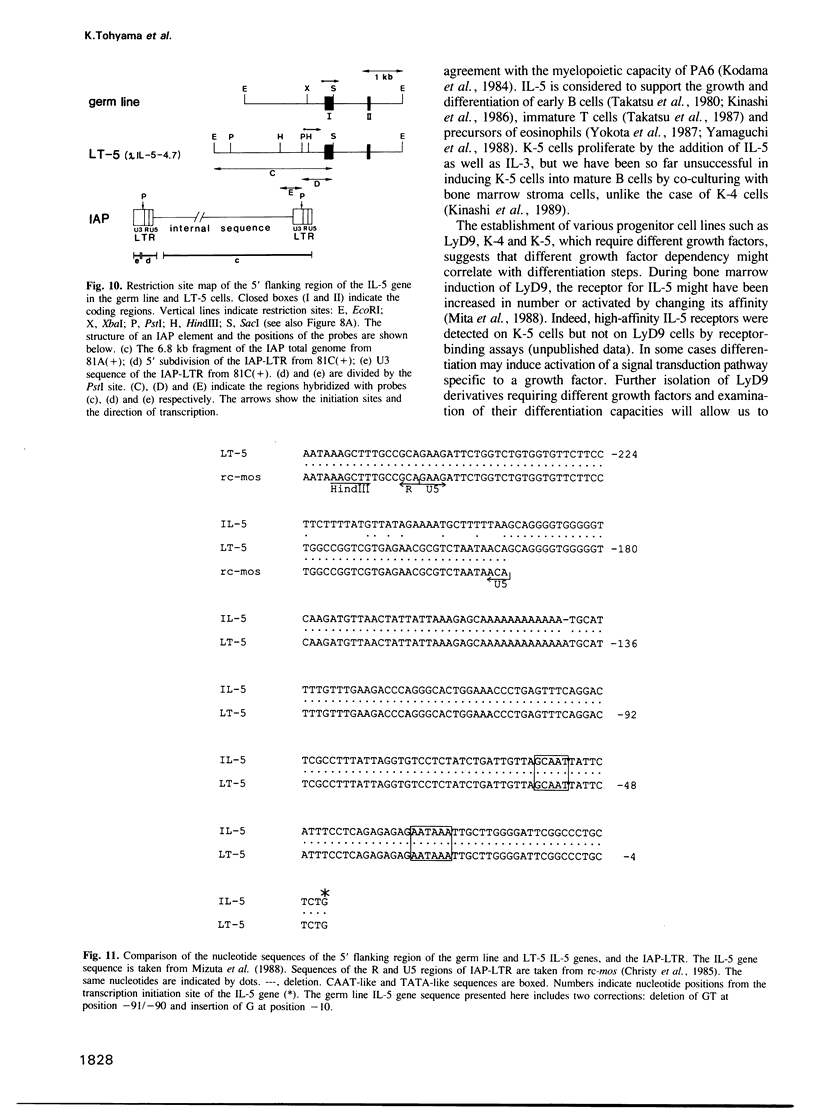
An interleukin-5 (IL-5)-dependent subclone, K-5, was established from an IL-3-dependent murine hemopoietic progenitor cell line by co-culturing with bone marrow stroma cells. K-5 cells were induced to differentiate into myeloid lineage cells by co-culturing with cloned PA6 stroma cells. By co-culturing with another cloned stroma cell (ST-2s10), K-5 cells gave rise to a factor-independent transformant cell line LT-5 which proliferated in an autocrine manner by secretion of IL-5 and produced tumors in nude mice. Molecular cloning of the IL-5 gene of LT-5 cells and the nucleotide sequencing of its 5' flanking region indicate that a transposition of an intracisternal A-particle (IAP) element to the 5' flanking region of the IL-5 gene is responsible for the constitutive expression of IL-5 mRNA of an aberrant size in LT-5 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. A., Pierce J. H., Watson C. J., Falco J., Ihle J. N., Paul W. E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987 Aug 28;50(5):809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Canaani E., Dreazen O., Klar A., Rechavi G., Ram D., Cohen J. B., Givol D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7118–7122. doi: 10.1073/pnas.80.23.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Ono M., Huang R. C. Terminally redundant sequences in cellular intracisternal A-particle genes. J Virol. 1981 May;38(2):680–687. doi: 10.1128/jvi.38.2.680-687.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Harada N., Takahashi T., Matsumoto M., Kinashi T., Ohara J., Kikuchi Y., Koyama N., Severinson E., Yaoita Y., Honjo T. Production of a monoclonal antibody useful in the molecular characterization of murine T-cell-replacing factor/B-cell growth factor II. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4581–4585. doi: 10.1073/pnas.84.13.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Kajigaya S., Suda T., Suda J., Saito M., Miura Y., Iizuka M., Kobayashi S., Minato N., Sudo T. A recombinant murine granulocyte/macrophage (GM) colony-stimulating factor derived from an inducer T cell line (IH5.5). Functional restriction to GM progenitor cells. J Exp Med. 1986 Oct 1;164(4):1102–1113. doi: 10.1084/jem.164.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Tohyama N., Tada T. Autocrine growth and tumorigenicity of interleukin 2-dependent helper T cells transfected with IL-2 gene. J Exp Med. 1989 Jan 1;169(1):13–25. doi: 10.1084/jem.169.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Kinashi T., Inaba K., Tsubata T., Tashiro K., Palacios R., Honjo T. Differentiation of an interleukin 3-dependent precursor B-cell clone into immunoglobulin-producing cells in vitro. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4473–4477. doi: 10.1073/pnas.85.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi T., Tashiro K., Inaba K., Takeda T., Palacios R., Honjo T. An interleukin-4-dependent precursor clone is an intermediate of the differentiation pathway from an interleukin-3-dependent precursor clone into myeloid cells as well as B lymphocytes. Int Immunol. 1989;1(1):11–19. doi: 10.1093/intimm/1.1.11. [DOI] [PubMed] [Google Scholar]
- Kodama H. A., Amagai Y., Koyama H., Kasai S. A new preadipose cell line derived from newborn mouse calvaria can promote the proliferation of pluripotent hemopoietic stem cells in vitro. J Cell Physiol. 1982 Jul;112(1):89–95. doi: 10.1002/jcp.1041120114. [DOI] [PubMed] [Google Scholar]
- Kodama H., Sudo H., Koyama H., Kasai S., Yamamoto S. In vitro hemopoiesis within a microenvironment created by MC3T3-G2/PA6 preadipocytes. J Cell Physiol. 1984 Mar;118(3):233–240. doi: 10.1002/jcp.1041180303. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Harada N., Naomi S., Hitoshi Y., Sakamoto K., Akagi M., Tominaga A., Takatsu K. Receptors for T cell-replacing factor/interleukin 5. Specificity, quantitation, and its implication. J Exp Med. 1988 Sep 1;168(3):863–878. doi: 10.1084/jem.168.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta T. R., Tanabe T., Nakakubo H., Noma T., Honjo T. Molecular cloning and structure of the mouse interleukin-5 gene. Growth Factors. 1988;1(1):51–57. doi: 10.3109/08977198809000246. [DOI] [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Nishikawa S., Ikuta K., Yamamura F., Naito M., Takahashi K., Nishikawa S. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J. 1988 May;7(5):1337–1343. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Karasuyama H., Rolink A. Ly1+ PRO-B lymphocyte clones. Phenotype, growth requirements and differentiation in vitro and in vivo. EMBO J. 1987 Dec 1;6(12):3687–3693. doi: 10.1002/j.1460-2075.1987.tb02702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stocking C., Löliger C., Kawai M., Suciu S., Gough N., Ostertag W. Identification of genes involved in growth autonomy of hematopoietic cells by analysis of factor-independent mutants. Cell. 1988 Jun 17;53(6):869–879. doi: 10.1016/s0092-8674(88)90329-7. [DOI] [PubMed] [Google Scholar]
- Takatsu K., Kikuchi Y., Takahashi T., Honjo T., Matsumoto M., Harada N., Yamaguchi N., Tominaga A. Interleukin 5, a T-cell-derived B-cell differentiation factor also induces cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4234–4238. doi: 10.1073/pnas.84.12.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu K., Tominaga A., Hamaoka T. Antigen-induced T cell-replacing factor (TRF). I. Functional characterization of a TRF-producing helper T cell subset and genetic studies on TRF production. J Immunol. 1980 May;124(5):2414–2422. [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Shadduck R. K. Purification and properties of L cell-derived colony-stimulating factor. J Lab Clin Med. 1979 Jul;94(1):180–193. [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Suda T., Suda J., Eguchi M., Miura Y., Harada N., Tominaga A., Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988 Jan 1;167(1):43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]
- Yokota T., Coffman R. L., Hagiwara H., Rennick D. M., Takebe Y., Yokota K., Gemmell L., Shrader B., Yang G., Meyerson P. Isolation and characterization of lymphokine cDNA clones encoding mouse and human IgA-enhancing factor and eosinophil colony-stimulating factor activities: relationship to interleukin 5. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7388–7392. doi: 10.1073/pnas.84.21.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]