Abstract
Adaptive immune response consists of many highly regulated, multistep cascades that protect against infection while preserving the health of autologous tissue. The proper initiation, maintenance, and resolution of such responses require the precise coordination of molecular and cellular signaling over multiple time and length scales orchestrated by lymphatic transport. In order to investigate these functions and manipulate them for therapy, a comprehensive understanding of how lymphatics influence immune physiology is needed. This review presents the current mechanistic understanding of the role of the lymphatic vasculature in regulating biomolecule and cellular transport from the interstitium, peripheral tissue immune surveillance, the lymph node stroma and microvasculature, and circulating lymphocyte homing to lymph nodes. This review also discusses the ramifications of lymphatic transport in immunity as well as tolerance and concludes with examples of how lymphatic-mediated targeting of lymph nodes has been exploited for immunotherapy applications.
Keywords: adaptive immune response, immune tolerance, immune physiology
1. INTRODUCTION
Although traditionally considered a conduit system responsible for maintaining tissue fluid balance, the lymphatics were recognized for their immunological transport role more than 40 years ago (1–3). Since then, the understanding of lymphatic transport and its effects on adaptive immune response, including effects on cell migration and trafficking as well as antigen presentation, has improved substantially. Indeed, lymph drainage affects more than just tissue edema. For example, lymph drainage facilitates both exogenous and self-antigen transport to lymph nodes to tune humoral response to immunization as well as regulatory T (Treg) cell function and immune tolerance (4); locally dampens antitumor immunity (5); and directs the remodeling of draining lymph nodes (4, 6, 7), tissues whose microstructure orchestrates the comingling of lymph and lymphocytes to facilitate adaptive immune response. The transport role of the lymphatics is thus intrinsically linked to lymphatic function in immune physiology. Accordingly, lymphatic drug targeting is increasingly utilized to enable the delivery of therapeutic agents to the local draining lymphatic basin and lymph nodes (8–11). The aims of this review are to integrate relevant developments in this area into a holistic perspective on the immune physiology of lymphatic transport and to highlight approaches that harness this role for immunotherapeutic applications.
2. IMMUNE PHYSIOLOGY OF LYMPHATIC TRANSPORT
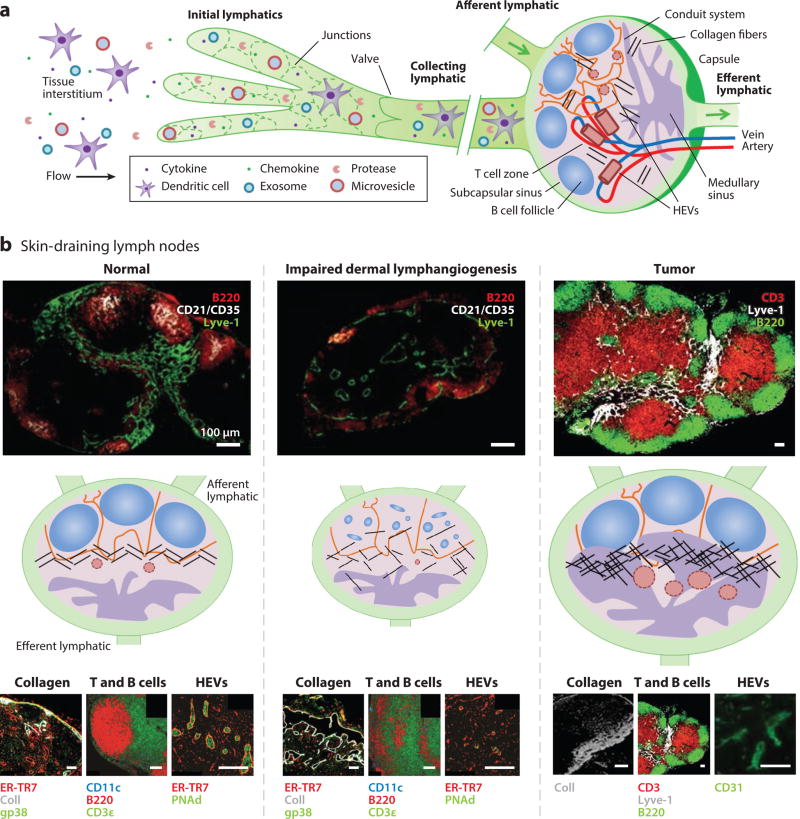
The cardiovascular system oxygenates and provides nutrients to interstitial tissues by plasma filtration at blood capillaries driven by Starling’s forces. This results in a net leakage of plasma into the interstitium that, if unchecked, causes fluid imbalance and tissue swelling. To resolve this mass transport problem, which is on the order of ~3 L of excess filtered blood plasma per day (12), the lymphatic system provides a drainage conduit (Figure 1) (13, 14) that, by virtue of cyclic pressure gradient variations (15), imparts a convective driving force for the removal of excess fluid from the interstitium. Thus, fluids and small molecules from interstitial tissues that drain into the lymphatic capillary plexus are concentrated as lymph (16, 17) and convected via the pumping function and valve-segmented lymphangion structure of larger lymphatic vessels to draining lymph nodes in a unidirectional fashion. The cumulative result is a convective driving force out of peripheral tissues into draining lymph nodes.
Figure 1.
Lymphatic transport–directed lymph node remodeling. (a) Lymphatic uptake of small molecules, particulates, and cells within the tissue interstitium results in carriage to draining lymph nodes, tissues whose microstructure orchestrates the comingling of lymph and lymphocytes to facilitate adaptive immunity and tolerance. The transport role of the lymphatics in the delivery of peripheral tissue–derived antigens, cytokines and chemokines, immune modulatory particles, and immune cells can thus modulate the immunological and biophysical microenvironment of draining lymph nodes to influence adaptive immune response in multiple ways. (b) Microstructures of skin-draining lymph nodes with (left) normal, (middle) impaired, and (right) tumor lymphatic transport are shown immunohistochemically and schematically, emphasizing representative collagen organization, T and B cell segmentation, and high endothelial venule (HEV) remodeling responses. Modified from References 4, 10, 13, and 14 with permission.
From an immunological standpoint, the net fluid transport process facilitated by lymphatics provides an efficient means to deliver antigen to immune cells of the adaptive immune system by both passive drainage and active cell-mediated transport mechanisms. This is because small-molecule protein antigens subjected to interstitial flow can drain into lymphatics and are delivered to dendritic cells, professional antigen-presenting cells that reside at high numbers within the lymph nodes [timescale: minutes to hours (18)]. In addition, lymph-borne antigen is transported to B cells directly in the case of small antigens (18) or by transfer from subcapsular macrophages (19). Alternatively, dendritic cells patrolling peripheral tissues can take up small-molecule or particulate antigen and chemotact toward lymphatic endothelial cell–expressed CCL21 [chemokine (C–C motif) ligand 21] (20) to enter the lymphatics and invade draining lymph nodes [timescale: hours to days (21)] to directly present (22) or transfer to stromal cells (23) major histocompatibility complex (MHC)-loaded peptide antigen. Because organized lymphocyte accumulation occurs in lymph nodes, which maintain mature naïve lymphocytes and initiate an adaptive immune response, these convective transport processes direct the delivery of antigen over multiple timescales to the major tissue sites of antigen presentation involved in the development of both immunity and tolerance (4, 5, 24–26).
The context of antigen presentation plays a critical role in determining the quality of induced adaptive immune response. First, dendritic cells that reside within lymph nodes and take up soluble antigen also express cytokines that differ from the antigen-bearing dendritic cells that infiltrate the lymph node after activation in the periphery (27), suggesting a distinct role for lymph-borne antigen and therefore lymphatic drainage in the fine-tuning of immune response. Second, from a simplified viewpoint, when antigen is presented in what might be considered steady-state conditions (e.g., in the absence of ancillary immune stimulation) by immature dendritic cells, antigen-specific T cell priming results in the development and expansion of Treg cells. These cells, again in highly simplistic terms, protect tissues from autoimmune reactions (28) but also contribute to tumor-associated immune suppression (29). Alternatively, if instead antigen is coincidentally encountered with immunological danger signal(s) (such as pathogen-associated molecular patterns or immune stimulatory cytokines) that result in dendritic cell activation/maturation, antigen presentation to cognate T cells directs their differentiation into immunogenic T cells that help B cell production of antibodies and expression of inflammatory cytokines in the direct clearance of target cells (30). The local immune signaling milieu at the time of antigen presentation thus plays a crucial role in directing the quality of adaptive immunity versus tolerance.
Lymphatics are also involved in directing adaptive immune response through modulation of the draining lymph node immunological microenvironment by providing a transport mechanism not only for antigen but also for endogenous immune modulators produced within the interstitium of peripheral tissues. For example, inflammatory cytokine levels in lymph-draining rheumatoid arthritis–afflicted joints of human patients are elevated 100-fold relative to serum (31). Exosomes and microparticles, which mediate intercellular transport of protein and ribonucleic/deoxyribonucleic acid (RNA/DNA) (32), are also drained from peripheral tissues via lymphatics to draining lymph nodes, where they regulate local tissue remodeling (33) and inflammatory signaling (34). Thus, the transport function of lymphatics can play multiple roles in the regulation of adaptive immunity.
3. LYMPHATIC TRANSPORT–DIRECTED REMODELING OF THE LYMPH NODE MICROENVIRONMENT AND ITS POTENTIAL INFLUENCE ON RESIDENT CELLS
Lymph nodes function as the body’s lymphocyte “transit hubs,” passage through which is intrinsically complex. Cells and lymph-borne solutes from local tissues are delivered and incompletely removed via unidirectional rail lines (lymphatics). Once in the lymph node, cells are loosely subdivided into terminals (T and B cell zones) while fluids and small molecules are shuttled intra–lymph nodally via trams (conduits). Gates [high endothelial venules (HEVs)] also facilitate entry of cells as well as exchange of solutes and fluids with the systemic blood circulation (Figure 1).
This complex and dynamic organization is critical to lymph node function in comingling small molecules and cells to facilitate signaling for adaptive immune response. For example, self-tolerance mechanisms fail, resulting in generalized autoimmunity in mice with a plt (paucity of lymph node T cells) mutation that lacks CCR7 (C–C chemokine receptor type 7) ligands (35). This is because CCL21 and CCL19, CCR7 ligands secreted by the stromal cells of the lymph node T cell zone that form the structural network that guides lymphocyte trafficking (36), are essential to the positioning of CCR7+ Treg, naïve T, and antigen-presenting cells in the lymph node paracortex (37). Disruption of lymph node architecture via pathogen-induced interference of homeostatic chemokine expression in infected lymph nodes also enhances Salmonella typhimurium virulence and has been hypothesized to represent a mechanism of immune suppression used by pathogens that primarily target lymphoid tissue (34). The organization of lymph nodes is thus crucially linked to their immunological functionality.
The cellular and molecular distribution profiles within lymph nodes are significantly influenced by lymphatic transport function. For example, in the absence of dermal lymphatics, disorganized stromal cell distributions, minimal B cell follicle definition, and smaller or collapsed HEVs (4) are found in skin-draining but not other lymph node localities (Figure 1), consistent with remodeling responses induced within draining lymph nodes when afferent lymph flow is occluded (6). These lymph node organizational changes correspond with abnormal distributions of CCL21 and CXCL13 [chemokine (C–X–C motif ) ligand 13] (4), chemokines that direct T and B cell positioning within the lymph node, respectively, as well as T cell zone fibroblastic reticular cells and reticular fibers (4), which regulate T cell migration (38). Together with in vitro observations of T zone fibroblastic reticular cell organization, proliferation, and secretion of CCL19 and CCL21, sensitivity to flow (39) suggests that lymphatic transport–regulated intranodal fluid flow may modulate chemokine-dependent organizational and structural remodeling within the lymph node interstitium.
In addition to flow putatively providing organizational cues, lymph-borne solutes and cells are implicated in directing lymph node remodeling. For example, vascular endothelial growth factor (VEGF) drained from the inflamed skin in a delayed-type hypersensitivity skin model induces lymphatic vessel remodeling and expansion within draining lymph nodes (40). Additionally, microparticles containing tumor necrosis factor secreted by peripheral tissue–resident mast cells and transported via lymphatics induce lymph node hypertrophy (34). Lymph-migrating dendritic cells also direct the remodeling of the lymph node fibroblastic reticular network after immunogenic challenge (41), and the extent of cellular remodeling of the lymph node interstitium is proportional to the number of mature lymph-transported dendritic cells (42). Dendritic cells also direct the proliferation of endothelial cells and HEV expansion within draining lymph nodes after either immunization or subcutaneous adoptive transfer of dendritic cells (43).
Given the importance of microstructural properties and cellular distributions in regulating lymph node efficacy at directing adaptive immune response and immunological tolerance, a potential pathophysiological role is suggested by the lymph node remodeling that accompanies numerous disease states. For example, sentinel, or tumor-draining, lymph nodes are exposed to a high concentration of lymph-transported molecules from the tumor interstitium by virtue of their proximity to growing tumors (Figure 1) (7, 8). Signaling pathways active within the local tumor microenvironment that result in tissue remodeling associated with cancer cell survival (44), invasion (45–47), and immune suppression (5) could therefore operate within tumor-draining lymph nodes. We recently analyzed lymph nodes from B16 melanoma–bearing mice with respect to tumor stage and found that melanoma lymphatic drainage was associated with alterations in lymph node hyaluronic acid and collagen content corresponding with physical adaptations that manifest in tumors (44, 48, 49), including increased intranodal pressures (7, 50, 51) and increased lymph node tissue stiffness and viscoelasticity (7). The potential for these and other remodeling responses to influence critical cellular processes in the lymph node, including cell proliferation, migration, and lymphocyte homing, is discussed below.
3.1. Proliferation of Lymph Node–Resident Cells
Cellular proliferation within lymph nodes, which is important in the expansion of antigen-specific lymphocytes (52) and stromal cells (53), as well as metastatic tumor formation by invading cancer cells (54), can be regulated by lymphatic-directed changes in lymph node hyaluronic acid abundance, extracellular matrix stiffness, and intranodal fluid flow profiles. Hyaluronic acid plays a key role in the survival and proliferation of human myeloma cells through local retention of the survival signal interleukin-6 (55). Hyaluronic acid induces strong proliferative responses in murine B, but not T, cells, indicating the potential for lymph node hyaluronic acid abundance to influence B cell proliferation and effector function (56). Low–molecular weight hyaluronic acid induces an increase in the proliferation of lymphatic endothelial cells and triggers their migration and organization into tubelike structures (57). Alterations in lymph node hyaluronic acid content, which were previously demonstrated to be associated with melanoma lymphatic drainage (7), therefore have the potential to influence the survival, proliferation, and functionality of lymph node resident cells.
Extracellular matrix stiffness is another aspect of the lymph node biophysical microenvironment that can influence cellular proliferation. Matrix stiffening resulting from increased collagen density had a negative effect on fibroblast proliferation (53). Stiffer scaffolds also showed increased resistance to fibroblast retraction, suggesting resistance to cell-directed remodeling (53). Substrate rigidity was also found to influence the proliferation of human T cells, with softer substrates resulting in higher naïve T cell proliferation when stimulated ex vivo (52). Similarly, murine melanoma proliferation was observed to increase in softer fibrin matrices, resulting in increased self-renewal and colony growth over time (57). However, there are also conflicting reports indicating that human breast and lung cancer cell proliferation was increased by two- and threefold, respectively, with an increase in substrate stiffness from 150 to 4,800 Pa (58). These results indicate the complexity and cell subtype dependency of stiffness-induced alterations in cell proliferation.
Changes in interstitial flow rates within lymph nodes as a result of remodeling also have the potential to affect the proliferation of resident cells. T cell zone fibroblastic reticular cell proliferation increases with flow rate in vitro (39). Flow rate also influences the proliferation of several types of cancer cells; a shear stress of 12 dyn/cm2 induces G0/G1 cell-cycle arrest, as opposed to static conditions inducing G2/M arrest, which inhibits cell differentiation (54). Overall, remodeling-induced physical changes in hyaluronic acid content, substrate stiffness, and intranodal flow may therefore regulate the proliferation of cells within the lymph node interstitium.
3.2. Intranodal Cell Migration
Cell migration within lymph nodes, which determines T and B cell zone distributions, stromal cell organization, and cancer cell invasion, is likely regulated by physical remodeling known to occur in lymph nodes, including changes in matrix stiffness, chemokine gradients, and intranodal pressure. Changes in extracellular matrix stiffness due to lymph node interstitial remodeling may influence cell migration by changing migratory force generation, migration persistence, and migration velocity. For example, increased substrate stiffness from 2,500 to 15,600 Pa caused primary human macrophages to increase their exerted force by threefold, indicating their adaptive capability to produce larger traction stresses in stiffer tissue microenvironments (59). In other research, neutrophils were observed to migrate more slowly but with a longer persistence time on stiffer substrates (60). The longer persistence time caused neutrophils to migrate an overall greater distance on stiffer substrates and with increased directionality, as opposed to the more random motion noted on softer substrates (60). In three-dimensional collagen matrices, fibroblast migration increased twofold with an increased substrate stiffness of 60 Pa due to a higher concentration of collagen (61). Lung and breast cancer cell velocities increased by ~15–20 µm/h when the extracellular matrix–conjugated polyacrylamide substrate stiffness was increased from 150 to 4,800 Pa (58). Changes in lymph node stiffness induced by lymphatic remodeling may therefore influence intra–lymph nodal migration of cells by altering traction forces, guiding cell directionality, and modifying cell velocity.
Flow (62), chemokine gradients (63, 64), and the coupled effects of flow on chemokine gradients (45, 65) within the remodeled lymph node interstitium likely also affect cell migration within lymph nodes. In one example, naïve T cell motility exhibited binary behavior, with an increased average locomotion of ~10 µm/min when the perfusion rate of explanted lymph nodes was increased above 40 µm/s, but with a cellular velocity of only 2–4 µm/min when perfusion rates were 1.3– 13 µm/s (62). Interstitial flow through three-dimensional collagen gels provoked an increase in matrix metalloproteinase–dependent fibroblast motility (66). Furthermore, mature dendritic cell secretion of CCL19 increased the polarization, motility, and maximum migration distance of coincubated naïve T cells in vitro (63). Additional studies have demonstrated that dendritic cells themselves migrate toward chemokine gradients of CCL19 and CCL21 as low as 50 and 100 nM, respectively (64). However, dendritic cells preferentially migrated toward CCL21 rather than CCL19 when both directional cues were present even at higher, equal concentrations (64). Cellular migration can also be directed by interstitial flow in response to secreted chemokine engagement with the surface-expressed receptor (for example, CCL21 and CCR7) via a mechanism termed autologous chemotaxis (45). Breast cancer cell migration and directionality were also found to be a function of flow velocity along streamlines (65). Thus, the velocity, directionality, and persistence of migrating lymphocytes and cancer cells may be affected by lymphatic-directed changes in intranodal flows and chemokine gradients.
Similarly, changes in lymph node pressure and pressure gradients induced by lymphatic-directed tissue remodeling can direct intranodal migration of cells. T cells derived from human peripheral blood, activated, and cultured under increased pressure conditions exhibited increased motility (67). When pressure gradients were applied to aggregates of human breast cancer cells, migration was also suppressed at localities within the aggregate that were exposed to lower pressures (68).
3.3. Cell Homing to Lymph Nodes via High Endothelial Venules
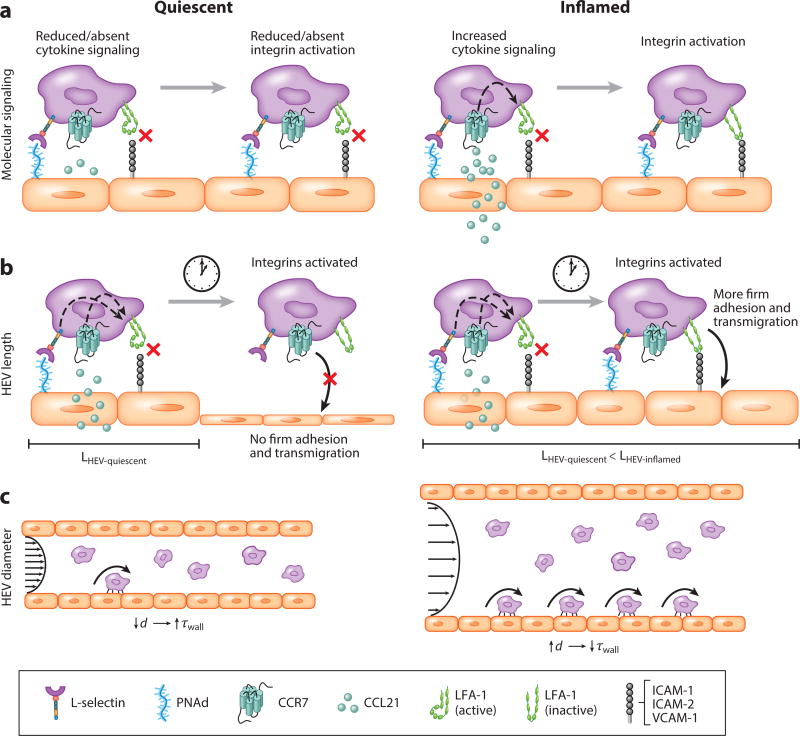
In addition to immigrating via lymphatic vessels, na¨ıve lymphocytes and other cells home to lymph nodes via specialized regions of lymph node vasculature termed HEVs (69). Early histological and electron microscopy studies revealed that during inflammatory events, lymphocytes migrate across the vessel wall in HEVs by penetrating junctions between neighboring endothelial cells (70). This highly orchestrated process is classically described in three interrelated steps: (a) selectin-mediated tethering and rolling of circulating lymphocytes on the vessel wall, (b) cytokine-mediated activation of integrins, presented on lymphocytes, and (c) integrin-mediated firm adhesion of lymphocytes to the endothelium and subsequent diapedesis.
In the first step of this process, L-selectin binds its endothelial ligand, peripheral node addressin (PNAd), via fast kinetic interactions, which allow cells to slow down relative to free flow velocity (Figure 2) (71–73). Expression of PNAd and consequential lymphocyte accumulation are lymphatic drainage dependent, as evidenced by a reduction in luminal PNAd expression following occlusion of the afferent lymphatic vessel in experimental models (74). Whereas the interaction of luminal PNAd with L-selectin is recognized as the direct mediator of lymphocyte tethering and rolling on the HEV, other, indirect, temporally or spatially regulated interactions have also been implicated in this tethering and rolling behavior. For instance, PNAd expressed on HEVs can also capture activated platelets via P-selectin, which in turn can interact with P-selectin glycoprotein ligand 1 ligands on circulating lymphocytes, enabling an indirect lymphocyte rolling effect (75). In addition to this indirect pathway, lymphocyte rolling is further complicated by locational variation in molecular expression. Whereas L-selectin–PNAd interactions mediate rolling behavior of lymphocytes in peripheral lymph nodes, HEVs of mesenteric lymph nodes facilitate rolling of lymphocytes via α4β7–MAdCAM-1 (mucosal addressin cellular adhesion molecule 1) interactions (76). Finally, molecular expression as it relates to lymphocyte trafficking across the HEV is temporally regulated. For example, developmental studies on murine models suggest a dynamic presentation of cellular adhesion molecules on the luminal surface of the HEV throughout growth. Initially, MAdCAM-1 dominates the adhesion of an α4β7-positive subset of cells to the HEV, yet 24 h after birth, a phenotypic switch enables L-selectin expressed on circulating lymphocytes to interact with PNAd (77).
Figure 2.
Inflammation-induced changes in cellular homing via high endothelial venules (HEVs). (a) In an inflamed HEV, an influx of cytokines expressed by or trafficked and translocated to the HEV enables increased integrin activation and engagement by circulating lymphocytes relative to quiescent HEVs. (b) Inflamed HEVs are longer; thus, they may support more adhesion of lymphocytes once they become fully activated relative to shorter, quiescent HEVs. (c) Increases in HEV diameter during inflammation and concomitant reductions in wall shear stress support greater frequencies of rolling cells relative to quiescent HEVs. Abbreviations: ICAM, intracellular adhesion molecule; LFA, lymphocyte function–associated antigen; PNAd, peripheral node addressin; VCAM, vascular cell adhesion molecule.
The second and third steps of lymphocyte transmigration demonstrate the strong interdependence of each part of this process, as integrin activation depends on cytokine signaling for successful firm adhesion formation (Figure 2). Lymphocyte function–associated antigen 1 (LFA-1), the integrin that firmly binds intracellular adhesion molecule (ICAM)-1, ICAM-2, or vascular cell adhesion molecule 1 (VCAM-1) expressed on the HEV endothelium, requires activation via membrane-associated cytokine signaling cascades (76, 78). In vivo intravital microscopy studies of murine models demonstrated that CCL21 is constitutively presented on the HEV lumen, and its interaction with CCR7 on circulating lymphocytes is required for LFA-1 activation and subsequent firm adhesion of naïve T cells to HEVs (79). In a plt mutant mouse strain employed due to its impaired T cell homing abilities, PNAd but not CCL21 is expressed on luminal HEVs and results in cell rolling but not firm adhesion (79). Interestingly, LFA-mediated cell arrest on HEVs of lymph nodes of plt mice can be restored via intracutaneous injection of CCL21 (79). Other cell type–specific molecular pairs have been identified as integrin activating, and their transport to the HEV lumen represents a critical step in physiological and pathophysiological processes (80, 81). Furthermore, although activation of LFA-1 by cytokines induces conformational changes necessary for binding ICAM-1, the engagement with this ligand is ultimately necessary for appropriate LFA-1 headpiece rearrangement for optimal firm adhesion mediation (82, 83).
Changes in transport and luminal presentation of integrin-activating cytokines and integrin ligands exemplify an important mechanism by which inflammation can alter lymphocyte trafficking across the HEV. Delivery of peripheral tissue–secreted cytokines to HEVs via draining lymphatics and the fibroblast reticular cell–based conduit system in lymph nodes enable remote signaling to HEVs that directs their adhesive capacity (84–86). This system for cytokine transport is essential to the observed “remote control” of the HEV via cytokine signaling. For example, Palframan et al. (87) demonstrated that monocyte chemoattractant protein 1 (MCP-1/CCL2) release in a model of skin inflammation resulted in transport of MCP-1/CCL2 to the draining lymph node and subsequent translocation to the luminal side of the HEV, which in turn led to activation of CCR2 signaling and subsequent integrin activation in adoptively transferred monocytes. Other inflammation-related changes, such as fever induction, increase the display of CCL21 and ICAM-1 on the HEV; both of these are necessary for the observation of temperature-induced increase in cell trafficking across the HEV (83).
Geometrical alterations in HEVs, such as changes in size and length, represent an additional consequence of inflammation that can affect lymphocyte trafficking to lymph nodes via biophysical effects. Upon immunization or infection, lymph nodes rapidly enlarge, subsequently inducing changes to the lymph node vasculature, specifically lengthening HEVs (88). The mechanism of expansion has been the subject of intense study; such research has emphasized the interplay among lymphocytes, dendritic cells, fibroblast reticular cells, and the expression of factors such as VEGF in proliferation of the endothelial cells that constitute the HEV (43, 89–92). The importance of such expansion in lymphocyte homing has been emphasized in recent research quantifying the capture efficiency of leukocyte-like HL-60 cells on various lengths of micropatterned selectin substrate under flow conditions (93). Because selectin-ligand bonds must counteract hydrodynamic shear forces of the blood vasculature, the density of adhesive ligands and the length of adhesive patches represent critical variables in the ability of cells to develop a sufficient number of bonds to counter such dispersive hydrodynamic forces (93). Varying the adhesive patch length can result in varied encounter time between a receptor on the circulating cell and its corresponding ligand, such that longer patch lengths enable longer encounter times and thus affect adhesive bond dynamics. Analogous assumptions as to the importance of selectin or selectin ligand patch length can be made in relation to HEV length; expansion of HEVs corresponds to an expansion in the length over which PNAd is expressed (92) and, thus, may influence lymphocyte capture via L-selectin-mediated rolling and the resulting transendothelial migration to accumulate within the lymph node (Figure 2). Furthermore, the influence of HEV length scales may be exacerbated by the synergistic effect of selectins, cytokines, and integrins in the transendothelial migration process in lymphocyte homing and accumulation. In both static and flow-based assays, Atarashi et al. (94) demonstrated that cross-linking P-selectin glycoprotein ligand 1, a P-selectin ligand on T helper 1 cells, increased adhesion frequency and strength to ICAM-1. When combined with cytokine-mediated integrin activation, cross-linking of P-selectin glycoprotein ligand 1 resulted in a greater-than-additive increase in ICAM-1 adhesion, emphasizing an important role for both cytokines and selectins in integrin activation (94). Because this selectin engagement–mediated intracellular signaling pathway presumably requires a finite threshold time for full integrin activation, in the presence of hemodynamic forces this activation time essentially translates to selectin engagement over a threshold length. In this context, length scales relate not only to the initiation of rolling adhesion but also to the formation of firm adhesions, further emphasizing the important role of HEV expansion to increased lymphocyte homing and accumulation in inflamed lymph nodes. We recently demonstrated that the persistence with which cells mediate rolling adhesion to P-selectin is cell subtype dependent (95). Whether the persistence of rolling adhesion to HEVs via other receptors diverges between lymph node–homing cell subtypes, and whether its combined effect with HEV expansion/contraction affects the recruitment of specific lymphocyte subtypes, remains to be elucidated.
Finally, changes in flow conditions have the potential to dramatically alter lymphocyte recruitment via the HEV. Kumar et al. (89) showed that, during inflammation, B cell–mediated HEV growth is manifested initially as increased HEV branching and later as elongation and circumferential growth of preexisting HEV segments. Flow through HEVs modeled as flow through a circular tube predicts that circumferential growth would result in a decrease in wall shear stress (96). This remodeling-induced shear stress reduction has potential consequences for lymphocyte homing and accumulation via HEV adhesive processes. Whereas the precise values of wall shear stress levels exhibited in HEVs of quiescent and inflamed lymph nodes have not yet been determined, one can infer, on the basis of studies of T lymphocyte interactions with PNAd-functionalized substrates under shear flow, that a decrease in shear stress up to a critical nonzero point can increase lymphocyte adhesion to the HEV (Figure 2) (97). These changes in lymphocyte recruitment may be due, in part, to shear stress–induced alterations in adhesive ligand expression. For example, Woolf et al. (98) demonstrated that, in a shear-free environment, CCL21 failed to activate integrins for successful T cell adhesion to ICAM-1, demonstrating an important role of shear stress in lymphocyte trafficking across the HEV. Regulation of biophysical parameters such as HEV length and shear stress level, compounded by changes in molecular expression and presentation directed by lymphatics, thus represents a critical aspect of lymph node remodeling that occurs during normal physiologic processes as well as diverse pathophysiological responses to influence lymph node cell homing.
4. IMPLICATIONS OF LYMPHATIC TRANSPORT IN IMMUNE RESPONSE
We now focus on the contribution of the transport role of lymphatics to adaptive immunity and tolerance. We do not elaborate on other immune modulatory roles of the lymphatic vasculature, including but not limited to the capacity of the lymphatic endothelium to directly present antigen to modulate T cell immunity and regulation (5, 23, 99–101) and its effects in cancer (5) and immune tolerance (5, 99, 100), which have been well reviewed elsewhere (102–104, 105), nor do we focus on the emerging concept of the lymphatic endothelium’s role as an “antigen archive” (105, 106).
4.1. Lymphedema-Associated Alterations in Immune Function and Response
Lymphedema, the failure of lymphatics to drain protein-rich interstitial fluid, can arise either as a congenital pathology (primary lymphedema) or as a result of therapy or injury (secondary lymphedema). The associated lymphostasis causes persistence and accumulation of antigen, foreign material, and immune complexes in the interstitium, which can cause chronic localized inflammation (107) in addition to fibrosis (4, 108, 109). This chronic inflammation also attenuates lymphatic contraction (90), hindering lymphatic flow to the lymph node and disrupting the trafficking of lymphocytes to the lymph node. Thus, lymphedema cumulatively results in a region of local immune suppression.
Accordingly, altered immunity has been reported in primary lymphedema (110), highlighting the importance of lymphatic pathways in adaptive immune response in a variety of pathologies. Supporting this concept are observations of edematous limbs being more prone to infection (111, 112) and skin malignancies occurring at higher rates in lymphedematous limbs compared with nonlymphedematous sites in transplant patients (113). Other examples of alterations in the immune response include delayed allergic contact dermatitis in postmastectomy lymphedema (114) and delayed graft rejection following ligation- or ablation-induced disruption of lymphatic function (115, 116). In the context of islet cell transplant, inhibition of lymphangiogenesis can also prolong allograft survival (117). However, lymphatic drainage appears to play complex roles in modulating immunity because corneal (118) and kidney (119) lymphangiogenesis is associated with graft rejection, whereas in the cornea, an absence of lymphatic drainage results in graft acceptance (118).
4.2. Lymphatic Transport as an Active Regulator of Adaptive Immune Response to Immunization
In immunology, the lymphatic system has largely been studied for its cell transport roles, so the functional contribution of lymphatic transport of dendritic cells from the periphery to draining lymph nodes to immune response to immunization has been investigated. In one study, when mice with the plt mutation, which leads to a loss of CCL21 and CCL19 expression in secondary lymphoid organs, were immunized subcutaneously with ovalbumin (OVA) as a model protein antigen in complete Freund’s adjuvant, proliferation and interleukin-2 production by T cells in draining lymph nodes remained intact (120). Interestingly, the proliferative and cytokine production responses of splenic T cells from immunized plt mice were greater than those of even wild-type (WT) mice, and when splenectomized, T cell responses in the draining lymph nodes of plt mice were lost (120). This finding implies that CCR7-directed dendritic cell trafficking to lymph nodes is not required for the development of T cell immunity in response to immunization. In another approach, the emigration of peripheral tissue–resident dendritic cells to draining lymph nodes was obstructed via treatment with anti-ICAM and anti-VCAM function-blocking antibodies (121). This study found trends that seemingly conflict with the prior report, as the blocking intervention significantly attenuated CD8 T cell responses to intradermally administered modified vaccinia Ankara (MVA)-based human nucleoprotein vaccine in mice adoptively transferred with CD8 T cells that recognize the H-2Db immunodominant human influenza A/NT/60/68 virus nucleoprotein epitope ASNENMDAM (121). However, inhibition of ICAM and VCAM may disrupt splenic responses via an unspecified mechanism in addition to dendritic cell–mediated transport of vaccine antigen to draining lymph nodes via the lymphatics, irrespective of the normal homing behavior by T cells reported with this intervention.
The idea that T cell immunity induced by immunization is lymph node independent is supported by analyses in alymphoplasia (aly/aly) mice (122), which are characterized by a complete lack of lymph nodes and Peyer’s patches, as well as structural alterations in the spleen and thymus due to a point mutation in the nuclear factor κB (NF-κB)-inducing kinase (123). Bone marrow chimeras were generated using irradiated aly/+ mice injected with bone marrow cells from aly/aly donor mice (aly/aly→aly/+) in order to restrict the NF-κB-inducing kinase to the hematopoietic system and vice versa (aly/+→aly/aly) (124). In distinct contrast to immune-deficient aly/aly mice, aly/+→aly/aly chimeras mounted robust T cell–driven autoimmune responses after subcutaneous immunization with myelin oligodendrocyte glycoprotein peptide in complete Freund’s adjuvant, even in mice lacking lymph nodes (124). Interestingly, T cell responses in splenectomized (aly/+aly/aly) chimeric mice, although delayed, also remained intact, with evidence of compensatory priming of T cell supported in the liver (124), supporting the concept that T cell immunity develops independently from dedicated secondary lymphoid structures. However, despite normal T cell immunity in immunized mice lacking lymph nodes, humoral immunity was disrupted (124). Specifically, titers of immunoglobulin M (IgM) were increased in immunized mice, whereas antigen-specific immunoglobulin G (IgG) was virtually absent (124), implying significant deficiencies in class switching and suggesting differential sensitivities of T cell versus B cell immunity to extra–lymph nodal priming.
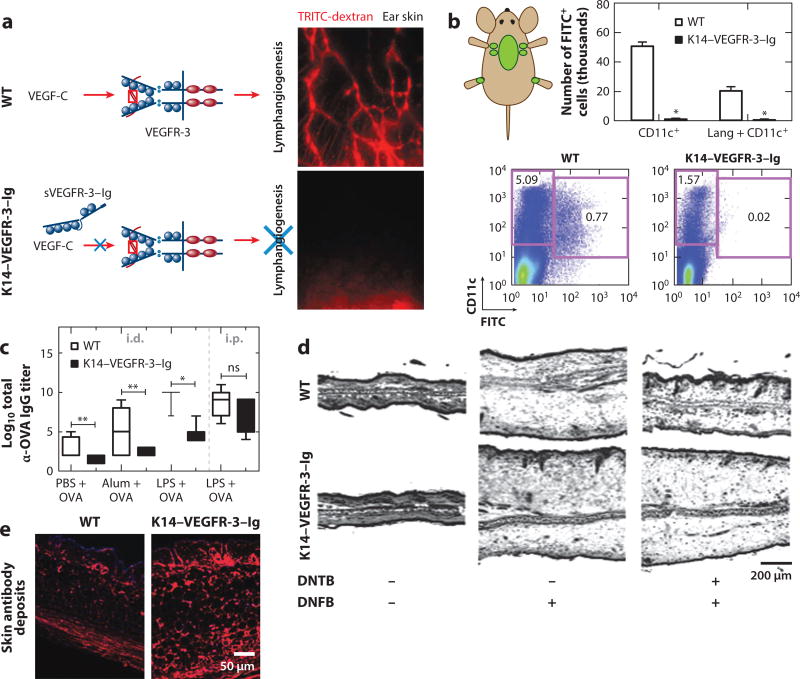
In addition to their cell transport roles, the lymphatics facilitate drainage of fluid from peripheral tissues to the lymph node, the function of which has been left largely unexplored in adaptive immune response. This is partly because mouse models of impaired lymphatic drainage require causative gene defects that may directly affect immunity, and inflammation associated with surgical or chemical disruption of lymphatic vessels also strongly affects immune responses. To overcome these limitations to evaluate the contribution of generalized lymphatic transport function to adaptive immune response to immunization, we implemented a K14–VEGFR-3–Ig mouse model that expresses soluble VEGF receptor 3 (VEGFR-3)–Ig via the keratin 14 (K14) promoter, which impairs VEGF-C signaling that results in defective lymphatic growth that is restricted to the skin (Figure 3) (125). As adults, these mice display no recognized physiological manifestations of the transgenic manipulation other than a paucity of initial dermal lymphatic capillaries and decreased fluid clearance from the skin, they survive up to 2 years, the lymph nodes are intact, and lymphatic vessels in other nondermal tissues appear normal. When we subjected the mice to passive and active antigen transport challenges from the skin, either using fluorescein isothiocyanate (FITC) painting, wherein soluble FITC is applied to the shaved dorsal skin of mice (4, 22), or intradermally injecting 1-µm-diameter fluorescent beads that are too large to be passively convected and instead require cell-mediated delivery to the lymph node (4, 5), transport of both FITC and dendritic cells in transgenic animals to draining lymph nodes was ablated (4). Thus, K14–VEGFR-3–Ig mice represent a highly suitable model to test the ramifications of lymphatic-mediated solute or cell-mediated antigen transport from peripheral skin tissues to draining lymph nodes in the development of adaptive immune response to dermal immune challenge.
Figure 3.
K14–VEGFR-3–Ig transgenic mice with (a) impaired dermal lymphangiogenesis (b) exhibit deficient antigen transport to draining lymph nodes from the skin (as measured by FITC painting). (c) In response to dermal immunization, humoral immunity is severely compromised in transgenic animals. (d ) K14–VEGFR-3–Ig mouse ears swell in response to dermal contact hypersensitivity challenge but cannot be pretolerized. H&E-stained cross sections of ears 48 h after challenge. (e) One-year-old K14–VEGFR-3–Ig mice exhibit autoimmune phenotypes, including antibody deposition (red) in the skin. Single asterisk, p < 0.05; double asterisks, p < 0.01 using Mann–Whitney. Abbreviations: DNFB, dinitrofluorobenzene; DNTB, dinitrothiocyanobenzene; FITC, fluorescein isothiocyanate; H&E, hematoxylin and eosin; i.d., intradermal; IgG, immunoglobulin G; i.p., intraperitoneal; LPS, lipopolysaccharide; ns, not significant; OVA, ovalbumin; PBS, phosphate-buffered saline; TRITC, tetramethylrhodamine; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; WT, wild type. Panel a modified from Reference 125 with permission. Panels b–e modified from Reference 4 with permission.
We compared the immune response to vaccination in transgenic K14–VEGFR-3–Ig animals with that of their WT littermates (4). To this end, we used a dermal or peritoneal immunization scheme consisting of OVA administered in conjunction with either soluble small-molecule Toll-like receptor ligand 4 lipopolysaccharide or alum, which forms an insoluble depot upon injection. Twenty-one days after immunization with a day 10 boost, T cell responses, including interferon-γ production with OVA restimulation and frequencies of MHCI OVA peptide SIINFEKL-specific T cells, in the K14–VEGFR-3–Ig mice appeared normal, despite proliferative responses by CD8+ and, more significantly, CD4+ T cells being slightly delayed. Further corroborating these observations was the finding that, when subjected to a dermal contact sensitization challenge via sensitization and recall with dinitrofluorobenzene (DNFB), T cell–mediated ear swelling was even more robust in transgenic relative to WT mice (Figure 3). Consistent with previous reports in plt mice (120), these data suggest compensatory mechanisms of T cell priming in K14–VEGFR-3–Ig mice and support the concept that T cell immunity in transgenic mice can develop independently from lymphatic-mediated transport.
When we evaluated humoral immunity in transgenic K14–VEGFR-3–Ig animals in response to OVA immunization, despite apparently normal T cell immunity in response to intradermal immunization, we found profound impairments in antigen-specific antibody titers (Figure 3) (4). Specifically, K14–VEGFR-3–Ig animals immunized with OVA and lipopolysaccharide exhibited serum IgG titers that were five or more orders of magnitude lower than those of WT animals. This deficiency was not the result of impairments of B cell function in transgenic animals per se, given that responses to intraperitoneal immunization were normal and B cells isolated from the lymph nodes and spleens of transgenic mice responded similarly to those from WT mice when stimulated ex vivo with lipopolysaccharide in terms of maturation as well as interferon-γ and IgM production. However, delays in CD4+ T cell priming, which plays a well-established role in mature B cell immune responses (126), may contribute. Conversely, the decreased ability of B cells, which normally have inhibitory roles in contact hypersensitivity reactions via inhibition of the elicitation phase by lymph node–resident B cells (127), to respond to intradermal immunization in the K14– VEGFR-3–Ig mice might also contribute to enhanced swelling in transgenic mice. These findings complement previous reports demonstrating the delivery of lymph-borne antigen to lymph node– resident B cells either directly through the conduits in the case of smaller antigens (18) or by transfer from subcapsular macrophages for larger antigens (19). The disorganized lymph node stroma and B cell follicles in transgenic mice (Figure 1) may also contribute to their impaired humoral immune responses to dermal vaccination, given recent reports of B cell priming and affinity maturation in response to subcutaneous challenge requiring the specialized topography of the lymph node (122). Cumulatively, these results indicate an essential role for lymphatic drainage function from the periphery in humoral immunity.
4.3. Lymphatic Transport in the Regulation of Immune Tolerance and Autoimmunity
Because the lymph node is an important site for the maintenance of self-tolerance (128), investigators have studied the contribution of drainage of self-antigen to draining lymph nodes from peripheral tissues via lymphatics to local immune tolerance. In early research, studies implementing a skin transplantation model where the blood, but not lymphatic, vasculature was connected to the surrounding tissue were used to investigate immune response to contact sensitization (1, 2). The skin sensitizer dansyl chloride, when injected in complete Freund’s adjuvant into alymphatic skin islands of guinea pigs, failed to induce tolerance; application to intact skin had the opposite effect. This finding suggests that lymphatics have a role in the development of immunologic tolerance. We also investigated tolerance to contact hypersensitivity (4) in K14–VEGFR-3–Ig animals, a model that is not complicated by surgically induced tissue damage and inflammation, which could also affect local immunity. In WT but not K14–VEGFR-3–Ig mice, pretreatment with dinitrothiocyanobenzene (DNTB, a tolerizing agent to DNFB) prevented DNFB-induced ear swelling (Figure 3), suggesting that transgenic mice are deficient in mechanisms of acquired tolerance against skin-encountered antigen. These corroborating results indicate that lymphatic transport to draining lymph nodes is essential to acquired peripheral tolerance.
We also explored the effect of chronically impaired lymphatic transport of endogenous self-antigen on autoimmune outcomes in K14–VEGFR-3–Ig compared with WT animals. Several hallmarks of autoimmunity in 1-year-old K14–VEGFR-3–Ig mice were observed, including indications of disrupted B cell Ig class switching, elevated serum titers of anti-double-stranded DNA antibodies, antibody deposits in the skin, and an increased frequency of mice with skin-reactive serum antibodies (Figure 3). B cell expression levels of B cell coreceptor CD19, which complexes with the antigen receptor of B cells to increase sensitivity to antigen-specific stimulation and whose increased expression is associated with increased autoantibody production (129) and autoimmunity (130), were also increased in skin-draining but not other lymph nodes or spleens of transgenic but not WT mice (4).
Given the emerging appreciation for the contribution of lymph node organization to peripheral tolerance, particularly with regard to the positioning of B cells and trafficking of T cells (128), hallmarks of autoimmunity observed in K14–VEGFR-3–Ig mice may also be related to the deficient lymphatic transport–related structural remodeling of skin-draining lymph nodes. For example, autoimmune phenotypes develop in mice lacking either CCR7 or CXCR5 (35, 131), the receptors for CCL21 and CXCL13, important in the segmentation of the T and B cell zones of the lymph node, respectively. The disruption of lymph node CCL21 and CXCL13 distributions and, as a result, tissue architecture and cellular trafficking that enhance S. typhimurium virulence were previously proposed as a mechanism of immune suppression used by pathogens that infect lymphoid tissue (34). Indeed, disruptions in the CCL21 and CXCR13 distributions resulting in B cell follicle disorganization restricted to the skin-draining lymph nodes of K14–VEGFR-3–Ig mice are associated with impaired dermal lymphatic transport (Figure 1). Furthermore, the upregulation of coreceptor CD19 expression and collapse of HEVs observed in these animals correspond directly to these transport deficiencies. Lower frequencies of Treg cells in skin-draining lymph nodes were also found, suggesting deficiencies in Treg cell homing to the skin-draining lymph nodes. Taken together with the increased CD19 expression by cells in the lymph nodes of K14–VEGFR-3–Ig mice, these data suggest that the B cell regulatory balance normally maintained by lymph nodes (122, 128) is dysregulated in the transgenic mice. Thus, in addition to enabling lymphborne antigen to flush through the B cell zone, coming in direct contact with B cells minutes after injection (18), lymphatic transport–directed lymph node structural organization represents a putative mechanism that supports the lymph node’s role in maintenance of immunological tolerance.
To investigate the role of lymphatics in local immune tolerance in a reverse model of heightened lymphangiogenesis, given that tumor expression of lymphangiogenic growth factor VEGF-C increases lymph drainage to the draining lymph node (5, 132), we explored the effect of tumor VEGF-C expression on preexisting immunity (5). Ten days prior to implantation of OVA-expressing B16F10 melanomas, we vaccinated mice with OVA and lipopolysaccharide, a treatment regimen that strongly inhibited WT tumor growth. Surprisingly, this immunization scheme had no effect on the growth of VEGF-C-overexpressing (lymphangiogenic) B16F10 melanomas expressing OVA. SIINFEKL-specific CD8+ T cell frequencies in both the tumor and tumor-draining lymph nodes (TDLNs) were also substantially decreased in animals bearing VEGF-C-overexpressing B16F10 melanomas, even while SIINFEKL-specific CD8+ T cells were circulating at similar levels in all vaccinated mice. Treatment with VEGFR-3-neutralizing antibody during tumor growth furthermore restored susceptibility to vaccination and increased the frequencies of SIINFEKL-specific CD8+ T cells in both the tumor and the TDLNs. The functional activation of tumor antigen–specific CD8+ T cells in TDLNs was also impaired in VEGF-C-overexpressing (lymphangiogenic) melanoma–bearing animals, as tumor infiltrates expressed less interferon-γ and lower levels of activation markers CD25 and CD69. The infiltrates were also more apoptotic compared with WT tumor infiltrates (5). These data demonstrate the capacity of VEGF-C-induced tumor lymphangiogenesis to suppress local immunity.
5. LYMPHATIC-TARGETED DRUG DELIVERY FOR LYMPH NODE IMMUNOMODULATION
Lymphatics and lymph nodes have emerged as therapeutic targets because, in addition to being both a frequent site of cancer metastasis and susceptible to infection by certain classes of pathogen, they play a central role in the regulation of adaptive immune response. Insights into the basic science of lymphatic immune physiology have thus provided important guidelines for the applicability and implementation of such schemes. Although targeting agents to lymphatics has been suggested (133), we focus in particular on therapeutic delivery to lymph nodes and highlight a few notable examples in cancer therapy (8, 10, 11, 134, 135), therapeutic immune suppression for transplantation and treatment of autoimmune diabetes (136, 137), and vaccines for infectious disease (135, 138, 139). Engineering design to achieve such targeting is not discussed here but has been reviewed in depth elsewhere (9, 30).
5.1. Cancer
Spontaneous development of antitumor immunity is associated with improved clinical outcome (140), suggesting that immunotherapy has the potential to increase cancer survival. Therefore, investigators have explored the benefit of lymph node targeting of subunit vaccines for cancer to achieve optimal immunotherapeutic efficacy (10, 11, 134, 135). However, the protective effect of vaccination can be dampened by the active suppression of antitumor immunity within regional TDLNs, despite the presence of functional effector T cells in the systemic circulation (141). Dendritic cell costimulation of T cells within TDLNs is inhibited (142), leading to less-effective priming of tumor antigen–specific cytotoxic T and T helper cells (143), even in the presence of highly immunogenic tumor antigens (144–147). Indeed, TDLNs often display an altered immunological microenvironment relative to non-tumor-associated lymph nodes, suggesting active regulation of the TDLN micromilieu by the tumor, whereas increased frequencies of TDLN-resident Treg cells predict poor patient outcome (148). Mitigating immune regulation locally within the tumor and TDLNs therefore represents a critical hurdle in effective management of cancer via immunotherapy.
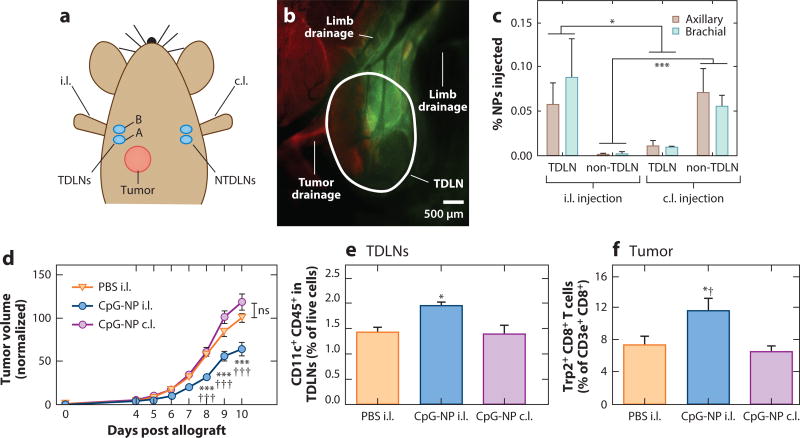
In support of the concept that immunomodulation within the TDLNs promotes antitumor immunity against endogenously produced tumor antigen draining via lymphatics to TDLN, we recently developed a proof-of-principle model to explore whether delivery of immunomodulatory agents to immune cells in TDLNs might enhance antitumor immune responses and reduce tumor burden. In this approach, we sought to induce dendritic cell maturation within TDLNs via delivery of CpG oligodeoxynucleotide (CpG), a Toll-like receptor 9 agonist that demonstrates strong T helper 1 potentiating activity (149). This was accomplished by leveraging a nanoparticle (NP) technology that, by virtue of its size and formulation [reviewed elsewhere (9)], accumulates rapidly and at appreciable concentrations in draining lymph nodes after injection in the dermis (8, 150, 151). Using the implantable B16F10 melanoma model, we compared treatment of tumor-bearing mice with NP conjugated to CpG (CpG-NP) in the ipsilateral limb (treating the TDLNs) relative to treatment with CpG-NP in the contralateral limb (treating the non-TDLNs). In response to daily treatment of the TDLNs with CpG-NP after tumor establishment, tumor growth was significantly reduced (Figure 4), whereas treatment with CpG-NP in the contralateral limb, plain NP, and free CpG in the ipsilateral limb had no effect. This finding suggests that the NP-mediated, targeted delivery of CpG to the TDLNs, rather than systemic immune activation, enhanced antitumor immune responses against the tumor. In support of the concept that reduced tumor growth resulted from CpG-NP’s immune modulatory activity within the TDLNs, strong changes in immune cell repertoires, including increased frequencies of mature dendritic cells within the TDLNs and increases in tumor antigen–specific CD8+ T cells in the tumor, were observed (Figure 4).
Figure 4.
Lymph node–targeted adjuvant immunotherapy slows tumor growth and changes the infiltrating lymphocyte profile in both the tumor and tumor-draining lymph nodes (TDLNs). (a) Location of tumor implantation with 5 × 10 B16-F10 cells relative to nanoparticle (NP) injection. (b,c) Lymphatic-draining NPs accumulate in the TDLNs after injection ipsilateral (i.l.) but not contralateral (c.l.) to the tumor, as measured by (c) a fluorescence plate reader or (b) fluorescence microscopy. Dextran, red; AF488-NP, green. (d) Daily CpG-NP treatment of the lymph node i.l. but not c.l. to a B16-F10 tumor, starting from days 4–9 post implantation, slows tumor growth and reshapes the immune milieu within (e) the TDLNs and (f) the tumor. Single asterisk, p < 0.05; triple asterisks, p < 0.001 with respect to the phosphate-buffered saline (PBS) control; single dagger, p < 0.05; triple daggers; p < 0.001 with respect to the same treatment in the c.l. limb. Abbreviations: ns, not significant; NTDLN, non-tumor-draining lymph node. Modified from Reference 8 with permission.
Implementing the same lymph node–targeting drug delivery technology, investigators evaluated the efficacy of TDLN- versus non-TDLN-targeted cancer vaccination in two tumor models in which NP-conjugated tumor antigen was codelivered with CpG-NP as adjuvant (10). Interestingly, although the TDLNs exhibited an immune-suppressed state, corroborating previous reports, TDLN-targeted vaccination outperformed vaccines delivered to non-TDLNs in terms of induction of potent cytotoxic CD8+ T cell responses both locally and systemically, as well as reducing the frequency of immune-suppressive myeloid-derived suppressor and Treg cells (10). Accordingly, TDLN targeting was associated with delayed disease progression induced by tumor vaccination (10). These findings underscore the potential for targeted immunotherapy to substantially improve efficacy in the treatment of cancer.
5.2. Transplantation and Autoimmune Diabetes
The lymph node’s role in the regulation of immunity versus tolerance has made it an attractive target for immunotherapeutic interventions aiming to achieve engraftment of transplanted tissues because T and dendritic cells participate in the rejection and acceptance of allotransplants (152, 153). To this end, the immune-suppressive drugs rapamycin and tacrolimus were encapsulated into micelles that, after injection in the dermis, resulted in accumulation in draining lymph nodes (136). With daily injection post transplant, MHC-mismatched skin allograft transplant survival was prolonged (136). More recently, mouse lymph nodes were shown to support the engraftment, growth, and function of directly injected hepatocytes, thymuses, and pancreatic islets (154). Lymph nodes thus represent attractive tissues to target both immunomodulatory agents and cells/tissues to prolong graft survival or improve graft function.
Emerging evidence also reinforces the potential for immunomodulation within lymph nodes regional to the pancreas in the treatment of autoimmune diabetes. For example, oral insulin–induced protection against autoimmune diabetes has been associated with the presence of interleukin-4-producing T cells of a helper 2 type in not only the pancreas but also the pancreatic lymph nodes (155). Moreover, treatment of nonobese diabetic mice with complete Freund’s adjuvant and exendin-4 as immunotherapy reversed new-onset diabetes by ~90%, corresponding with an increase in the frequency of Treg cells in pancreatic lymph nodes (156). Immunotherapy via phosphatidylserine liposomes, which accumulated in the pancreatic lymph nodes and pancreas after intraperitoneal administration, was capable of inducing tolerogenic dendritic cells that impaired the proliferation of autoreactive T cells in vitro, expanding antigen-specific CD4+ T cells in vivo and decreasing diabetes incidence (137). Immunomodulation within pancreatic lymph nodes thus appears to have significant potential to ameliorate autoimmune diabetes immunotherapy.
5.3. Infectious Disease
Recent observations of lymphatic transport contributing to the robust development of humoral immunity to immunization (4) suggest that lymph node–targeted immunotherapeutic approaches are suitable for infectious disease applications where neutralizing antibodies are desired. To this end, several approaches have illustrated the potent efficacy of lymph node targeting in boosting immunogenicity. Phage presentation of lymph node–homing peptides administered intravenously dramatically increased the humoral immune response, as indicated by increased antiphage serum antibody titers (157). Direct injection of OVA and Toll-like receptor 3 ligand poly(inosinic:cytidylic acid)–encapsulating microparticles into lymph nodes also significantly enhanced the resulting antibody titers relative to intramuscular administration (158). More recent research exploring the influence of molded particle size, shape, and aspect ratio on dendritic cell uptake and lymph node delivery and persistence of antigen post administration demonstrated the benefit of particle-mediated antigen conjugation in OVA immunization schemes, resulting in significantly improved OVA-specific antibody titers compared with free antigen either alone or in its nonconjugated form (159).
T cell immunity induced by vaccination has also been improved via targeted lymph node delivery. For example, in addition to boosting humoral immune response, intranodal injection of OVA and poly(inosinic:cytidylic acid)–encapsulating microparticles significantly increased the frequency of and cytokine production by SIINFEKL-specific CD8+ T cells induced by immunization (158). Codelivery of CpG and various MHCI peptides to draining lymph nodes as albumin-hitchhiking lipid-modified amphiphiles also demonstrated similar trends (11). Similar to lymph node–targeted delivery of protein antigen via conjugation to micelles boosting induced cellular immune responses post administration in the skin (160), NP conjugation–mediated increases in OVA delivery to lymph nodes and uptake by resident dendritic cells after intrapulmonary immunization with CpG resulted in dramatically enhanced antigen-specific CD8+ and CD4+ T cell immunity and CD8+ T cell memory (5). These increases in splenic and lung effector responses induced by lymph node–targeted vaccination were capable of protecting animals from infection with a recombinant strain of influenza H1N1 PR8, which expresses SIINFEKL in the neuraminidase stalk (5). The potency of both systemic and localized T helper 1 immunity induced by intrapulmonary immunization with CpG coadministered with the tuberculosis antigen Ag85B was also significantly improved by lymph node targeting via NP conjugation and enhanced the protective effect of Ag86B immunization against aerosol Mycobacterium tuberculosis challenge (10). Similarly, antigen and dual Toll-like receptor ligand poly(inosinic:cytidylic acid)– and monophosphoryl lipid A–loaded interbilayer cross-linked multilamellar vesicles that increased payload delivery to lymph nodes after intrapulmonary immunization to induce effector memory mucosal immunity conferred protection from infection with simian immunodeficiency virus (SIV) gag–expressing vaccinia virus (135). Lymph node–targeted vaccination schemes thus appear promising in providing superior protection against infectious disease.
6. CONCLUSIONS AND FUTURE PERSPECTIVES
As the nascent field of immunoengineering continues to grow, the challenge in understanding and appreciating the physiology of the immune system remains significant. Whereas single-factor mechanisms at the genetic, molecular, and cellular levels have provided and will continue to provide significant insight, the interplay of these factors in a temporally and locoregionally regulated fashion is often overlooked. However, it is increasingly clear that immune response and regulation depend on the coordination of intercellular signaling events that transpire over diverse time and length scales and are significantly regulated by lymphatics. An interdisciplinary approach coupling molecular, cellular, and tissue immunology with lymphatic transport considerations will continue to provide insight into complex immunological processes and systems as well as design criteria for advanced drug delivery and tissue engineering approaches in immunoengineering.
Acknowledgments
The writing of this review was supported by National Science Foundation Award 1342194; a grant from the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology; a Cell and Tissue Engineering National Institutes of Health Biotechnology training grant (T32 GM-008433); and Public Health Services grant UL1TR000454 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Advancing Translational Sciences.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any memberships, affiliations, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Friedlaender MH, Baer H. Immunologic tolerance: role of the regional lymph node. Science. 1972;176:312–14. doi: 10.1126/science.176.4032.312. [DOI] [PubMed] [Google Scholar]
- 2.Friedlaender MH, Chisari FV, Baer H. The role of the inflammatory response of skin and lymph nodes in the induction of sensitization to simple chemicals. J. Immunol. 1973;111:164–70. [PubMed] [Google Scholar]
- 3.Silberberg-Sinakin I, Thorbecke GJ, Baer RL, Rosenthal SA, Berezowsky V. Antigen-bearing Langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell. Immunol. 1976;25:137–51. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J. Immunol. 2012;189:2181–90. doi: 10.4049/jimmunol.1103545. Demonstrates in vivo the contribution of lymphatic transport to humoral immunity and acquired tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1:191–99. doi: 10.1016/j.celrep.2012.01.005. Shows that melanoma VEGF-C overexpression causes tumor- and draining lymph node–localized immune suppression despite systemic antitumor immunity. [DOI] [PubMed] [Google Scholar]
- 6.Mebius RE, Streeter PR, Brevé J, Duijvestijn AM, Kraal G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell Biol. 1991;115:85–95. doi: 10.1083/jcb.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohner NA, McClain J, Tuell SL, Warner A, Smith B, et al. Lymph node biophysical remodeling is associated with melanoma lymphatic drainage. FASEB J. 2015;29:4512–22. doi: 10.1096/fj.15-274761. Demonstrates that melanoma-draining lymph nodes exhibit elevated collagen and hyaluronic acid levels and increased stiffness and viscoelasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35:814–24. doi: 10.1016/j.biomaterials.2013.10.003. Shows that tumor-draining lymph node adjuvant targeting improves the efficacy of immunotherapy. [DOI] [PubMed] [Google Scholar]
- 9.Thomas SN, Schudel A. Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr. Opin. Chem. Eng. 2015;7:65–74. doi: 10.1016/j.coche.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeanbart L, Ballester M, de Titta A, Corthesy P, Romero P, et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol. Res. 2014;2:436–47. doi: 10.1158/2326-6066.CIR-14-0019-T. Shows that the efficacy of tumor vaccination is improved by targeting to lymph nodes draining solid tumors. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, et al. Structure-based programming of lymphnode targeting in molecular vaccines. Nature. 2014;507:519–22. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherwood L. Human Physiology: From Cells to Systems. San Francisco: Cengage Learning; 2014. [Google Scholar]
- 13.Rizwan A, Bulte C, Kalaichelvan A, Cheng M, Krishnamachary B, et al. Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Sci. Rep. 2015;5:10002. doi: 10.1038/srep10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ondondo B, Jones E, Hindley J, Cutting S, Smart K, et al. Progression of carcinogen-induced fibrosarcomas is associated with the accumulation of naive CD4+ T cells via blood vessels and lymphatics. Int. J. Cancer. 2014;134:2156–67. doi: 10.1002/ijc.28556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negrini D, Moriondo A, Mukenge S. Transmural pressure during cardiogenic oscillations in rodent diaphragmatic lymphatic vessels. Lymphat. Res. Biol. 2004;2:69–81. doi: 10.1089/lrb.2004.2.69. [DOI] [PubMed] [Google Scholar]
- 16.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012;92:1005–60. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Shibata M, Kamiya A. Mechanism of macromolecule concentration in collecting lymphatics in rat mesentery. Microvasc. Res. 1997;54:193–205. doi: 10.1006/mvre.1997.2043. [DOI] [PubMed] [Google Scholar]
- 18.Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–76. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–14. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 20.Haessler U, Pisano M, Wu M, Swartz MA. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. PNAS. 2011;108:5614–19. doi: 10.1073/pnas.1014920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomura M, Hata A, Matsuoka S, Shand FH, Nakanishi Y, et al. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci. Rep. 2014;4:6030. doi: 10.1038/srep06030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005;5:617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 23.Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J. Exp. Med. 2014;211:1153–66. doi: 10.1084/jem.20132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009;206:2937–46. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clement CC, Cannizzo ES, Nastke M-D, Sahu R, Olszewski W, et al. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PLOS ONE. 2010;5:e9863. doi: 10.1371/journal.pone.0009863. Demonstrates that lymph is enriched in self-antigen generated by physiological tissue catabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 2003;4:733–39. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 28.Gratz IK, Campbell DJ. Organ-specific and memory treg cells: specificity, development, function, and maintenance. Front. Immunol. 2014;5:333. doi: 10.3389/fimmu.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–60. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 31.Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44:541–49. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. Shows that lymph-draining joints of rheumatoid arthritis patients is highly enriched in immunomodulatory cytokines and chemokines. [DOI] [PubMed] [Google Scholar]
- 32.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 34.Kunder CA, St John AL, Li G, Leong KW, Berwin B, et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 2009;206:2455–67. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davalos-Misslitz AC, Rieckenberg J, Willenzon S, Worbs T, Kremmer E, et al. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur. J. Immunol. 2007;37:613–22. doi: 10.1002/eji.200636656. [DOI] [PubMed] [Google Scholar]
- 36.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 2009;9:618–29. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunol. 2006;7:344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 38.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 2009;183:4273–83. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- 40.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–67. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acton SE, Farrugia AJ, Astarita JL, Mourão-Sá D, Jenkins RP, et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 2014;514:498–502. doi: 10.1038/nature13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martín-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 2003;198:615–21. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J. Exp. Med. 2006;203:1903–13. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–38. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 46.Shieh AC, Rozansky HA, Hinz B, Swartz MA. Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res. 2011;71:790–800. doi: 10.1158/0008-5472.CAN-10-1513. [DOI] [PubMed] [Google Scholar]
- 47.Barcus CE, Keely PJ, Eliceiri KW, Schuler LA. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J. Biol. Chem. 2013;288:12722–32. doi: 10.1074/jbc.M112.447631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Insana MF, Pellot-Barakat C, Sridhar M, Lindfors KK. Viscoelastic imaging of breast tumor microenvironment with ultrasound. J. Mammary Gland Biol. Neoplasia. 2004;9:393–404. doi: 10.1007/s10911-004-1409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutmann R, Leunig M, Feyh J, Goetz AE, Messmer K, et al. Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Res. 1992;52:1993–95. [PubMed] [Google Scholar]
- 50.Nathanson SD, Mahan M. Sentinel lymph node pressure in breast cancer. Ann. Surg. Oncol. 2011;18:3791–96. doi: 10.1245/s10434-011-1796-y. [DOI] [PubMed] [Google Scholar]
- 51.Nathanson SD, Shah R, Chitale DA, Mahan M. Intraoperative clinical assessment and pressure measurements of sentinel lymph nodes in breast cancer. Ann. Surg. Oncol. 2014;21:81–85. doi: 10.1245/s10434-013-3249-2. [DOI] [PubMed] [Google Scholar]
- 52.O’Connor RS, Hao X, Shen K, Bashour K, Akimova T, et al. Substrate rigidity regulates human T cell activation and proliferation. J. Immunol. 2012;189:1330–39. doi: 10.4049/jimmunol.1102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abreu EL, Palmer MP, Murray MM. Collagen density significantly affects the functional properties of an engineered provisional scaffold. J. Biomed. Mater. Res. A. 2010;93:150–57. doi: 10.1002/jbm.a.32508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang S-F, Chang CA, Lee D-Y, Lee P-L, Yeh Y-M, et al. Tumor cell cycle arrest induced by shear stress: roles of integrins and Smad. PNAS. 2008;105:3927–32. doi: 10.1073/pnas.0712353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent T, Jourdan M, Sy MS, Klein B, Mechti N. Hyaluronic acid induces survival and proliferation of human myeloma cells through an interleukin-6-mediated pathway involving the phosphorylation of retinoblastoma protein. J. Biol. Chem. 2001;276:14728–36. doi: 10.1074/jbc.M003965200. [DOI] [PubMed] [Google Scholar]
- 56.Rafi A, Nagarkatti M, Nagarkatti PS. Hyaluronate-CD44 interactions can induce murine B-cell activation. Blood. 1997;89:2901–8. [PubMed] [Google Scholar]
- 57.Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat. Commun. 2014;5:4619. doi: 10.1038/ncomms5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tilghman RW, Cowan CR, Mih JD, Koryakina Y, Gioeli D, et al. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLOS ONE. 2010;5:e12905. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hind LE, Dembo M, Hammer DA. Macrophage motility is driven by frontal-towing with a force magnitude dependent on substrate stiffness. Integr. Biol. Quant. Biosci. Nano Macro. 2015;7:447–53. doi: 10.1039/c4ib00260a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, et al. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114:1387–95. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–35. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang JH, Cárdenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, et al. Requirements for T lymphocyte migration in explanted lymph nodes. J. Immunol. 2007;178:7747–55. doi: 10.4049/jimmunol.178.12.7747. [DOI] [PubMed] [Google Scholar]
- 63.Kaiser A, Donnadieu E, Abastado JP, Trautmann A, Nardin A. CC chemokine ligand 19 secreted by mature dendritic cells increases naive T cell scanning behavior and their response to rare cognate antigen. J. Immunol. 2005;175:2349–56. doi: 10.4049/jimmunol.175.4.2349. [DOI] [PubMed] [Google Scholar]
- 64.Haessler U, Pisano M, Wu M, Swartz MA. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. PNAS. 2011;108:5614–19. doi: 10.1073/pnas.1014920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polacheck WJ, Charest JL, Kamm RD. Interstitial flow influences direction of tumor cell migration through competing mechanisms. PNAS. 2011;108:11115–20. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi ZD, Ji XY, Qazi H, Tarbell JM. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1225–34. doi: 10.1152/ajpheart.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galimberti M, Tolić-Nørrelykke IM, Favillini R, Mercatelli R, Annunziato F, et al. Hypergravity speeds up the development of T-lymphocyte motility. Eur. Biophys. J. 2006;35:393–400. doi: 10.1007/s00249-006-0046-x. [DOI] [PubMed] [Google Scholar]
- 68.Tien J, Truslow JG, Nelson CM. Modulation of invasive phenotype by interstitial pressure-driven convection in aggregates of human breast cancer cells. PLOS ONE. 2012;7:e45191. doi: 10.1371/journal.pone.0045191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 70.Marchesi VT, Gowans JL. The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscope study. Proc. R. Soc. B. 1964;159:283–90. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 71.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–60. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 72.Kansas GS, Ley K, Munro JM, Tedder TF. Regulation of leukocyte rolling and adhesion to high endothelial venules through the cytoplasmic domain of L-selectin. J. Exp. Med. 1993;177:833–38. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subramanian H, Grailer JJ, Ohlrich KC, Rymaszewski AL, Loppnow JJ, et al. Signaling through L-selectin mediates enhanced chemotaxis of lymphocyte subsets to secondary lymphoid tissue chemokine. J. Immunol. 2012;188:3223–36. doi: 10.4049/jimmunol.1101032. [DOI] [PubMed] [Google Scholar]
- 74.Mebius RE, Streeter PR, Brevé J, Duijvestijn AM, Kraal G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell Biol. 1991;115:85–95. doi: 10.1083/jcb.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–55. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- 76.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 77.Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3 cells to colonize lymph nodes. PNAS. 1996;93:11019–24. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boscacci RT, Pfeiffer F, Gollmer K, Sevilla AI, Martin AM, et al. Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing. Blood. 2010;116:915–25. doi: 10.1182/blood-2009-11-254334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stein JV, Rot A, Luo Y, Narasimhaswamy M, Nakano H, et al. The CC chemokine thymus- derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 2000;191:61–76. doi: 10.1084/jem.191.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okada T, Ngo VN, Ekland EH, Förster R, Lipp M, et al. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J. Exp. Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, et al. The CCR7 ligand ELC (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 2001;193:1105–12. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shamri R, Grabovsky V, Gauguet J-M, Feigelson S, Manevich E, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 83.Chen Q, Fisher DT, Clancy KA, Gauguet J-MM, Wang W-C, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat. Immunol. 2006;7:1299–308. doi: 10.1038/ni1406. [DOI] [PubMed] [Google Scholar]
- 84.Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int. Immunol. 2008;20:1483–87. doi: 10.1093/intimm/dxn110. [DOI] [PubMed] [Google Scholar]
- 85.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 2000;192:1425–40. doi: 10.1084/jem.192.10.1425. Demonstrates a transport role of lymph node conduits and their molecular size-dependent profiles of exclusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu TT, Browning JL. Role of the lymphotoxin/LIGHT system in the development and maintenance of reticular networks and vasculature in lymphoid tissues. Front. Immunol. 2014;5:47. doi: 10.3389/fimmu.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 2001;194:1361–74. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar V, Chyou S, Stein JV, Lu TT. Optical projection tomography reveals dynamics of HEV growth after immunization with protein plus CFA and features shared with HEVs in acute autoinflammatory lymphadenopathy. Front. Immunol. 2012;3:282. doi: 10.3389/fimmu.2012.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar V, Scandella E, Danuser R, Onder L, Nitschké M, et al. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell-lymphotoxin-dependent pathway. Blood. 2010;115:4725–33. doi: 10.1182/blood-2009-10-250118. [DOI] [PubMed] [Google Scholar]
- 90.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J. Immunol. 2006;177:3369–79. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 91.Shrestha B, Hashiguchi T, Ito T, Miura N, Takenouchi K, et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J. Immunol. 2010;184:4819–26. doi: 10.4049/jimmunol.0903063. [DOI] [PubMed] [Google Scholar]
- 92.Chyou S, Benahmed F, Chen J, Kumar V, Tian S, et al. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J. Immunol. 2011;187:5558–67. doi: 10.4049/jimmunol.1101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tong Z, Cheung LS-L, Stebe KJ, Konstantopoulos K. Selectin-mediated adhesion in shear flow using micropatterned substrates: Multiple-bond interactions govern the critical length for cell binding. Integr. Biol. 2012;4:847–56. doi: 10.1039/c2ib20036h. [DOI] [PubMed] [Google Scholar]
- 94.Atarashi K, Hirata T, Matsumoto M, Kanemitsu N, Miyasaka M. Rolling of Th1 cells via P-selectin glycoprotein ligand 1 stimulates LFA-1-mediated cell binding to ICAM-1. J. Immunol. 2005;174:1424–32. doi: 10.4049/jimmunol.174.3.1424. [DOI] [PubMed] [Google Scholar]
- 95.Oh J, Edwards EE, McClatchey PM, Thomas SN. Analytical cell adhesion chromatography reveals impaired persistence of metastatic cell rolling adhesion to P-selectin. J. Cell Sci. 2015;128:3731–43. doi: 10.1242/jcs.166439. Shows that the persistence of selectin-mediated rolling adhesion is cell subtype dependent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hell. J. Cardiol. 2005;46:9–15. [PubMed] [Google Scholar]
- 97.Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature. 1996;379:266–69. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- 98.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat. Immunol. 2007;8:1076–85. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 99.Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012;120:4772–82. doi: 10.1182/blood-2012-04-427013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat. Commun. 2015;6:6771. doi: 10.1038/ncomms7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, et al. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J. Immunol. 2014;192:5002–11. doi: 10.4049/jimmunol.1302492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol. Res. 2014;2:701–7. doi: 10.1158/2326-6066.CIR-14-0115. [DOI] [PubMed] [Google Scholar]
- 103.Fletcher AL, Malhotra D, Turley SJ. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol. 2011;32:12–18. doi: 10.1016/j.it.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Investig. 2014;124:943–52. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirosue S, Dubrot J. Modes of antigen presentation by lymph node stromal cells and their immunological implications. Front. Immunol. 2015;6:446. doi: 10.3389/fimmu.2015.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat. Commun. 2014;5:3989. doi: 10.1038/ncomms4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ridner SH. Pathophysiology of lymphedema. Semin. Oncol. Nurs. 2013;29:4–11. doi: 10.1016/j.soncn.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 108.Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am. J. Pathol. 2010;176:1122–29. doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghanta S, Cuzzone DA, Torrisi JS, Albano NJ, Joseph WJ, et al. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H1065–77. doi: 10.1152/ajpheart.00598.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baird JB, Charles JL, Streit TG, Roberts JM, Addiss DG, Lammie PJ. Reactivity to bacterial, fungal, and parasite antigens in patients with lymphedema and elephantiasis. Am. J. Trop. Med. Hyg. 2002;66:163–69. doi: 10.4269/ajtmh.2002.66.163. [DOI] [PubMed] [Google Scholar]
- 111.Rossy K. Lymphedema. Medscape. 2013 Jan 8; http://emedicine.medscape.com/article/1087313-overview.
- 112.Carlson JA. Lymphedema and subclinical lymphostasis (microlymphedema) facilitate cutaneous infection, inflammatory dermatoses, and neoplasia: a locus minoris resistentiae. Clin. Dermatol. 2014;32:599–615. doi: 10.1016/j.clindermatol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 113.Bordea C, Wojnarowska F, Morris PJ. Multiple cutaneous malignancies arising in limbs with signs of lymphatic insufficiency in transplant patients. Br. J. Plast. Surg. 1999;52:619–22. doi: 10.1054/bjps.1999.3200. [DOI] [PubMed] [Google Scholar]
- 114.Mallon E, Powell S, Mortimer P, Ryan TJ. Evidence for altered cell-mediated immunity in post-mastectomy lymphoedema. Br. J. Dermatol. 1997;137:928–33. [PubMed] [Google Scholar]
- 115.Stark RB, Dwyer EM, Deforest M. Effect of surgical ablation of regional lymph nodes on survival of skin homografts. Ann. N. Y. Acad. Sci. 1960;87:140–48. [Google Scholar]
- 116.Lambert PB, Frank HA, Bellman S, Farnsworth D. The role of the lymph trunks in the response to allogeneic skin transplants. Transplantation. 1965;3:62–73. doi: 10.1097/00007890-196501000-00009. [DOI] [PubMed] [Google Scholar]
- 117.Yin N, Zhang N, Xu J, Shi Q, Ding Y, Bromberg JS. Targeting lymphangiogenesis after islet transplantation prolongs islet allograft survival. Transplantation. 2011;92:25–30. doi: 10.1097/TP.0b013e31821d2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, et al. Alternatively spliced vascular endothelial growth factor receptor 2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009;15:1023–30. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 2004;15:603–12. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 120.Mori S, Nakano H, Aritomi K, Wang CR, Gunn MD, Kakiuchi T. Mice lacking expression of the chemokines Ccl21-ser and Ccl19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 2001;193:207–18. doi: 10.1084/jem.193.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teoh D, Johnson LA, Hanke T, McMichael AJ, Jackson DG. Blocking development of a CD8+ T cell response by targeting lymphatic recruitment of APC. J. Immunol. 2009;182:2425–31. doi: 10.4049/jimmunol.0803661. [DOI] [PubMed] [Google Scholar]
- 122.Hofmann J, Greter M, Du Pasquier L, Becher B. B cells need a proper house, whereas T cells are happy in a cave: the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol. 2010;31:144–53. doi: 10.1016/j.it.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 123.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding NF-κB-inducing kinase. Nat. Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 124.Greter M, Hofmann J, Becher B. Neo-lymphoid aggregates in the adult liver can initiate potent cell-mediated immunity. PLOS Biol. 2009;7:e1000109. doi: 10.1371/journal.pbio.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–73. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 127.Watanabe R, Fujimoto M, Ishiura N, Kuwano Y, Nakashima H, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am. J. Pathol. 2007;171:560–70. doi: 10.2353/ajpath.2007.061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 129.Saito E, Fujimoto M, Hasegawa M, Komura K, Hamaguchi Y, et al. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J. Clin. Investig. 2002;109:1453–62. doi: 10.1172/JCI15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J. Immunol. 2000;165:6635–43. doi: 10.4049/jimmunol.165.11.6635. [DOI] [PubMed] [Google Scholar]
- 131.Wengner AM, Höpken UE, Petrow PK, Hartmann S, Schurigt U, et al. CXCR5- and CCR7-dependent lymphoid neogenesis in a murine model of chronic antigen-induced arthritis. Arthritis Rheum. 2007;56:3271–83. doi: 10.1002/art.22939. [DOI] [PubMed] [Google Scholar]
- 132.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 2007;170:774–86. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schudel A, Kassis T, Dixon JB, Thomas SN. S-Nitrosated polypropylene sulfide nanoparticles for thiol-dependent transnitrosation and toxicity against adult female filarial worms. Adv. Healthc. Mater. 2015;4:1484–90. doi: 10.1002/adhm.201400841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Investig. 2015;125:2532–46. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci. Transl. Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dane KY, Nembrini C, Tomei AA, Eby JK, O’Neil CP, et al. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J. Control. Release. 2011;156:154–60. doi: 10.1016/j.jconrel.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 137.Pujol-Autonell I, Serracant-Prat A, Cano-Sarabia M, Ampudia RM, Rodriguez-Fernandez S, et al. Use of autoantigen-loaded phosphatidylserine-liposomes to arrest autoimmunity in type 1 diabetes. PLOS ONE. 2015;10:e0127057. doi: 10.1371/journal.pone.0127057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, et al. Nanoparticle conjugation of antigen enhances cytotoxic T cell responses in pulmonary vaccination. PNAS. 2011;108:e989–97. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ballester M, Nembrini C, Dhar N, de Titta A, de Piano C, et al. Nanoparticle conjugation and pulmonary delivery enhance the protective efficacy of Ag85B and CpG against tuberculosis. Vaccine. 2011;29:6959–66. doi: 10.1016/j.vaccine.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 140.Tefany FJ, Barnetson RS, Halliday GM, McCarthy SW, McCarthy WH. Immunocytochemical analysis of the cellular infiltrate in primary regressing and non-regressing malignant melanoma. J. Investig. Dermatol. 1991;97:197–202. doi: 10.1111/1523-1747.ep12479662. [DOI] [PubMed] [Google Scholar]
- 141.Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Investig. 2011;121:2350–60. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cochran AJ, Morton DL, Stern S, Lana AM, Essner R, Wen DR. Sentinel lymph nodes show profound downregulation of antigen-presenting cells of the paracortex: implications for tumor biology and treatment. Mod. Pathol. 2001;14:604–8. doi: 10.1038/modpathol.3880358. [DOI] [PubMed] [Google Scholar]
- 143.Pinzon-Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol. Cell Biol. 2005;83:451–61. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 144.Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, et al. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int. J. Cancer. 2001;92:703–11. doi: 10.1002/1097-0215(20010601)92:5<703::aid-ijc1250>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 145.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–58. [PubMed] [Google Scholar]
- 146.Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, et al. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J. Exp. Med. 1998;188:1641–50. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jarmalavicius S, Welte Y, Walden P. High immunogenicity of the human leukocyte antigen peptidomes of melanoma tumor cells. J. Biol. Chem. 2012;287:33401–11. doi: 10.1074/jbc.M112.358903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mohos A, Sebestyén T, Liszkay G, Plótár V, Horváth S, et al. Immune cell profile of sentinel lymph nodes in patients with malignant melanoma—FOXP3+ cell density in cases with positive sentinel node status is associated with unfavorable clinical outcome. J. Transl. Med. 2013;11:43. doi: 10.1186/1479-5876-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004;4:249–58. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 150.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release. 2006;112:26–34. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 151.Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLOS ONE. 2013;8:e61646. doi: 10.1371/journal.pone.0061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Coates PT, Colvin BL, Hackstein H, Thomson AW. Manipulation of dendritic cells as an approach to improved outcomes in transplantation. Expert Rev. Mol. Med. 2002;4:1–21. doi: 10.1017/S1462399402004283. [DOI] [PubMed] [Google Scholar]
- 153.Ehser S, Chuang JJ, Kleist C, Sandra-Petrescu F, Iancu M, et al. Suppressive dendritic cells as a tool for controlling allograft rejection in organ transplantation: promises and difficulties. Hum. Immunol. 2008;69:165–73. doi: 10.1016/j.humimm.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 154.Komori J, Boone L, DeWard A, Hoppo T, Lagasse E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat. Biotechnol. 2012;30:976–83. doi: 10.1038/nbt.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ploix C, Bergerot I, Fabien N, Perche S, Moulin V, Thivolet C. Protection against autoimmune diabetes with oral insulin is associated with the presence of IL-4 type 2 T cells in the pancreas and pancreatic lymph nodes. Diabetes. 1998;47:39–44. doi: 10.2337/diab.47.1.39. [DOI] [PubMed] [Google Scholar]
- 156.Tian B, Hao J, Zhang Y, Tian L, Yi H, et al. Upregulating CD4+CD25+FOXP3+ regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy. Transplantation. 2009;87:198–206. doi: 10.1097/TP.0b013e3181933261. [DOI] [PubMed] [Google Scholar]
- 157.Trepel M, Arap W, Pasqualini R. Modulation of the immune response by systemic targeting of antigens to lymph nodes. Cancer Res. 2001;61:8110–12. [PubMed] [Google Scholar]
- 158.Jewell CM, Lopez SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. PNAS. 2011;108:15745–50. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mueller SN, Tian S, DeSimone JM. Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Mol. Pharm. 2015;12:1356–65. doi: 10.1021/mp500589c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eby JK, Dane KY, O’Neil CP, Hirosue S, Swartz MA, Hubbell JA. Polymer micelles with pyridyl disulfide-coupled antigen travel through lymphatics and show enhanced cellular responses following immunization. Acta Biomater. 2012;8:3210–17. doi: 10.1016/j.actbio.2012.06.007. [DOI] [PubMed] [Google Scholar]