Abstract
Purpose of Review
The purpose of this review is to provide a broad overview of current trends in stem cell research and its applications in cardiovascular medicine. Researches on different stem cell sources, their inherent characteristics, and the limitations they have in medical applications are discussed. Additionally, uses of stem cells for both modeling and treating cardiovascular disease are discussed, taking note of the obstacles these engineered interventions must overcome to be clinically viable.
Recent Findings
Tissue engineering aims to replace dysfunctional tissues with engineered constructs. Stem cell technologies have been a great enabling factor in working toward this goal.
Summary
Many tissue-engineered products are in development that utilize stem cell technology. Although promising, some refinement must be made to these constructs with respect to safety and functionality. A deeper understanding of basic differentiation and tissue developmental mechanisms is required to allow these engineered tissues to be translated into the clinic.
Keywords: Stem cells, Cardiovascular, Regenerative medicine, Induced pluripotent stem cells, Embryonic stem cells, Tissue engineering
Introduction
Cardiovascular disease is globally the leading cause of mortality with an estimated 17 million annual deaths [1]. This is approximately 30% of reported annual deaths and is expected to have an increased incidence through the year 2030 [1, 2]. In the USA, there is an estimated total of 27 million individuals afflicted nationwide [1]. As people age, their natural ability to repair and regulate homeostasis of the cardiovascular system declines [3]. This natural decline in cardiovascular health is exacerbated by environmental factors, where clinical intervention will eventually be needed [3, 4]. One of the issues with current therapies for the treatment of cardiovascular disease is that different patients will respond in varying levels to the drugs administered. This discrepancy arises due to unique cellular and genetic conditions underlying these diseases that are specific to the individual. It is for this reason that the concept of personalized medicine has gained attraction in recent years by both physicians and researchers alike. The purposes of this review are to provide some insight into the advancements of stem cell technologies and examine their applications in cardiovascular medicine. In this review, we will discuss different stem cell sources and their current applications, while taking note of the limitations of each. This review takes the stance that induced pluripotent stem cell technology provides an exciting avenue for developing therapeutics with the aim of making personalized medicine through regenerative therapy a reality.
Embryonic Stem Cells
Brief History of Embryonic Stem Cells
Embryonic stem cells (ESCs) are cells derived from the inner cell mass of the preimplantation blastocyst that retain the ability to differentiate into all three germ layers [5]. Human ESCs have been derived from the human embryo and have displayed pluripotency [6]. Theoretically, ESCs are capable of being expanded in culture indefinitely, which is due to their active telomerase enzymes that prevent telomere shortening, senescence, and rapid apoptosis [7]. These are exciting features of ESCs because it not only allows for the generation of varied cell types for genetic modeling but also for the potential of generating a nearly unlimited supply of cells for use. This aspect makes it particularly attractive for use in cell-based therapies which require large cell numbers. Though human ESCs offered much promise in the years since they were first isolated by Thomson in 1998, public opinion heavily influenced how these cells ultimately could be used [8]. Because the establishment of an ESC line requires the destruction of the developing human embryo, the country was faced with a new ethical dilemma regarding the use of this cell source for research. This national dialogue resulted in a restricted progress in ESC research since there could be no new ESC lines generated with federal funds as declared by the Dickey-Wicker amendment in 1996, which is still in effect to this day. However, subsequent presidential administrations have made suggestions on how to work within the guidelines of Dickey-Wicker while impeding scientific progress as little as possible [9]. Currently, the excess unused eggs from in vitro fertilization (IVF) are the main source of new ESC lines in the USA as they fall into a special category that is permissible under current law [10].
The use of leftover IVF embryos alleviated some of the restrictions ESC research faced previously in that it allowed for a greater number of cell lines with a wide variety of genetic backgrounds to be tested for pathology in disease and necessary pathways in development. Healthy cell lines may also be generated for biobanking-defined human leukocyte antigen (HLA) lines for cell therapies [11]. However, storage protocols must be optimized as current methodologies have led to overall low viability rates for these embryos after thawing [12]. One reason an embryo may not be selected for IVF is that it is deemed to be of low “quality” as determined by the physical characteristics of the embryo that have been associated with low efficacy for implantation in utero [13–15]. Different quality blastocysts have been tested and, surprisingly, are able to give rise to high quality cells that display proper ESC morphology and expression patterns [16, 17]. However, there are some debates regarding the efficiency differences between cell lines in ESC derivation [18, 19]. These cells also show varying capacity to differentiate into cardiomyocytes expressing proper markers regardless of original blastocyst quality [20].
Differentiation of ESCs
The development of protocol for differentiation of ESCs into cardiomyocytes in vitro has been largely based on observations of cardiac development pathways in vivo. These pathways include activities of Wnt, activin/Nodal/TGFβ, BMP, and FGF [21]. A typical protocol may include the following: ESC differentiation into cardiac mesoderm through addition of BMP4, Nodal/activin A, and Wnt/β-catenin; subsequent inhibition of both Wnt/β-catenin and Nodal/activin A to yield cardiac progenitor cells; continued inhibition of Wnt/β-catenin and addition of FGF to induce differentiation into cardiomyocytes; and addition of Wnt/β-catenin, IGF, NRG, FGF1, Notch1, and periostin to support further proliferation [21, 22, 23••, 24].
The generation of cardiomyocytes from ESCs may be a potential therapy for patients that have suffered from myocardial infarction, one occurrence of which can destroy as much as one billion cardiomyocytes [21]. Progress has been made in the development of functional cardiac tissue in vitro. Stevens et al. aimed to create 3D cardiac tissue consisting of ESC-derived cells alone, in a scalable method. To do so, ESCs were cultured and induced to differentiate into cardiomyocytes (through genetic pathways similar to those previously discussed), which were then incubated on a rotating orbital shaker. This yielded an aggregate of disc-shaped cardiac tissue in the center of each plate, which showed synchronous and spontaneous beating. The individual cells were shown to be electrochemically coupled to one another, resembling the functionality that occurs in normal cardiac tissue. This indicates that the experimentally developed cardiomyocytes could be able to functionally integrate with host cardiac tissue [25]. In fact, this functional integration was demonstrated to occur when cardiomyocytes derived from human ESCs were implanted into pig and guinea pig hearts [26, 27]. Cardiomyocyte derived from ESCs has allowed for great advancements in the field of regenerative medicine but is currently limited in their potential for therapies.
Adult Stem Cells
Many tissues retain the ability to regenerate upon damage well into adulthood [28–30]. This regenerative capability is due to somatic stem cells, which retain the ability to differentiate into various cell types of their respective tissues. This is known as multipotency, and while these cells are thought to be more limited in their differentiation potential than ESCs, they do come with some added benefits. These cells are autologous which reduces the need for immunosuppression for potential therapies. In addition, these cells are derived from an adult individual and thus circumvent the ethical issues surrounding human ESC use.
For cardiovascular regenerative medicine, a candidate autologous stem cell type lies in the bone marrow. Bone marrow-derived stem cells (BMSCs) have been shown to aid in the regeneration of vasculature [31, 32], but their contribution to new cardiomyocyte generation remains controversial [33, 34]. These cells have even been shown to generate perfusable vascular networks for in vitro tissue models [35]. BMSCs have also been used to alleviate limb ischemia. In one study, rats received intravenous injections of bone marrow mesenchymal stem cells. It was found that the BMSCs significantly increased capillary density and improved renal function via an activation of the PI3K-Akt signaling pathway [36]. In another study, the effects of BMSCs in repairing acute ischemic-related renal damage were assessed by injecting Lin(−)Sca-1+ c-Kit+ BMSCs into an area of acute tubular necrosis in mice [37]. The cells differentiated into renal tubular epithelial cells, repairing the ischemic renal tubular injury. Both of these studies illustrated the potential of BMSC therapy in alleviating the effects of ischemia. However, bone marrow extraction is an invasive and painful procedure, and the availability of BMSCs varies from person to person and is further impaired in older or diseased patients. There is little known about the regulatory mechanisms for BMSCs. For these reasons, correcting ischemic damage through BMSC therapy has limited use in clinical applications.
Alternatively, adipose-derived mesenchymal stem cells (ADMSCs) have also been tested for use in cardiovascular regeneration [38–40]. These cells have the benefit of being easily extractable and in ample supply for most patients. Cardiac resident adipose tissue has also been implicated in normal heart repair and homeostasis [39, 41]. ADMSCs have been tested in donors of different health statuses to assess potential functional differences [42]. Because cardiovascular disease is often a consequence of the presence of risk factors such as hypertension and diabetes, ADMSCs must be tested in this context to paint a more accurate picture of their potential as a therapeutic. It was noted that even elderly ADMSCs that are healthy were able to produce functional vascular grafts. However, cells derived from diabetics caused thrombosis in the mouse model [42]. Additionally, the diabetic ADMSCs showed impaired remodeling of the implant due to a decreased capacity for fibrinolysis when compared to healthy ADMSC populations.
Induced Pluripotent Stem Cells
Although human ESCs have been crucial in understanding basic biology surrounding pluripotency and differentiation, current laws restricting their use hinder their efficacy for cell-based therapies. Autologously derived adult stem cells such as BMSCs seem to get around some of the ethical and compatibility issues faced by ESCs but ultimately are limited in that these cells are difficult to isolate or will be in scarce supply for elderly or diseased patients who would be prime candidates for regenerative therapy. An ideal cell source for regenerative medicine needs to be readily available, immunologically compatible, and not ethically dubious to obtain. In 2006, Shinya Yamanaka described successful reprogramming of human somatic cells into a pluripotent state that was similar to ESCs in both its phenotype and transcriptome [43••]. This was accomplished by using retroviral transduction of what have become known as the Yamanaka factors (Oct3/4, c-MYC, Klf4, Sox-2). Yamanaka also demonstrated the differentiation potential of these cells into various lineages via quantification of cell identifying lineage-specific marker expression. The advent of these induced pluripotent stem cells (iPSCs) generated a large interest in the stem cell and regenerative medicine communities because it opened many avenues for research and therapeutics.
Reprogramming of Somatic Cells
The introduction of the exogenous Yamanaka factors into dermal fibroblasts resulted in these cells taking on an ESC-like morphology in that they form rounded colonies. These transcription factors modulate pathways involved in development and metabolism in order to generate and maintain a pluripotent state [44]. Specifically, Oct4, Sox2, and Klf4 were found to regulate developmental pathways while c-Myc regulates metabolism. Subsequent studies show that while c-Myc is dispensable for reprogramming, it does increase the speed at which this process takes place [45]. The exact mechanism of reprogramming is still being actively investigated, although some reports utilizing histone deacetylase inhibitors, or other small molecules that influence chromatin remodeling proteins, have increased the efficiency of reprogramming using only Oct4 and Sox2 overexpression [46, 47]. These findings suggest that chromatin modification is a key component of this process. Elucidating the mechanistic effects of cell reprogramming could help in increasing the efficiency of the overall process. While the genetic mechanisms are being worked out, researchers are adapting methodologies of cell culture and Yamanaka factor introduction to optimize reprogramming protocols.
The original protocol for generating iPSCs relied upon using an integrating viral vector and mouse embryonic fibroblasts (MEF) as feeder cells to aid in reprogramming. Both of these cause significant concerns for translation of these cells into regenerative therapies in human. The presence of xenogenic feeder cells complicates the isolation of human cells, and any residual MEF cells in an implanted tissue could cause unwanted immune reactions and thereby harm the engrafted construct. In addition, the integrated DNA that supplied the Yamanaka factors could have off-target effects, depending on where it was inserted. To address this, protocols using Matrigel® have been developed for feeder-free culture of iPSCs so as to assure that the final differentiated cells are purely human derived [48]. A few alternatives to integrating viral vector for introducing Yamanaka factors have been studied. Among these are small molecules, ready-made proteins and CRISPR systems for knocking in and out constitutively expressed Yamanaka genes [49–51]. The important theme in these different reprogramming protocols is that they produce iPSCs without integrating vectors. Although these protocols are innovative and may be advantageous over existing protocols, the most efficient way to accomplish this goal currently is by using a nonintegrating Sendai virus as the vector for the reprogramming factors [52, 53]. Using Sendai virus for reprogramming is straightforward, has a high transduction efficiency, and is nonintegrating. These qualities make it ideal for clinically relevant uses such as modeling and treating cardiovascular diseases.
Differentiating into Cardiovascular Lineages
Different stimuli can be employed to differentiate iPSCs into the desired lineage. Included within these parameters will be both biochemical and physical cues, as well as general media components, and each of these components must be optimized for efficient differentiation. Specifically, cardiovascular cell differentiation relies upon temporally controlled activation of the Wnt signaling pathway in order to generate multipotent, brachyury+ mesodermal progenitor cells that can be further differentiated into vascular endothelial cells, vascular smooth muscle cells, or cardiomyocytes [22, 54, 55]. Provision of a small molecule agonist of the Wnt pathway in a temporal-specific manner can markedly improve the generation of various cells of the cardiovascular lineage.
Cardiomyocyte differentiation relies on both up- and downregulation of Wnt signaling. Inhibition of Wnt signaling must be done after they reach the brachyury+ progenitor phase in order to generate high cardiomyocyte yields [22]. This can be accomplished via shRNA knockdown of WNT or small molecule inhibitors [23••, 24]. Utilizing small molecule inhibitors would be better suited for regenerative medicine applications as it does not alter the genome of these cells in any way. Reports of using this method of controlling the Wnt pathway, cultured in insulin and growth factor free medium, have boasted ∼90% purity in their differentiated cell populations [23••]. This is a marked increase from the original embryoid body differentiation protocols that would see relatively low cardiomyocyte yields from those populations [56, 57]. Smooth muscle and endothelial cells can be derived from these brachyury+ progenitors without subsequent inhibition of Wnt signaling. These progenitor cells can be cultured on Matrigel® and placed in either commercially available vascular smooth muscle or endothelial cell media, respectively, in order to derive these lineages. Endothelial cells can be further matured into venous or arterial phenotypes using biomimetic bioreactors to provide physical cues in the form of fluid shear stress, which has been shown to have profound effects on vascular endothelial cell behavior [58•, 59, 60]. Differentiated endothelial cells exposed to the fluid shear stress were found to have upregulated vasoprotective markers KLF2 and 4 in addition to upregulating NOTCH signaling that is associated with mature arterial endothelial cells. This type of maturation event will be very important for certain applications of these differentiated cells which will be discussed in the next section.
Applications of iPSCs for Cardiovascular Regenerative Medicine
Modeling Cardiovascular Disease with iPSCs
An attractive feature of iPSCs is that they retain all genetic traits of the cell donor, and this can be leveraged to model diseases in vitro. This method is advantageous, compared to using somatic myocardium, because iPSCs have a larger replicative capacity and are much more readily available than donor heart tissue. iPSC technology has been used to study hypertrophic cardiomyopathy (HCM) where cellular phenotype was shown to be conserved in iPSC differentiated cardiomyocytes [61]. This model was the first to show that RAS signaling was perturbed in human cardiomyocytes with HCM causing mutation in PTPN11. Other genetic backgrounds of HCM have been assessed using iPSC to gain insights into the diseases’ electrical alterations [62, 63••]. These studies showed that high calcium concentration during diastole causes a progression of HCM phenotype in cardiomyocytes with a mutated MYH7. Human iPSC-based disease modeling provided a unique perspective over mouse models since the structure of sarcomeric proteins between mouse and human is quite different [64]. These differences cause alternate cellular phenotypes in mouse and human like sarcomeric backgrounds with the same mutation [65].
Vascular diseases such as supravalvular aortic stenosis (SVAS) can also be modeled with hiPSC technology [66]. SVAS is characterized by haplodeficiency of elastin and eventually the reduction in luminal diameter due to proliferating vascular smooth muscle cells in the supravalvular aortic region. This phenotype was recapitulated using iPSC-derived smooth muscle cells in that they were noted to have an increased proliferation rate as compared to wild-type cells. The iPSC modeling system also revealed a disorganization of contractile bundles in SVAS smooth muscle cells, indicating that the cellular phenotype is particularly robust in terms of modeling SVAS. iPSC modeling systems offer a dual capacity for investigators in that they cannot only offer a platform for gaining valuable insights into the mechanisms of disease progression but can also be used as a tool for drug screening to treat the disease [67]. Drug screens on human cells will give a better picture of how human cells will react to treatment than using mouse models. This concept may also be applied in the clinic as a means of personalized medicine to determine what drugs are the most effective for an individual’s cells [68, 69, 70••].
Tissue Engineering and Regenerative Therapies
Stem cells also offer a great platform for developing clinical interventions to treat cardiovascular disease. A few examples currently in the clinical trial stage are listed in Table 1. The goal of regenerative medicine is to replace or restore nonfunctional tissues. A very exciting, albeit ambitious, approach is to generate entire hearts for transplant patients [71, 72]. This methodology aims to address two major issues transplant recipients face: the lack of availability of organs and complications due to immune rejection. The perfusion decellularization method seeks to remove all endogenous cells within a mature adult heart while leaving behind the extracellular matrix that keeps the organ geometry and vascular structure intact. This allows investigators to utilize xenogenic organs of similar size to help fill the clinical demand. Different methodologies for recellularizing the extracellular matrix (ECM) scaffold have been explored where it was noted that re-endothelialization prior to parenchymal recellularization increased cell infiltration and ventricular functionality [72]. Human iPSC-derived cardiac progenitors have been used to repopulate mouse heart ECM scaffolds. This strategy resulted in proper endothelial and myocardial differentiation and localization [73]. However, arrhythmic beating and poor contractile force were noted in the construct. This implies that, although the ECM can direct proper differentiation of progenitors, the tissue may not be maturing properly. This could be due to missing mechanical or electrical cues that are necessary for functional tissue maturation and myocyte coupling. Additionally, other cell types may be necessary for maturation of functional heart tissue such as cardiac fibroblasts [74, 75]. More basic biology of its constituent components must be understood in terms of how to generate physiologically relevant vasculature, myocardium, and pacemakers before the dream of generating a fully functional heart can be realized.
Table 1.
Cardiovascular stem cell therapies in the USA
| Disease | Type | Status | Time frame | Cell source | Phase | Delivery mechanism | Clinical trial ID |
|---|---|---|---|---|---|---|---|
| Ischemic cardiomyopathy | Interventional | Completed | September 12, 2005–June 05, 2015 | Autologous BMSC | 1 | Intramyocardial injection | NCT00203203 |
| Chronic ischemic left ventricular dysfunction | Interventional | Ongoing | December 02, 2013– | Allogenic hMSC | 2 | Transendocardial injection | NCT02013674 |
| General aging | Interventional | Ongoing | July 18, 2016– | Allogenic hMSC | 2 | Intravenous injection | NCT02065245 |
| Coronary artery disease | Interventional | Ongoing | April 10, 2006– | Autologous BMSC-derived aldehyde dehygrogenase bright cells | 1 | Intramyocardial injection | NCT00314366 |
| Ischemic cardiomyopathy | Interventional | Completed | May 15, 2007–October 15, 2014 | Autologous c-kit+ cardiac stem cells | 1 | Intracoronary injection | NCT00474461 |
| Peripheral arterial disease | Interventional | Recruiting | April 25, 2016– | Autologous ADSC | 1 | Intravenous injection or intramuscular injection | NCT02756884 |
| Congenital heart disease | Interventional | Ongoing | January 29, 2008– | BMSC | 2 | Cardiomyoplasty | NCT01034007 |
| Myocardial infarction | Interventional | Completed | July 2014–April 2016 | Allogenic mesenchymal bone marrow cells | 3 | Intravenous injection | NCT02672267 |
| Acute myocardial infarction | Interventional | Ongoing | June 2014– | Allogenic cardiac stem cells | 2 | Intracoronary infusion | NCT02439398 |
| Cardiomyopathy | Observational | Recruiting | June 2014– | hiPSC | – | – | NCT02417311 |
| Chronic myocardial ischemia | Interventional | Recruiting | January 2016– | Autologous MSC | 2 | Intramyocardial injection | NCT02462330 |
| Cell type | No. of trials | % Observational | % Interventional | ||||
| hiPSC | 5 | 100 | 0 | ||||
| ADSC/BMSC | 41 | 4.9 | 95.1 |
Examples of recently completed or ongoing stem cell-based therapy trials. Total trials listed on clinicaltrials.gov for cardiovascular diseases are given for each cell type. Currently, there are no clinical trials using embryonic stem cells in the USA, so this category was omitted from this table
Each of these components of healthy heart tissue can be generated individually in order to provide insights into the mechanisms that facilitate proper development and to potentially provide therapeutic interventions at a smaller scale. Tissue-engineered vascular grafts (TEVG) aim to provide fully functional vasculature for patients in need of a bypass surgery [76••]. Methods have been attempted to use artificial scaffolds of different porosity to generate vessels in situ [77–79]. These grafts showed infiltration of native endothelial and vascular smooth muscle cells. Although there was some remodeling and ECM deposition, the artificial polylactic acid scaffolds remained largely intact after 12 months. It was noted that these acellular scaffolds showed macrophage infiltration, which has positive implications for vascular remodeling by influencing vascular cell behavior and ECM deposition [80–82]. In addition, these scaffolds also need to be preseeded with autologous bone marrow-derived endothelial cells. Ideally, a TEVG would utilize a cell source that is less invasive to collect. Some labs are utilizing iPSC technology to develop engineered blood vessels. Two-millimeter-thick polyglycolic acid (PGA) scaffolds have been utilized to develop TEVG by seeding them with vascular smooth muscle cells (VSMC) [76••, 83]. Static culture for approximately 8 weeks on this scaffold yielded an implantable vessel with inherent ECM composition and is perfusable in vivo. These vessels do not harbor sufficient strength to withstand burst pressure which may be due to lack of sufficient collagen deposition and/or suboptimal remodeling efficiency. These vessels also must be coated with endothelial cells to prevent thrombogenicity and be able to interact with circulating leukocytes. Endothelial specification must be taken into account as arterial endothelium behaves much differently than venous [84, 85]. Endothelial cells contain robust mechanical sensing mechanisms that influence gene expression and remodeling, and these abilities have been leveraged to specify differentiation into an arterial-like phenotype via biomimetic luminal flow [58•, 86].
Cardiac patches offer a great therapeutic to aid repair after myocardial infarction by reducing scar tissue formation and global remodeling [87–89]. Similar to what is seen in whole organ engineering attempts, poor electrical coupling of cells is seen within cardiac patches [90]. The lack of electrical coupling of these cells is a recurring issue that researchers are attempting to circumvent via biomaterial and cell maturational interventions. Nanostructural modifications of the scaffolds used in generating these tissues have provided improvements in tissue morphology and electrical coupling of mature cardiomyocytes [90–92]. Additionally, biomaterial modifications aimed at mimicking natural ECM protein and polysaccharide composition have been developed to aid progenitor cell differentiation into cardiomyocytes [93]. The modified hyaluronic acid patches showed an increased expression of cardiac markers, including the gap junction protein connexin43, as compared to other synthetic scaffolds. However, testing of the signal propagation characteristics of these differentiated cardiac cells is needed to confirm if this expression is sufficient to allow synchronous contraction with native myocardium. As an alternative approach, cardiac patches may also be used as a mode of delivery for cardiac progenitor cells (CPC) [94]. In this way, the functionality of the patch tissue itself is not a factor because the progenitor cells will translocate from the pericardium into the infarct site where they will mediate repair.
Stem cells may also be used to generate biological pacemakers, which is a crucial development for whole organs to be viable for translation. The sinoatrial node (SAN) is thought to be the initiator of the electrical signal that controls beating [95]. Current strategies for generating biological pacemakers aim to functionally mimic nodal cells of the SAN. Human mesenchymal stem cells have been transduced to express the pacemaker-specific HCN2 ion channel and subsequently expressed cardiac gap junction proteins [96, 97]. It is important to note that these cells are not pacemakers within themselves but rather provide a mechanism of depolarization for the resident cardiomyocytes coupled to the HCN2+ hMSC via the If current generated by HCN2. This multicell setup initiated subsequent action potential firing when resident cardiomyocytes became hyperpolarized between beats in a canine model [98], although it is unclear if pacing was due entirely to implanted cells. Researchers have attempted to isolate nodal-like cells from the heterogeneous mixture of hESC-derived cardiomyocytes but ultimately have been unable to show their ability to regulate sustained contraction in vivo [99, 100, 101••]. Transcription factors Shox2 and Tbx18 have each been implicated in the maintenance of cardiac pacing in nodal cells and have been used to generate autonomous pacemakers from mouse stem cells [102, 103]. Although these cells were found to have pacing capabilities, they still must be further refined to fully recapitulate physiologic pacing and be shown to maintain pacing functionality long term.
Conclusion
The ability to generate cells of the cardiovascular system allows for more relevant in vitro disease modeling systems and designing of tissue-engineered therapeutics. Various cell sources have been assessed for generating cardiac, endothelial, and smooth muscle cells with robust phenotypes. Studies with embryonic stem cells offered great insights into the methodologies that can reproducibly differentiate into these lineages although their use was limited due to political and ethical concerns of the public. Autologous adult stem cell sources are very attractive, from a therapeutics perspective, due to their inherent immune compatibility with the host. However, they are not always abundant or the most accessible. In cases where the cells can be extracted, there are still issues with the maintenance of these cells due to the lack of knowledge of the basic biology for BMSCs. iPSCs represent the most versatile stem cells in that they have the combined attributes of being autologous, easily accessible, and not ethically dubious. These qualities make them a prime candidate for both experimental studies in different genetic backgrounds and engineering therapies as outlined in Fig. 1. Reprogramming and differentiation to a specific cell lineage requires time, so interventions using this cell source would be limited to nonurgent treatments as patients would need to wait for weeks for the tissue to mature. Still, stem cells show great promise in recapitulating the physiology and architecture of complex tissues, though more fine-tuning of the differentiation protocol is necessary to become more efficient in cardiomyocyte generation. In order to accomplish this goal, more of the basic biology of stem cells must be pursued so that these therapeutics approaches can become more robust. An interdisciplinary approach is necessary to accomplish the goal of personalized regenerative medicine. Collaborations between stem cell biologists, bioengineers, and material scientists will offer the most efficient path to translation for these projects. The expertise from these fields will allow researchers to discern and recapitulate the proper cell environments to allow for the maturation of these tissues that will yield in physiologically relevant implants.
Fig. 1.
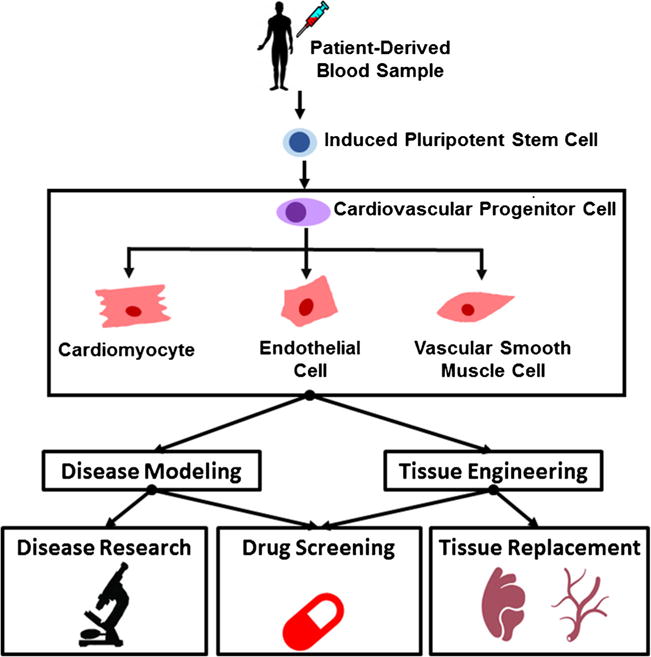
Schematic of stem cell applications in cardiovascular medicine. Schematic depiction of iPSCs derived from peripheral blood that are differentiated into a cardiovascular lineage. These cells can be utilized for either tissue engineering constructs to replace dysfunctional vasculature or myocardium for clinical interventions or drug screening in complex, 3D tissues. 2D cell culture also allows for modeling of disease with these cells and high-throughput drug screening
Acknowledgments
This work was supported by 1R01HL116705-01, 1R01HL132130-01, DOD 11959515, and Connecticut’s Regenerative Medicine Research Fund (CRMRF) 12-SCB-YALE-06, 15-RMB-YALE-08 (all to YQ).
Footnotes
This article is part of the Topical Collection on Regenerative Medicine
Compliance with Ethical Standards
Conflict of Interest Christopher W. Anderson, Nicole Boardman, Jiesi Luo, Jinkyu Park, and Yibing Qyang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2016 update. A report from the American Heart Association. Circulation. 2016;133:38–48. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Ding D, Zhang Y, Yang Y, Li Q, Chen X, et al. Prediction of the risk of mortality using risk score in patients with coronary heart disease. Oncotarget [Internet] 2016 Nov 7; doi: 10.18632/oncotarget.13166. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27835576. [DOI] [PMC free article] [PubMed]
- 4.Veronesi G, Tunstall-Pedoe H, Ferrario MM, Kee F, Kuulasmaa K, Chambless LE, et al. Combined effect of educational status and cardiovascular risk factors on the incidence of coronary heart disease and stroke in European cohorts: implications for prevention. Eur J Prev Cardiol [Internet] 2016 Nov 11; doi: 10.1177/2047487316679521. 2047487313505821. Available from: http://cpr.sagepub.com/content/early/2013/09/17/2047487313505821.abstract. [DOI] [PubMed]
- 5.Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, et al. Embryonic stem cell lines derived from human blastocysts. Science [Internet] 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145%5Cn. http://dx.doi.org/10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 6.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 7.Liu CC, Ma DL, Yan T-D, Fan X, Poon Z, Poon L-F, et al. Distinct responses of stem cells to telomere uncapping—a potential strategy to improve the safety of cell therapy. Stem Cells [Internet] 2016;34(10):2471–84. doi: 10.1002/stem.2431. [DOI] [PubMed] [Google Scholar]
- 8.Mclaren A. Ethical and social considerations of stem cell research. Nature [Internet] 2001;414(6859):129–31. doi: 10.1038/35102194%5Cn. http://www.ncbi.nlm.nih.gov/pubmed/11689959. [DOI] [PubMed] [Google Scholar]
- 9.Bobrow JC. The ethics and politics of stem cell research. Trans Am Ophthalmol Soc [Internet] 2005;103:138–41–2. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1447568&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 10.Lyerly AD, Faden RR. Willingness to donate frozen embryos for stem cell research. Science (80−) 2007;317:46–7. doi: 10.1126/science.1145067. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H, Behr B, Reddy VV, Hughes M, Pan Y, Baker J. Human embryonic stem cell lines with lesions in FOXP3 and NF1. 2016:1–13. doi: 10.1371/journal.pone.0151836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjogren A, Hardarson T, Andersson K, Caisander G, Lundquist M, Wikland M, et al. Human blastocysts for the development of embryonic stem cells. Reprod Biomed Online. 2004;9(3):326–9. doi: 10.1016/s1472-6483(10)62149-9. [DOI] [PubMed] [Google Scholar]
- 13.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 14.Ménézo Y, Veiga A, Benkhalifa M. Improved methods for blastocyst formation and culture. Hum Reprod [Internet] 1998;13(Suppl 4):256–65. doi: 10.1093/humrep/13.suppl_4.256. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10091076. [DOI] [PubMed] [Google Scholar]
- 15.Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71(5):836–42. doi: 10.1016/s0015-0282(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 16.Raya A, Rodríguez-Pizà I, Arán B, Consiglio A, Barri PN, Veiga A, et al. Generation of cardiomyocytes from new human embryonic stem cell lines derived from poor-quality blastocysts. Cold Spring Harb Symp Quant Biol. 2008;73:127–35. doi: 10.1101/sqb.2008.73.038. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, et al. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006 Sep;24:2669–76. doi: 10.1634/stemcells.2006-0377. [DOI] [PubMed] [Google Scholar]
- 18.Mitalipova M, Calhoun J, Shin S, Wininger D, Schulz T, Noggle S, et al. Human embryonic stem cell lines derived from discarded embryos. Stem Cells. 2003;21:521–6. doi: 10.1634/stemcells.21-5-521. [DOI] [PubMed] [Google Scholar]
- 19.Stojkovic M, Lako M, Stojkovic P, Stewart R, Przyborski S, Armstrong L, et al. Derivation of human embryonic stem cells from day-8 blastocysts recovered after three-step in vitro culture. Stem Cells [Internet] 2004;22(5):790–7. doi: 10.1634/stemcells.22-5-790. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15342943. [DOI] [PubMed] [Google Scholar]
- 20.Oh SK, Kim HS, Ahn HJ, Seol HW, Kim YY, Park YB, et al. Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells [Internet] 2005;23(2):211–9. doi: 10.1634/stemcells.2004-0122. Available from: http://doi.wiley.com/10.1634/stemcells.2004-0122%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/15671144. [DOI] [PubMed] [Google Scholar]
- 21.Später D, Hansson EM, Zangi L, Chien KR. How to make a cardiomyocyte. Development [Internet] 2014;141(23):4418–31. doi: 10.1242/dev.091538. Available from: http://dev.biologists.org/content/141/23/4418.abstract. [DOI] [PubMed] [Google Scholar]
- 22.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci [Internet] 2012;109(27):E1848–57. doi: 10.1073/pnas.1200250109. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc [Internet] 2013;8:162–75. doi: 10.1038/nprot.2012.150. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3612968&tool=pmcentrez&rendertype=abstract. This paper describes a protocol for the modulation of a key pathway in cardiac differentiation to efficiently derive cardiomyocytes from iPSC and ESCs that is now widely used. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren Y, Lee MY, Schliffke S, Paavola J, Amos PJ, Ge X, et al. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol [Internet] Elsevier Ltd. 2011;51(3):280–7. doi: 10.1016/j.yjmcc.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A [Internet] 2009;15(6):1211–22. doi: 10.1089/ten.tea.2008.0151. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2774496&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marbán E, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111(1):11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 27.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol [Internet] 2004;22(10):1282–9. doi: 10.1038/nbt1014. Available from: http://dx.doi.org/10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Both SK, Yang F, Cui FZ, Pan J, Meijer GJ, et al. Concise review: cell-based strategies in bone tissue engineering and regenerative medicine. Stem Cells Transl Med. 2014;3(1):98–107. doi: 10.5966/sctm.2013-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther [Internet] 2016;7(1):131. doi: 10.1186/s13287-016-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154(4):827–42. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res [Internet] 1999;85(3):221–8. doi: 10.1161/01.res.85.3.221. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10436164. [DOI] [PubMed] [Google Scholar]
- 32.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest [Internet] 2001;107(11):1355–6. doi: 10.1172/JCI12150. Available from: http://www.jci.org/articles/view/12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transpl [Internet] 2003;7(Suppl 3):86–8. doi: 10.1034/j.1399-3046.7.s3.13.x. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12603699. [DOI] [PubMed] [Google Scholar]
- 34.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature [Internet] 2004;428(6983):664–8. doi: 10.1038/nature02446. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3736322&tool=pmcentrez&rendertype=abstract. [DOI] [PubMed] [Google Scholar]
- 35.Rüger BM, Breuss J, Hollemann D, Yanagida G, Fischer MB, Mosberger I, et al. Vascular morphogenesis by adult bone marrow progenitor cells in three-dimensional fibrin matrices. Differentiation. 2008;76(7):772–83. doi: 10.1111/j.1432-0436.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 36.Jia X, Pan J, Li X, Li N, Han Y, Feng X, et al. Bone marrow mesenchymal stromal cells ameliorate angiogenesis and renal damage via promoting PI3k-Akt signaling pathway activation in vivo. Cytotherapy [Internet] 2016;18(7):838–45. doi: 10.1016/j.jcyt.2016.03.300. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1465324916303425. [DOI] [PubMed] [Google Scholar]
- 37.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest [Internet] 2003;112(1):42–9. doi: 10.1172/JCI17856. Available from: http://www.jci.org/cgi/content/abstract/112/1/42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siciliano C, Chimenti I, Bordin A, Ponti D, Iudicone P, Peruzzi M, et al. The potential of GMP-compliant platelet lysate to induce a permissive state for cardiovascular transdifferentiation in human mediastinal adipose tissue-derived mesenchymal stem cells. Biomed Res Int. 2015;2015 doi: 10.1155/2015/162439. Article ID 162439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayes-Genis A, Soler-Botija C, Farré J, Sepúlveda P, Raya A, Roura S, et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. J Mol Cell Cardiol [Internet] Elsevier Ltd. 2010;49(5):771–80. doi: 10.1016/j.yjmcc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Pittenger MF, Mackay AM, Beck S, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science (80−) 1999 Apr;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 41.Ahima RS, Flier JS, Flier JS, Spiegelman BM, Mohamed-Ali V, et al. Adipose tissue as an endocrine organ. Trends Endocrinol Metab [Internet] 2000;11(8):327–32. doi: 10.1016/s1043-2760(00)00301-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10996528. [DOI] [PubMed] [Google Scholar]
- 42.Krawiec JT, Weinbaum JS, Liao H-T, Ramaswamy AK, Pezzone DJ, Josowitz AD, et al. In vivo functional evaluation of tissue-engineered vascular grafts fabricated using human adipose-derived stem cells from high cardiovascular risk populations. Tissue Eng Part A. 2016;22(9–10):765–75. doi: 10.1089/ten.tea.2015.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell [Internet] 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867407014717. This is the first development of human induced pluripotent stem cells from adult fibroblasts and has established the foundation for human disease modeling and cell-based therapies. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Huang J, Chen T, Wang Y, Xin S, Li J, et al. Yamanaka factors critically regulate the developmental signaling network in mouse embryonic stem cells. Cell Res. 2008;18(12):1177–89. doi: 10.1038/cr.2008.309. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol [Internet] 2008;26(1):101–6. doi: 10.1038/nbt1374. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18059259. [DOI] [PubMed] [Google Scholar]
- 46.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–75. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 47.Feng B, Jiang J, Kraus P, Ng J-HH, Heng J-CDC, Chan Y-SS, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat Cell Biol [Internet] 2009;11:197–203. doi: 10.1038/ncb1827. VN-r(2) Available from: http://dx.doi.org/10.1038/ncb1827. [DOI] [PubMed] [Google Scholar]
- 48.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A [Internet] 2009;106(37):15720–5. doi: 10.1073/pnas.0908450106. Available from: http://www.pnas.org/content/106/37/15720.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell [Internet] Elsevier Inc. 2009;4(6):472–6. doi: 10.1016/j.stem.2009.05.005. Available from: http://dx.doi.org/10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian E, Sun G, Sun G, Chao J, Ye P, Warden C, et al. Small-molecule-based lineage reprogramming creates functional astrocytes. Cell Rep [Internet] Elsevier Company. 2016;16(3):781. doi: 10.1016/j.celrep.2016.06.042. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2211124716307963%5Cn http://www.ncbi.nlm.nih.gov/pubmed/27396343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kehler J, Greco M, Martino V, Pachiappan M, Yokoe H, Chen A, et al. RNA-generated and gene-edited induced pluripotent stem cells for disease modeling and therapy. J Cell Physiol [Internet] 2016 doi: 10.1002/jcp.25597. [DOI] [PubMed] [Google Scholar]
- 52.Itoh M, Kawagoe S, Tamai K, Okano HJ, Nakagawa H. Integration-free T cell-derived human induced pluripotent stem cells (iPSCs) from a patient with recessive dystrophic epidermolysis bullosa (RDEB) carrying two compound heterozygous mutations in the COL7A1 gene. Stem Cell Res [Internet] The Authors. 2016;17(1):32–5. doi: 10.1016/j.scr.2016.05.003. Available from: http://linkinghub. elsevier.com/retrieve/pii/S1873506116300344. [DOI] [PubMed] [Google Scholar]
- 53.Itoh M, Kawagoe S, Okano HJ, Nakagawa H. Integration-free T cell-derived human induced pluripotent stem cells (iPSCs) from a healthy individual: WT-iPSC1. Stem Cell Res [Internet] The Authors. 2016;17(1):22–4. doi: 10.1016/j.scr.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Lian X, Bao X, Al-Ahmad A, Liu J, Wu Y, Dong W, et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports [Internet] The Authors. 2014;3(5):804–16. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai H, Gao Y, Arzigian M, Wojchowski DM, Wu WS, Wang ZZ. BMP4 regulates vascular progenitor development in human embryonic stem cells through a Smad-dependent pathway. J Cell Biochem. 2010;109(2):363–74. doi: 10.1002/jcb.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kehat I, Kenyagin-Karsenti D. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest [Internet] 2001;108(3):363–4. doi: 10.1172/JCI12131. Available from: http://www.jci.org/cgi/content/abstract/108/3/407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell [Internet] Elsevier Inc. 2011;8(2):228–40. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 58•.Sivarapatna A, Ghaedi M, Le AV, Mendez JJ, Qyang Y, Niklason LE. Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor. Biomaterials [Internet] Elsevier Ltd. 2015;53:621–33. doi: 10.1016/j.biomaterials.2015.02.121. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0142961215002598. This paper shows how physical cues such as flow rate are capable of maturing stem cell derived endothelial cells into specified subtypes for the generation of functional tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baeyens N, Schwartz MA. Biomechanics of vascular mechanosensation and remodeling. Mol Biol Cell [Internet] 2016;27(1):7–11. doi: 10.1091/mbc.E14-11-1522. Available from: http://www.molbiolcell.org/content/27/1/7.abstract%5Cn http://www.molbiolcell.org/content/27/1/7.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-notch signaling pathways. Arterioscler Thromb Vasc Biol. 2009;29(12):2125–31. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
- 61.Carvajal-Vergara X, Sevilla A, Souza SLD, Ang Y, Schaniel C, Lee D, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature [Internet] Nature Publishing Group. 2010;465(7299):808–12. doi: 10.1038/nature09005. Available from: http://dx.doi.org/10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han L, Li Y, Tchao J, Kaplan AD, Lin B, Li Y, et al. Study familial hypertrophic cardiomyopathy using patient-specific induced pluripotent stem cells. Cardiovasc Res. 2014;104(2):258–69. doi: 10.1093/cvr/cvu205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell [Internet] Elsevier Inc. 2013;12(1):101–13. doi: 10.1016/j.stem.2012.10.010. Available from: http://dx.doi.org/10. 1016/j.stem.2012.10.010. This is the first paper describing familiar hypertrophic cardiomyopathy with myosin muation using patient induced pluripotent stem cell approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lompre AM, Mercadier JJ, Wisnewsky C, Bouveret P, Pantaloni C, D’Albis A, et al. Species- and age-dependent changes in the relative amounts of cardiac myosin isoenzymes in mammals. Dev Biol [Internet] 1981;84(2):286–90. doi: 10.1016/0012-1606(81)90396-1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20737866. [DOI] [PubMed] [Google Scholar]
- 65.Lowey S, Lesko LM, Rovner AS, Hodges AR, White SL, Low RB, et al. Functional effects of the hypertrophic cardiomyopathy R403Q mutation are different in an alpha- or beta-myosin heavy chain backbone. J Biol Chem. 2008;283(29):20579–89. doi: 10.1074/jbc.M800554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ge X, Ren Y, Bartulos O, Lee MY, Yue Z, Kim KY, et al. Modeling supravalvular aortic stenosis syndrome with human induced pluripotent stem cells. Circulation. 2012;126(14):1695–704. doi: 10.1161/CIRCULATIONAHA.112.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Misra A, Sheikh AQ, Kumar A, Luo J, Zhang J, Hinton RB, et al. Integrin β3 inhibition is a therapeutic strategy for supravalvular aortic stenosis. J Exp Med [Internet] 2016;213(3):451–63. doi: 10.1084/jem.20150688. Available from: http://jem.rupress.org/content/213/3/451?etoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.del Alamo JC, Lemons D, Serrano R, Savchenko A, Cerignoli F, Bodmer R, et al. High throughput physiological screening of iPSC-derived cardiomyocytes for drug development. Biochim Biophys Acta - Mol Cell Res [Internet] Elsevier BV. 2016;1863(7):1717–27. doi: 10.1016/j.bbamcr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tzatzalos E, Abilez OJ, Shukla P, Wu JC. Engineered heart tissues and induced pluripotent stem cells: macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliv Rev [Internet] Elsevier BV. 2016;96:234–44. doi: 10.1016/j.addr.2015.09.010. Available from: http://dx.doi.org/10.1016/j.addr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70••.Dash BC, Levi K, Schwan J, Luo J, Bartulos O, Wu H, et al. Tissue-engineered vascular rings from human iPSC-derived smooth muscle cells. Stem Cell Reports [Internet] The Authors. 2016;7(1):19–28. doi: 10.1016/j.stemcr.2016.05.004. This is the first publication describing the utilization of 3D vascular tissue rings to study vascular disease using patient induced pluripotent stem cell derived vascular smooth muscle cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med [Internet] 2008;14(2):213–21. doi: 10.1038/nm1684. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18193059. [DOI] [PubMed] [Google Scholar]
- 72.Robertson MJ, Dries-Devlin JL, Kren SM, Burchfield JS, Taylor DA. Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS One [Internet] 2014;9(2):e90406. doi: 10.1371/journal.pone.0090406. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3937369&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu T-Y, Lin B, Kim J, Sullivan M, Tobita K, Salama G, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun [Internet] Nature Publishing Group. 2013;4:2307. doi: 10.1038/ncomms3307. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23942048. [DOI] [PubMed] [Google Scholar]
- 74.Kamkin A, Kiseleva I, Isenberg G, Wagner KD, Günther J, Theres H, et al. Cardiac fibroblasts and the mechano-electric feedback mechanism in healthy and diseased hearts. Prog Biophys Mol Biol. 2003;82(1–3):111–20. doi: 10.1016/s0079-6107(03)00009-9. [DOI] [PubMed] [Google Scholar]
- 75.Kiando SR, Tucker NR, Castro-Vega L-J, Katz A, D’Escamard V, Tréard C, et al. PHACTR1 is a genetic susceptibility locus for fibromuscular dysplasia supporting its complex genetic pattern of inheritance. PLOS Genet [Internet] 2016;12(10):e1006367. doi: 10.1371/journal.pgen.1006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76••.Gui L, Dash BC, Luo J, Qin L, Zhao L, Yamamoto K, et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials [Internet] Elsevier Ltd. 2016;102:120–9. doi: 10.1016/j.biomaterials.2016.06.010. This is the first report of tissue-engineered blood vessels using vascular smooth muscle cells derived from human induced pluripotent stem cells and sets the stage for autologous iPSC-based vessel repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patterson JT, Gilliland T, Maxfield MW, Church S, Shinoka T, Breuer CK. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regen Med. 2013;7(3):409–19. doi: 10.2217/rme.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tara S, Kurobe H, Rocco KA, Maxfield MW, Best CA, Yi T, et al. Well-organized neointima of large-pore poly(L-lactic acid) vascular graft coated with poly(L-lactic-co-ε-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(L-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis [Internet] 2014;237(2):684–91. doi: 10.1016/j.atherosclerosis.2014.09.030. Available from: http://www.sciencedirect.com/science/article/pii/S0021915014014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurobe H, Maxfield MW, Tara S, Rocco KA, Bagi PS, Yi T, et al. Development of small diameter nanofiber tissue engineered arterial grafts. PLoS One [Internet] 2015;10(4):e0120328. doi: 10.1371/journal.pone.0120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mirensky TL, Nelson GN, Brennan MP, Roh JD, Hibino N, Yi T, et al. Tissue-engineered arterial grafts: long-term results after implantation in a small animal model. J Pediatr Surg [Internet] Elsevier Inc. 2009;44(6):1127–33. doi: 10.1016/j.jpedsurg.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 81.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood [Internet] 2000;96(1):34–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10891427. [PubMed] [Google Scholar]
- 82.Hibino N, Yi T, Duncan DR, Rathore A, Dean E, Naito Y, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. Faseb J [Internet] 2011;25(12):4253–63. doi: 10.1096/fj.11-186585. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3236622&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao J, Niklason L, Langer R. Surface modification of polyglycolic acid meshes increases the seeding density and spreading of smooth muscle cells. J Biomed Mater Res. 1998;42:417–24. doi: 10.1002/(sici)1097-4636(19981205)42:3<417::aid-jbm11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 84.Rufaihah AJ, Huang NF, Kim J, Herold J, Volz KS, Park TS, et al. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Am J Transl Res. 2013;5(1):21–35. [PMC free article] [PubMed] [Google Scholar]
- 85.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 86.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10(1):53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hashizume R, Fujimoto KL, Hong Y, Guan J, Toma C, Tobita K, et al. Biodegradable elastic patch plasty ameliorates left ventricular adverse remodeling after ischemia-reperfusion injury: a preclinical study of a porous polyurethane material in a porcine model. J Thorac Cardiovasc Surg [Internet] Elsevier Inc. 2013;146(2):391–9. doi: 10.1016/j.jtcvs.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hashizume R, Hong Y, Takanari K, Fujimoto KL, Tobita K, Wagner WR. The effect of polymer degradation time on functional outcomes of temporary elastic patch support in ischemic cardiomyopathy. Biomaterials [Internet] Elsevier Ltd. 2013;34(30):7353–63. doi: 10.1016/j.biomaterials.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei HJ, Chen CH, Lee WY, Chiu I, Hwang SM, Lin WW, et al. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29(26):3547–56. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem Biophys Res Commun. 2007;361(4):847–53. doi: 10.1016/j.bbrc.2007.07.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu S, Liu X, Hu T, Chu PK, Ho JPY, Chan YL, et al. A biomimetic hierarchical scaffold: natural growth of nanotitanates on three-dimensional microporous Ti-based metals. Nano Lett. 2008;8(11):3803–8. doi: 10.1021/nl802145n. [DOI] [PubMed] [Google Scholar]
- 92.Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol [Internet] Nature Publishing Group. 2011;6(11):720–5. doi: 10.1038/nnano.2011.160. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3208725&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang MC, Wang SS, Chou NK, Chi NH, Huang YY, Chang YL, et al. The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin-polysaccharide cardiac patches in vitro. Biomaterials [Internet] Elsevier Ltd. 2009;30(22):3757–65. doi: 10.1016/j.biomaterials.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 94.Jang J, Park H-J, Kim S-W, Kim H, Park JY, Na SJ, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials [Internet] Elsevier Ltd. 2016;112:264–74. doi: 10.1016/j.biomaterials.2016.10.026. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0142961216305695. [DOI] [PubMed] [Google Scholar]
- 95.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47(4):658–87. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 96.Potapova I, Plotnikov A, Lu Z, Danilo P, Valiunas V, Qu J, et al. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res. 2004;94(7):952–9. doi: 10.1161/01.RES.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]
- 97.Valiunas V, Doronin S, Valiuniene L, Potapova I, Zuckerman J, Walcott B, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol [Internet] 2004;555(Pt 3):617–26. doi: 10.1113/jphysiol.2003.058719. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14766937%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1664864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Plotnikov AN, Shlapakova I, Szabolcs MJ, Danilo P, Lorell BH, Potapova IA, et al. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116(7):706–13. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 99.Scavone A, Capilupo D, Mazzocchi N, Crespi A, Zoia S, Campostrini G, et al. Embryonic stem cell-derived CD166+ precursors develop into fully functional sinoatrial-like cells. Circ Res. 2013;113(4):389–98. doi: 10.1161/CIRCRESAHA.113.301283. [DOI] [PubMed] [Google Scholar]
- 100.Zhang H, Lau DH, Shlapakova IN, Zhao X, Danilo P, Robinson RB, et al. Implantation of sinoatrial node cells into canine right ventricle: biological pacing appears limited by the substrate. Cell Transplant. 2011;20(11–12):1907–14. doi: 10.3727/096368911X565038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101••.Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol [Internet] Nature Publishing Group. 2016;35:1–16. doi: 10.1038/nbt.3745. This paper is the first to demonstrate pacemaking ability of sinoatrial node cells derived from human iPSCs. [DOI] [PubMed] [Google Scholar]
- 102.Ionta V, Liang W, Kim EH, Rafie R, Giacomello A, Marbán E, et al. SHOX2 overexpression favors differentiation of embryonic stem cells into cardiac pacemaker cells, improving biological pacing ability. Stem Cell Reports. 2015;4(1):129–42. doi: 10.1016/j.stemcr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McNally EM, Svensson EC. Setting the pace: Tbx3 and Tbx18 in cardiac conduction system development. Circ Res. 2009;104(3):285–7. doi: 10.1161/CIRCRESAHA.109.193680. [DOI] [PMC free article] [PubMed] [Google Scholar]


