Abstract
Altered brain somatostatin functions recently appeared as key elements for the pathogenesis of stress-related neuropsychiatric disorders. The hippocampus exerts an inhibitory feedback on stress but the mechanisms involved remain unclear. We investigated herein the role of hippocampal somatostatin receptor subtypes in both stress response and behavioral emotionality using C57BL/6, wild type and sst2 or sst4 knockout mice. Inhibitory effects of hippocampal infusions of somatostatin agonists on stress-induced hypothalamo-pituitary-adrenal axis (HPA) activity were tested by monitoring peripheral blood and local hippocampus corticosterone levels, the latter by using microdialysis. Anxiolytic and antidepressant-like effects were determined in the elevated-plus maze, open field, forced swimming, and stress-sensitive beam walking tests. Hippocampal injections of somatostatin analogs and sst2 or sst4, but not sst1 or sst3 receptor agonists produced rapid and sustained inhibition of HPA axis. sst2 agonists selectively produced anxiolytic-like behaviors whereas both sst2 and sst4 agonists had antidepressant-like effects. Consistent with these findings, high corticosterone levels and anxiety were found in sst2KO mice and depressive-like behaviors observed in both sst2KO and sst4KO strains. Both hippocampal sst2 and sst4 receptors selectively inhibit stress-induced HPA axis activation but mediate anxiolytic and antidepressive effects through distinct mechanisms. Such results are to be accounted for in development of pathway-specific somatostatin receptor agents in the treatment of hypercortisolism (Cushing’s disease) and stress-related neuropsychiatric disorders.
INTRODUCTION
Stress has an important role in the pathogenesis of neuropsychiatric disorders such as major depression (Lin and Sibille, 2015a; McEwen et al, 2015; Pittenger and Duman, 2008). The main effect of stress is to activate the hypothalamo-pituitary-adrenal axis (HPA), leading to release of glucocorticoids from adrenal glands into the blood. Because of their liposolubility, glucocorticoids reach the brain by crossing the blood–brain barrier and bind to glucocorticoid and mineralocorticoid receptors (Joels, 2008). The hippocampus contains the highest concentration of glucocorticoid receptors and has a key role for negative feedback regulations on the HPA axis (Herman et al, 2005; McEwen et al, 2015). High glucocorticoid levels contribute to hippocampal dysfunctions and may lead to hippocampal atrophy as observed in major depression or Cushing’s disease (Andela et al, 2015; Sheline, 1996). Ablation of the dorsal hippocampus or lateral fornix disrupts the diurnal corticosteroid rhythm and elevates resting corticosteroids in rodents (Fendler et al, 1961). The intrinsic hippocampal mechanisms responsible for this effect remain unclear, but transynaptic glutamatergic projections from GABA neurons in the peri-paraventricular region and the bed nucleus of the stria terminalis may be involved (Herman and Mueller, 2006; Radley and Sawchenko, 2011). As mineralocorticoid and glucocorticoid receptors are predominantly expressed in principal cells, previous studies concerning corticosteroid effects on neuronal activity in the hippocampus focused mainly on these neurons (Joels, 2008). However, chronic stress also impairs GABAergic functions in the hippocampus by affecting the integrity of parvalbumin-expressing neurons (Hu et al, 2010) and somatostatin (SOM)-expressing cells (Czeh et al, 2015). In vivo, rapid SOM release occurs in the dorsal dentate gyrus when acute stress or dexamethasone is applied to animals (Arancibia et al, 2001), suggesting a role for SOM in the hippocampal regulatory feedback on stress. In addition, mice lacking SOM exhibit elevated behavioral emotionality, high basal plasma corticosterone (CORT) levels, and reduced gene expression of BDNF, cortistatin and GAD67, together recapitulating behavioral, neuroendocrine and molecular features of human depression (Lin and Sibille, 2015a).
Five somatostatin receptor subtypes have been described, sst1, sst2, sst3, sst4, and sst5. The development of selective SOM analogs (Rohrer et al, 1998) and the availability of genetically modified animal models (Zeyda and Hochgeschwender, 2008) has contributed to a better understanding of individual sst receptor characteristics and their roles in brain functions and disorders, including anxiety and depression (Engin et al, 2008; Engin and Treit, 2009; Epelbaum et al, 2009; Viollet et al, 2000). Recent studies identified a causal role for frontal and cingular SOM cells in modulating behavioral emotionality in mice (Lin and Sibille, 2015a, 2015b; Soumier and Sibille, 2014) and showed that SOM anxiolytic effects are mediated by sst2 receptors expressed in the amygdala and the septum of the rat brain (Yeung et al, 2011; Yeung and Treit, 2012). While all sst receptor subtypes (except for sst5) are present in the hippocampus, SOM has been shown to modulate learning and memory formation through sst2 and sst4 receptors selectively (Gastambide et al, 2009, 2010; Guillou et al, 1993). The present study investigated the role of hippocampal SOM and its receptors in stress response and emotionality, an issue that has so far remained unexplored.
MATERIALS AND METHODS
Experimental Goals
The aims of the present study were first to determine whether any of the four sst receptor subtypes (sst1-4) expressed in the hippocampus inhibits stress-induced activation of the HPA axis and then, to investigate whether such inhibitory control could have anxiolytic or antidepressant properties. Intrahippocampal injections of selective sst receptor agonists were given prior to a stress event and plasma CORT elevation was measured. Given that plasma levels do not always parallel brain concentrations of glucocorticoids, microdialysis was conducted to assess CORT levels after sst agonist infusions and acute stress directly in the hippocampus. Then, independent groups of mice received sst agonists intrahippocampally and anxiety or depressive-like behaviors were assessed. Finally, further hippocampal microdialysis assays and emotional evaluations were conducted in sst2 or sst4 knockout mice.
Animals
Male mice of the C57Bl/6 J strain were obtained from Charles River Laboratories (L’Arbresle, France). sst2 knockout (sst2KO) strain was originally generated by Zheng et al, (Zheng et al, 1997). sst4 knockout (sst4KO) mice were produced by integration (knockin) of LacZ gene into the sstr4 gene (Helyes et al, 2009). All lines were backcrossed to the C57BL/6 J background (N>11). KO and wild-type male littermates were obtained by intercrossing heterozygous +/− mice. Animals were bred in an animal facility equipped with an artificial 12 h light/dark cycle (7:00AM on). Mice were 4–8-month-old at the beginning of the experiments. All experiments were conducted between 7 h 30 min and 12 h, and in compliance with the directive 2010/63/EU, Animal Care and Use Committee (Bordeaux) and approved under the number 5012098-A.
Surgery
Mice were anesthetized with a ketamine (1 mg/kg body weight)-xylazine (10 mg/kg body weight) mix and placed on a stereotaxic frame. A microdialysis guide-cannulae (CMA/7 microdialysis probe, CMA microdialysis, Kista, Sweden) was implanted at the following coordinates from the bregma: AP: −2.2 mm; L: ±1.35 mm; V: 1 mm. The laterality of the implantation was randomized between right and left side of the brain. Guide cannulae were fixed to the skull with dental cement (Palavit G, Promodentaire) and 3 screws (stainless steel, Ø =0.5 mm, L=1 mm; FOM2000). For behavioral experiments, mice were implanted at the same coordinates with stainless steel guide-cannulae (Le Guellec tubular components, Douarnenez, France). Sterile stylets were inserted in the cannulae to maintain patency. Mice were allowed to recover from surgery for 1 week before experiments.
Drugs
Somatostatin analogs (RC160 and octreotide, TOCRIS, Lille, France), as well as selective agonists for sst1 (L-797,591 Rahway, NJ, USA) sst2 (L-054,264, TOCRIS, France), sst3 (L-796,778 Rahway) or sst4 (L-803,087, TOCRIS) were all used at the dose of 5 nmol. For microdialysis experiments, all drugs were diluted in artificial cerebrospinal fluid (aCSF) containing 10% of dimethylsulfoxide (Vehicle, Veh) and were delivered into the hippocampus via the microdialysis probe within 15 min. For other experiments, the drugs were prepared at the concentration of 5 nmol/0.5 μl based on previous experiments (Gastambide et al, 2010) and were delivered into the hippocampus within 5 min in freely moving animals as previously described (Martel et al, 2006). Injections were given 15 min before testing sessions.
Microdialysis
Microdialysis was performed in freely moving animals to measure CORT levels in the dorsal hippocampus 1 h before and 3 h after acute stress as previously described (Dorey et al, 2012). The experiment was conducted by squad of two animals belonging to controls or experimental groups, respectively. The day before the experiment, animals were individually placed in a 30 cm-diameter chamber made of clear plexiglass walls and equipped with a grid floor. Animals had ad libitum access to their habitual chow and water. A microdialysis probe (CMA/7 membrane length 1 mm; Ø=0.24 mm; CMA Microdialysis) was inserted into the guide-cannulae. The membrane was 1 mm longer than the guide-cannulae to be directly in contact with the hippocampal tissue. Probe was continuously perfused with sterile, filtered Dulbecco’s solution (mock CSF, Sigma, Saint-Quentin Fallavier, France) at a rate of 0.1 μl/min for fluid equilibration during one night. After the equilibration phase, baseline dialysates were collected every 15 min with a flow rate of 1 μl/min during 1 h. After baseline establishment, three inescapable foot-shocks (0.9 mA; 1 s) were applied from the grid floor within 1 min to provide stress. For pharmacological experiments, the perfusion was switched to another syringe containing the drug or the vehicle for 15 min after baseline. At the end of the injection, syringes were switched back to aCSF and the stress procedure was applied. The dialysates were collected every 15 min during 3 h after stress. Samples were stored at −80 °C until free CORT determination.
Plasma Corticosterone Collection
Mice received an intrahippocampal injection of either one sst agonist or Veh solution in their home-cage. Fifteen minutes after the injection, 3 foot-shocks were delivered as described above and 15 min later blood was collected from the retro-orbital vessel under anaesthesia (isofluran) by a capillary tube. After centrifugation at 3500 rpm for 10 min at 4 °C, the supernatant was stored at −80 °C until ELISA assay. An enzyme immunoassay commercial kit (Correlate-EIA, Assay Designs, Ann Arbor, MI) was used to measure plasma or hippocampal CORT concentrations. The sensitivity of the ELISA assay is 18.6 pg/ml. Baseline sample concentration was >10-fold above the sensitivity threshold. The data are expressed in pg/ml/15 min for CSF and in ng/ml for plasma.
Behavior
Motion recording during intrahippocampal microdialysis
Each mouse was video recorded and its activity was quantified by videotracking (Viewpoint, Champagne au Mont d’Or, France). Three displacement thresholds were determined from pilot trials to quantify resting or very small motions, small motions and large motions, respectively, as function of pixel displacements/16 ms. Changes of movement types were counted as a motion unit and analyzed per period of 15 min to assess the evolution of the activity from the baseline to the end of the post-stress evaluation.
Elevated Plus-Maze
The elevated plus-maze (EPM) apparatus was composed of grey polyvinylcarbonate and consisted of four arms surrounding a central platform. Each arm was 30 cm long, 7 cm wide, and 60cm above the ground. The four arms joined at the center on a 7 cm square platform. Two opposite arms of the plus maze (‘closed arms’) were bordered by 24 cm-high sidewalls opened on the top, and the two other arms (‘open arms’) were exposed to a 70 lux light intensity. At the beginning of each test, the mouse was placed in the center of the maze in a cylinder (7 cm in diameter, 17 cm high) for 30 s. Then, the cylinder was removed and the animal was allowed to explore all arms of the maze freely for 8 min. An entry was counted when the mouse entered an arm with all 4 feet. The number of entries, times in open and closed arms, latencies to entry were measured.
Open-Field
The open-field was a white circular arena (1 m diameter) surrounded by a 25 cm-high wall made of opaque plexiglass. One lamp, 2 m above the apparatus, provided 70 lux of illumination distributed equally over the entire surface of the apparatus. Animals were placed facing to the wall and were allowed to freely explore the apparatus for 5 min. The floor was virtually divided into a periphery annulus 12 cm wide and a central zone. Exploration parameters were quantified by videotracking.
Forced Swim Test
Mice were placed into a glass cylinder (diameter 15 cm, height 29 cm) containing 20 cm of water at 25±1 °C. The protocol used is that initially described by Porsolt et al. (Porsolt et al, 1977), in which the animal is maintained in water during 6 min. Given that mice are very active during the first 2 minutes and inactive during the last 2 minutes of FST, the times spent in swimming, climbing, or immobility were analyzed during the last 4 minutes for pharmacology experiments or during the first 4 minutes in knockout experiments, in order to unmask, respectively, antidepressant and depressive-like effects.
Stress-Sensitive Beam Walking Test
Mice were placed at the extremity of a 1 m-long beam, 80 cm high from the floor. The start platform was narrow (3 × 4 cm) and exposed to intense light (120 lux) to encourage the animal to walk through the beam to reach the dark compartment. The floor was lined with foam to prevent hurting if animals fall down. The time to cross the beam and the number of hesitations (stop or slowing down) were recorded and quantified by the videotrack system. Animals were first trained for 3 days (3 trials/day) with beams of increased difficulties as a function of their shape and size: 10 mm square beam, 15 mm round beam, 8 mm square beam. A cutoff was applied if the mouse fell down from the beam or did not cross the beam in 2-min time. The next day, mice were injected with sst2 or sst4 agonists or vehicle 15 min before receiving a foot-shock stress as described above. Fifteen minutes later, mice were tested on the beams experienced during training and an additional beam (10 mm round) was introduced. Results are the mean of two successive trials for each beam. Rationales and the data regarding stress sensitivity of the task are presented in Supplementary Information 3.
Histology
At the end of the experiments, mice were anesthetized with 27 mg/0.5 ml of Sodium Pentobarbital and quickly decapitated. Entire heads were immersed in a solution of 10% formaldehyde at 4 °C for 2 weeks. The brain was removed from the skull and was then cryoprotected and coronally sectioned into slices (60 μm thickness). A Thionin acetate stain was used to check the exactness the implantations.
Statistical Analysis
Statistical analyses were performed using the Statview 5.0 software (Statistical Analysis System Institute Inc, NC, USA). The data were analyzed using ANOVA to determine main factor effects and followed by appropriate post hoc tests. Mean group comparisons were also performed using Student’s t-tests.
RESULTS
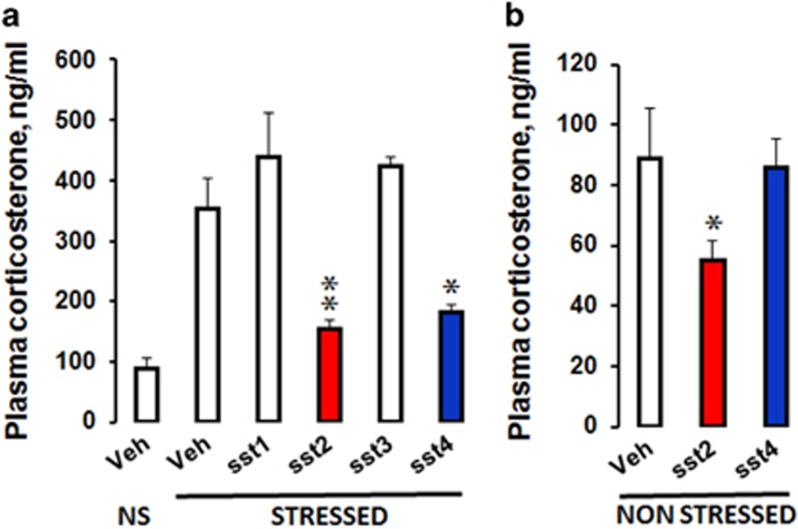
Plasma CORT levels increased by 4-fold following stress exposure relative to non-stressed conditions (Figure 1). Stress-induced CORT levels were lower following intrahippocampal injections of L-054,264 (t(11)=3.98; P=0.002) or L-803,087 (t(9)=2.94; P=0.016) but not of L-797,591 or L-796-778 agonists relative to stressed controls, indicating that hippocampal sst2 and sst4, but not sst1 or sst3 receptors, contribute to inhibit stress-induced activation of the HPA axis. L-054,264 also reduced basal CORT levels significantly (t(8)=1.92; P=0.04), whereas L-803,087 was inefficient.
Figure 1.
Hippocampal infusions of sst2 and sst4 receptor agonists prior to an acute stress decrease the reactivity of the HPA axis. (a) Animals received hippocampal infusion of Veh. (NS, non-stressed, N=5; and stressed, N=6), sst1 (N=4), sst2 (N=7), sst3 (N=4), or sst4 (N=5) receptor agonists, 15 min before acute foot-shock stress. Plasma was collected 15 min after stress for ELISA analysis. Both sst2 and sst4 receptor agonists attenuated CORT response to acute stress exposure (P=0.0022 and P=0.0164, respectively), whereas other agonists had no effect. The sst2 agonist (N=5; P=0.04) but not the sst4 agonist (N=5) also reduced basal CORT levels in NS mice (b). The data are expressed in ng/ml, means+SEM *P<0.05, **P<0.01 relative to stressed Veh or to NS Veh, respectively in A and B (Student’s t-test).
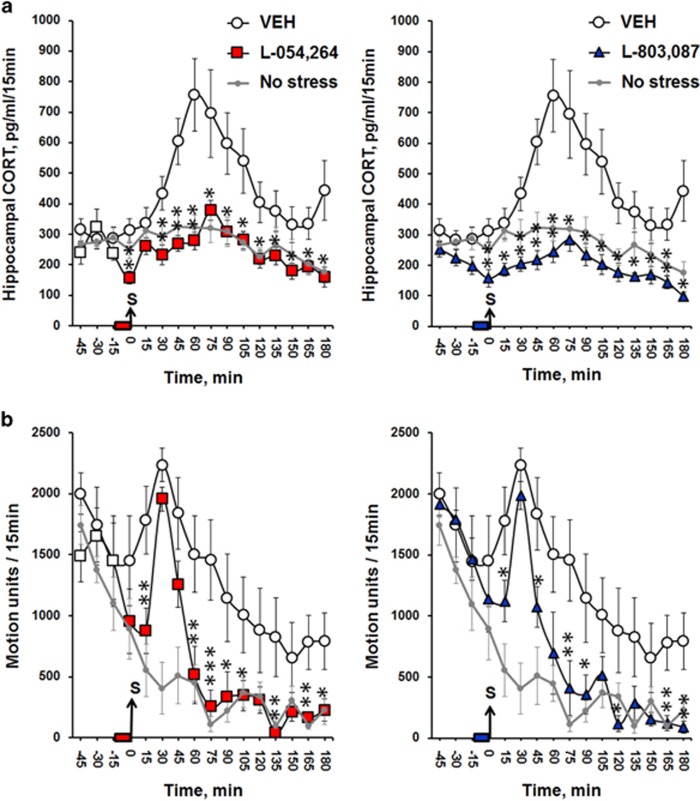
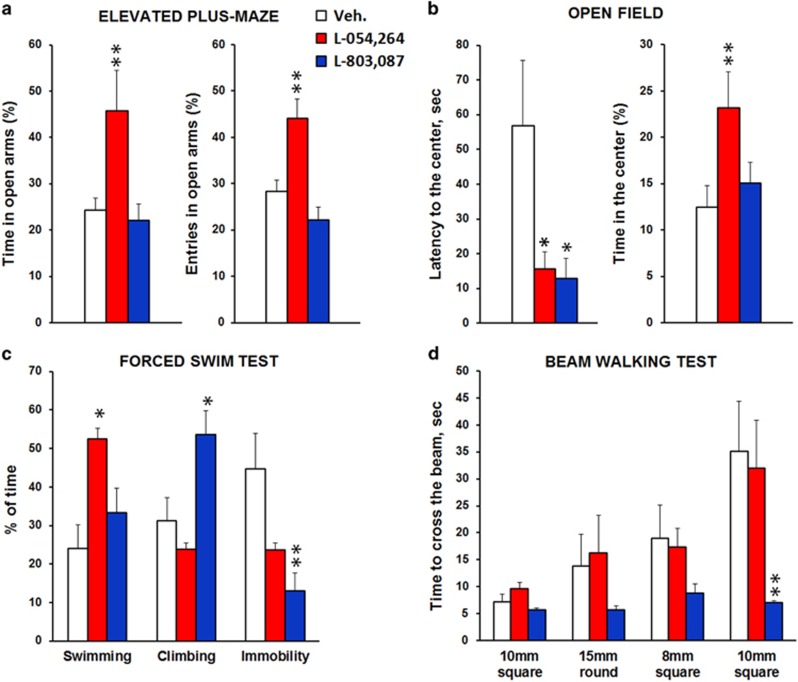
Hippocampal CORT concentrations sampled by microdialysis rose within 1 h following stress onset in control mice (Figure 2) and progressively returned to baseline levels within the next 2 h. SOM analogs RC160 and octreotide, known to target sst2-3-5 receptor subtypes attenuated stress-induced increase in hippocampal CORT levels (Supplementary Information 2; treatment effects Fs⩾4.10; Ps⩽0.05 for both analogs). The non-peptidic agonists L-054,264 and L-803,087 (Figure 3) targeting sst2 and sst4, respectively, fully blocked the elevation of CORT levels in the hippocampus, following acute stress (treatment effects: Fs⩾7.61; Ps⩽0.01 for both agonists). Inhibitory effects started immediately after infusion (Ps⩽0.01 for both agonists) and lasted for at least 3 h post stress onset (Ps⩽0.05 for both agonists). As indicated on Figure 2b, stress induced a transient peak of hyperactivity in all groups. Then, locomotor activity in stressed mice infused with either the sst2 or sst4 agonist rapidly recovered the level of non-stressed controls and was significantly lower relative to stressed controls (treatment effects: Fs⩾6.18; Ps⩽0.02 for both agonists), indicating that both sst2 and sst4 receptors attenuate stress-induced reactivity. However, further behavioral investigations revealed that each of these agonists alleviates stress-induced emotional responses differently (Figure 3). L-054,264 selectively reduced anxiety-like behaviors as assessed by exploration levels of open places either in the EPM (P⩽0.009 for both entries and time in open arms) or in the open-field (P=0.01). In the EPM, L-054,264 also shortened the latency to enter an open arm (P=0.001). Both L-054,264 and L-803,087 modified behaviors in the FST but the latter increased the attempts to escape by climbing on the wall of the glass cylinder (P=0.04), whereas the former increased the time spent in swimming (P=0.02). Finally, only L-803,087 improved performance in the beam walking test. The sst4 agonist prevented both stress-induced hesitations (P=0.007, data not shown) and increases in time (P<0.01) to cross the beams as a function of difficulty and novelty, further highlighting that hippocampal sst2 and sst4 receptors control different emotional behaviors.
Figure 2.
Hippocampal concentrations of CORT and general motion activity are decreased by infusions of both sst2 and sst4 receptor agonists. (a) Time-course evolution of CORT level in the dorsal hippocampus sampled every 15 min for 1 h before and 3 h following acute stress. The bar on the time scales indicates the infusion of the sst agonist. The grey arrow indicates when the acute foot-shock stress (S) was applied. Both sst2 (red square; N=10) and sst4 (blue triangle; N=7) receptor agonists rapidly decreased hippocampal CORT levels (within 15 min) and inhibited CORT elevation in response to acute stress as compared with stressed Veh (white circle; N=16). The dots and gray lines represent the measures performed on controls, which did not received foot-shocks (No stress, N=7). Results are expressed as mean±SEM hippocampal CORT concentration in pg/ml/15 min. (b) Time-course evolution of stress-induced activity. Both agonists decreased stress reactivity in a long-lasting manner. Results are expressed as mean±SEM of motion units/15 min. *P<0.05, **P<0.01, compared with stressed Veh with Student’s t-tests. A full color version of this figure is available at the Neuropsychopharmacology journal online.
Figure 3.
Hippocampal sst2 and sst4 receptors regulate emotional behaviors differently. Elevated-plus maze, open field, FST, and beam walking tests were performed on the same animals with intra-hippocampal Veh (N=10), sst2 (L-054,264; N=8) or sst4 (L-803,087, N=8) agonist infusions. (a) Anxiety-like behavior evaluated in the elevated-plus maze showed that infusion of L-054,264 increased the time spent and the open arms entries, whereas infusion of L-803,087 had no effect. The data are expressed as mean percentage of time and entries (+SEM) related to total time spent or entries in all arms during the test. (b) Anxiety-like behavior evaluated in the open-field showed that both L-054,264 and L-803,087 decreased latency to reach the center of the arena but only L-054,264 increased the time spent in the center area. The data are expressed as mean latency to reach the center in seconds (+SEM) and mean percentage of time spent in the center (+SEM) relatively to the entire arena. (c) Recording of the last 4 minutes in the FST, showed that L-054,264 increased the time spent in swimming, whereas no effect was observed on climbing nor, albeit almost significant, on immobility (P=0.07). L-803,087 decreased immobility to the benefit of increased climbing but not of swimming responses. Results are expressed in percentage of time spent (+SEM) for each behavior. (d) Beam Walking testing showed that the time to cross the beams was decreased by L-803,087 and reached a significant level in the hardest difficulty tested. Square or round and the associated number indicate the shape and the section size of the beam. The data are expressed as mean time (second +SEM) to cross the beam. *P<0.05 and **P<0.01 relative to the Veh group using Student’s t-tests.
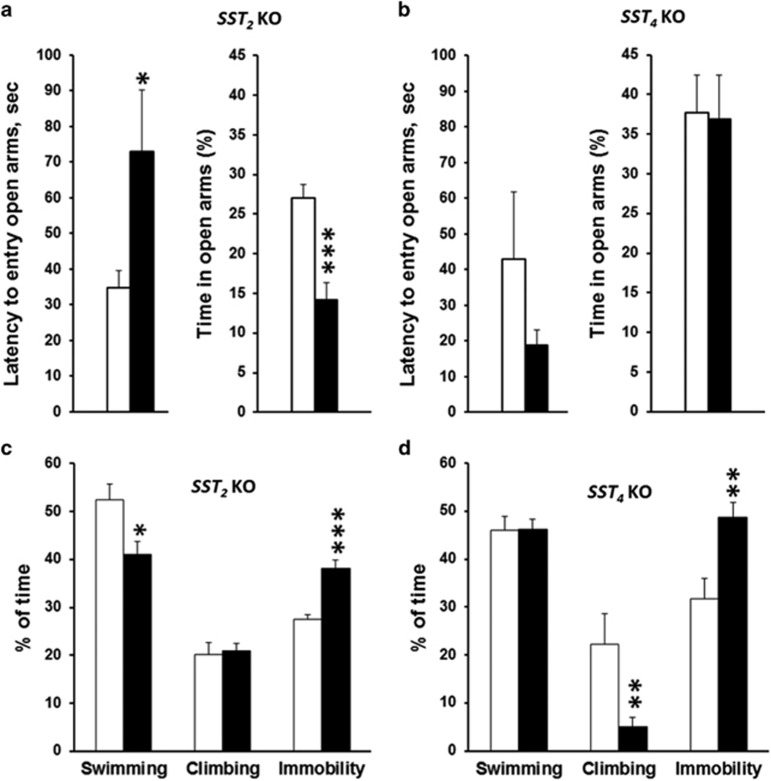
Consistent with such findings, behaviors of sst2KO and sst4KO mice in the EPM and in the FST were altered in an opposite manner (Figure 4). Relative to wild-type littermates, sst2KO mice displayed increased latency to enter an open arm (P=0.04) and lower exploration time in open arms (P=0.001), whereas no significant effect was detected between sst4KO and wild type. In the FST, both sst2KO and sst4KO mice displayed increased immobility relative to their wild-type littermates (P=0.001 and P=0.004, respectively). However, swimming decreased selectively in sst2KO mice (P=0.002), whereas climbing decreased selectively in sst4KO mice (P=0.02), indicating that active behaviors are qualitatively altered depending on genotypes.
Figure 4.
sst2 and sst4 receptor gene deletion differently modulate anxiety- and depressive-like behaviors. Elevated-plus maze testing aimed at evaluating anxiety-like behaviors showed that the latency to enter an open arm (seconds) was increased while percentage of time spent in open arms was decreased in sst2KO mice (a) but not in sst4KO mice (b) relative to their WT littermates. sst2KO, N=12; WT, N=12; sst4KO, N=10 WT N=8. Recording of the first 4 minutes of the FST (aimed at evaluating depressive-like behavior) revealed increased immobility in both sst2KO (c) and sst4KO (d) mice. Active behaviors differed between genotypes in that swimming decreased in sst2KO, whereas climbing was affected in sst4KO mice. Sst2KO, N=12; WT, N=12; sst4KO, N=10; WT, N=10. *P<0.05, **P<0.01, ***P<0.001 relative to wild-type littermates with Student’s t-tests.
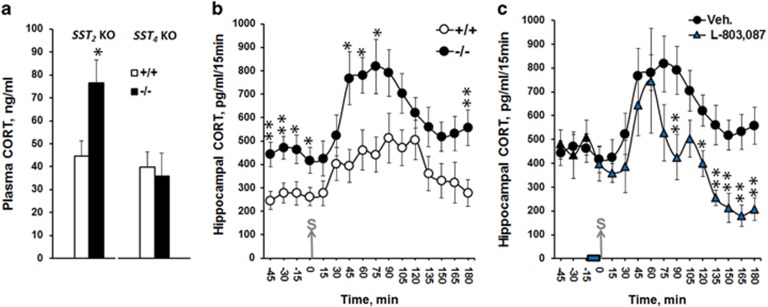
Basal levels of plasma CORT were significantly higher in sst2KO mice (P=0.04) than in WT littermates and consistently, basal CORT levels in hippocampal dialysates were also higher in sst2KO mice (Figure 5). A significant genotype effect was observed both before (F(1.16)=9.91; P=0.006) and after stress exposure (F(1,16)=6.86; P=0.019). The time lag to produce a significant increase (45 min, P=0.009) was shorter, and the maximal (P=0.02) and recovery (P=0.01) levels of CORT concentrations were significantly higher in sst2KO than in WT, indicating that the HPA axis is constitutively upregulated and is more reactive to stress in the absence of sst2 receptors. No change in CORT levels was observed in sst4KO mice (P=0.78). Finally, and to further investigate the inhibitory control induced by sst4 receptor activation, intrahippocampal injections of L-803,087 were tested in sst2KO mice (Figure 5c). L-803,087 lowered stress-induced elevation of hippocampal CORT levels in sst2KO mice (F(1,12)=5.76; P=0.03), thus confirming that hippocampal sst4 receptors contribute to the inhibition of the HPA axis. However, the inhibitory effect of L-803,087 occurred only later (+1h30), whereas it occurred immediately after stress onset in experiments with C57BL/6 mice (see Figure 2), indicating that the presence of hippocampal sst2 receptors is required for the fast-acting inhibition of the HPA axis by somatostatinergic neurotransmission.
Figure 5.
sst2 gene deletion leads to elevated plasma and hippocampal CORT concentrations and L-803,087 decreases CORT levels only during recovery of acute stress. (a) Basal plasma CORT concentration measured in sst2KO mice (−/−, N=8) was higher than in wild-type mice (+/+, N=5) but not in sst4KO mice (−/−N=8; +/+ N=12). (b) Levels of CORT concentration measured in hippocampal dialysates were also higher in sst2KO (N=9) than wild-type (N=9) mice, both before and after the application of electric foot-shocks. (c) Hippocampal infusion of the sst4 agonist L-803,087 in sst2KO mice (N=5) did not alter the early increase in CORT levels (from 0 to 90 min) following acute stress. However, L-803,087 infusion accelerated recovery (from 90 to 180 min) and even decreased baseline CORT levels, as compared with Veh-infused sst2KO mice(N=9). The bar on the time scale indicates when L-803,087 was infused. The grey arrow indicates the time when acute stress was applied. The data are expressed as mean CORT concentration (ng/ml for plasma levels or pg/ml/15 min for intrahippocampal levels)±SEM *P<0.05, **P<0.01 vs wild-type groups or relative to the Veh group using Student’s t-tests.
DISCUSSION
The hippocampus is important for episodic and spatial memory but is now also recognized for its role in mood regulation (McEwen et al, 2015). There is evidence for a connection between hippocampal-HPA axis dysregulations and stress-related illnesses such as depression, generalized anxiety, post-traumatic stress disorders, and memory deficits (Lucassen et al, 2014). Chronic stress exposure (Sapolsky, 2000), acute exposure to psychogenic stressors (Popoli et al, 2012) or acute therapies with high-dose of corticoids (Brown, 2009) negatively impact hippocampal neuroplasticity, neurogenesis, and neuronal or glial survival in relationship with depression (Banasr and Duman, 2007; Banasr et al, 2011; Czeh and Lucassen, 2007; Pittenger and Duman, 2008). Studies have also linked depression to an increase in the excitatory-inhibitory ratio, which may be attributed to a loss of GABAergic neurons. Parvalbumin and SOM subpopulations in both the dorsal and ventral hippocampus are particularly vulnerable to chronic stress exposure (Czeh et al, 2015). We observed a causal inhibitory effect of RC160, octreotide, and selective sst2 or sst4 receptor agonists on the acute stress response, as measured by alterations in both plasma and hippocampal CORT levels, demonstrating the critical role by SOM neurotransmission in inhibitory hippocampal feedback on the HPA axis. The levels and the time course of stress-induced increase in hippocampal CORT were comparable to previous experiments using the same protocol (Chauveau et al, 2010; Dorey et al, 2012; Tronche et al, 2010). Consistent with the finding that acute stress or dexamethasone produces rapid SOM release in the hippocampus (Arancibia et al, 2001), SOM inhibitory effects were detectable within 15 min after infusion, indicating that activation of the hippocampal somatostatinergic network by glucocorticoids controls the stress-response rapidly. sst2 and sst4 receptor agonists produced stronger effects than RC160 and octreotide, in agreement with better stability and longer half-life than peptidergic analogs (Rohrer et al, 1998). Mice reacted to footshocks by expressing a transient peak of locomotor activity, which was comparable in all groups, indicating that sst receptor functions do not alter sensory thresholds in this context. Both sst2 and sst4 receptor agonists reduced locomotor activity, an inhibitory effect, which occurred rapidly and persisted for the entire experiment. The time course of decreased activity in mice infused with sst2 or sst4 agonists suggests that the effect represents a more complete adaptation (habituation) to the aversive context than to be a motor deficiency. Accordingly, L-054,264 had no effect and L-803,087 improved performance in the beam walking test. Both agonists also had similar inhibitory effects on immobility in the FST. Interestingly, in this latter test, they produced different active behaviors, implying that distinct mechanisms underlie their behavioral effects. The sst2 receptor agonist increased swimming behavior without affecting climbing behavior, a type of behavioral pattern that is characteristic of serotoninergic activity. In sharp contrast, the sst4 receptor agonist increased climbing behavior without affecting swimming as produced by antidepressants with noradrenergic activity (Detke et al, 1995; Reneric et al, 2001). Though this dissociation is less clear in mice than in rats, the behavioral profile produced by L-054,264 (decreased immobility/increased swimming) was similar to that previously observed in rats that received intracerebroventricular injections of SOM (Engin et al, 2008) or L-779,976, another sst2 selective agonist (Engin and Treit, 2009). In addition, the hippocampal injection of sst2, but not the sst4 agonist, also produced anxiolytic-like behavior in EPM and open field testing, corroborating the idea that hippocampal sst2 and sst4 regulate different emotional responses. These results indicate that the affinities for the sst receptor subtypes of L-054,264 (1250-fold superior for sst2 than for sst4) and L-803,087 (6700-fold superior for sst4 than for sst2) are sufficient to produce selective behavioral changes (Rohrer et al, 1998; Yang et al, 1998).
Consistent with the pharmacological results and previous studies (Viollet et al, 2000), sst2KO, but not sst4KO, mice displayed increased anxiety-like behaviors. CORT levels were also constitutively higher in sst2KO mice in agreement with the increased ACTH release as previously described in these mice (Viollet et al, 2000) and the increased basal level of CORT reported in SOMKO mice (Lin and Sibille, 2015a), an effect undetected in sst4KO mice. In addition, increased immobility in the FST associated with decreased swimming in sst2KO mice, whereas climbing was decreased in sst4KO mice corroborate the idea that sst2 and sst4 regulate emotionality by different mechanisms. The recent data showing increased immobility in the FST in sst4KO mice, whereas intraperitoneal treatments with J-2156, another highly selective sst4 agonist, decreased immobility in the tail suspension test in C57BL/6 mice, support the idea that the sst4 receptor subtype is involved in depression-like behaviors (Scheich et al, 2016). We previously showed that injections of sst4 agonists in the dorsal hippocampus produced a shift from flexible cognitive to habit memory systems to rescue task performance (Gastambide et al, 2009) as similarly produced by stress (Schwabe and Wolf, 2013). Together with the present results, this suggests that dorsal hippocampal sst4 receptors control both emotional and cognitive responses to cope with stressful situations.
Previous results showed that the functionality of hippocampal sst4 receptors is lost, following pharmacological blockade or knockout of sst2 receptors. L-803,087-mediated increases in glutamatergic excitability and bursting frequency in hippocampal CA1 region, as well as L-803,087-mediated shifts toward the use of striatal memory systems are both blocked by sst2 antagonists or sst2 gene deletion (Cammalleri et al, 2006; Gastambide et al, 2010; Moneta et al, 2002). In line with these results, we show here that in the absence of sst2 receptors, the sst4-mediated inhibition on the HPA axis is lost during induction of the stress response, while it remains effective during the stress recovery phase. On basis of immunohistochemistry detections (Gastambide et al, 2010), binding experiments (Viollet et al, 2000) and mRNA mapping (Vanetti et al, 1994) in the mouse brain, the non-overlapping distribution of sst2 and sst4, respectively found in the dentate gyrus and in the CA1 subfield suggests that interactions between these sst receptor functions occur indirectly. The non-overlapping distribution may also account for the differential effects of sst2 and sst4 on anxiety-like and depressive-like behaviors. Insofar as the role of the CA1 subfield in emotions remains unclear, the present results suggest that dentate gyrus sst2 and CA1 sst4 receptors conjointly control behavior in response to glucocorticoids by providing anxiolytic and antidepressant effects. Given the general consensus for involvement of the ventral hippocampus in regulation of stress responses and emotionality, it is interesting that the effects were produced by injections into the dorsal hippocampus. Whether the effects are relative to spread of injections in the ventral hippocampus and/or to intrahippocampal communication remains to be determined. Notwithstanding, a recent study demonstrated that optogenetic control of granule cells activity in the dorsal dentate gyrus selectively results in a dramatic increase in exploratory behavior in novel environments while affecting the ventral dentate gyrus causes an equally robust anxiolytic-like behavior (Kheirbek et al, 2013).
In conclusion, SOM is a key regulator of the HPA axis activity not only at the pituitary level but also at the brain level, and besides cortical, hypothalamic, amygdalar and septal involvements, hippocampal SOM neurotransmission critically controls both HPA function and emotionality through sst2 and sst4 receptors. This must be taken into account for the development and management of multiselective sst receptor ligands such as pasireotide, which is currently used to control hypercortisolism in the Cushing’s disease, a syndrome most often associated with anxiety, irritability, depression, and memory troubles (Colao et al, 2014).
FUNDING AND DISCLOSURE
This study was supported by the Région Aquitaine and by the Programme Interdisciplinaire du CNRS ‘Longévité et Vieillissement’. The authors declare no conflict of interest.
Acknowledgments
We thank the CPN and the INCIA small animal facility staffs for technical assistance and animal care. We thank Christophe Tronche, Una Avdic (Erasmus fellow), Marie Mennesson and Bertrand Beauchoux for their assistance during experiments. We also thank Dr Gary Gilmour for helpful comments on the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A et al (2015). Mechanisms in endocrinology Cushing's syndrome causes irreversible effects on the human brain: a systematic review of structural and functional magnetic resonance imaging studies. Eur J Endocrinol 173: R1–R14. [DOI] [PubMed] [Google Scholar]
- Arancibia S, Payet O, Givalois L, Tapia-Arancibia L (2001). Acute stress and dexamethasone rapidly increase hippocampal somatostatin synthesis and release from the dentate gyrus hilus. Hippocampus 11: 469–477. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS (2007). Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets 6: 311–320. [DOI] [PubMed] [Google Scholar]
- Banasr M, Dwyer JM, Duman RS (2011). Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr Opin Cell Biol 23: 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES (2009). Effects of glucocorticoids on mood, memory, and the hippocampus treatment and preventive therapy. Ann NY Acad Sci 1179: 41–55. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Cervia D, Dal Monte M, Martini D, Langenegger D, Fehlmann D et al (2006). Compensatory changes in the hippocampus of somatostatin knockout mice: upregulation of somatostatin receptor 2 and its function in the control of bursting activity and synaptic transmission. Eur J Neurosci 23: 2404–2422. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Tronche C, Pierard C, Liscia P, Drouet I, Coutan M et al (2010). Rapid stress-induced corticosterone rise in the hippocampus reverses serial memory retrieval pattern. Hippocampus 20: 196–207. [DOI] [PubMed] [Google Scholar]
- Colao A, Boscaro M, Ferone D, Casanueva FF (2014). Managing Cushing's disease: the state of the art. Endocrine 47: 9–20. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ (2007). What causes the hippocampal volume decrease in depression? Eur Arch Psychiatry Clin Neurosci 257: 250–260. [DOI] [PubMed] [Google Scholar]
- Czeh B, Varga ZKK, Henningsen K, Kovacs GL, Miseta A, Wiborg O (2015). Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus 25: 393–405. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I (1995). Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121: 66–72. [DOI] [PubMed] [Google Scholar]
- Dorey R, Pierard C, Chauveau F, David V, Beracochea D (2012). Stress-induced memory retrieval impairments: different time-course involvement of corticosterone and glucocorticoid receptors in dorsal and ventral hippocampus. Neuropsychopharmacology 37: 2870–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Stellbrink J, Treit D, Dickson CT (2008). Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience 157: 666–676. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D (2009). Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology 206: 281–289. [DOI] [PubMed] [Google Scholar]
- Epelbaum J, Guillou J-L, Gastambide F, Hoyer D, Duron E, Viollet C (2009). Somatostatin, Alzheimer's disease and cognition: an old story coming of age? Prog Neurobiol 89: 153–161. [DOI] [PubMed] [Google Scholar]
- Fendler K, Karmos G, Telegdy G (1961). Effect of hippocampal lesion on pituitary-adreenal function. Acta Physiol Acad Sci Hung 20: 293. [PubMed] [Google Scholar]
- Gastambide F, Lepousez G, Viollet C, Loudes C, Epelbaum J, Guillou J-L (2010). Cooperation between hippocampal somatostatin receptor subtypes 4 and 2: functional relevance in interactive memory systems. Hippocampus 20: 745–757. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Viollet C, Lepousez G, Epelbaum J, Guillou J-L (2009). Hippocampal SSTR4 somatostatin receptors control the selection of memory strategies. Psychopharmacology 202: 153–163. [DOI] [PubMed] [Google Scholar]
- Guillou JL, Micheau J, Jaffard R (1993). Effects of intrahippocampal injections of somatostatin and cysteamine on spatial discrimination-learning in mice. Psychobiology 21: 265–271. [Google Scholar]
- Helyes Z, Pinter E, Sandor K, Elekes K, Banvolgyi A, Keszthelyi D et al (2009). Impaired defense mechanism against inflammation, hyperalgesia, and airway hyperreactivity in somatostatin 4 receptor gene-deleted mice. Proc Natl Acad Sci USA 106: 13088–13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Mueller NK (2006). Role of the ventral subiculum in stress integration. Behav Brain Res 174: 215–224. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czeh B, Fluegge G, Zhang W (2010). Stress Impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 35: 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M (2008). Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol 583: 312–321. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE et al (2013). Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77: 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, Sibille E (2015. a). Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry 20: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, Sibille E (2015. b). Transcriptome changes induced by chronic psychosocial/environmental or neuroendocrine stressors reveal a selective cellular vulnerability of cortical somatostatin (SST) neurons, compared with pyramidal (PYR) neurons. Mol Psychiatry 20: 285–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Pruessner J, Sousa N, Almeida OFX, Van Dam AM, Rajkowska G et al (2014). Neuropathology of stress. Acta Neuropathol 127: 109–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel G, Millard A, Jaffard R, Guillou JL (2006). Stimulation of hippocampal adenylyl cyclase activity dissociates memory consolidation processes for response and place learning. Learning Mem 13: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD (2015). Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41: 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneta D, Richichi C, Aliprandi M, Dournaud P, Dutar P, Billard JM et al (2002). Somatostatin receptor subtypes 2 and 4 affect seizure susceptibility and hippocampal excitatory neurotransmission in mice. Eur J Neurosci 16: 843–849. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS (2008). Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33: 88–109. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G (2012). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M (1977). Behavioral despair in mice - primary screening-test for antidepressants. Arch Int Pharmacodyn Ther 229: 327–336. [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE (2011). A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci 31: 9683–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneric JP, Bouvard M, Stinus L (2001). Idazoxan and 8-OH-DPAT modify the behavioral effects induced by either NA, or 5-HT, or dual NA/5-HT reuptake inhibition in the rat forced swimming test. Neuropsychopharmacology 24: 379–390. [DOI] [PubMed] [Google Scholar]
- Rohrer SP, Birzin ET, Mosley RT, Berk SC, Hutchins SM, Shen DM et al (1998). Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science 282: 737–740. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2000). Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57: 925–935. [DOI] [PubMed] [Google Scholar]
- Scheich B, Gaszner B, Kormos V, Laszlo K, Adori C, Borbely E et al (2016). Somatostatin receptor subtype 4 activation is involved in anxiety and depression-like behavior in mouse models. Neuropharmacology 101: 204–215. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT (2013). Stress and multiple memory systems: from 'thinking' to 'doing’. Trends Cogn Sci 17: 60–68. [DOI] [PubMed] [Google Scholar]
- Sheline YI (1996). Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Mol Psychiatry 1: 298–299. [PubMed] [Google Scholar]
- Soumier A, Sibille E (2014). Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 39: 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche C, Lestage P, Louis C, Carrie I, Beracochea D (2010). Pharmacological modulation of contextual ‘episodic-like’ memory in aged mice. Behav Brain Res 215: 255–260. [DOI] [PubMed] [Google Scholar]
- Vanetti M, Ziolkowska B, Wang X, Horn G, Hollt V (1994). mRNA distribution of two isoforms of somatostatin receptor 2 (mSSTR2A and mSSTR2B) in mouse brain. Brain Res Mol Brain Res 27: 45–50. [DOI] [PubMed] [Google Scholar]
- Viollet C, Vaillend C, Videau C, Bluet-Pajot MT, Ungerer A, L'Heritier A et al (2000). Involvement of sst2 somatostatin receptor in locomotor, exploratory activity and emotional reactivity in mice. Eur J Neurosci 12: 3761–3770. [DOI] [PubMed] [Google Scholar]
- Yang LH, Guo LQ, Pasternak A, Mosley R, Rohrer S, Birzin E et al (1998). Spiro 1H-indene-1,4 '-piperidine derivatives as potent and selective non-peptide human somatostatin receptor subtype 2 (sst(2)) agonists. J Med Chem 41: 2175–2179. [DOI] [PubMed] [Google Scholar]
- Yeung M, Engin E, Treit D (2011). Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology 216: 557–567. [DOI] [PubMed] [Google Scholar]
- Yeung M, Treit D (2012). The anxiolytic effects of somatostatin following intra-septal and intra-amygdalar microinfusions are reversed by the selective sst2 antagonist PRL2903. Pharmacol Biochem Behav 101: 88–92. [DOI] [PubMed] [Google Scholar]
- Zeyda T, Hochgeschwender U (2008). Null mutant mouse models of somatostatin and cortistatin, and their receptors. Mol Cell Endocrinol 286: 18–25. [DOI] [PubMed] [Google Scholar]
- Zheng H, Bailey A, Jiang MH, Honda K, Chen HY, Trumbauer ME et al (1997). Somatostatin receptor subtype 2 knockout mice are refractory to growth hormone-negative feedback on arcuate neurons. Mol Endocrinol 11: 1709–1717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.