Abstract
Introduction
Sigma-1 receptors (Sig-1Rs) are molecular chaperones that reside mainly in the endoplasmic reticulum (ER) but exist also in the proximity of the plasma membrane. Sig-1Rs are highly expressed in the CNS and are involved in many cellular processes including cell differentiation, neuritogenesis, microglia activation, protein quality control, calcium-mediated ER stress and ion channel modulation. Disturbance in any of the above cellular processes can accelerate the progression of many neurological disorders; therefore, the Sig-1R has been implicated in several neurological diseases.
Areas covered
This review broadly covers the functions of Sig-1Rs including several neurodegenerative disorders in humans and drug addiction-associated neurological disturbance in the case of HIV infection. We discuss how several Sig-1R ligands could be utilized in therapeutic approaches to treat those disorders.
Expert opinion
Emerging understanding of the cellular functions of this unique transmembrane chaperone may lead to the use of new agents or broaden the use of certain available ligands as therapeutic targets in those neurological disorders.
Keywords: Alzheimer’s disease, amyotrophic lateral sclerosis, frontotemporal lobar degeneration, HIV-associated neurodementia, neurodegenerative diseases, Parkinson’s disease, psychiatric disorders, sigma-1 receptor
1. Introduction
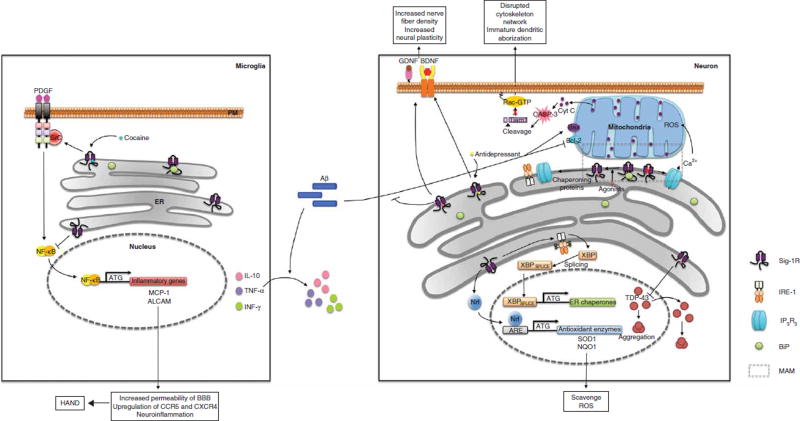
The sigma-1 receptor (Sig-1R) is a non-opioid type of receptor [1] that interacts with endogenous steroids. It was later identified as a ligand-responsive endoplasmic reticulum (ER) chaperone residing specifically at the ER–mitochondrion interface referred to as the mitochondria-associated ER membrane (MAM) [2]. At the MAM, Sig-1Rs chaperone the activity of inositol 1,4,5-trisphosphate type 3 (IP3) receptors [2] and the IRE-1 ER stress sensor [3] and regulate such diverse processes as calcium signaling, lipid biosynthesis, Rac GTPase effector Tiam1 [4] and apoptosis (Figure 1). In addition, Sig-1Rs are known to protect cells from oxidative stress [5,6] and ER-mediated cellular stress, and are known to participate in the unfolded protein response (UPR). Interestingly, the Sig-1R not only exists in the MAM but also translocates via ligand stimulation to other parts of cells or neurons where Sig-1Rs can interact with and modulate the functions of several receptors, ion channels or kinases on the plasma membrane [7,8]. This property of the Sig-1R was designated as ‘inter-organelle signaling modulation’ [9]. The functions of Sig-1Rs thus relate to their actions locally at the MAM but also remotely at other parts of the cell or neuron.
Figure 1. Sig-1R functions in the brain and the potential therapeutic benefits provided by the ligands are shown.
Sig-1Rs physically interact with BiP under resting state conditions. On stimulation, Sig-1Rs disassociate from BiP and interact with several client proteins to exert chaperone activity, regulate calcium homeostasis and maintain mitochondrial activity. Sig-1R ligands promote neural plasticity via secretion of neural trophic factors or stabilization of the cytoskeletal networks. In the event of cellular stress, Sig-1Rs activate signaling pathways that increase the transcription of ER chaperones and antioxidant enzymes. Ligand stimulation also alleviates protein aggregations as well as neuroinflammation.
ALCAM: Activated leukocyte adhesion molecule; BDNF: Brain-derived neurotrophic factor; BiP: Binding immunoglobulin protein; ER: Endoplasmic reticulum; GDNF: Glial-derived neurotrophic factor; IRE-1: Inositol-requiring enzyme 1; MAM: Mitochondria-associated ER membrane; MCP-1: Monocyte chemoattractant protein-1; NQO1: NAD(P)H dehydrogenase, quinone 1; Nrf2: Nuclear factor erythroid 2-related factor; PDGF: Platelet-derived growth factor; ROS: Reactive oxygen species Sig-1R: Sigma-1 receptor; SOD1: Superoxide dismutase 1; XBP-1: X-box binding protein 1.
In this review, we have highlighted the works that present the potential of Sig-1R ligands in ameliorating the progression of common neurological disorders. In particular, we are interested in how the Sig-1R is involved in the onset or progression of neuronal dysfunctions arising from motor neuron diseases (MNDs), neurodegenerative disorders particularly the Alzheimer’s disease (AD) and Parkinson’s disease (PD), psychiatric disorders and last, HIV-associated neurodementia (HAND). We aim to examine these aspects individually and attempt to pinpoint what could be the most promising approaches for future therapeutic interventions.
2. Neurodegenerative disorders
2.1 Motor neuron diseases
MNDs are a group of progressive neurological disorders that are caused by deterioration of the nerve cells that control muscle movement. They affect 1 – 5 out of 100,000 people in the world and can be classified into sporadic or inherited types. To date, most of the MNDs still lack effective therapeutic strategies. Genetic alterations in a number of genes including the open reading frame C9orf72, superoxide dismutase 1 (SOD1), transactivating response element (TAR) DNA binding protein (TARDBP, also known as TDP-43), RNA-binding protein fused in sarcoma, valosin-containing protein, SQSTM1/p62 and Profilin1 have been linked to the genetic heterogeneity and etiological diversity in the pathogenesis of the familial MND and allied dementia [10].
Scientists have placed Sig-1Rs in the spotlight to address the onset and pathogenesis of motor neuron (MN)-related disorders. Two recent amyotrophic lateral sclerosis (ALS) review articles highlight the Sig-1R as one of the genes involved in the pathogenesis of ALS and bring out the concept that SIGMAR1 heterogeneity might be related to the pathogenesis of ALS [11,12]. A pioneer genetic study analyzing different ethnic groups reveals that SIGMAR1 mutations are linked to several familial MNDs. In 2010, Luty et al. [13] conducted a series of mutation screenings of candidate genes that cosegregate with the disease phenotype in the Australian-origin frontotemporal lobar degeneration (FTLD)-MND lineage (family Aus-14) and identified a nonpolymorphic mutation (c.672*51G > T) in the 3′-untranslated region (UTR) of the SIGMAR1 gene. Their findings support that SIGMAR1 is a causative gene for familial FTLD-MND. Further, they found that the Sig-1R agonist opipramol had significant effects on TDP-43 localization. Changes in TDP-43 localization patterns by Sig-1R ligand treatments suggest that these compounds may have direct therapeutic potential for TDP-43 proteinopathies that are associated with FTLD-MND (Figure 1). In 2011, Al-Saif et al. [14] conducted homozygosity mapping and direct sequencing in an extended consanguinity family from Saudi Arabia and found a mutation at the E102Q locus in the SIGMAR1 gene that was accountable for the onsets of juvenile ALS. The E102Q-mutant Sig-1Rs showed aberrant membrane distribution patterns when compared to wild-type Sig-1Rs. A recent paper also found that overexpressing SIGMAR1 gene-variant E102Q aggravates mitochondrial damages and leads to aberrant TDP-43 localization [15]. The above three reports strengthen the argument that SIGMAR1 is associated with MNDs. Conversely, Belzil et al. [16] reported that variations in the 3′-UTR of the SIGMAR1 gene are not associated with FTLD-MND pathogenesis in the Caucasian cohort populations whom they screened. In this study, the authors sequenced the UTR coding regions of the SIGMAR1 gene in a targeted Caucasian population, of which 25 individual familial ALS patients had a history of cognitive impairments. Surprisingly, they identified one variant (c.672* 43G > T) in the 3′-UTR of SIGMAR1 in one patient among the large populations, and the same variant was also identified in one individual out of the 190 matched controls. Hence, the authors suggested that this variant is not the cause of ALS in this particular pedigree. Interestingly, 52% of affected individuals carried a hexanucleotide repeat expansion in C90RF72. Given the fact that both SIGMAR1 and C90RF72 are close to each other on chromosome 9p, the authors argued that the 3′-UTR SIGMAR1 variant identified by Luty et al. actually segregated with C90RF72 expansions; thus, further studies in different populations are warranted to support the assertion that SIGMAR1 gene is causative of ALS in certain pedigrees.
Abnormal intracellular accumulations of misfolded proteins in the brain are pathological hallmarks of most neurodegenerative diseases. A growing body of evidence suggests that Sig-1R maintains protein quality by regulating protein degradation and stability [3,17]. In addition, various studies have shown that Sig-1R ligands exert ameliorating effects on proteinopathy-associated neurodegenerative diseases. Since ligand activation may promote and stabilize Sig-1R oligomers, thus conferring improved chaperone functionality to the Sig-1R [18] and attention has been focused on Sig-1R protein modifications and the resultant effects in ALS progression. Indeed, abnormal Sig-1R accumulation is found in the neuronal nuclear inclusions in many neurodegenerative diseases [13,19]. Sig-1R participation in the degradation of misfolded protein via the endoplasmic reticulum-associated degradation machinery linked to the ubiquitin-mediated UPR indicates that Sig-1Rs may be part of the innate cellular responses to counteract the pathological mechanisms and promote survival in affected MNs.
High levels of Sig-1Rs are found in the MNs in the spinal cord and brainstem regions [20,21]. Remarkably, although the precursor MNs are known to be present in the spinal cord at early developmental stages, Sig-1R expression was not detectable in the MNs prior to E15, but was more intense at E18 [22]. Notably, synaptic cholinergic dysfunction and diminution in postsynaptic cholinergic-related structure was observed in parallel to early loss of Sig-1R immunoreactivity in lumbar MNs from the young ALS mice [23]. Behavioral studies evaluating motor coordination using the rotarod performance test revealed that Sig-1R knockout (KO) mice stayed on for a shorter time than wild-type mice, implying that the Sig-1R plays a role in the motor behavior [21]. It was also found that Sig-1R agonist PRE-084 improved locomotor performance outcomes in the SOD1 (G93A) mouse model of ALS. PRE-084 administration also significantly preserved the MNs and neuromuscular connections in the lumbar spinal cord of the SOD1 transgenic mice [24]. These positive results suggested that Sig-1R ligands could be applied to prolong the lifespan in ALS patients. In fact, KO of Sig-1Rs exacerbates ALS progression in the SOD1 (G93A) ALS mouse model [25]. Similar results were obtained by Ono et al. [26] using the same ALS mouse model and the NSC34 cell line model. Although the Sig-1R agonist SA4503 did not delay the onset time of ALS, it suppressed the progression of ALS and significantly extended the lifespan of SOD1 (G93A) mice.
Several proteins have been found to exhibit proteinopathies in MND patients. Typically, these proteins have the propensity to form abnormal aggregates and inclusion bodies in the affected neurons. Sig-1Rs were found to abnormally redistribute in α-MN of ALS patients, form ubiquitinated aggregates that lead to UPR and interact with another ALS-8-associated ER protein, vesicle-associated membrane protein-associated protein B (VAPB). Additionally, overall Sig-1R levels were significantly reduced in the ALS patient samples [27]. The authors also confirmed our previous findings [4] that depletion of Sig-1R leads to increased oxidative stress and abnormal mitochondrial membrane potential, thus triggering cytochrome c release and elevated caspase-3 cleavages. Interestingly, pharmacological activation of Sig-1R using PRE-084 exerts a similar neuroprotective effect by reducing abnormal VAPB aggregates [27]. Another study confirmed that the Sig-1R is beneficial to MN survival and motor behavior via the modulation of astrocytosis and macrophage/microglia activation [28]. Using a spontaneous MN degeneration wobbler mouse model that is not linked to SOD1 mutation, the authors discovered that chronic treatment of PRE-084 enhanced brain-derived neurotrophic factor (BDNF)-mediated trophic support to improve MN survival and ameliorate ALS symptoms. However, one recent study reported that when attempting to combine the treatments of PRE-084 and a natural polyphenol resveratrol (RSV) in the ALS mouse model, the combinatory therapy approach did not show a synergistic effect [29]. The absence of a synergistic effect may be indicative of a common protective mechanism since RSV is known to suppress oxidative stress and protect cellular functions, which are also recognized as Sig-1R functions.
Taken together, the increasing evidence supports that Sig-1Rs are involved in the progression of several MNDs. The onset of some familial MNDs may be due to genetic mutations in SIGMAR1 causing inadequate MN function, and others may be related to the formation of certain proteinopathies due to the loss of Sig-1R function in aged MNs. Sig-1R ligands apparently display therapeutic potential to treat these disorders.
2.2 Alzheimer’s disease
AD is the most prevalent neurodegenerative disorder associated with dementia [30]. Although AD pathology is complex and its etiology remains inconclusive, hallmarks of human AD include progressive cognitive decline that follows chronic neuroinflammation and the emergence of hyperphosphorylated Tau protein aggregates and amyloid β (Aβ) plaques [31,32]. It has been proposed that Sig-1Rs comprise part of the endogenous cellular defense against neurodegenerative disorders such as AD and parkinsonism [33,34]. Calcium signaling at the MAM, in particular, appears to play an important role in AD pathophysiology and the Sig-1R may be responsible for neuroprotective regulatory functions in this subcompartment [35]. Indeed, neuroimaging studies revealed that Sig-1Rs are present in lower density in brains from AD patients relative to the brains of healthy individuals [36]. Moreover, there is emerging evidence that certain polymorphisms of the SIGMAR1 gene, especially when present alongside the known AD risk factor APOE4, are linked to the onset of AD neurodegeneration [37,38]. These findings suggest that Sig-1Rs prevent or mitigate AD pathology and therefore present as a promising therapeutic target.
Understanding the pathophysiology of AD is important for devising efficacious treatment strategies. Aβ plaque deposits, neurofibrillary tangles (NFTs) and neuroinflammation all play critical and perhaps interrelated roles in the progression of AD [39]. Aβ deposits in brain tissue have received the most attention in the investigation of AD onset and progression. Ab plaques are large aggregates of misfolded amyloid protein fragments of varying lengths. Aβ25–35 fragments are implicated in the pathophysiology of AD and possess multiple neurotoxic mechanisms including the disruption of calcium homeostasis, production of reactive oxygen species (ROS), hyperactivation of NMDA channels and promotion of proapoptotic signaling through the upregulation of Bax (Figure 1). Injections of Aβ25–35 fragments in mice and rats induce an increase in intracellular calcium concentrations, memory deficits, and AD-like pathology in brain tissue [40,41]. A selective Sig-1R agonist, afobazole, was reported to prevent Bax increase and promote calcium homeostasis in rat cortical neurons. Following incubation with Aβ25 – 35, however, this observation was not extended to the pan-selective Sig-1R agonist, 1,3-di-O-tolylguanidine (DTG) [40]. Another group reported that the high affinity Sig-1R agonists (+)-pentazocine (PTZ), SA4503 and PRE-084 each prevent Aβ25–35-induced memory impairments, as did specific neurosteroids that are known to interact with the Sig-1R such as dehydroepiandrosterone (DHEA) [41]. Pharmacologic stimulation of Sig-1Rs is also reported to alleviate oxidative stress and indeed, small interfering RNA (siRNA) knockdown or KO of Sig-1Rs results in elevated ROS in mouse or rat tissue; these effects have been shown to confer susceptibility to oxidative cytotoxicity in vitro [4,6,42]. Aβ1 – 42 fragments also increase the production of intracellular ROS, which has been shown to inhibit long-term potentiation (LTP) in rat hippocampal slices. PRE-084 rescues LTP suppression by Aβ 1 – 42 in part by activation of the nuclear factor erythroid 2-related factor (Nrf2) redox signaling pathway; however, whether pharmacological stimulation of the Sig-1R is sufficient to overcome oxidative stress mediated by all pathogenic amyloid species involved in AD progression remains to be clarified [43]. It has been proposed that Sig-1Rs are able to signal through the Nrf2 transcriptional pathway, in light of the finding that (+)-PTZ treatment increases cellular mRNA of the housekeeping antioxidant proteins NQO1 and SOD1 [6].
NFTs are composed primarily of hyperphosphorylated Tau protein fragments in aggregates known as paired helical filaments (PHFs) [44]. Normal tau proteins stabilize the structure of microtubules in neurons; however, in disease models misfolded and cleaved tau fragments are unable to perform their regular function and promote the nucleation and accumulation of PHFs on the surface of lysosomes, thereby blocking the lysosome-autophagy pathway [45]. Loss of Sig-1Rs in cell lines has recently been demonstrated to cause deficits in endolysosomal autophagy pathways; however, whether Sig-1R agonists could abrogate NFT accumulation in AD models remains to be addressed [46]. Moreover, PHFs are known to be heavily ubiquitinated; however, it is somewhat controversial whether or not normal tau proteins are substrates of the ubiquitin-proteasome pathway and it is unclear if proteasomal degradation participates in the cellular response against NFT accumulation [47–49]. The Sig-1R participates in the UPR and displays molecular chaperone activity, indicating that it may help to clear protein accumulations that mediate cellular stress [2,3,17,19]. Aβ25–35 injection in mice was demonstrated to promote the aberrant hyperphosphorylation of tau proteins and the development of Aβ1 – 42 fragments in the hippocampus. These effects were dramatically attenuated by a mixed muscarinic/Sig-1R agonist, ANAVEX2–73 [50]. Donepezil is the most widely prescribed compound for AD and, although its proposed mechanism of action is acetylcholine esterase inhibition, it is also a potent Sig-1R agonist. One group reported that donepezil does indeed bind to Sig-1Rs in human male brains and that the agonist potentiates neural growth factor-mediated neurite outgrowth via the action of Sig-1Rs and IP3 receptors [51,52]. Thus, future investigations may emphasize ligand cocktails that stimulate an array of receptor types, with the Sig-1R among them, in order to modulate cellular response mechanisms.
AD pathology may also be caused by chronic inflammation of the CNS [32]. AD patients have higher proinflammatory cytokines and a large population of either ‘primed’ microglia or bona fide active microglia in the M1 phenotype. Early neuroinflammation may be beneficial to the CNS since it can promote the phagocytosis of Aβ plaques and degenerated neurons. However, the reactivity of microglia appears to be poorly regulated, and in later stages of the disease these phagocytic cells accelerate the atrophy of brain areas through the mediation of synaptic and neuronal phagocytosis. The presence of Aβ plaques has been the traditional marker of AD; however, it is now under consideration that neuroinflammatory processes precede the arrival of amyloid deposits. Both microglia and astrocytes express abundant Sig-1R, and the pharmacological activation of Sig-1Rs was shown to modulate neuroimmune responses [53–55]. Indeed, we previously reported that KO of Sig-1R causes dramatic upregulation of several immune response proteins [5]. Sig-1R agonist DTG administration reduces the secretion of inflammatory cytokines including TNF-a, INF-γ and IL-10 and is able to suppress the release of cytotoxic ROS associated with activated microglia and macrophages (Figure 1) [55]. Activation of Sig-1Rs in primary rat microglia by DTG induced potent suppression of microglia activation through the inhibition of cytokine release, cytoskeletal rearrangement and migration. In agreement with other reports of Sig-1R mechanisms of regulation, DTG suppressed both short-and long-term intracellular calcium increases associated with activation of microglia [55]. It is, therefore, very interesting to understand how pharmacological stimulation of Sig-1Rs may prevent immune effector cell-mediated atrophy of the CNS; however, suppressing the innate immune system in early stages of AD may promote the accumulation of Ab deposits.
Although there are several potentially promising avenues for Sig-1R intervention in AD pathology, critical issues need to be further evaluated. Perhaps Sig-1R agonists can prevent Aβ25–35-mediated cell damage and exert beneficial immunomodulatory effects on CNS phagocytes, though the long-term and total biological effects of immunomodulation are unknown in AD. Although the emerging data on Sig-1R ligand-based therapies in the treatment of AD are promising, it is unclear if molecules that exclusively target Sig-1Rs will be sufficient to overcome AD pathology and retard neurodegeneration. Further, if the cause of AD in specific cases is indeed linked to inheritance of defective SIG-MAR1 gene variants, it is also unclear if agonist-based treatment regimens would exert any observable effects on AD pathophysiology.
2.3 Parkinson’s disease
PD is second in prevalence only to AD among neurodegenerative disorders, with approximately three-quarters of parkinsonism reportedly due to neuronal proteinopathies [56]. The causes of familial and sporadic PD are still unclear; however, genetic, aging and environmental risk factors have been identified. As with many neurodegenerative disorders, oxidative stress, insoluble protein aggregations and neuroinflammation are reported to contribute to the pathogenesis of PD [57]. Parkinson’s pathobiology is classically characterized as the progressive loss of dopaminergic neurons in the substantia nigra leading to the hallmark tremors of the limbs and loss of motor control observed in clinical settings. More recently, our understanding of PD has expanded to include several nonmotor symptoms (NMSs) that often precede motor symptoms such as depression, autonomic dysfunction and cognitive decline [58]. These NMSs are linked to the emergence of Lewy bodies, inclusion bodies primarily composed of α-synuclein, in nerve cells [59]. A few early studies have investigated the role of Sig-1Rs in mouse models of PD: previously for the amelioration of dyskinesias associated with L-dopa treatment and more recently for their apparent neurorestorative and protective properties. Additionally, Sig-1R binding potential as evaluated by positron emission tomography is reduced in brain areas affected by PD pathology, thus emphasizing the potential role of Sig-1Rs [60].
Most pharmacological studies involving the Sig-1R in the context of PD have been focused on the alleviation of L-dopa-induced dyskinesias. The Sig-1R antagonist BMY-14802 suppressed L-dopa induction of dyskinesias; however, it is unclear if this is due to nonspecific actions of the ligand [61]. Dextromethorphan, a Sig-1R agonist and NMDA receptor ligand was also able to suppress L-dopa-associated involuntary movements; however, NMDA antagonists were unable to block these effects, thereby indicating Sig-1R mechanisms are likely involved [62]. The paradigm of inhibiting Sig-1R function may not be the most advantageous strategy for treating PD. As described in this review, several neuroprotective functions of the Sig-1R have been thoroughly evaluated; compromising Sig-1Rs may potentiate neurodegeneration by sensitizing neurons to stressors present in PD such as protein accumulations and excitotoxicity. Indeed, siRNA knockdown of Sig-1Rs in Chinese hamster ovarian cells sensitized them to dopamine excitotoxicity and in vitro studies have demonstrated that Sig-1R ligands confer protection against glutamate excitotoxicity [42,63]. Ideally, future therapies would center on slowing or halting the pathogenesis of PD rather than alleviating symptoms associated with PD.
As mentioned, microglia and astrocytes express abundant Sig-1R, and pharmacological stimulation thereof has been shown to regulate the secretion of cytokines and other biological factors. Neuroinflammation, as in AD, is recognized to play roles in the neurodegeneration associated with PD [57,64]. Pharmacological stimulation of the Sig-1R in order to evoke antiapoptotic and neuromodulatory effects is an attractive potential therapy design. The Sig-1R agonist PRE-084 was shown to promote the upregulation of BDNF and glial-derived neurotrophic factor both in the striatum and substantia nigra in a 6-hydroxydopamine lesion mouse model of PD (Figure 1) [65]. These increases in nourishing brain factors were accompanied by suppression of microglial activation and increased nerve fiber density in the damaged brain regions relative to animals that received saline injections. Along with these neurobiological improvements, animals that received PRE-084 displayed improved forelimb use, indicating a neurorestorative role for the Sig-1R. These early findings point to an optimistic future for PD therapies but require further studies before any conclusions can be drawn.
PD shares specific pathophysiological hallmarks of AD. Specifically, neuroinflammation, oxidative stress, calcium dysregulation and apoptosis are all cellular processes that may be targeted by Sig-1R stimulation in order to prevent or slow neurodegeneration in PD. The role of the Sig-1R in removing pathological protein accumulations is not well defined; however, the importance of Sig-1Rs in both UPR and autophagy points to its potential for therapeutic intervention. Pharmacological manipulation of the Sig-1R to modulate any of these listed factors could be viable for developing future treatment strategies for PD.
3. Roles of Sig-1R in neuropsychiatric disorders
The role of Sig-1R in the etiology and treatment of psychiatric conditions including schizophrenia, depression, anxiety, dementia and drug addiction has recently gained much interest.
Schizophrenia is a major disorder of the CNS with poorly understood etiology that is characterized by delusions, hallucinations, disorganized speech and behavior, in addition to a variety of other negative symptoms such as inexpressive faces, few gestures, general disinterest and inability to feel pleasure [66]. It has also been observed that schizophrenia has a heritability rate of 80%, demonstrating a large genetic role in disease transmission [67]. Recently, researchers have identified a specific Sig-1R polymorphism associated with individuals diagnosed with schizophrenia in a Japanese cohort. However, another study found no association between specific genetic polymorphisms and disease [68]. Therefore, a meta-analysis was performed and the Gln2Pro polymorphism in the SIG-MAR1 gene was found to confer a small but significantly elevated susceptibility to schizophrenia. Individuals with this polymorphism exhibited decreased prefrontal activity compared to Gln/Gln genotypes with or without a schizophrenia diagnosis, providing a potential mechanism for the increased disease prevalence found in the Gln2Pro population [66]. Characterizing the specific functional variations resulting from Sig-1R polymorphisms has presented a challenge. For example, the Sig-1R Gln2Pro polymorphism has been shown to have no effect on the Sig-1R binding potential of certain Sig-1R agonist [69]. Thus, the polymorphism is unlikely to be associated with Sig-1R–related treatment outcomes in this population. Further characterization of this polymorphism is essential to its legitimacy as a potential schizophrenia risk conferring mutation.
Inasmuch as Sig-1R has shown to enhance the function of NMDA receptor [33,70,71], it is reasonable that Sig-1R has been speculated to relate to cognitive function. Indeed, recent evidences suggested a relation between Sig-1R and the cognitive aspect of schizophrenia [72]. In the case of certain compounds, agonist stimulation of the Sig-1R apparently enhanced cognitive function, while decreasing negative symptom severity [72]. Similar cognition findings have been replicated using the high-affinity Sig-1R ligand treatment in mice following the model of phencyclidine-treatment-induced cognitive deficits [73]. On the other hand, another study suggests otherwise [74].
Major depressive disorder (MDD) is one of the most prevalent conditions in the developed world with a lifetime prevalence of 16.2% [75]. Decreased cognitive function is one of the hallmarks of MDD and results in a mixture of physiological and behavioral changes. It has been increasingly suggested that chronic stress may result in neuroanatomical changes in the amygdala, hippocampus and prefrontal cortex, which are major emotional and cognitive control centers in the brain [76]. Sig-1R polymorphisms have also been associated with an elevated risk of MDD in a Japanese cohort [77]. Interestingly, the rs1800866 polymorphism only demonstrated an association with disease susceptibility, but not fluvoxamine or sertraline treatment efficacy, and is suggestive that rs1800866 plays a role in depression pathophysiology [77]. The role of Sig-1R in the etiology of depression is primarily thought to be related to its role in NMDA receptor modulation. Biochemically, Sig-1Rs binds IP3 receptors on the ER. Altering Sig-1R activity results in perturbations of the cellular calcium homeostasis that is important to AMPA signal induction and subsequent NMDA stimulation. In animal studies, SA4503, a Sig-1R agonist, potentiated the antidepressant-like effect of amantadine, a NMDA receptor antagonist, in a forced swimming test [78]. SA4503 has also been shown to have Sig-1R–dependent antidepressant-like effects in a tail-suspension test [79]. The antidepressant-like effect of igmesine, a Sig-1R agonist, treatment was found to be dependent on intracellular calcium modulation, as L-type or N-type voltage-dependent calcium channel antagonist treatment negated its effect [80]. Fluvoxamine, a current selective serotonin reuptake inhibitor (SSRI) with, however, high affinity for Sig-1R, has also been shown to elevate Sig-1R plasma levels in patients with late–life MDD, although this increase of Sig-1Rs did not correlate with symptom improvement [81]. This demonstrates a double effect of fluvoxamine in the stimulation of Sig-1R, as both a direct agonist and the promotion of Sig-1R expression. However, the mechanism of increased Sig-1R expression requires further exploration. The therapeutic efficacy of some SSRIs may result from Sig-1R–dependent neural modulation [82]. The affinities of various SSRIs for the Sig-1R have been characterized and are as follows: fluvoxamine (Ki = 36 nM), sertraline (Ki = 57 nM), fluoxetine (Ki = 120 nM) and citalopram (Ki = 292 nM) [83]. However, large-scale, double-blind, placebo-controlled studies across multiple countries on the effect of Sig-1R agonists fluoxetine and igmesine on MDD have yielded mixed results in the UK outpatient population [84]. DHEA treatment has also been known to alleviate some depressive symptoms in individuals with MDD. The efficacy of this neurosteroid treatment is increasingly thought to be due to stimulation of Sig-1R and subsequent enhancement of noradrenaline and serotonin neurotransmission, resulting in improved cognition [85]. Other researchers have demonstrated that the activity of DHEA is dependent on Sig-1R stimulation and subsequent synaptic changes via PKC and CaM kinase II [86]. In animal studies, it was found that DHEAs and pregnenolone sulfate are released during the tail-suspension tests [87]. Exogenous treatment with either of these Sig-1R agonists confers antidepressant-like effects. Further, these researchers demonstrated that these benefits were Sig-1R–dependent as prophylactic treatment with either BD1047 or progesterone prevented the effect [87]. This further demonstrates that many psychoactive substances are partially dependent on the activity of Sig-1R signaling. Overall, the role of Sig-1R agonists in the treatment of depression is quite promising. Already, a variety of SSRI treatments have been shown to have partial efficacy via Sig-1R activation. Further, antidepressant therapies that confer Sig-1R activity seem to have the additional benefit of improved cognitive function. However, the mixed results in clinical trials may signify the heterogeneity of the disease in differing populations. As we further characterized the pathophysiology of depression, we may better identify deeper level of molecular mechanisms whereby Sig-1Rs participate in the biological process of the development of depression.
Reductions in neural plasticity and dendritic spine density have been implicated in both schizophrenia and depression [88]. We have previously shown that Sig-1R plays an important role in growth factor-induced neurite outgrowth [89]. Further, Sig-1R knockdown neurons exhibit a reduction in dendritic density and plasticity [4]. The expression of BDNF largely mediates the development and maintenance of dendritic spines. Antidepressant treatments are known to induce BDNF formation and subsequent dendritic spine development. This is important therapeutically, as BDNF KO mice demonstrated reduced response to fluoxetine, a Sig-1R agonist (Figure 1) [90]. BDNF-induced neuritogenesis is an important therapeutic target of Sig-1R agonist treatments that warrants further exploration.
4. Sig-1R in drug addiction and HAND
It is well established that the neuropharmacological effects of certain drugs are mediated via Sig-1R. For instance, the early activation of the Sig-1R is integral in establishing the rewarding effects of cocaine in conditioned place preference studies [91]. In another behavioral model, Sig-1R activity was found to positively correlate with the development of cocaine-induced locomotor sensitization [92]. More recently, dynamic interactions between Sig-1Rs and voltage-gate potassium channels (Kv)1.2 potassium channels have been demonstrated to shape cocaine-induced behavioral and neuronal responses [8]. Interestingly, the Sig-1R–Kv1.2 interaction intensifies during cocaine withdrawal, suggesting that disruption of this complex may be an avenue in the treatment of cocaine addiction. Application of Sig-1R antagonists as well as antisense oligodeoxynucleotides were effective in reducing the severity and duration of cocaine-induced convulsions [93], suggesting a role of Sig-1Rs in mediating certain side effects of cocaine. Sig-1Rs also interact directly with methamphetamine, not only enhancing the locomotor response to methamphetamine but also enhancing the behavioral response [94]. However, methamphetamine-induced behavior and rewarding effects were attenuated on co-application of SSRIs, including fluoxetine and fluvoxamine [95]. These SSRIs are known to possess Sig-1R agonist activity. This would, therefore, suggest that the Sig-1R reduces the effects of methamphetamine administration. Thus, there is an apparent discrepancy in Sig-1R’s role in mediating methamphetamine response.
In addition to methamphetamine and cocaine, Sig-1Rs have been implicated in alcohol abuse. Mice that received Sig-1R antagonists decreased their alcohol intake, whereas those receiving the Sig-1R agonists increased ethanol consumption [96]. Sig-1Rs have also been shown to modulate ethanol-induced neuroplasticity and therefore alter behavioral responses to alcohol consumption [97]. Taken together, these results suggest the Sig-1R as a potential therapeutic target in disrupting the underlying pathological mechanism by which drug abuse functions.
Drug abuse has been intricately tied to HIV infection and associated neurological disturbance, since the beginning of the AIDS epidemic. HAND is a well-known complication of HIV infection in ~ 10 – 24% of those who acquire the infection [98]. The pathogenesis by which HIV induces encephalopathy includes infection of the microglia and macrophages that enter the CNS. Subsequently, the immune system is activated and neurotoxins are released, resulting in destruction of the brain parenchyma. HAND can also present as dementia, in which basic daily functions of living are impaired. Studies have shown that the Sig-1R agonist 4-phenyl-1-(4-phenylbutyl) piperidine reduces HIV protein gp120-induced neurotoxicity via regulation of B-cell lymphoma2 expression; thus, Sig-1R agonists may be applied to slow progression of HAND in drug abuse patient populations [99].
Pathologically, HAND can be visualized as disruption of the blood–brain barrier, astrocyte activation and aberrant neuronal anatomy [100]. The advent of highly active antiretroviral therapy (HAART) has improved the longevity of those infected with HIV and has reduced the incidence of opportunistic infections. However, the incidence of HAND has actually increased in those patients who are using cocaine while receiving HAART. Abuse of cocaine has been associated with increased seroprevalence and progression of HIV [101]. These observations led to the hope that therapeutic targets against the Sig-1R can reduce the progression of cocaine-mediated HAND. Although a Sig-1R target would not prevent the physiological process by which HAND functions, it would delay neurological destruction in cocaine abusers, and thus prolong the asymptomatic phase of the disease.
Cocaine-induced upregulation of HIV-1 expression involves Sig-1R [54]. Further, cocaine potentiates HIV-associated monocyte adhesion and migration via upregulation of adhesion molecules which in turn involves activation of Sig-1R [100]. Specifically, in response to cocaine, Sig-1R translocates from MAM to the plasma membrane to interact with and activate c-Src and platelet-derived growth factor receptor in sequence which eventually lead to the transcription of the chemokine monocyte chemoattractant protein-1 (MCP-1) and activated leukocyte adhesion molecule (ALCAM) (Figure 1). Both MCP-1 and ALCAM are instrumental in enhancing neuronal inflammation [102]. Application of a Sig-1R antagonist BD1047 has been shown to decrease cocaine-induced MCP-1 expression. Thus, Sig-1R is intimately related to the activation of neural inflammation and thus the application of a Sig-1R antagonist could serve as a therapeutic measure in slowing the progression of disease in HIV-infected cocaine users [103].
5. Conclusion
An increasing number of reports demonstrate that Sig-1R ligands exert therapeutic functions (Table 1); still, the nature of each ligand and the pathogenesis of the diseases they are deployed against may determine the therapy efficacies. Thorough characterizations of the Sig-1R agonists and antagonists will help answer the role of Sig-1Rs in the benefits of treating certain neurological disorders. Additionally, future studies determining the treatment timeline and strategizing potential combinatory targets are necessary for achieving a better therapeutic efficacy.
Table 1.
Summary of the Sig-1R ligands in therapeutic potentials.
| Sig-1R ligand | Function | Diseases | Cellular mechanisms | Therapeutic effects |
|---|---|---|---|---|
| AC915 | Antagonist | FTLD-MND | Decrease TDP-43 localization [13] | |
| Afobazole | Agonist | AD | Decrease Bax [40] Reduce AB25–35-mediated increases in intracellular Ca2+ and NO [40] | |
| AZ66 | Drug addiction | Diminish the stimulatory effect of methamphetamine and reduce behavioral sensitization [94] | ||
| BD1008 | Antagonist | Cocaine-induced convulsions and lethality | Antagonism of Sig-1R [93] | |
| BD1047 | Antagonist | Cocaine-mediated HAND | Decrease NF-κB activated monocyte chemoattractant protein-1 [103] | |
| BMY-14802 | Antagonist | PD | Suppresses L-Dopa-induced dyskinesia [61] | |
| Citalopram | Uncharacterized | Depression | SSRI [82] | |
| Dehydroepiandrosterone | Agonist | Depression | Induces LTP via CaM Kinase II and PKC activation [86] | |
| Schizophrenia | Improve PCP-induced cognitive deficits [73] | |||
| Dextromethorphan | Agonist | PD | Suppresses L-Dopa-induced dyskinesia [62] | |
| Dimemorfan | Agonist | AD | Attenuates AB25–35-induced memory impairment [108] | |
| Donepezil | Agonist | AD | Blocks the suppressive action of AB1–42 fragments on LTP [43] | |
| Blocks AB25–35-induced memory impairment [109] | ||||
| Schizophrenia | Improve PCP-induced cognitive deficits in mice [110] | |||
| E-5842 | Antagonist | Schizophrenia | Reduces tardive dyskinesia [111] | |
| Fluvoxamine | Agonist | Depression | Increase plasma levels of Sig-1R [82] | |
| Schizophrenia | Improves cognitive functions [82] | |||
| Improves cognitive functions [72,73] | ||||
| Fluoxetine | Agonist | Depression | SSRI [82] | |
| Haloperidol | Antagonist | FTLD-MND | Regulate TDP-43 localization [13] | |
| Igmesine | Agonist | Depression | Intracellular Ca2+ modulation [80] | |
| Opipramol | Agonist | FTLD-MND | Regulate TDP-43 localization [13] | |
| Panamesine (EMD57445) | Antagonist | Schizophrenia | Improves positive symptoms of schizophrenia [112] | |
| (+)-Pentazocine | Agonist | AD | Prevent AB 25–35-induced memory impairment [41] | |
| 4-phenyl-1-(4-phenylbutyl) piperidine | Agonist | HIV | Change B-cell lymphoma 2 expression [99] | |
| Pregnenolone sulfate | Agonist | AD | Blocks AB25–35-mediated loss of hippocampal pyramidal cells [113] | |
| Depression | Induces LTP via CaM Kinase II and PKC activation [86] | |||
| (+/−)PPCC | Uncharacterized | Depression | Cognitive improvements following cholinergic depletion [114] | |
| PRE-O84 | Agonist | Huntington’s Disease | Calpastatin and NF-κB [115] | |
| ALS | Induce clearance of mutant vesicle-associated membrane protein-associated protein B aggregates [27] | |||
| Preserve MN and neuromuscular connections in the lumbar spinal cord [24] | ||||
| Promote the phosphorylation of Akt and ERK-½ [26] | ||||
| AD | Reduce reactive astrocytes in wobbler mice [28] | |||
| Block suppressive action of AB1–42 fragments on LTP [43] | ||||
| PD | Partial restoration of nerve fiber density in 6-OHDA lesions along with upregulation of brain-derived neurotrophic factor and glial derived neurotrophic factor [65] | |||
| Schizophrenia | Reverse NMDA antagonist dizocilpine’s effect [116] | |||
| SA4503 | Agonist | AD | Prevent AB 25–35-induced memory impairment [41] | |
| ALS | Preserve MN functions [26] | |||
| Schizophrenia | Reverse NMDA antagonist dizocilpine’s effect [116] | Improve PCP-induced cognitive deficits [73] | ||
| Depression | Potentiate NMDA antagonist amantadine effect [78] | |||
| Sertraline | Antagonist | Depression | Antagonizing Sig-1R [117] | SSRI [82] |
6-OHDA: 6-hydroxydopamine; AB: Amyloid b; AD: Alzheimer’s disease; ALS: Amyotrophic lateral sclerosis; FTLD: Frontotemporal lobar degeneration; HAND: HIV-associated neurodementia; LTP: Long-term potentiation; MN: Motor neuron; MND: Motor neuron disease; NO: Nitric oxide; PCP: Phencyclidine; PD: Parkinson’s disease; Sig-1R: Sigma-1 receptor; SSRI: Selective serotonin reuptake inhibitor; TDP: Transactivating response element DNA binding protein.
6. Expert opinion
The relatively recent finding that the Sig-1R is a ligand-operated ER chaperone has sparked several investigations into molecule-based therapies for diverse neurological disorders. Despite the promising results from many of these studies, mechanisms by which the Sig-1R ligands in question elicit their effects need to be totally clarified. Nevertheless, the potential for Sig-1R pharmacological stimulation to impede neurodegeneration or, in more exciting studies, the regeneration of neurons lost to neurodegenerative pathophysiology warrant further study. Moreover, there are an increasing number of reports that identify mutations in the SIGMAR1 gene that are associated with disease.
In order to develop efficacious treatment strategies for these neurodegenerative diseases, we must first understand how and why these diseases manifest. Without this key piece of the puzzle, even our most intelligent therapeutic approaches will continue to only address a portion of the issue; however, it becomes increasingly clear that the pathology of diseases such as AD, PD and MNDs involve widespread nervous system dysfunction. Interestingly, Sig-1Rs have been reported to attenuate several of the pathological hallmarks shared by many neurodegenerative disorders, that is, inflammation (microglia activation), protein accumulations and oxidative stress to varying degrees in cellular and in vivo models. These broadly therapeutic effects certainly qualify the Sig-1R as a target in future therapies and are especially enticing when one begins to consider the possibility of additive or synergistic drug effects. Indeed, until we elucidate the root causes of these neurological disorders, perhaps ligand cocktail therapies may be an avenue to devise treatment strategies that are as sophisticated as the disease processes they attempt to combat.
The findings that implicate SIGMAR1 polymorphisms that cosegregate or associate with neurological disease point to another interesting direction for Sig-1R research. If defective copies of SIGMAR1 cause or potentiate the emergence of disease, further investigation into the role of Sig-1R in disease may highlight crucial cellular defense pathways. Further, if specific mutations in SIGMAR1 are indeed causative and critical in certain incidences of disease, perhaps future gene therapies to compensate for these mutations would be able to reduce the pathophysiology of neurological disorders. The idea of gene therapy is particularly interesting, because in the case of disease resulting from mutation, pharmacological stimulation of the mutated protein may have little or no effect.
So far, Sig-1R agonist-based therapies exert the most pronounced effects in already deteriorating systems; however, there are no convincing findings that these same agonists prevent the onset of neurological disease. Not only does this highlight the complex etiology of neurological disorders, it may also be indicative that the Sig-1R plays only a subsidiary role in mitigating stress that can be covered by other ER chaperones. Thus, combining Sig-1R with other non-Sig-1R-mediated effects is a potential direction, in which multiple treatments may compensate for the shortages within. It would also be helpful to investigate the best stage to introduce Sig-1R stimulation in various pathological conditions to maximize their potential therapeutic efficacies. Perhaps the weakest link in the Sig-1R studies is the lack of a primary mechanism through which the agonists/antagonists are working since many of the ligands also have affinity to other targets. In general, activation of Sig-1Rs exerts neuroprotective effects in neurons but tends to poise adverse effects in activated glial cells. This may explain why agonists are beneficial in certain conditions, whereas antagonists are preferred in other cases. Therefore, care needs to be taken when strategizing therapeutic treatments. Moreover, agonist stimulation of Sig-1Rs is reported to mitigate cognitive deficits after the onset of many of the diseases covered in this review; however, Sig-1R agonists do not appear to enhance cognitive abilities when no deficits are present. Although this observation is likely unrelated to the disease pathologies themselves, it provides perspective into another possible therapeutic use for targeting Sig-1Rs.
The UPR has recently received much attention in many diseases including the neurodegenerative diseases. Since the discovery of Sig-1R as an ER chaperone and its role in participating in protein quality control, attention has been focused on this function and diseases related to its disruption. Accumulating evidence demonstrated that loss function of Sig-1R leads to imbalanced ROS [2,3,104,105], suggesting the following perturbed UPR and ER overload responses that are exacerbated by ER stress. Failure of UPR and the consequence of dysregulated autophagy and mitophagy are often claimed in AD, PD and MNDs [106,107]. Thus, Sig-1R modulation upstream of the UPR may be an early event to catch in future approaches. This gives the impression that Sig-1R stimulation would then be only beneficial when cells are faced with stress loads that overwhelm their endogenous management abilities. Further elucidation of disease causes may provide insight into how and when to stimulate Sig-1Rs, perhaps along with other ligands that activate other receptors for cooperative effects.
Highlights.
Inter-organelle signaling and associated modulation may play important roles in neurodegenerative diseases and psychiatric disorders.
The ER-mitochondrion interface is critical in these diseases and may serve as a “hub” for integrating the signaling events locally and remotely.
The sigma-1 receptor chaperone at the ER-mitochondrion interface participates in the signaling modulation locally, but also remotely by acting as a messenger between this interface and other parts of neurons, thereby potentially influencing the cause or disease processing of these neuronal disorders.
Acknowledgments
S-YA Tsai, and MJ Pokrass have equally contributed to this work.
The authors were supported by the Intramural research program NIDA, NIH/DHHS.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1••.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240(4849):219–21. doi: 10.1126/science.2832949. This was the first report of Sigma-1 receptor (Sig-1R) binding to steroids. [DOI] [PubMed] [Google Scholar]
- 2••.Hayashi T, Su T-P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131(3):596–610. doi: 10.1016/j.cell.2007.08.036. This paper was the first to identify Sig-1R as an endoplasmic reticulum (ER) chaperone. [DOI] [PubMed] [Google Scholar]
- 3••.Mori T, Hayashi T, Hayashi E, Su T-P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013;8(10):e76941. doi: 10.1371/journal.pone.0076941. This paper first reported Sig-1R regulating ER stress vis IRE-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Tsai S-Y, Hayashi T, Harvey BK, et al. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1-GTP pathway. Proc Natl Acad Sci USA. 2009;106(52):22468–73. doi: 10.1073/pnas.0909089106. This paper was the first report identifying the mechanism of Sig-1R regulating neuritogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai S-Y, Rothman RK, Su T-P. Insights into the Sigma-1 receptor chaperone’s cellular functions: a microarray report. Synapse. 2012;66(1):42–51. doi: 10.1002/syn.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal A, Fontanilla D, Gopalakrishnan A, et al. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol. 2012;682(1–3):12–20. doi: 10.1016/j.ejphar.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz JL, Su T-P, Hiranita T, et al. A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals (Basel) 2011;4(6):880–914. doi: 10.3390/ph4060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Kourrich S, Hayashi T, Chuang J-Y, et al. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152(1–2):236–47. doi: 10.1016/j.cell.2012.12.004. This paper reported how cocain stimulates Sig-1R to shape neural plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Su T-P, Hayashi T, Maurice T, et al. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31(12):557–66. doi: 10.1016/j.tips.2010.08.007. This is an excellent review of Sig-1Rs signaling pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617–28. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- 11.Fecto F, Siddique T. SIGMAR1 mutations, genetic heterogeneity at the chromosome 9p locus, and the expanding etiological diversity of amyotrophic lateral sclerosis. Ann Neurol. 2011;70(6):867–70. doi: 10.1002/ana.22648. [DOI] [PubMed] [Google Scholar]
- 12.Iguchi Y, Katsuno M, Ikenaka K, et al. Amyotrophic lateral sclerosis: an update on recent genetic insights. J Neurol. 2013;260(11):2917–27. doi: 10.1007/s00415-013-7112-y. [DOI] [PubMed] [Google Scholar]
- 13.Luty AA, Kwok JB, Dobson-Stone C, et al. Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann Neurol. 2010;68(5):639–49. doi: 10.1002/ana.22274. [DOI] [PubMed] [Google Scholar]
- 14•.Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011;70(6):913–19. doi: 10.1002/ana.22534. The above two papers by Luty et al., and Al-Saif et al., were the first two reports discovering the association of SIGMAR1 mutations and motor neuron disease. [DOI] [PubMed] [Google Scholar]
- 15.Tagashira H, Shinoda Y, Shioda N, et al. Methyl pyruvate rescues mitochondrial damage caused by SIGMAR1 mutation related to amyotrophic lateral sclerosis. Biochim Biophys Acta. 2014;1840(12):3320–34. doi: 10.1016/j.bbagen.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Belzil VV, Daoud H, Camu W, et al. Genetic analysis of SIGMAR1 as a cause of familial ALS with dementia. Eur J Hum Genet. 2013;21(2):237–9. doi: 10.1038/ejhg.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi T, Hayashi E, Fujimoto M, et al. The lifetime of UDP-galactose: ceramide galactosyltransferase is controlled by a distinct endoplasmic reticulum-associated degradation (ERAD) regulated by sigma-1 receptor chaperones. J Biol Chem. 2012;287(51):43156–69. doi: 10.1074/jbc.M112.380444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gromek KA, Suchy FP, Meddaugh HR, et al. The oligomeric states of the purified sigma 1 receptor are stabilized by ligands. J Biol Chem. 2014;289(29):20333–44. doi: 10.1074/jbc.M113.537993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miki Y, Mori F, Kon T, et al. Accumulation of the sigma-1 receptor is common to neuronal nuclear inclusions in various neurodegenerative diseases. Neuropathology. 2014;34(2):148–58. doi: 10.1111/neup.12080. [DOI] [PubMed] [Google Scholar]
- 20.Alonso G, Phan V, Guillemain I, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97(1):155–70. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 21.Mavlyutov TA, Epstein ML, Andersen KA, et al. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons An anatomical and behavioral study. Neuroscience. 2010;167(2):247–55. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavlyutov TA, Epstein ML, Liu P, et al. Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N,N’-dimethyltryptamine forming enzyme, indole-N-methyl transferase. Neuroscience. 2012;206:60–8. doi: 10.1016/j.neuroscience.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casas C, Herrando-Grabulosa M, Manzano R, et al. Early presymptomatic cholinergic dysfunction in a murine model of amyotrophic lateral sclerosis. Brain Behav. 2013;3(2):145–58. doi: 10.1002/brb3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancuso R, Olivan S, Rando A, et al. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurotherapeutics. 2012;9(4):814–26. doi: 10.1007/s13311-012-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mavlyutov TA, Epstein ML, Verbny YI, et al. Lack of sigma-1 receptor exacerbates ALS progression in mice. Neuroscience. 2013;240:129–34. doi: 10.1016/j.neuroscience.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ono Y, Tanaka H, Takata M, et al. SA4503, a sigma-1 receptor agonist, suppresses motor neuron damage in in vitro and in vivo amyotrophic lateral sclerosis models. Neurosci Lett. 2014;559:174–8. doi: 10.1016/j.neulet.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Prause J, Goswami A, Katona I, et al. Altered localization, abnormal modification and loss of function of sigma receptor-1 in amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22(8):1581–600. doi: 10.1093/hmg/ddt008. [DOI] [PubMed] [Google Scholar]
- 28•.Peviani M, Salvaneschi E, Bontempi L, et al. Neuroprotective effects of the Sigma-1 receptor (S1R) agonist PRE-084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiol Dis. 2014;62:218–32. doi: 10.1016/j.nbd.2013.10.010. This report described the potential use of Sig-1R agonists as a therapeutic target. [DOI] [PubMed] [Google Scholar]
- 29.Mancuso R, Del Valle J, Morell M, et al. Lack of synergistic effect of resveratrol and sigma-1 receptor agonist (PRE-084) in SOD1G93A ALS mice: overlapping effects or limited therapeutic opportunity? Orphanet J Rare Dis. 2014;9:78. doi: 10.1186/1750-1172-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thies W, Bleiler L. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–45. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Price JL, McKeel DW, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30(7):1026–36. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurice T, Su T-P. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Hayashi T, Tsai S-Y, Mori T, et al. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin Ther Targets. 2011;15(5):557–77. doi: 10.1517/14728222.2011.560837. This is an excellent review on Sig-1R’s cellular mechanisms and its implications on treating psychiatric disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedskog L, Pinho CM, Filadi R, et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci USA. 2013;110(19):7916–21. doi: 10.1073/pnas.1300677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishina M, Ohyama M, Ishii K, et al. Low density of sigma1 receptors in early Alzheimer’s disease. Ann Nucl Med. 2008;22(3):151–6. doi: 10.1007/s12149-007-0094-z. [DOI] [PubMed] [Google Scholar]
- 37.Maruszak A, Safranow K, Gacia M, et al. Sigma receptor type 1 gene variation in a group of polish patients with Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:432–8. doi: 10.1159/000101990. [DOI] [PubMed] [Google Scholar]
- 38.Fehér Á, Juhász A, László A, et al. Association between a variant of the sigma-1 receptor gene and Alzheimer’s disease. Neurosci Lett. 2012;517(2):136–9. doi: 10.1016/j.neulet.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Mrak RE. Neuropathology and the neuroinflammation idea. J Alzheimers Dis. 2009;18(3):473–81. doi: 10.3233/JAD-2009-1158. [DOI] [PubMed] [Google Scholar]
- 40.Behensky AA, Yasny IE, Shuster AM, et al. Afobazole activation of sigma-1 receptors modulates neuronal responses to amyloid-beta25–35. J Pharmacol Exp Ther. 2013;347(2):468–77. doi: 10.1124/jpet.113.208330. [DOI] [PubMed] [Google Scholar]
- 41•.Maurice T, Su T-P, Privat A. Sigma1 receptor agonists and neurosteroids attenuate beta25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83(2):413–28. doi: 10.1016/s0306-4522(97)00405-3. This report shows that Sig-1R agonists can attenuate Aβ25–35-induced amnesia in the animal model. [DOI] [PubMed] [Google Scholar]
- 42.Mori T, Hayashi T, Su T-P. Compromising sigma-1 receptors at the endoplasmic reticulum render cytotoxicity to physiologically relevant concentrations of dopamine in a nuclear factor-kB/Bcl-2-dependent mechanism: potential relevance to Parkinson’s disease. J Pharmacol Exp Ther. 2012;341(3):663–71. doi: 10.1124/jpet.111.190868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solntseva EI, Kapai NA, Popova OV, et al. The involvement of sigma1 receptors in donepezil induced rescue of hippocampal LTP impaired by beta-amyloid peptide. Brain Res Bull. 2014;106:56–61. doi: 10.1016/j.brainresbull.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Brion J-P. Immunological demonstration of tau protein in neurofibrillary tangles of Alzheimer’s disease. J Alzheimers Dis. 2006;9(3 Suppl):177–85. doi: 10.3233/jad-2006-9s321. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Martinez-Vicente M, Kriiger U, et al. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet. 2009;18(21):4153–70. doi: 10.1093/hmg/ddp367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vollrath JT, Sechi A, Dreser A, et al. Loss of function of the ALS protein SigR1 leads to ER pathology associated with defective autophagy and lipid raft disturbances. Cell Death Dis. 2014;5:e1290. doi: 10.1038/cddis.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science. 1987;235(4796):1641–4. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- 48.David DC, Layfield R, Serpell L, et al. Proteasomal degradation of tau protein. J Neurochem. 2002;83(1):176–85. doi: 10.1046/j.1471-4159.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- 49.Feuillette S, Blard O, Lecourtois M, et al. Tau is not normally degraded by the proteasome. J Neurosci Res. 2005;80(3):400–5. doi: 10.1002/jnr.20414. [DOI] [PubMed] [Google Scholar]
- 50.Lahmy V, Meunier J, Malmström S, et al. Blockade of Tau hyperphosphorylation and Abeta1–42 generation by the aminotetrahydrofuran derivative ANAVEX2–73, a mixed muscarinic and sigma1 receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2013;38(9):1706–23. doi: 10.1038/npp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishima T, Nishimura T, Iyo M, et al. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: role of sigma-1 receptors and IP3 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1656–9. doi: 10.1016/j.pnpbp.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa M, Sakata M, Ishii K, et al. High occupancy of sigma1 receptors in the human brain after single oral administration of donepezil: a positron emission tomography study using [11C] SA4503. Int J Neuropsychopharmacol. 2009;12(8):1127–31. doi: 10.1017/S1461145709990204. [DOI] [PubMed] [Google Scholar]
- 53.Ruscher K, Shamloo M, Rickhag M, et al. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134(Pt 3):732–46. doi: 10.1093/brain/awq367. [DOI] [PubMed] [Google Scholar]
- 54.Gekker G, Hu S, Sheng WS, et al. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. Int Immunopharmacol. 2006;6(6):1029–33. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 55•.Hall AA, Herrera Y, Ajmo CT, et al. Sigma receptors suppress multiple aspects of microglial activation. Glia. 2009;57(7):744–54. doi: 10.1002/glia.20802. This is the report describing how Sig-1R suppresses microglia activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savica R, Grossardt BR, Bower JH, et al. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol. 2013;70(7):859–66. doi: 10.1001/jamaneurol.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao H-M, Zhang F, Zhou H, et al. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 2011;119(6):807–14. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sung VW, Nicholas AP. Nonmotor symptoms in Parkinson’s disease: expanding the view of Parkinson’s disease beyond a pure motor, pure dopaminergic problem. Neurol Clin. 2013;31(3 Suppl):S1–16. doi: 10.1016/j.ncl.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 59.Recasens A, Dehay B, Bové J, et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014;75(3):351–62. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 60.Mishina M, Ishiwata K, Ishii K, et al. Function of sigma1 receptors in Parkinson’s disease. Acta Neurol Scand. 2005;112(2):103–7. doi: 10.1111/j.1600-0404.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- 61.Paquette MA, Foley K, Brudney EG, et al. The sigma-1 antagonist BMY-14802 inhibits L-DOPA-induced abnormal involuntary movements by a WAY-100635-sensitive mechanism. Psychopharmacology (Berl) 2009;204(4):743–54. doi: 10.1007/s00213-009-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paquette MA, Brudney EG, Putterman DB, et al. Sigma ligands, but not N-methyl-D-aspartate antagonists, reduce levodopa-induced dyskinesias. Neuroreport. 2008;19(1):111–15. doi: 10.1097/WNR.0b013e3282f3b0d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lesage AS, De Loore KL, Peeters L, et al. Neuroprotective sigma ligands interfere with the glutamate-activated NOS pathway in hippocampal cell culture. Synapse. 1995;20(2):156–64. doi: 10.1002/syn.890200210. [DOI] [PubMed] [Google Scholar]
- 64.Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S210–12. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- 65••.Francardo V, Bez F, Wieloch T, et al. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain. 2014;137(Pt 7):1998–2014. doi: 10.1093/brain/awu107. This report highlighted the therapeutic potential of Sig-1R ligands on Parkinson’s disease. [DOI] [PubMed] [Google Scholar]
- 66.Ohi K, Hashimoto R, Yasuda Y, et al. The SIGMAR1 gene is associated with a risk of schizophrenia and activation of the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(5):1309–15. doi: 10.1016/j.pnpbp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47(3):210–20. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- 68.Uchida N, Ujike H, Nakata K, et al. No association between the sigma receptor type 1 gene and schizophrenia: results of analysis and meta-analysis of case-control studies. BMC Psychiatry. 2003;3:13. doi: 10.1186/1471-244X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishikawa M, Ishiwata K, Ishii K, et al. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: a positron emission tomography study using [11C] SA4503. Biol Psychiatry. 2007;62(8):878–83. doi: 10.1016/j.biopsych.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Monnet FP, Morin-Surun MP, Leger J, et al. Protein kinase C-dependent potentiation of intracellular calcium influx by sigma1 receptor agonists in rat hippocampal neurons. J Pharmacol Exp Ther. 2003;307(2):705–12. doi: 10.1124/jpet.103.053447. [DOI] [PubMed] [Google Scholar]
- 71.Balasuriya D, Stewart AP, Edwardson JM. The sigma-1 receptor interacts directly with GluN1 but not GluN2A in the GluN1/GluN2A NMDA receptor. J Neurosci. 2013;33(46):18219–24. doi: 10.1523/JNEUROSCI.3360-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iyo M, Shirayama Y, Watanabe H, et al. Fluvoxamine as a sigma-1 receptor agonist improved cognitive impairments in a patient with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(4):1072–3. doi: 10.1016/j.pnpbp.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 73••.Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2007;32(3):514–21. doi: 10.1038/sj.npp.1301047. This report shows how Sig-1R agonist fluvoxamine can alleviate PCP-induced cognitive deficits. [DOI] [PubMed] [Google Scholar]
- 74.Niitsu T, Fujisaki M, Shiina A, et al. A randomized, double-blind, placebo-controlled trial of fluvoxamine in patients with schizophrenia: a preliminary study. J Clin Psychopharmacol. 2012;32(5):593–601. doi: 10.1097/JCP.0b013e3182664cfc. [DOI] [PubMed] [Google Scholar]
- 75.Trivedi MH, Lin EHB, Katon WJ. Consensus recommendations for improving adherence, self-management, and outcomes in patients with depression. CNS Spectr. 2007;12(8 Suppl 13):1–27. [PubMed] [Google Scholar]
- 76.Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45(7):797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 77.Kishi T, Yoshimura R, Okochi T, et al. Association analysis of SIGMAR1 with major depressive disorder and SSRI response. Neuropharmacology. 2010;58(7):1168–73. doi: 10.1016/j.neuropharm.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 78.Skuza G, Rogóz Z. A potential antidepressant activity of SA4503, a selective sigma 1 receptor agonist. Behav Pharmacol. 2002;13(7):537–43. doi: 10.1097/00008877-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Ukai M, Maeda H, Nanya Y, et al. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol Biochem Behav. 1998;61(3):247–52. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 80.Urani A, Romieu P, Portales-Casamar E, et al. The antidepressant-like effect induced by the sigma(1) (sigma(1)) receptor agonist igmesine involves modulation of intracellular calcium mobilization. Psychopharmacology (Berl) 2002;163(1):26–35. doi: 10.1007/s00213-002-1150-y. [DOI] [PubMed] [Google Scholar]
- 81.Shimizu H, Takebayashi M, Tani M, et al. Sigma-1 receptor concentration in plasma of patients with late-life depression: a preliminary study. Neuropsychiatr Dis Treat. 2013;8:1867–72. doi: 10.2147/NDT.S53386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stahl SM. Antidepressant treatment of psychotic major depression: potential role of the sigma receptor. CNS Spectr. 2005;10(4):319–23. doi: 10.1017/s1092852900022641. [DOI] [PubMed] [Google Scholar]
- 83.Narita N, Hashimoto K, Tomitaka S, et al. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol. 1996;307(1):117–19. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- 84.Volz H-P, Stoll KD. Clinical trials with sigma ligands. Pharmacopsychiatry. 2004;37(Suppl 3):S214–20. doi: 10.1055/s-2004-832680. [DOI] [PubMed] [Google Scholar]
- 85.Van Broekhoven F, Verkes RJ. Neurosteroids in depression: a review. Psychopharmacology (Berl) 2003;165(2):97–110. doi: 10.1007/s00213-002-1257-1. [DOI] [PubMed] [Google Scholar]
- 86.Moriguchi S, Yamamoto Y, Ikuno T, et al. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. J Neurochem. 2011;117(5):879–91. doi: 10.1111/j.1471-4159.2011.07256.x. [DOI] [PubMed] [Google Scholar]
- 87.Dhir A, Kulkarni S. Involvement of sigma (sigma1) receptors in modulating the anti-depressant effect of neurosteroids (dehydroepiandrosterone or pregnenolone) in mouse tail-suspension test. J Psychopharmacol. 2008;22(6):691–6. doi: 10.1177/0269881107082771. [DOI] [PubMed] [Google Scholar]
- 88.Penzes P, Cahill ME, Jones KA, et al. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–93. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takebayashi M, Hayashi T, Su T-P. Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J Pharmacol Exp Ther. 2002;303(3):1227–37. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- 90.Chen Z-Y, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romieu P, Phan VL, Martin-Fardon R, et al. Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26(4):444–55. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 92•.Liu Y, Matsumoto RR. Alterations in fos-related antigen 2 and sigma1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther. 2008;327(1):187–95. doi: 10.1124/jpet.108.141051. This was the first report describing cocaine-mediated Sig-1R gene and protein expression. [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto RR, Hewett KL, Pouw B, et al. Rimcazole analogs attenuate the convulsive effects of cocaine: correlation with binding to sigma receptors rather than dopamine transporters. Neuropharmacology. 2001;41(7):878–86. doi: 10.1016/s0028-3908(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 94.Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43–51. doi: 10.1208/s12248-011-9311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rahmadi M, Mori T, Kanazawa M, et al. Involvement of sigma 1 receptor in the SSRI-induced suppression of the methamphetamine-induced behavioral sensitization and rewarding effects in mice. Nihon Shinkei Seishin Yakurigaku Zasshi. 2013;33(2):49–56. [PubMed] [Google Scholar]
- 96.Sabino V, Cottone P, Blasio A, et al. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36(6):1207–18. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nagel BJ, Schweinsburg AD, Phan V, et al. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139(3):181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grant I, Atkinson JH, Hesselink JR, et al. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections Studies with neuropsychologic testing and magnetic resonance imaging. Ann Intern Med. 1987;107(6):828–36. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, Shi Y, Qiao L, et al. Sigma-1 receptor agonists provide neuroprotection against gp120 via a change in bcl-2 expression in mouse neuronal cultures. Brain Res. 2012;1431:13–22. doi: 10.1016/j.brainres.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 100.Yao H, Kim K, Duan M, et al. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci. 2011;31(16):5942–55. doi: 10.1523/JNEUROSCI.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goodkin K, Shapshak P, Metsch LR, et al. Cocaine abuse and HIV-1 infection: epidemiology and neuropathogenesis. J Neuroimmunol. 1998;83(1–2):88–101. doi: 10.1016/s0165-5728(97)00225-7. [DOI] [PubMed] [Google Scholar]
- 102•.Buch S, Yao H, Guo M, et al. Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Curr HIV Res. 2012;10(5):425–8. doi: 10.2174/157016212802138823. This review descibes the association of Sig-1R and cocaine-enhanced HIV progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eugenin EA, Osiecki K, Lopez L, et al. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsai S-Y, Hayashi T, Mori T, et al. Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem. 2009;9(3):184–9. doi: 10.2174/1871524910909030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meunier J, Hayashi T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J Pharmacol Exp Ther. 2010;332(2):388–97. doi: 10.1124/jpet.109.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wager K, Russell C. Mitophagy and neurodegeneration: the zebrafish model system. Autophagy. 2013;9(11):1693–709. doi: 10.4161/auto.25082. [DOI] [PubMed] [Google Scholar]
- 107.Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24(1):69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang H-H, Chien J-W, Chou Y-C, et al. Anti-amnesic effect of dimemorfan in mice. Br J Pharmacol. 2003;138(5):941–9. doi: 10.1038/sj.bjp.0705117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meunier J, Ieni J, Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25–35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br J Pharmacol. 2006;149(8):998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kunitachi S, Fujita Y, Ishima T, et al. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent subchronic administration of donepezil: role of sigma-1 receptors. Brain Res. 2009;1279:189–96. doi: 10.1016/j.brainres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 111.Albayrak Y, Hashimoto K. Beneficial effects of the sigma-1 agonist fluvoxamine for tardive dyskinesia in patients with postpsychotic depressive disorder of schizophrenia: report of 5 cases. Prim Care Companion CNS Disord. 2012;14:6. doi: 10.4088/PCC.12br01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frieboes RM, Murck H, Wiedemann K, et al. Open clinical trial on the sigma ligand panamesine in patients with schizophrenia. Psychopharmacology (Berl) 1997;132(1):82–8. doi: 10.1007/s002130050323. [DOI] [PubMed] [Google Scholar]
- 113.Yang R, Chen L, Wang H, et al. Anti-amnesic effect of neurosteroid PREGS in Abeta25–35-injected mice through sigma1 receptor-and alpha7nAChR-mediated neuroprotection. Neuropharmacology. 2012;63(6):1042–50. doi: 10.1016/j.neuropharm.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 114.Antonini V, Marrazzo A, Kleiner G, et al. Anti-amnesic and neuroprotective actions of the sigma-1 receptor agonist (−)-MR22 in rats with selective cholinergic lesion and amyloid infusion. J Alzheimers Dis. 2011;24(3):569–86. doi: 10.3233/JAD-2011-101794. [DOI] [PubMed] [Google Scholar]
- 115.Hyrskyluoto A, Pulli I, Törnqvist K, et al. Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: involvement of calpastatin and the NF-kB pathway. Cell Death Dis. 2013;4:e646. doi: 10.1038/cddis.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maurice T, Phan VL, Urani A, et al. Differential involvement of the sigma(1) (sigma(1)) receptor in the anti-amnesic effect of neuroactive steroids, as demonstrated using an in vivo antisense strategy in the mouse. Br J Pharmacol. 2001;134(8):1731–41. doi: 10.1038/sj.bjp.0704355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ishima T, Fujita Y, Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol. 2014;727:167–73. doi: 10.1016/j.ejphar.2014.01.064. [DOI] [PubMed] [Google Scholar]