Abstract
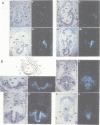
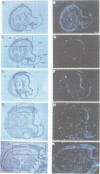
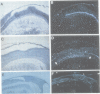
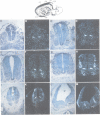
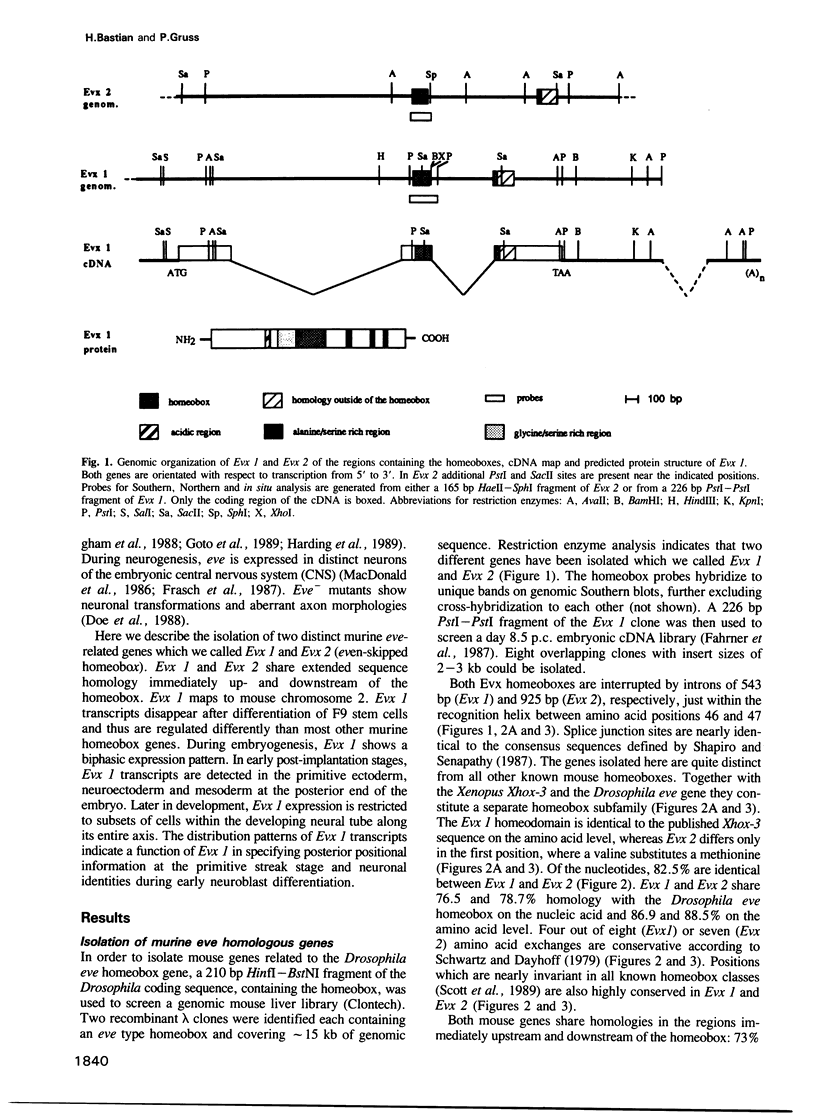
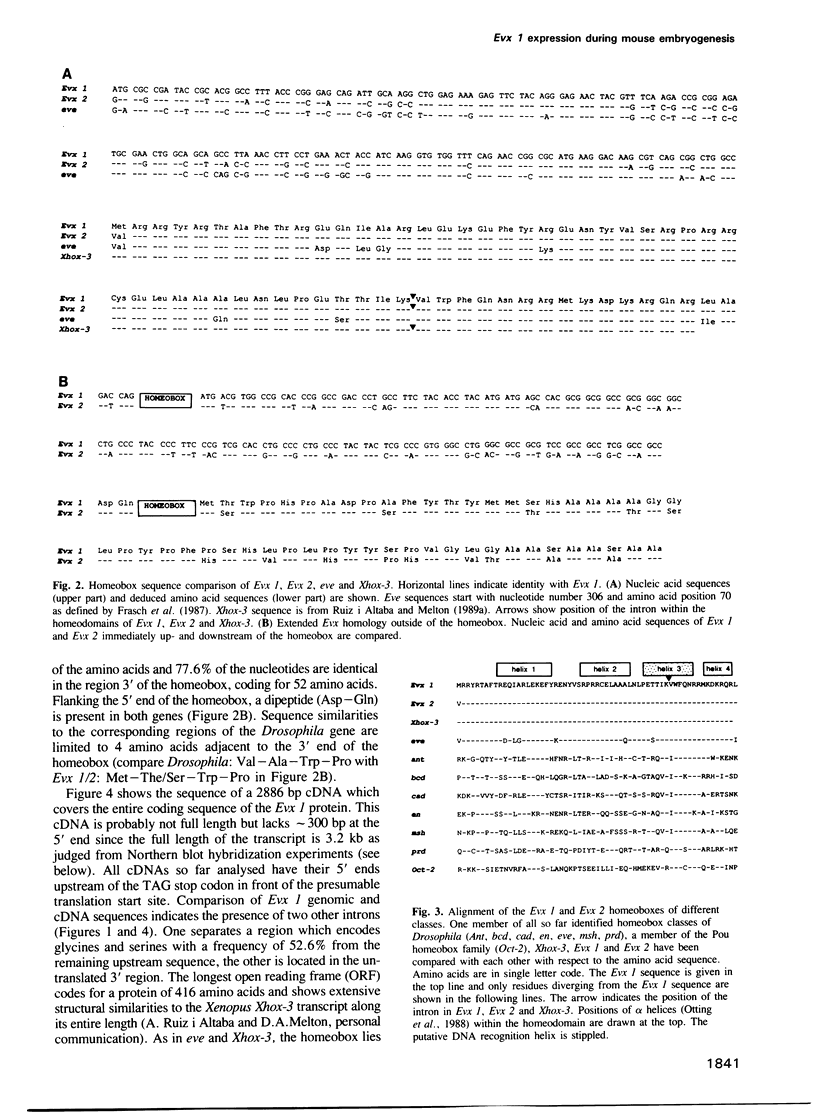
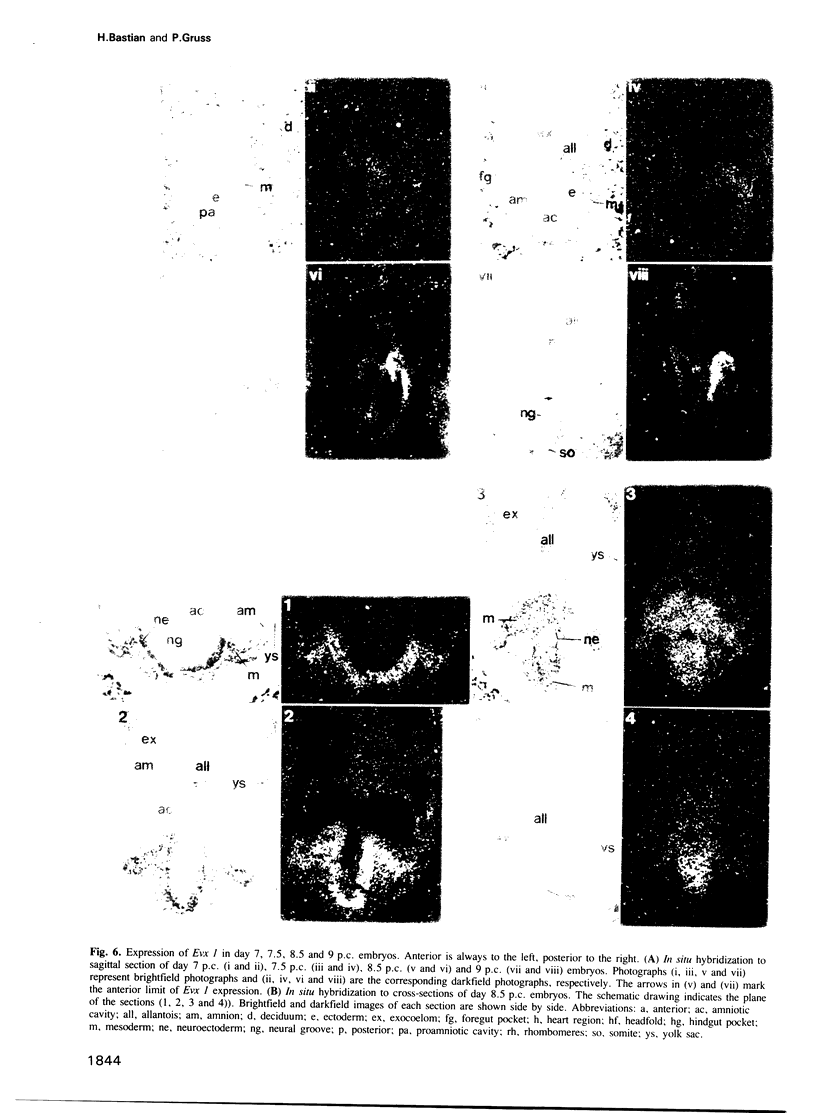
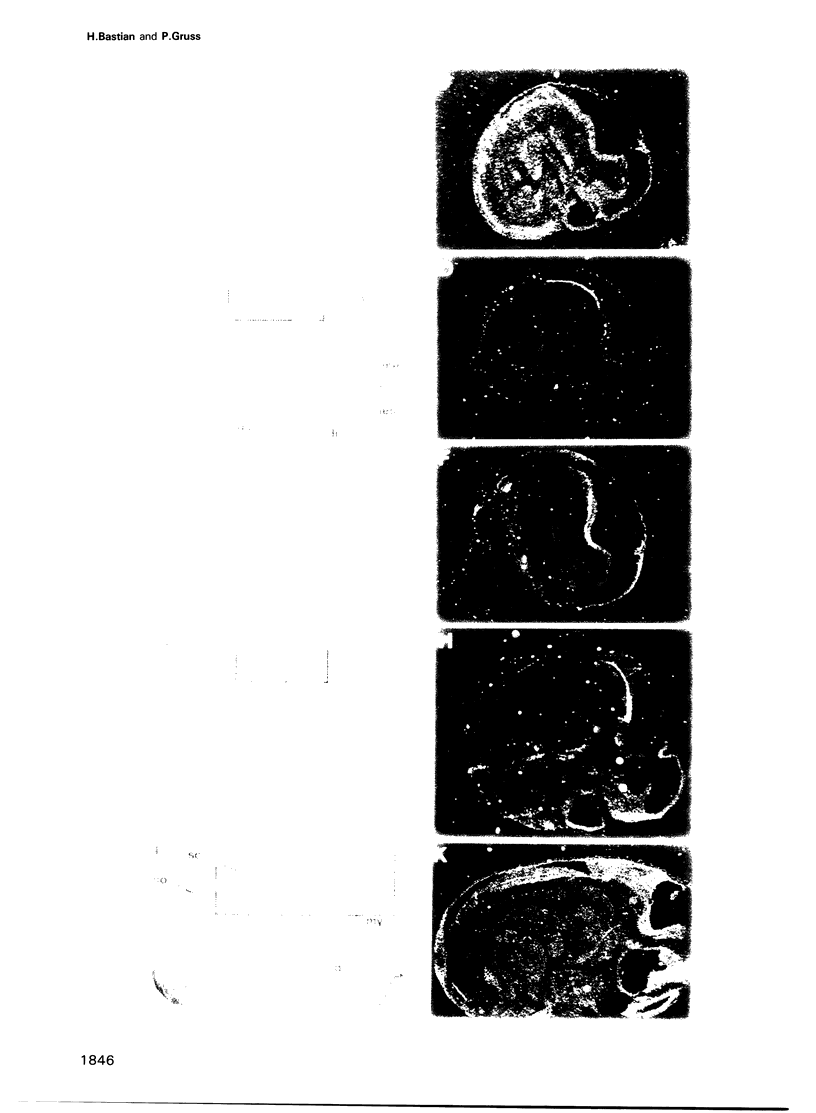
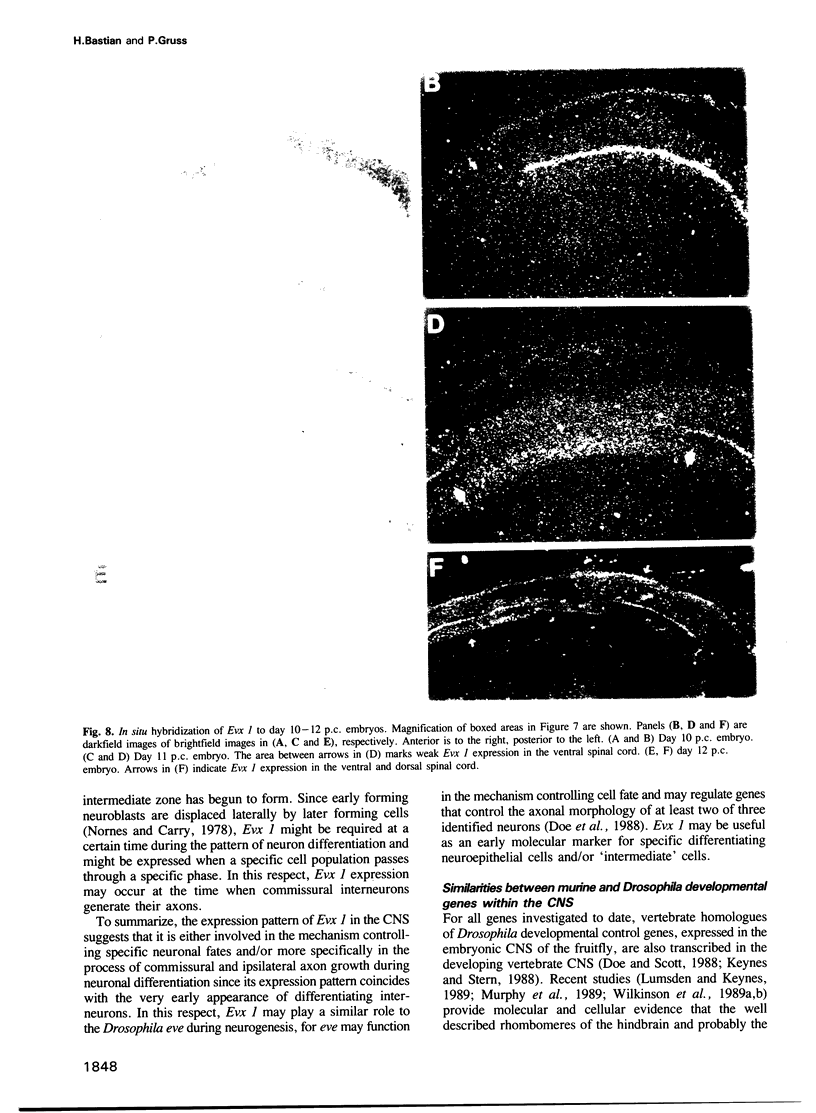
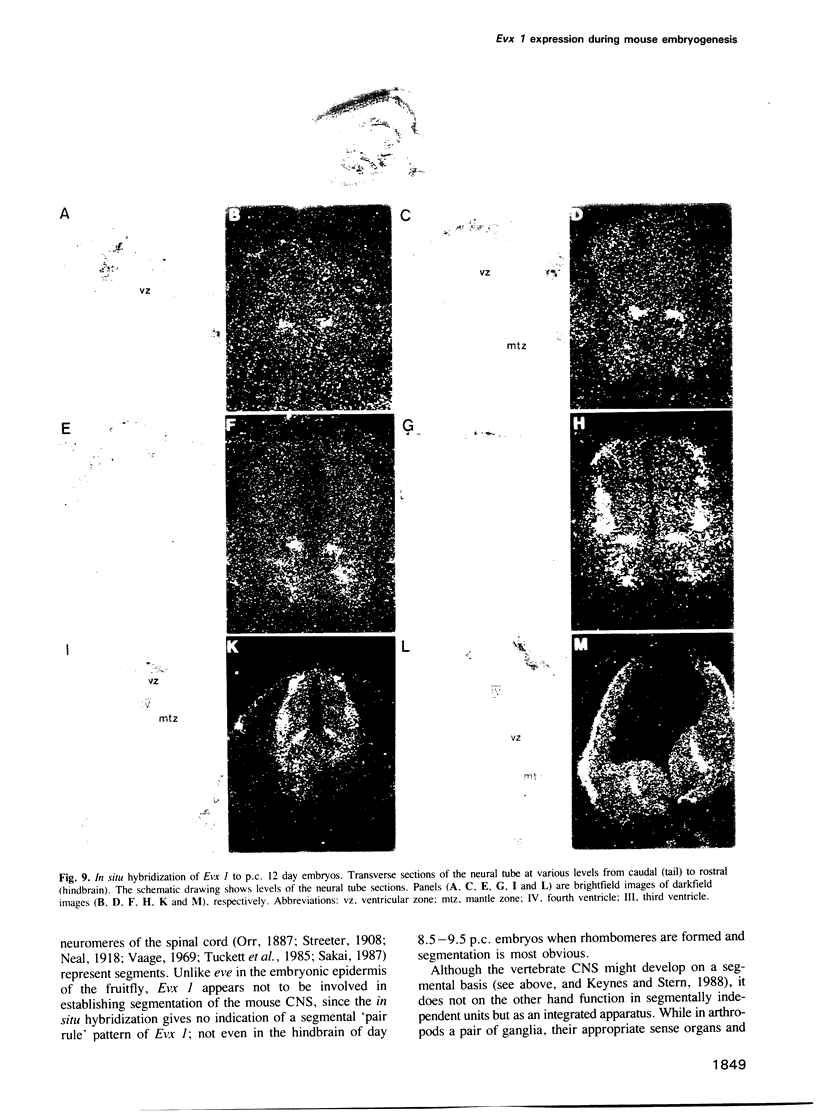
Using the Drosophila even-skipped (eve) homeobox as a probe, we have isolated two murine genes, Evx 1 and Evx 2, from a genomic library. Evx 1, Evx 2, eve and the Xenopus Xhox-3 constitute a family of related genes based on similar homeodomain sequences. In addition, Evx 1 and Evx 2 share extended amino acid conservation outside of the homeobox. The Evx 1 protein consists of 416 amino acids as deduced from the longest open reading frame of Evx 1 cDNAs. Evx 1 is located 3.7 cM from the Hox 5 locus on mouse chromosome 2. It is expressed in undifferentiated F9 stem cells but not in cells differentiated with retinoic acid and cAMP. During embryogenesis, Evx 1 shows a biphasic expression pattern. From days 7 to 9 p.c. Evx 1 expression emerges at the posterior end of the embryo within the primitive ectoderm, and later in the mesoderm and neuroectoderm. From days 10 to 12.5 p.c. Evx 1 transcripts are restricted to specific cells within the neural tube and hindbrain along their entire lengths and coincides temporally, as well as spatially, with maturation of early forming interneurons, possibly commissural interneurons. The early and late transcription pattern is compatible with a role of Evx 1 in specifying posterior positional information along the embryonic axis similar to the Xenopus Xhox-3 and in specifying neuronal cell fates within the differentiating neural tube in analogy to eve in the embryonic central nervous system of Drosophila, respectively.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Balling R., Deutsch U., Gruss P. undulated, a mutation affecting the development of the mouse skeleton, has a point mutation in the paired box of Pax 1. Cell. 1988 Nov 4;55(3):531–535. doi: 10.1016/0092-8674(88)90039-6. [DOI] [PubMed] [Google Scholar]
- Balling R., Mutter G., Gruss P., Kessel M. Craniofacial abnormalities induced by ectopic expression of the homeobox gene Hox-1.1 in transgenic mice. Cell. 1989 Jul 28;58(2):337–347. doi: 10.1016/0092-8674(89)90848-9. [DOI] [PubMed] [Google Scholar]
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Tjian R. A purified Drosophila homeodomain protein represses transcription in vitro. Cell. 1989 Aug 11;58(3):433–440. doi: 10.1016/0092-8674(89)90424-8. [DOI] [PubMed] [Google Scholar]
- Bopp D., Burri M., Baumgartner S., Frigerio G., Noll M. Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell. 1986 Dec 26;47(6):1033–1040. doi: 10.1016/0092-8674(86)90818-4. [DOI] [PubMed] [Google Scholar]
- Bopp D., Jamet E., Baumgartner S., Burri M., Noll M. Isolation of two tissue-specific Drosophila paired box genes, Pox meso and Pox neuro. EMBO J. 1989 Nov;8(11):3447–3457. doi: 10.1002/j.1460-2075.1989.tb08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri M., Tromvoukis Y., Bopp D., Frigerio G., Noll M. Conservation of the paired domain in metazoans and its structure in three isolated human genes. EMBO J. 1989 Apr;8(4):1183–1190. doi: 10.1002/j.1460-2075.1989.tb03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. W., Goetz J., Wright C. V., Fritz A., Hardwicke J., De Robertis E. M. Differential utilization of the same reading frame in a Xenopus homeobox gene encodes two related proteins sharing the same DNA-binding specificity. EMBO J. 1988 Jul;7(7):2139–2149. doi: 10.1002/j.1460-2075.1988.tb03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D., Graham E., Sime C., Hill R. A gene with sequence similarity to Drosophila engrailed is expressed during the development of the neural tube and vertebrae in the mouse. Development. 1988 Oct;104(2):305–316. doi: 10.1242/dev.104.2.305. [DOI] [PubMed] [Google Scholar]
- Davis C. A., Joyner A. L. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988 Dec;2(12B):1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Oliver G., Wright C. V. Determination of axial polarity in the vertebrate embryo: homeodomain proteins and homeogenetic induction. Cell. 1989 Apr 21;57(2):189–191. doi: 10.1016/0092-8674(89)90954-9. [DOI] [PubMed] [Google Scholar]
- Dearolf C. R., Topol J., Parker C. S. The caudal gene product is a direct activator of fushi tarazu transcription during Drosophila embryogenesis. Nature. 1989 Sep 28;341(6240):340–343. doi: 10.1038/341340a0. [DOI] [PubMed] [Google Scholar]
- Deutsch U., Dressler G. R., Gruss P. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell. 1988 May 20;53(4):617–625. doi: 10.1016/0092-8674(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Scott M. P. Segmentation and homeotic gene function in the developing nervous system of Drosophila. Trends Neurosci. 1988 Mar;11(3):101–106. doi: 10.1016/0166-2236(88)90154-3. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Smouse D., Goodman C. S. Control of neuronal fate by the Drosophila segmentation gene even-skipped. Nature. 1988 May 26;333(6171):376–378. doi: 10.1038/333376a0. [DOI] [PubMed] [Google Scholar]
- Dressler G. R., Gruss P. Do multigene families regulate vertebrate development? Trends Genet. 1988 Aug;4(8):214–219. doi: 10.1016/s0168-9525(88)80003-9. [DOI] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprey P., Chowdhury K., Dressler G. R., Balling R., Simon D., Guenet J. L., Gruss P. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev. 1988 Dec;2(12A):1647–1654. doi: 10.1101/gad.2.12a.1647. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Davidson D. Somitogenesis in amphibia. IV. The dynamics of tail development. J Embryol Exp Morphol. 1983 Aug;76:157–176. [PubMed] [Google Scholar]
- FUJITA S. ANALYSIS OF NEURON DIFFERENTIATION IN THE CENTRAL NERVOUS SYSTEM BY TRITIATED THYMIDINE AUTORADIOGRAPHY. J Comp Neurol. 1964 Jun;122:311–327. doi: 10.1002/cne.901220303. [DOI] [PubMed] [Google Scholar]
- Fahrner K., Hogan B. L., Flavell R. A. Transcription of H-2 and Qa genes in embryonic and adult mice. EMBO J. 1987 May;6(5):1265–1271. doi: 10.1002/j.1460-2075.1987.tb02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone M. S., Baron A., Gaunt S. J., Mattei M. G., Duboule D. Hox-5.1 defines a homeobox-containing gene locus on mouse chromosome 2. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4760–4764. doi: 10.1073/pnas.85.13.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frasch M., Hoey T., Rushlow C., Doyle H., Levine M. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 1987 Mar;6(3):749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M., Warrior R., Tugwood J., Levine M. Molecular analysis of even-skipped mutants in Drosophila development. Genes Dev. 1988 Dec;2(12B):1824–1838. doi: 10.1101/gad.2.12b.1824. [DOI] [PubMed] [Google Scholar]
- Goto T., Macdonald P., Maniatis T. Early and late periodic patterns of even skipped expression are controlled by distinct regulatory elements that respond to different spatial cues. Cell. 1989 May 5;57(3):413–422. doi: 10.1016/0092-8674(89)90916-1. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Harding K., Hoey T., Warrior R., Levine M. Autoregulatory and gap gene response elements of the even-skipped promoter of Drosophila. EMBO J. 1989 Apr;8(4):1205–1212. doi: 10.1002/j.1460-2075.1989.tb03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. P., Melton D. A. Microinjection of synthetic Xhox-1A homeobox mRNA disrupts somite formation in developing Xenopus embryos. Cell. 1988 Jun 3;53(5):687–697. doi: 10.1016/0092-8674(88)90087-6. [DOI] [PubMed] [Google Scholar]
- Hoey T., Levine M. Divergent homeo box proteins recognize similar DNA sequences in Drosophila. Nature. 1988 Apr 28;332(6167):858–861. doi: 10.1038/332858a0. [DOI] [PubMed] [Google Scholar]
- Hoey T., Warrior R., Manak J., Levine M. DNA-binding activities of the Drosophila melanogaster even-skipped protein are mediated by its homeo domain and influenced by protein context. Mol Cell Biol. 1988 Nov;8(11):4598–4607. doi: 10.1128/mcb.8.11.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Holley J. A., Nornes H. O., Morita M. Guidance of neuritic growth in the transverse plane of embryonic mouse spinal cord. J Comp Neurol. 1982 Mar 10;205(4):360–370. doi: 10.1002/cne.902050405. [DOI] [PubMed] [Google Scholar]
- Ingham P. W., Baker N. E., Martinez-Arias A. Regulation of segment polarity genes in the Drosophila blastoderm by fushi tarazu and even skipped. Nature. 1988 Jan 7;331(6151):73–75. doi: 10.1038/331073a0. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Martin G. R. En-1 and En-2, two mouse genes with sequence homology to the Drosophila engrailed gene: expression during embryogenesis. Genes Dev. 1987 Mar;1(1):29–38. doi: 10.1101/gad.1.1.29. [DOI] [PubMed] [Google Scholar]
- Lumsden A., Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989 Feb 2;337(6206):424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Ingham P., Struhl G. Isolation, structure, and expression of even-skipped: a second pair-rule gene of Drosophila containing a homeo box. Cell. 1986 Dec 5;47(5):721–734. doi: 10.1016/0092-8674(86)90515-5. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- Moffie D., Hamburger H. L. Pain and neuroma formation in Wallenberg's lateral medullary syndrome. Clin Neurol Neurosurg. 1986;88(3):217–221. doi: 10.1016/s0303-8467(86)80033-6. [DOI] [PubMed] [Google Scholar]
- Murphy P., Davidson D. R., Hill R. E. Segment-specific expression of a homoeobox-containing gene in the mouse hindbrain. Nature. 1989 Sep 14;341(6238):156–159. doi: 10.1038/341156a0. [DOI] [PubMed] [Google Scholar]
- Nornes H. O., Carry M. Neurogenesis in spinal cord of mouse: an autoradiographic analysis. Brain Res. 1978 Dec 22;159(1):1–6. doi: 10.1016/0006-8993(78)90105-1. [DOI] [PubMed] [Google Scholar]
- Nornes H. O., Das G. D. Temporal pattern of neurogenesis in spinal cord of rat. I. An autoradiographic study--time and sites of origin and migration and settling patterns of neuroblasts. Brain Res. 1974 Jun 14;73(1):121–138. doi: 10.1016/0006-8993(74)91011-7. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Kluding H., Jürgens G. Genes affecting the segmental subdivision of the Drosophila embryo. Cold Spring Harb Symp Quant Biol. 1985;50:145–154. doi: 10.1101/sqb.1985.050.01.020. [DOI] [PubMed] [Google Scholar]
- Otting G., Qian Y. Q., Müller M., Affolter M., Gehring W., Wüthrich K. Secondary structure determination for the Antennapedia homeodomain by nuclear magnetic resonance and evidence for a helix-turn-helix motif. EMBO J. 1988 Dec 20;7(13):4305–4309. doi: 10.1002/j.1460-2075.1988.tb03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. H., Martin-Blanco E., Coleman K. G., Poole S. J., Ellis M. C., Kornberg T. B., Goodman C. S. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989 Sep 8;58(5):955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Schafer B., Goodman C. S., Holmgren R. The role of segment polarity genes during Drosophila neurogenesis. Genes Dev. 1989 Jun;3(6):890–904. doi: 10.1101/gad.3.6.890. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Robert B., Sassoon D., Jacq B., Gehring W., Buckingham M. Hox-7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J. 1989 Jan;8(1):91–100. doi: 10.1002/j.1460-2075.1989.tb03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Bimodal and graded expression of the Xenopus homeobox gene Xhox3 during embryonic development. Development. 1989 May;106(1):173–183. doi: 10.1242/dev.106.1.173. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Interaction between peptide growth factors and homoeobox genes in the establishment of antero-posterior polarity in frog embryos. Nature. 1989 Sep 7;341(6237):33–38. doi: 10.1038/341033a0. [DOI] [PubMed] [Google Scholar]
- SIDMAN R. L., MIALE I. L., FEDER N. Cell proliferation and migration in the primitive ependymal zone: an autoradiographic study of histogenesis in the nervous system. Exp Neurol. 1959 Oct;1:322–333. doi: 10.1016/0014-4886(59)90024-x. [DOI] [PubMed] [Google Scholar]
- Sakai Y. Neurulation in the mouse. I. The ontogenesis of neural segments and the determination of topographical regions in a central nervous system. Anat Rec. 1987 Aug;218(4):450–457. doi: 10.1002/ar.1092180414. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schughart K., Kappen C., Ruddle F. H. Duplication of large genomic regions during the evolution of vertebrate homeobox genes. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7067–7071. doi: 10.1073/pnas.86.18.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa L. D., Silan C. M., Justice M. J., Mercer J. A., Bauskin A. R., Ben-Neriah Y., Duboule D., Hastie N. D., Copeland N. G., Jenkins N. A. A molecular genetic linkage map of mouse chromosome 2. Genomics. 1990 Mar;6(3):491–504. doi: 10.1016/0888-7543(90)90479-e. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Gillespie L. L., Godsave S. F., Isaacs H. V., Paterno G. D. The role of fibroblast growth factor in early Xenopus development. Development. 1989;107 (Suppl):141–148. doi: 10.1242/dev.107.Supplement.141. [DOI] [PubMed] [Google Scholar]
- Smart I. H. Proliferative characteristics of the ependymal layer during the early development of the spinal cord in the mouse. J Anat. 1972 Apr;111(Pt 3):365–380. [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Cooke J., Green J. B., Howes G., Symes K. Inducing factors and the control of mesodermal pattern in Xenopus laevis. Development. 1989;107 (Suppl):149–159. doi: 10.1242/dev.107.Supplement.149. [DOI] [PubMed] [Google Scholar]
- Stern C. D., Keynes R. J. Spatial patterns of homeobox gene expression in the developing mammalian CNS. Trends Neurosci. 1988 May;11(5):190–192. doi: 10.1016/0166-2236(88)90120-8. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Tam P. P. The histogenetic capacity of tissues in the caudal end of the embryonic axis of the mouse. J Embryol Exp Morphol. 1984 Aug;82:253–266. [PubMed] [Google Scholar]
- Tuckett F., Lim L., Morriss-Kay G. M. The ontogenesis of cranial neuromeres in the rat embryo. I. A scanning electron microscope and kinetic study. J Embryol Exp Morphol. 1985 Jun;87:215–228. [PubMed] [Google Scholar]
- Wentworth L. E. The development of the cervical spinal cord of the mouse embryo. II. A Golgi analysis of sensory, commissural, and association cell differentiation. J Comp Neurol. 1984 Jan 1;222(1):96–115. doi: 10.1002/cne.902220109. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Chavrier P., Bravo R., Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989 Feb 2;337(6206):461–464. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Behringer R. R., Mostoller M. P., Brinster R. L., Palmiter R. D. Transgenic mice overexpressing the mouse homoeobox-containing gene Hox-1.4 exhibit abnormal gut development. Nature. 1989 Feb 2;337(6206):464–467. doi: 10.1038/337464a0. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Cho K. W., Oliver G., De Robertis E. M. Vertebrate homeodomain proteins: families of region-specific transcription factors. Trends Biochem Sci. 1989 Feb;14(2):52–56. doi: 10.1016/0968-0004(89)90043-1. [DOI] [PubMed] [Google Scholar]