Abstract
Objectives:
This trial investigated whether probiotics improved mood, stress and anxiety in a sample selected for low mood. We also tested whether the presence or severity of irritable bowel syndrome symptoms, and levels of proinflammatory cytokines, brain-derived neurotrophic factor and other blood markers, would predict or impact treatment response.
Method:
Seventy-nine participants (10 dropouts) not currently taking psychotropic medications with at least moderate scores on self-report mood measures were randomly allocated to receive either a probiotic preparation (containing Lactobacillus helveticus and Bifidobacterium longum) or a matched placebo, in a double-blind trial for 8 weeks. Data were analysed as intent-to-treat.
Results:
No significant difference was found between the probiotic and placebo groups on any psychological outcome measure (Cohen’s d range = 0.07–0.16) or any blood-based biomarker. At end-point, 9 (23%) of those in the probiotic group showed a ⩾60% change on the Montgomery–Åsberg Depression Rating Scale (responders), compared to 10 (26%) of those in the placebo group (, p = ns). Baseline vitamin D level was found to moderate treatment effect on several outcome measures. Dry mouth and sleep disruption were reported more frequently in the placebo group.
Conclusions:
This study found no evidence that the probiotic formulation is effective in treating low mood, or in moderating the levels of inflammatory and other biomarkers. The lack of observed effect on mood symptoms may be due to the severity, chronicity or treatment resistance of the sample; recruiting an antidepressant-naive sample experiencing mild, acute symptoms of low mood, may well yield a different result. Future studies taking a preventative approach or using probiotics as an adjuvant treatment may also be more effective. Vitamin D levels should be monitored in future studies in the area. The results of this trial are preliminary; future studies in the area should not be discouraged.
Keywords: Probiotic, mood, anxiety, stress, gut, inflammation, immune function, vitamin D
The idea that supplemented probiotic bacteria – ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ (Sanders, 2008: 58) – could be used as a treatment for major depressive disorder (MDD) was suggested, in modern times, by Logan and Katzman (2005). A key concept behind this review was that nutritional influences on depression are underestimated and some strains of probiotic bacteria may correct underlying biological factors associated with depression. Indeed, probiotic strains have been shown to lower proinflammatory cytokines (Lin et al., 2008) and oxidative stress (Kullisaar et al., 2003) in humans, both of which are associated with mood disorders (Ascoli et al., 2016; Dowlati et al., 2010; Stefanescu and Ciobica, 2012). Gut–brain axis research suggests that bacteria in the gastrointestinal (GI) tract also communicate with the central nervous system in the absence of an immune response (Cryan and Dinan, 2012; Keightley et al., 2015).
Many papers have explored the links among gut microbes, brain and behaviour (e.g. Collins et al., 2012; Mayer, 2011; Thakur et al., 2014). A number of narrative reviews suggest manipulation of gut microbiota composition (usually via ingestion of probiotic supplements) as an intervention for psychological outcomes (e.g. Bested et al., 2013; Cryan and Dinan, 2012; Dinan et al., 2013; Keightley et al., 2015). Dinan et al. (2013) coined the term ‘psychobiotics’ to describe probiotic bacteria that produces a health benefit in patients with psychiatric illness. However, these reviews rely mainly on evidence from animal studies in which certain probiotic strains have affected emotional behaviour and brain activity (e.g. Bravo et al., 2011; Messaoudi et al., 2010). For example, Messaoudi et al. (2010) found that Lactobacillus helveticus and Bifidobacterium Longum – the probiotic strains used in this study – had beneficial effects on conditioned defensive burying in rats.
Tillisch et al. (2013) provided the first demonstration that probiotics affect brain activity in healthy humans using functional magnetic resonance imaging (fMRI). A recent systematic review found that the human research assessing the impact of probiotics on psychological outcomes is limited, and that the few existing human trials assessed the effect of various probiotic strains on mood in healthy samples only (Romijn and Rucklidge, 2015). For example, L. helveticus and B. longum improved mood and anxiety symptoms in a sample of 55 healthy participants over a 30-day intervention period (Messaoudi et al., 2010). As all existing trials were in healthy human subjects, the review concluded that trials in populations selected for a level of psychological distress, or with diagnosed clinical psychiatric disorders, were necessary to advance the field (Romijn and Rucklidge, 2015).
Since the 2015 review, Steenbergen et al. (2015) reported that a multispecies probiotic formulation reduced cognitive reactivity to sad mood significantly more than placebo in a healthy sample; however, there was no effect of probiotics on Beck Depression Inventory (BDI) scores in this study. Akkasheh at al. (2016) reported a group difference in BDI score after supplementation with probiotics or placebo, adjuvant to citalopram, in a sample with diagnosed MDD; however, this result no longer reached significance when controlling for confounders (Akkasheh et al., 2016).
The present trial was a randomized controlled trial testing probiotic bacteria as a primary treatment for mood and other psychological outcomes in a sample selected for low mood. Based on the existing evidence from gut–brain axis research, and on models linking mood disorders with inflammation, immune activation and the state of the gut microbiota, the trial was designed to ascertain whether a specific probiotic formulation containing L. helveticus and B. longum – previously found to improve emotional behaviour in animals and psychological outcomes and humans – would improve psychological outcomes in our sample.
This study also aimed to examine whether the levels of blood biomarkers would predict or impact treatment response. Given the association between depression and inflammation, and the effects of probiotics on proinflammatory cytokines, we elected to measure a subset of inflammatory markers (interleukin [IL]-1β, IL-6 and tumour necrosis factor alpha [TNF-α]). Although this particular probiotic formulation has not been tested for effects on inflammatory markers, several strains of Lactobacillus have displayed anti-inflammatory properties in vitro in human intestinal epithelial cells (Wallace et al., 2003), and B. longum R0175 has been found to improve symptoms of ulcerative colitis in humans, suggesting potential anti-inflammatory properties (Haskey and Dahl, 2009). We also measured brain-derived neurotrophic factor (BDNF), as a decrease in inflammation and oxidative stress may lead to increased BDNF, which is a potential factor in the development and maintenance of depression (Berk et al., 2011). Emerging evidence suggests that vitamin D is an important modifier of the effects of the intestinal flora on inflammatory processes (Ly et al., 2011), which is interesting given the association between vitamin D and low mood (Anglin et al., 2013), and could be an effect moderator in any interventional research using probiotics for low mood; we therefore measured blood levels of vitamin D. In addition to measuring biomarkers of inflammation, we also aimed to ascertain whether presence, severity or change in irritable bowel syndrome (IBS) symptoms would predict or impact treatment response. There is evidence of high comorbidity of MDD with IBS (Fond et al., 2014) suggesting some possible common factors in aetiology, such as an increase in systemic inflammation, which has been observed in both disorders (Dowlati et al., 2010; Liebregts et al., 2007). It was therefore anticipated that symptoms of gut disorder may be an important moderator of treatment response when testing a gut-directed intervention for mood symptoms.
Materials and methods
Design
The trial used a double-blind, randomized, placebo-controlled design, in which participants were assigned (1:1) to receive either the active probiotic or a placebo for 8 weeks. The trial was prospectively registered on the Australian New Zealand Clinical Trials Registry (Ref#: ACTRN12613000438752; www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364005&;isReview=true). No changes were made after trial registration was completed. The study received ethical approval from the national New Zealand Southern Health and Disability Ethics Committee (URA/12/05/013) and the Human Ethics Committee of the University of Canterbury (HEC 2012/67).
Participants
Participants were recruited in Canterbury, New Zealand, via self-referral. Screening was completed using an online survey (http://bit.ly/UCnutritionresearch). If a potential participant was ineligible, they were directed to local resources for mental health care. Inclusion criteria were as follows: (1) either ⩾11 on the Quick Inventory of Depressive Symptomatology (QIDS-SR16) or ⩾14 on the depression subscale of the Depression, Anxiety and Stress Scale (DASS-42); (2) aged 16+ at the time of screening; and (3) free of any psychiatric medication for at least 4 weeks prior to the trial. Participants who were currently undergoing regular psychological therapy were allowed to participate and continue with their therapy, provided that they had already been receiving therapy for 6 months or more prior to beginning the trial. Exclusion criteria were as follows: any neurological disorder; renal, hepatic, cardiovascular or respiratory disease; any serious medical condition with major medical interventions anticipated during the trial; pregnancy or breastfeeding; use of any supplement considered potentially antidepressant (e.g. St John’s Wort, 5-HTP, SAMe); serious risk of suicide or violence; and current or recent probiotic or antibiotic use. Participants were asked not to consume any other probiotic supplements during the course of the trial. If an antibiotic or antidepressant course was begun by any patient during the course of the trial, then that patient was considered to have violated trial protocol.
Interventions
The test product was a probiotic formula developed by Lallemand Health Solutions (Mirabel, Quebec, Canada), which contains freeze-dried L. helveticus R0052 (strain I-1722 in the French National Collection of Cultures of Microorganisms [CNCM], Institut Pasteur, Paris, France) and B. longum R0175 (CNCM strain I-3470) bacteria at a dosage of three billion colony-forming units (⩾3 × 109 CFU) per 1.5 g sachet. Excipients used were as follows: xylitol, maltodextrin, plum flavour and malic acid. Bacterial enumeration of the product confirmed that the sachets contained ⩾3 × 109 CFU at the beginning and end of the study. The placebo product contained only the excipients and was sensorially identical to the active product. Both products were room temperature stable and in the form of orally dispersible powder in plain sachets.
Measures
Demographic information
Date of birth, ethnicity, household income, occupation and highest educational qualification were requested at screening. Occupation data were used to calculate a score of socio-economic status (SES) based on the New Zealand socio-economic index (NZSEI-06) (Milne et al., 2013).
Montgomery–Åsberg Depression Rating Scale
The Montgomery–Åsberg Depression Rating Scale (MADRS) is a well-validated 10-item clinician-rated diagnostic questionnaire designed to measure the severity of depressive episodes (Montgomery and Åsberg, 1979). The maximum possible score is 60. Severity cut-offs used were as follows: none/recovered (0–8), mild (9–17), moderate (18–34) and severe (⩾35) (Müller et al., 2003); response was defined as >60% reduction in score from baseline. The patients were rated in person at baseline and 8 weeks.
Improved Clinical Global Impressions scale
The Improved Clinical Global Impressions (iCGI) scale includes two clinician-rated measures that assess clinical severity on a 7-point scale at the time of rating (severity scale, ICGI-S) and treatment response from baseline on a 13-point scale (improvement scale, iCGI-I) (Kadouri et al., 2007). The patients were rated at baseline and 8 weeks.
QIDS-SR16
The QIDS-SR16 is a 16-item self-rated questionnaire designed to assess the severity of depressive symptoms (Rush et al., 2003). Scores range from 0 to 27. Severity cut-offs include none (<6), mild (6–10), moderate (11–15), severe (16–20) and very severe (⩾21). The patients were rated at screening, baseline and every 2 weeks throughout the study.
Global Assessment of Functioning
The Global Assessment of Functioning (GAF) is a clinician-rated measure on which the social, occupational and psychological functioning of adults is rated from 1–100, with a higher score indicative of better overall functioning (Caldecott-Hazard and Hall, 1995). The patients were rated at baseline and 8 weeks.
DASS-42
DASS-42 is a 42-item self-report questionnaire designed to assess current severity of symptoms relating to depression, anxiety and stress (Lovibond and Lovibond, 1995). The patients were rated at screening, baseline and 8 weeks.
Irritable Bowel Syndrome Symptom Severity Scale
Irritable Bowel Syndrome Symptom Severity Scale (IBS-SSS) is a five 100-point sliding scale that assesses the severity and frequency of abdominal pain, the severity of abdominal distention, dissatisfaction with bowel habits and interference with quality of life (Francis et al., 1997). Scores range from 0 to 500. Severity cut-offs include remission/no IBS symptoms (<75), mild (75–175), moderate (175–300) and severe (>300). The patients were rated at baseline and 8 weeks.
Biomarkers
Blood samples were collected at baseline and 8 weeks to measure levels of high-sensitivity C-reactive protein (hsCRP), IL-1β, IL-6, TNF-α, vitamin D and BDNF. Testing was completed at Canterbury Health Laboratories. Plasma 25 hydroxycholecalciferol vitamin D was measured using high-performance liquid chromatography (HPLC)-tandem mass spectrometry. HsCRP was measured using latex-enhanced nephelometry. Serum IL-1β, IL-6, TNF-α and BDNF were measured using commercially available enzyme-linked immunosorbent assays (ELISA; R&D Systems). For the hsCRP assays, specimens were collected from patients who had not recently (within the last 4 days) suffered illness or had overt signs of inflammation. HsCRP and vitamin D testing was completed immediately after specimen collection. For the BDNF, IL-1β, IL-6 and TNF-α assays, blood was centrifuged (Eppendorf 5810R centrifuge at 2500 rpm for 10 min) and serum aliquoted immediately after specimen collection and stored at −80°C until batch testing could be completed.
Monitoring
A checklist of common side effects associated with taking medications (e.g. headaches, rash, nausea) was used every 2 weeks to monitor for adverse events. Participants were also asked to estimate their intake of substances (caffeine, nicotine, alcohol and street drugs) every 2 weeks. Participants were asked every 2 weeks to self-report how many doses of the study product they had taken and missed.
Randomization and blinding
Randomization was completed by a research assistant not otherwise involved in the study: a table of numbers representing probiotic and placebo was randomized (1:1) in blocks of 10 using www.randomization.com. Sachets (probiotic and placebo) were pre-packaged according to the randomization code. This meant that participants, clinicians and raters remained blind to the allocated group of each participant until after the database was locked and data analysis was completed.
Study procedure
Eligible participants attended a meeting at the University of Canterbury conducted by a clinical psychologist, trainee clinical psychologist or a trained research assistant. After informed consent was obtained, clinician-rated measures were rated based on a personal interview. Blood samples were collected, and participants completed self-report questionnaires on a computer-based survey. Participants were allocated to the next sequentially numbered bag. During the 8-week intervention period, participants were asked to take one sachet at the same time each day, preferably before a meal, by pouring the orally dispersible powder from the sachet directly into the mouth where it rapidly dissolved. Participants were monitored every 2 weeks by an online questionnaire. The same procedure was followed at the final assessment.
Sample size
Due to the lack of any pilot data, the target sample size of 40 per group was selected based on the size of similar studies in the literature and what would identify a clinically meaningful group difference. Randomized controlled trials using probiotics and assessing psychological outcomes typically have a sample size of 40–75 (Akkasheh et al., 2016; Dickerson et al., 2014; Diop et al., 2008; Messaoudi et al., 2010). Recruiting 80 participants in total would be sufficient to show a moderate effect size (>0.60) for the comparison of probiotic and placebo as statistically significant (two-tail α = 0.05) with 80% power.
Statistical analysis
The primary outcome measures defined a priori were the MADRS, the iCGI and the QIDS-SR16. IBM SPSS Statistics version 20 was used for all statistical analyses. Baseline variables were compared between treatment groups using t-tests, chi square tests and Mann–Whitney U tests. Changes from baseline to the end of treatment were compared using analysis of covariance (ANCOVA), with the baseline level as the covariate. Changes in measures (iCGI-I ratings) were compared using one-way analysis of variance (ANOVA). Efficacy analyses were completed by an independent statistician. The treatment effects were summarized by mean differences and 95% confidence intervals derived from the ANCOVA/ANOVA models. Adverse events occurring in ⩾5% of the sample were compared between groups using Fisher’s exact tests. Analyses of all psychological outcome measures were completed on an intention-to-treat basis. The last-observation-carried-forward technique was used to impute missing values. Secondary analyses were undertaken on all psychological outcomes using the per protocol set, defined as those randomized participants who took their allocated product for the full intervention period (⩾80% compliance) and did not violate the study protocol. Biomarker variables were analysed per protocol. Skewed variables were log10 transformed. Changes from baseline to the end of treatment were compared using ANCOVA, with the baseline level as the covariate. For log10 transformed data, differences derived from the ANCOVA models for each treatment group were back transformed for display as geometric mean ratios, with the ratio of these used for the inter group comparison. Data for non-transformed biomarker variables were summarized by mean differences and 95% confidence intervals derived from the ANCOVA models. All significance tests were two-tailed and p-values <0.05 were considered statistically significant.
Exploratory analyses
Hierarchical multiple regression was used to examine whether treatment response was moderated by baseline levels of biomarkers. The baseline value of the dependent variable was entered (with any variable found to be correlated with biomarker levels), followed by experimental group and moderator variable. The interaction between the predictor moderator variable and group was then added into the model as the critical term identifying the presence of a moderating effect. Significant interaction effects were examined by calculating regression slopes one standard deviation above and below the mean of the moderator; simple slope analyses were conducted to examine whether the regression slopes differed significantly from zero.
Results
Study population
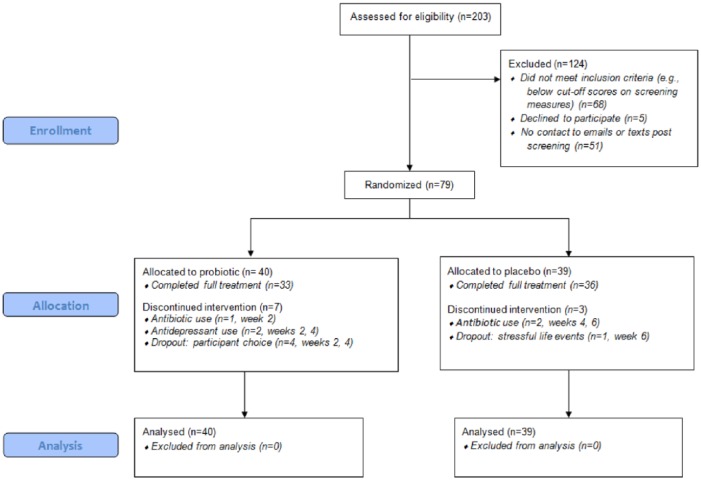
From 203 self-referrals between May 2013 and May 2014, 79 adults (age ⩾ 16 years) with at least moderate low mood completed informed consent and were assigned to the probiotic group (n = 40) or placebo group (n = 39). Seven people from the probiotic group and three people from the placebo group dropped out prior to finishing the trial (see Figure 1 for CONSORT diagram), resulting in 10 baseline observations being carried forward for outcome measures rated at baseline and 8 weeks (MADRS, CGI, GAF, DASS-42, IBS-SSS). In addition, 8 mid-trial observations were carried forward on the QIDS-SR16 (taken every 2 weeks throughout the study). Overall, the two groups were well matched in baseline characteristics (Table 1). Six participants were undergoing long-term psychotherapy during the trial: three from the probiotic group and three from the placebo group. No participant discontinued their psychotherapy during the trial. There was a significant imbalance in history of antidepressant usage, with 70% of the probiotic group self-reporting prior antidepressant usage compared with 46% of the placebo group, , p < 0.05. The probiotic group scored significantly higher on the IBS-SSS at baseline (median = 105) than the placebo group (median = 75) (Mann–Whitney U test), U = 558.5, Z = −2.17, p < 0.05. No other significant differences were found.
Figure 1.
CONSORT flow diagram.
Table 1.
Baseline demographic and clinical characteristics of study participants (n = 79).
| Characteristic | Probiotic group (n = 40) | Placebo group (n = 39) |
|---|---|---|
| Demographics | ||
| Age, years, mean (SD) | 35.8 (14) | 35.1 (14.5) |
| Male, n (%) | 8 (20) | 9 (23) |
| Socioeconomic status,a mean (SD) | 45.0 (11.5) | 41.6 (13.2) |
| Education, n (%) | ||
| No high school certificate | 3 (8) | 7 (18) |
| Completed high school | 13 (33) | 10 (26) |
| Post-secondary (e.g. trade certificate) | 15 (38) | 13 (33) |
| University degree | 9 (23) | 9 (23) |
| Ethnic origin, n (%) | ||
| New Zealanders of European descent | 30 (75) | 31 (80) |
| NZ Maori | 2 (5) | 1 (3) |
| Other | 8 (20) | 7 (18) |
| Clinical | ||
| Baseline MADRS score, mean (SD) | 28.3 (6.1) | 27.0 (6.3) |
| Chronic low mood,b,c n (%) | 31 (78) | 24 (62) |
| Anxiety disorder,c n (%) | ||
| Current | 20 (50) | 16 (41) |
| Past | 21 (53) | 18 (46) |
| Alcohol/substance misuse or dependence,c n (%) | ||
| Current | 4 (10) | 1 (3) |
| Past | 6 (15) | 8 (21) |
| Any co-occurring disorder,c n (%) | ||
| Current | 23 (57) | 18 (46) |
| Past | 24 (60) | 21 (54) |
| History of antidepressant use,c n (%) | 28 (70) | 18 (46)* |
| Current therapy,d n (%) | 3 (8) | 3 (8) |
| IBS severity,e median (IQR) | 105 (53–252) | 75 (28–138)* |
| Severity of IBS by category, n (%) | ||
| None | 16 (40) | 19 (49) |
| Mild | 10 (25) | 15 (39) |
| Moderate | 8 (20) | 5 (13) |
| Severe | 6 (15) | 0 (0) |
MADRS: Montgomery–Åsberg Depression Rating Scale; IBS: irritable bowel syndrome; SD: standard deviation; IQR: interquartile range.
Based on the New Zealand Socio-Economic Index 2006 (NZSEI-06) (Milne et al., 2013).
Chronic depression defined as >2 years continuous symptoms for the current episode of low mood.
Established using self-report and retrospective self-report.
Currently receiving any form of psychotherapy (self-report) – note that must be regularly for >6 months in order to meet entry criteria.
Score on Irritable Bowel Syndrome Symptom Severity Scale at baseline (0–500).
Significantly different from probiotic group, p < 0.05.
Psychological efficacy outcomes
Intent-to-treat analysis (n = 79) showed no significant group differences on any outcome measure (Table 2). Secondary analyses based on the per protocol sample (n = 69) yielded similar results, with no primary or secondary outcome approaching significance (data not shown). Further analyses controlling for history of antidepressant usage, season at recruitment, and presence/severity of IBS at baseline revealed very similar results to the primary analyses, with no variable tested having a significant effect on any outcome. At end-point, 9 (23%) of those in the probiotic group showed a ⩾60% change on the MADRS (responders), compared to 10 (26%) of those in the placebo group (, p = ns).
Table 2.
Baseline and post 8-week data on psychological outcome measures in intent-to-treat population (n = 79).
| Outcome variable | Probiotic |
Placebo |
Difference [95% CI] | p | ESb d | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | Post mean (SD) | Change from baselinea | Baseline mean (SD) | Post mean (SD) | Change from baselinea | ||||
| MADRS | 28.3 (6.1) | 19.3 (8.9) | −8.7 | 27.0 (6.3) | 17.6 (9.5) | −9.7 | −1.0 [−4.9, 3.0] | 0.62 | 0.11 |
| iCGI-S | 3.8 (0.5) | 2.9 (1.1) | −0.9 | 3.66 (0.6) | 2.7 (1.0) | −0.9 | −0.1 [−0.5, 0.4] | 0.75 | 0.07 |
| iCGI-Ic | 2.4 (2.2) | 2.4 | 2.7 (2.0) | 2.7 | −0.3 [−1.2, 0.6] | 0.52 | 0.16 | ||
| QIDS-SR16 | 15.2 (3.9) | 8.5 (5.5) | −6.3 | 13.2 (3.8) | 8.0 (4.8) | −5.6 | 0.7 [−1.5, 2.8] | 0.53 | 0.15 |
| GAF | 61.2 (5.6) | 67.5 (8.5) | 6.0 | 63.1 (5.4) | 69.3 (7.1) | 6.6 | 0.7 [−2.6, 3.9] | 0.68 | 0.1 |
| DASS | 64.3 (23.9) | 37.9 (29.0) | −23.4 | 50.0 (20.6) | 29.5 (20.8) | −23.6 | −0.2 [−10.6, 10.1] | 0.97 | 0.01 |
| Depression | 24.2 (9.1) | 13.2 (11.6) | −9.9 | 19.0 (10.5) | 10.3 (8.8) | −9.9 | −0.03 [−4.1, 4.1] | 0.99 | 0.003 |
| Anxiety | 15.2 (10.4) | 8.9 (9.6) | −5.2 | 10.6 (6.6) | 6.0 (6.0) | −5.7 | −0.4 [−3.5, 2.6] | 0.78 | 0.07 |
| Stress | 25.0 (9.2) | 15.9 (10.4) | −8.1 | 20.4 (8.4) | 13.2 (8.3) | −8.3 | −0.2 [−4.0, 3.6] | 0.92 | 0.02 |
ES: effect size; MADRS: Montgomery–Åsberg Depression Rating Scale; iCGI: improved Clinical Global Impression Scale (Severity/Improvement scale); QIDS-SR16: Quick Inventory of Depressive Symptoms; GAF: Global Assessment of Functioning; DASS: Depression, Anxiety and Stress Scale.
Adjusted for baseline.
Cohen’s d effect size: measured as the mean difference in change divided by the pooled standard deviation of the change, based on values adjusted for baseline.
Assesses change only, therefore not measured at baseline.
Biomarkers
Baseline blood data were available for 77 participants (probiotic, n = 38; placebo, n = 39) (Table 3). In total, 58 of the 77 participants (75%) had at least one marker of inflammation (CRP, IL-1β, IL-6 or TNF-α) elevated outside the reference range at baseline (reference ranges shown in Table 3). There were no significant differences in biomarker levels between the active treatment and placebo groups at baseline.
Table 3.
Baseline biomarker levels for all participants for whom baseline blood data were available (n = 77).
| Biomarker | Sample with baseline blood data (n = 77) |
|||
|---|---|---|---|---|
| GM | Range | Deficient (below reference range): n (%) | Elevated (above reference range): n (%) | |
| CRP (mg/L), reference range: 0–3 | 1.6 | 0.2–37.3 | NA | 23 (30) |
| IL-1β (pg/mL), reference range: 0–0.201 | 0.2 | 0–1.7 | NA | 49 (64) |
| IL-6 (pg/mL), reference range: 0.447–9.960 | 1.9 | 0.4–10.0 | 2 (3) | 3 (4) |
| TNF-α (pg/mL), reference range: 0.550–2.816 | 0.9 | 0.1–3.6 | 14 (18) | 4 (5) |
| BDNF (ng/mL), reference range: 6.2–42.6 | 25.3 | 5.2–48.2 | 1 (1) | 2 (3) |
| Vitamin D (nmol/L), reference range: 50–150 | 52.7 | 11–119 | 31 (40) | 0 (0) |
Abbreviations: GM = geometric mean; CRP = C-reactive protein; IL = interleukin; TNF = tumor necrosis factor; BDNF = brain derived neurotrophic factor.
Both baseline and end-point blood data were available for 65 participants (probiotic, n = 29; placebo, n = 36); however, due to some test results falling outside of the range of the assays used, per protocol sets differed for hsCRP (n = 55), IL-1β (n = 63) and IL-6 (n = 62). No significant group differences were observed in change in the level of any biomarker over the intervention period (Tables 4 and 5). Controlling for time of sample collection had no significant effect on any biomarker outcome.
Table 4.
Baseline to post 8-week biomarker levels in per protocol population using log10 transformed data.
| Biomarker (n; active treatment:placebo) | Probiotic |
Placebo |
GM ratiob |
p c |
|---|---|---|---|---|
| GM ratioa | GM ratioa | Probiotic vs placebo [95% CI] | ||
| CRP (55; 24:31) | 0.83 | 0.88 | 0.93 [0.72, 1.57] | 0.75 |
| IL-1β (63; 27:36) | 0.86 | 0.85 | 1.02 [0.53, 1.82] | 0.95 |
| IL-6 (62; 29:33) | 1.10 | 0.93 | 1.20 [0.66, 1.05] | 0.12 |
| TNF-α (65; 29:36) | 1.09 | 1.09 | 1.01 [0.70, 1.40] | 0.96 |
GML: geometric mean; BL: baseline; CRP: C-reactive protein; IL: interleukin; TNF: tumour necrosis factor; ANCOVA: analysis of covariance.
Post:pre. Based on log10 scale; back transformed for display.
Based on log10 scale, adjusted for baseline and back transformed.
Significance value derived from the ANCOVA models based on log10 scale.
Table 5.
Baseline to post 8-week biomarker levels in per protocol population.
| Biomarker (n; active treatment:placebo) | Probiotic |
Placebo |
Difference [95% CI] | p | ESb d | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) | Post mean (SD) | Change from BLa | Baseline mean (SD) | Post mean (SD) | Change from BLa | ||||
| Vitamin D, nmol/L (65; 29:36) | 57.7 (22.1) | 57.1 (21.8) | −1.16 | 60.4 (24.3) | 57.1 (23.0) | −2.90 | 1.71 [−6.79, 10.20] | 0.69 | 0.10 |
| BDNF, ng/mL (64; 28:36) | 27.6 (9.0) | 27.7 (6.2) | 0.95 | 25.7 (6.3) | 25.6 (8.9) | −0.79 | 1.75 [−2.16, 5.66] | 0.38 | 0.23 |
SD: standard deviation; BL: baseline; BDNF: brain-derived neurotrophic factor.
Adjusted for baseline.
Cohen’s d effect size: measured as the mean difference in change divided by the pooled standard deviation of the change, based on values adjusted for baseline.
Monitoring
There were significant group differences in the number of occurrences of two adverse events; however, in both cases, there were more occurrences in the placebo group than the probiotic group (Fisher’s exact tests: dry mouth, p < 0.05; sleep disruption, p < 0.05). The rates of all other adverse events did not differ between the groups (Table 6). There were three serious adverse events over the course of the trial, all of which were suicide attempts by one participant from the placebo group. There were no serious adverse events in the probiotic group.
Table 6.
Treatment-emergent adverse events reported by at least 5% of participants during the trial, by treatment group.
| Probiotic group (n = 40) | Placebo group (n = 39) | p | |
|---|---|---|---|
| Constipation | 7 | 11 | 0.29 |
| Change in appetite | 7 | 10 | 0.42 |
| Nausea | 6 | 7 | 0.77 |
| Loss of libido | 5 | 6 | 0.76 |
| Weight gain | 4 | 7 | 0.35 |
| Dry mouth | 2 | 8 | 0.048* |
| Nightmares | 6 | 4 | 0.74 |
| Abdominal pain | 5 | 4 | 1.00 |
| Anxiety | 5 | 4 | 1.00 |
| Urinary retention | 5 | 4 | 1.00 |
| Gastrointestinal disturbances | 7 | 2 | 0.15 |
| Headache | 4 | 5 | 0.74 |
| Agitation | 4 | 3 | 1.00 |
| Skin rash | 4 | 2 | 0.68 |
| Blurred vision | 2 | 3 | 0.68 |
| Inability to achieve orgasm | 3 | 2 | 1.00 |
| Sleep disruption | 0 | 5 | 0.03* |
| Sedation/lethargy | 1 | 3 | 0.36 |
p < 0.05.
Overall, adherence to the study protocol was good, with 97% of required doses taken as measured by post-study sachet counts. There was no significant difference in compliance rates between groups (number who took a minimum of 95% of required doses; , p = ns). During the study period, there were no group differences in rates of alcohol misusers (defined as more than two standard drinks per day for women and three standard drinks per day for men; n = 4 [10%] for probiotic group, n = 2 [5%] for placebo group; p = ns), smokers (n = 8 [20%] for probiotic group, n = 10 [35%] for placebo group; p = ns) or cannabis users (n = 6 [15%] for probiotic group, n = 3 [10%] for placebo group; p = ns).
Exploratory analyses
We examined baseline biomarker levels as possible moderators of treatment effect to attempt to provide insights in this very new area of research. Two participants were excluded from the analyses as they did not provide baseline blood data. Significant interaction effects were found between vitamin D and group on several outcome measures. The interaction between group and vitamin D explained significant amounts of variance in iCGI-S (ΔR2 = 0.07, p < 0.05), QIDS-SR16 (ΔR2 = 0.05, p < 0.05) and GAF (ΔR2 = 0.06, p < 0.05) scores over and above the baseline value of the dependent variable, season at time of recruitment (control variable), and the main effects of group and vitamin D. Simple slope analyses revealed that the association between baseline vitamin D and change on these measures was significant for the probiotic group (iCGI-S, b = 0.017, t = 2.26, p < .05; QIDS-SR16, b = 0.082, t = 2.60, p < 0.05; GAF, b = −0.124, t = 2.66, p < 0.05) but not for the placebo group (iCGI-S, b = −0.005, t = 0.76, p = ns; QIDS-SR16, b = −0.002, t = 0.07, p = ns; GAF, b = 0.022, t = 0.47, p = ns). Among those randomized to the probiotic group, those who had high vitamin D at baseline showed greater improvement in mood and functioning than those who had low vitamin D at baseline.
Discussion
Existing evidence suggests that depression is associated with increased permeability of the gut wall (Maes et al., 2012), increased immune and inflammatory activation (Berk et al., 2013; Maes, 2011) and gut disorders such as IBS (Fond et al., 2014). These underlying biological factors support a proposal made by Logan and Katzman (2005) that probiotics could be used as a treatment for depression. This study aimed to undertake preliminary testing of probiotics as a treatment for low mood. The lack of effect of probiotics on psychological outcomes in the present study is perhaps not surprising given the incipient nature of this area of research. There are many possible reasons for the lack of effect; some signals from our exploratory analyses may help to break some ground in this field.
First, it is possible that the findings of this study are restricted by the length of the intervention period or sample size. To our knowledge, the only previous trial to have assessed mood outcomes after probiotic administration in a symptomatic sample is a trial by Akkasheh et al. (2016), which reported positive effects of probiotics on BDI scores compared with placebo (although this effect was attenuated by confounders) in a sample of 40 participants over 8 weeks; however, this trial used probiotics as an adjuvant treatment alongside citalopram. It is possible that probiotics would take longer than 8 weeks to effect changes in mood when used as a primary treatment in a symptomatic sample, as in the present study. Given that most modern antidepressant studies have reported effect sizes of 0.2–0.4 (Vöhringer and Ghaemi, 2011), it is likely that our study was underpowered to detect an effect of probiotics. However, we think it unlikely that the lack of any observable group difference in this study was an issue of power alone, given the remarkably similar change in outcomes in both groups (p-value range = 0.52–0.99). To detect a change at this level would have required a sample of thousands, which would have produced a clinically irrelevant result. In addition, a marginally bigger change was observed in the placebo group than the probiotic group on several outcome measures, which also argues against the lack of observed effect being an issue of power alone. We therefore consider that there were probably multiple factors responsible for the lack of observable effect.
The lack of effect on inflammatory markers is somewhat unexpected given the elevated inflammatory profile of our sample, and given the probable anti-inflammatory effects of some probiotics, including some Lactobacillus strains and B. Longum (Haskey and Dahl, 2009; Wallace et al., 2003). It is possible that these tests were underpowered and may have reached significance in a larger sample. In addition, although we controlled for time of sample collection in our analyses, it would have been preferable to collect samples at the same time for each participant.
The severity and chronicity of the current sample is another consideration: mean baseline MADRS score in the present sample was high in the moderate range and almost 70% of the sample reported continuous low mood lasting more than 2 years. It is possible that probiotics may be more effective in shorter term or in less severe low mood. Baseline severity and chronicity were not reported by Akkasheh et al. (2016), so no comparison can be drawn.
Recruiting participants not currently receiving pharmacological treatment for their low mood was intended to provide insight into the efficacy of probiotics as a primary, rather than adjunctive, treatment for low mood. However, recruiting a sample of unmedicated participants may have increased the overall treatment resistance of the sample, given that a majority of participants had received antidepressant medications prior to the study and still presented with significant, chronic low mood (self-report). There was an imbalance in prior antidepressant usage between the groups (more of the probiotic group had used antidepressants in the past), which may indicate a higher level of treatment resistance among the probiotic group. Although controlling for the group difference in prior antidepressant usage had no effect on outcomes in this study, it is possible that the overall treatment resistance of the sample is not well represented by prior antidepressant use. We did not formally assess treatment resistance; a standardized measure assessing treatment resistance (e.g. Antidepressant Treatment History Form; Sackeim, 2001) may have been valuable in interpreting the results of this trial.
Another potential confounder was that we allowed concurrent therapy on condition that the therapy had been ongoing for 6 months. This decision was taken on the basis of dose–response research that suggests that sudden improvements during long-term psychotherapy (more than 15 sessions) are only as likely as spontaneous remission (see Hansen et al., 2002 for a review). Although it is possible that the inclusion of these participants may have skewed our results, we believe this is unlikely given that only 6 participants (8%) were receiving concurrent psychotherapy, and there was an equal number of these participants in each treatment group (any potential effect on study outcomes was randomized out between the treatment groups).
The probiotic formulation used in this trial has shown positive effects on both emotional behaviour in animal studies (Arseneault-Breard et al., 2012; Messaoudi et al., 2010) and psychological outcomes in a human study (Messaoudi et al., 2011). However, despite the existing evidence to support the efficacy of the study product, it remains possible that different strains, more CFU or more strains combined into a single product may be more effective. It is important that the results of the current study are not generalized to all potential probiotic strains.
Our exploratory analyses appear to show a moderating effect of vitamin D on treatment response. In the probiotic group, participants with high levels of vitamin D at the beginning of the study experienced significantly greater improvement on several psychological outcomes over time than those with low vitamin D at baseline. Baseline vitamin D had no effect on responding in the placebo group. Given the modulatory effects of vitamin D on the immune system (Battersby et al., 2012) and its role in immune cell development and function (Griffin et al., 2003), it is possible that the vitamin D status of the host could have an effect on the relationship between the gut microbiota and the immune system: low vitamin D could limit response to probiotic treatment as any changes to the microbiome composition would not necessarily be translated to the immune system. Although this mechanism is unsupported by the lack of any significant effect of probiotics on inflammatory markers in the present study, it is possible that measuring markers of cell-mediated immunity may have been more appropriate. However, it is equally possible that there are other mechanisms that we have yet to consider.
The current study assessed whether probiotics affect mood and other psychological outcomes when used as a primary treatment in a sample selected for low mood. This study has several limitations which should be addressed in future research. Measures of body mass index (BMI), body fat percentage, dietary intake and physical activity should be taken in future probiotics studies. The lack of intestinal microbiome analysis is a significant limitation of the current study, as we are unable to determine whether this probiotic formulation colonized effectively. It is possible that the lack of observed effect of probiotics on psychological outcomes is due to the size of the sample, the length of the intervention period, the severity, chronicity or treatment resistance of the sample, or the strains used. It is equally possible that probiotics are more appropriate as an adjuvant treatment for low mood or depression, as suggested by Logan and Katzman (2005). This is supported by the recent positive trial by Akkasheh et al. (2016) which showed that probiotics were beneficial to patients with MDD adjuvant to citalopram. Preventative trials giving probiotics to those at risk of gut dysbiosis (e.g. those born by caesarean section or bottle fed in infancy), which record any impact on mental health outcomes in later life, may also prove valuable. Pärtty et al. (2015) provided probiotics or a placebo for 6 months in infancy and found a group difference in occurrences of attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) at 13 years of age. Early exposure to probiotics may also be protective for developing depression; further longitudinal studies are warranted.
In testing probiotics as a primary treatment for low mood, further studies testing different probiotic strains, for longer intervention periods and in different populations (e.g. less severe/chronic low mood, antidepressant-naive) are important next steps in this very new area of research. Despite the lack of any observable effect of probiotics on psychological or biological outcomes in this study, future studies are strongly encouraged, and should take into account the findings of this study in relation to vitamin D, low levels of which could hinder the efficacy of probiotic supplements for low mood. The continued investigation of probiotics as a potential primary or adjuvant treatment for mood is especially important in light of the emerging research suggesting a novel biological aetiology of mood disorders, such as the relationship with inflammatory/immune response and mitochondrial dysfunction (see Kaplan et al. (2015) for a review). The treatments outlined in the clinical practice guidelines for mood disorders recently published by the Royal Australian and New Zealand College of Psychiatrists (Malhi et al., 2015) adhere very much to the conventional model for treatment of mood disorders; this new understanding of the aetiology of mental disorders necessitates a new paradigm for treatment. This area of research is in its infancy and, given the enormous burden of mood disorders on Western societies, the importance of continuing to explore the therapeutic potential of probiotics for low mood should not be underestimated.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Romijn has received research funding from Lallemand Health Solutions. None of the other authors have any conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research funding from Lallemand Health Solutions which provided the intervention products and paid for the blood testing. Lallemand Health Solutions was not involved with the design, execution, analysis or writing of this article. This work was also supported by a PhD scholarship awarded to the first author through a private donation from Marie Lockie.
References
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. (2016) Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 32: 315–320. [DOI] [PubMed] [Google Scholar]
- Anglin RE, Samaan Z, Walter SD, et al. (2013) Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. British Journal of Psychiatry 202: 100–107. [DOI] [PubMed] [Google Scholar]
- Arseneault-Breard J, Rondeau I, Gilbert K, et al. (2012) Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. British Journal of Nutrition 107: 1793–1799. [DOI] [PubMed] [Google Scholar]
- Ascoli BM, Géa LP, Colombo R, et al. (2016) The role of macrophage polarization on bipolar disorder: Identifying new therapeutic targets. Australian and New Zealand Journal of Psychiatry 0004867416642846. [DOI] [PubMed] [Google Scholar]
- Battersby AJ, Kampmann B, Burl S. (2012) Vitamin D in early childhood and the effect on immunity to Mycobacterium tuberculosis. Clinical and Developmental Immunology 2012: 430972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza A, et al. (2011) Pathways underlying neuroprogression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience and Biobehavioral Reviews 35: 804–817. [DOI] [PubMed] [Google Scholar]
- Berk M, Williams LJ, Jacka FN, et al. (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Medicine 11: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested AC, Logan AC, Selhub EM. (2013) Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part III: Convergence toward clinical trials. Gut Pathogens 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America 108: 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldecott-Hazard S, Hall R. (1995) Outcome assessment in depressed hospitalized patient. Journal of the Florida Medical Association 82: 24–29. [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. (2012) The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology 10: 735–742. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. (2012) Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience 13: 701–712. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Stallings C, Origoni A, et al. (2014) Effect of probiotic supplementation on Schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. The Primary Care Companion to CNS Disorders 16: e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Stanton C, Cryan JF. (2013) Psychobiotics: A novel class of psychotropic. Biological Psychiatry 74: 720–726. [DOI] [PubMed] [Google Scholar]
- Diop L, Guillou S, Durand H. (2008) Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: A double-blind, placebo-controlled, randomized trial. Nutrition Research 28: 1. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, et al. (2010) A meta-analysis of cytokines in major depression. Biological Psychiatry 67: 446–457. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Hamdani N, et al. (2014) Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. European Archives of Psychiatry and Clinical Neuroscience 1–10. [DOI] [PubMed] [Google Scholar]
- Francis C, Morris J, Whorwell P. (1997) The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Alimentary Pharmacology & Therapeutics 11: 395–402. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Xing N, Kumar R. (2003) Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annual Review of Nutrition 23: 117–145. [DOI] [PubMed] [Google Scholar]
- Hansen NB, Lambert MJ, Forman EM. (2002) The psychotherapy dose-response effect and its implications for treatment delivery services. Clinical Psychology: Science and Practice 9: 329–343. [Google Scholar]
- Haskey N, Dahl WJ. (2009) Synbiotic therapy improves quality of life and reduces symptoms in pediatric ulcerative colitis. ICAN: Infant, Child, & Adolescent Nutrition 1: 88–93. [Google Scholar]
- Kadouri A, Corruble E, Falissard B. (2007) The improved Clinical Global Impression Scale (iCGI): Development and validation in depression. BMC Psychiatry 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BJ, Rucklidge JJ, McLeod K, et al. (2015) The emerging field of nutritional mental health: Inflammation, the microbiome, oxidative stress, and mitochondrial function. Clinical Psychological Science 3: 964–980. [Google Scholar]
- Keightley PC, Koloski NA, Talley NJ. (2015) Pathways in gut-brain communication: Evidence for distinct gut-to-brain and brain-to-gut syndromes. Australian and New Zealand Journal of Psychiatry 49: 207–214. [DOI] [PubMed] [Google Scholar]
- Kullisaar T, Songisepp E, Mikelsaar M, et al. (2003) Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. British Journal of Nutrition 90: 449–456. [DOI] [PubMed] [Google Scholar]
- Liebregts T, Adam B, Bredack C, et al. (2007) Immune activation in patients with irritable bowel syndrome. Gastroenterology 132: 913–920. [DOI] [PubMed] [Google Scholar]
- Lin YP, Thibodeaux CH, Pena JA, et al. (2008) Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflammatory Bowel Diseases 14: 1068–1083. [DOI] [PubMed] [Google Scholar]
- Logan AC, Katzman M. (2005) Major depressive disorder: Probiotics May be an adjuvant therapy. Medical Hypotheses 64: 533–538. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. (1995) Manual for the Depression Anxiety Stress Scales. Sydney, NSW: Psychology Foundation. [Google Scholar]
- Ly NP, Litonjua A, Gold DR, et al. (2011) Gut microbiota, probiotics, and vitamin D: Interrelated exposures influencing allergy, asthma, and obesity? Journal of Allergy and Clinical Immunology 127: 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. (2011) Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry 35: 664–675. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis J-C, et al. (2012) Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. Journal of Affective Disorders 141: 55–62. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Bassett D, Boyce P, et al. (2015) Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Australian and New Zealand Journal of Psychiatry 49: 1087–1206. [DOI] [PubMed] [Google Scholar]
- Mayer EA. (2011) Gut feelings: The emerging biology of gut-brain communication. Nature Reviews Neuroscience 12: 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, et al. (2010) Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. British Journal of Nutrition 105: 755–764. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Violle N, Bisson J-F, et al. (2011) Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2: 256–261. [DOI] [PubMed] [Google Scholar]
- Milne B, Byun U, Lee A. (2013) New Zealand Socio-Economic Index 2006. Wellington, New Zealand: Statistics New Zealand. [Google Scholar]
- Montgomery SA, Åsberg M. (1979) A new depression scale designed to be sensitive to change. British Journal of Psychiatry 134: 382–389. [DOI] [PubMed] [Google Scholar]
- Müller MJ, Himmerich H, Kienzle B, et al. (2003) Differentiating moderate and severe depression using the Montgomery–Åsberg depression rating scale (MADRS). Journal of Affective Disorders 77: 255–260. [DOI] [PubMed] [Google Scholar]
- Pärtty A, Kalliomäki M, Wacklin P, et al. (2015) A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatric Research 77: 823–828. [DOI] [PubMed] [Google Scholar]
- Romijn AR, Rucklidge JJ. (2015) Systematic review of evidence to support the theory of psychobiotics. Nutrition Reviews 73: 675–693. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, et al. (2003) The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry 54: 573–583. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. (2001) The definition and meaning of treatment-resistant depression. Journal of Clinical Psychiatry 62: 10–17. [PubMed] [Google Scholar]
- Sanders ME. (2008) Probiotics: Definition, Sources, Selection, and Uses. Clinical Infectious Diseases 46: S58–S61. [DOI] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, Van Hemert S, et al. (2015) A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain, Behavior, and Immunity 48: 258–264. [DOI] [PubMed] [Google Scholar]
- Stefanescu C, Ciobica A. (2012) The relevance of oxidative stress status in first episode and recurrent depression. Journal of Affective Disorders 143: 34–38. [DOI] [PubMed] [Google Scholar]
- Thakur AK, Shakya A, Husain GM, et al. (2014) Gut-microbiota and mental health: Current and future perspectives. Journal of Pharmacology and Clinical Toxicology 2: 1016. [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, et al. (2013) Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144: 1394–1401.e1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vöhringer PA, Ghaemi SN. (2011) Solving the antidepressant efficacy question: Effect sizes in major depressive disorder. Clinical Therapeutics 33: B49–B61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TD, Bradley S, Buckley ND, et al. (2003) Interactions of lactic acid bacteria with human intestinal epithelial cells: Effects on cytokine production. Journal of Food Protection 66: 466–472. [DOI] [PubMed] [Google Scholar]