Abstract
Objective
Trichomonas vaginalis (TV) is the world’s most common curable sexually transmitted infection (STI) and has implications for reproductive health in women. We determined incidence and correlates of TV in an HIV-uninfected peripartum cohort.
Methods
Women participating in a prospective study of peripartum HIV acquisition in Western Kenya were enrolled during pregnancy and followed until 9 months postpartum. TV was assessed every 1–3 months using wet mount microscopy. Correlates of incident TV were determined using Cox proportional hazards models.
Results
Among 1271 women enrolled, median age was 22 years (interquartile range [IQR] 19–27) and gestational age was 22 weeks (IQR 18–26); most (78%) were married and had uncircumcised male partners (69%). Prevalent TV was detected in 81 women (6%) at enrolment. Among women without TV at enrolment, 112 had TV detected during 1079 person-years of follow-up (10.4 per 100 person-years). After adjustment for socio-economic factors, male partner circumcision status, pregnancy status and other STIs, TV incidence was higher during pregnancy than postpartum (22.3 vs 7.7 per 100 person-years, adjusted hazard ratio [aHR] 3.68, 95% CI 1.90–7.15, p<0.001). Women with circumcised male partners had a 58% lower risk of incident TV compared to women with uncircumcised partners (aHR 0.42, 95% CI 0.23–0.76, p=0.004). Employed women had lower risk of incident TV than unemployed women (aHR 0.49, 95% CI 0.31–0.79, p=0.003); recent STI was associated with increased TV risk (aHR 2.97, 95% CI 1.49–5.94, p=0.002).
Conclusion
TV was relatively common in this peripartum cohort. Male circumcision may confer benefits in preventing TV.
Keywords: Trichomonas vaginalis, incidence, reproductive health, female, male circumcision, peripartum period
INTRODUCTION
Trichomonas vaginalis is the most common curable sexually transmitted infection (STI) worldwide, with regional prevalence rates in women ranging from 5% in the Western Pacific to 18% in Africa.1–5 Each year, 25 million T. vaginalis infections occur during pregnancy, with women in sub-Saharan Africa disproportionately affected compared to other regions.6,7 T. vaginalis is associated with pelvic inflammatory disease and risk of HIV acquisition in women.8–12 Several individual studies13–19 and two meta-analyses20,21 implicate T. vaginalis infection in poor infant outcomes including preterm birth, small-for-gestational-age, premature rupture of membranes and low birth weight.
Hormonal changes during pregnancy and postpartum may alter susceptibility to T. vaginalis infection or persistence of infections.2,22 Changes in sexual behaviour may also influence T. vaginalis exposure during and after pregnancy. Cross-sectional studies have estimated T. vaginalis prevalence between 15–32% in peripartum African cohorts, and older age, younger age at sexual debut, higher education, being married and comorbid STIs have been all been associated with prevalent T. vaginalis infection.23–26 In non-pregnant African women, male partner circumcision status has been associated with lower T. vaginalis incidence; however, this relationship has not been evaluated in pregnant and postpartum populations.23,27 In addition, there are limited data on T. vaginalis incidence and cofactors among pregnant and postpartum African women.28,29 We evaluated incidence and correlates of T. vaginalis among pregnant and postpartum women in Western Kenya.
METHODS
Design & participants
We used data from a longitudinal study investigating rates and cofactors of acute HIV infection among HIV-uninfected women at two rural antenatal care (ANC) clinics in Western Kenya (the Mama Salama Study) conducted between May 2011 and July 2014. Recruitment, eligibility criteria, and follow-up procedures for the parent study have been previously described.30 Briefly, pregnant women were eligible if they were ≥14 weeks gestation, were ≥14 years old, had negative rapid HIV test at enrolment or within the 3 months prior, planned to remain in the study area for the duration of pregnancy through 9 months postpartum, were willing to have a home visit, and were not enrolled in another research study.
Ethics approval
All study procedures were approved by the Kenyatta National Hospital/University of Nairobi Ethical Research Committee (#P11/4/2010) and the University of Washington Institutional Review Board (#38472A) prior to study initiation.
Study procedures
Women attended follow-up visits during pregnancy (20, 24, 32, 36 weeks gestation) and postpartum (2, 6, 10, 14 weeks; 6 and 9 months). At each study visit, standardized questionnaires were administered by study nurses on sociodemographic factors, reproductive history, sexual behaviour, hormonal contraception and condom use, medical history and genital symptoms. Male partner characteristics were reported by participating women. Women self-collected vaginal swabs, had physical exams at each study visit and had pelvic exams at enrolment, 28 weeks gestation, 6 weeks postpartum, and 6 months postpartum. Swabs were not collected during menstruation. All women with T. vaginalis infection were treated with metronidazole (one dose of 2 grams) per the World Health Organization and Kenya Ministry of Health’s national treatment guidelines for laboratory-confirmed genital infections in pregnancy.w1 w2 Antimicrobials were administered at no cost as part of the study. Women were counselled to have their partner come to the study clinic for complete STI testing and treatment.w3
Laboratory procedures
C. trachomatis and N. gonorrhoeae were assessed at enrolment using endocervical samples for nucleic acid amplification tests (NAAT) with the APTIMA Combo 2 Assay (HOLOGIC/GEN-PROBE, Inc, San Diego, CA). Syphilis serology was based on rapid plasma reagin (RPR) tests conducted at enrolment as part of routine ANC and abstracted from maternal child health booklets, or conducted by study staff if the test was not performed. Candidiasis and bacterial vaginosis (BV) were assessed at every study visit. Candidiasis was detected by direct microscopy after addition of 10% KOH. Vaginal Gram stained slides were evaluated using Nugent’s criteria, with BV defined as a score of 7–10. T. vaginalis was assessed at every study visit and infection was determined by detection of motile trichomonads using wet mount microscopy. All laboratory procedures were performed by study laboratory technicians, who had received standardized training on microscopy. Quality assurance was performed monthly by comparing results in a subset of samples with an external lab. Genital symptoms for T. vaginalis were defined as abnormal discharge, foul odour, vaginal itching, or vaginal burning.
Statistical analysis
HIV-uninfected women with baseline T. vaginalis assessment and at least one reassessment of T. vaginalis at study follow-up were included in the analyses. Women who acquired HIV infection during the study were excluded to control for potential underlying differences in that group. Incident T. vaginalis was defined as a new infection detected among women who did not have prevalent T. vaginalis at enrolment. Timing of incident T. vaginalis was estimated to be at the midpoint between the pre-infection visit and the visit at which the infection was detected.
Cox proportional hazards models were used to determine correlates of time-to-first incident T. vaginalis infection. All models used robust standard errors. Variables associated with T. vaginalis (alpha = 0.10) in the univariate analyses were included in the multivariate models and were tested for collinearity. Potential correlates included sociodemographic and sexual partnership characteristics, hormonal contraception and condom use, other curable STIs (C. trachomatis, N. gonorrhoeae and syphilis), sexual behaviours, and vaginal washing or drying practices. Unprotected sex, number of sexual partners, BV, candidiasis, vaginal washing and drying, and postpartum hormonal contraception use were included as time-varying. Women were classified as hormonal contraception nonusers if no hormonal contraception was ever used, including women that did not use another form of contraception.
We conducted sensitivity analyses to reduce potential misclassification of undetected prevalent infections as incident infections due to low sensitivity of wet mount microscopy.w4-w6 We repeated all analyses excluding women with T. vaginalis detected at enrolment and/or at first post-enrolment visit to evaluate whether T. vaginalis incidence and correlates would remain similar to our primary analyses. Data were analysed using Stata 13.1/MP for Windows (Stata Corporation, College Station, TX).
RESULTS
Overall, 1271 women (97% of women in the parent study) met criteria for inclusion for this analysis; women were excluded due to HIV infection (n=25) and lack of T. vaginalis results (n=8). Most women were married (78%), median age was 22 years (interquartile range [IQR] 19–27), median gestational age at enrolment was 22 weeks (IQR 16–26) and median level of highest education completed was 8 years (IQR 7–10) (Table 1). Among women with male partners, median partnership duration was 4 years (IQR 1–7) and one-third (31%) reported their male partner was circumcised. No male partners were reported to have been circumcised during follow-up. At enrolment, about half (55%) of women reported condomless sexual intercourse in the last 30 days. Bacterial vaginosis (23%) and candidiasis (25%) were the most common genital infections detected at enrolment. Other curable STIs were detected in 109 (9%) women (C. trachomatis 5%, N. gonorrhoeae 3% and syphilis 1%).
Table 1.
Characteristics of study population1
| N (Percentage) or Median (Interquartile range) | ||
|---|---|---|
|
| ||
| Characteristic | All women n=1271 |
|
| Demographic
| ||
| Age (years) | 22 | (19–27) |
| Highest level of education (years) | 8 | (7–10) |
| ≤8 years of completed education | 813 | (64%) |
| Currently married | 997 | (78%) |
| Duration of partnership (years)2 | 4 | (1–7) |
| Partner ≥10 years older2 | 181 | (18%) |
| Employed | 557 | (44%) |
| Crowding (≥3 people/room) | 414 | (33%) |
|
Sexual behaviour and partner characteristics | ||
| Number of sexual acts (last 30 days) | 1 | (0–4) |
| Any reported condomless sex (last 30 days) | 690 | (55%) |
| Number of sexual partners (last 30 days) | 1 | (1–1) |
| Circumcised male partner2,3 | 353 | (31%) |
| HIV-infected partner2,3 | 17 | (2%) |
|
Gynaecological history | ||
| Number of children | 2 | (1–3) |
| <2 years since last birth4 | 164 | (19%) |
| Any reported vaginal washing (last week) | 762 | (60%) |
| Any reported vaginal drying (last week) | 224 | (18%) |
| Self-reported history of STIs | 81 | (6%) |
| Postpartum hormonal contraceptive use5 | ||
| Implant | 114 | (9%) |
| Injectable | 181 | (15%) |
| Oral | 49 | (4%) |
| None | 901 | (72%) |
|
STI diagnosis | ||
| Trichomonas vaginalis | 81 | (6%) |
| Non-TV curable STIs6 | 85 | (9%) |
| Chlamydia trachomatis | 70 | (6%) |
| Neisseria gonorrhoeae | 31 | (2%) |
| Syphilis | 10 | (1%) |
| Bacterial vaginosis | 288 | (23%) |
| Candidiasis | 312 | (25%) |
Missing data not shown all characteristics assessed at baseline unless indicated
Among women reporting current relationship
Male circumcision and HIV status of male partners reported by female partner
Among women with ≥1 children
Among postpartum women only; current use at 9 months postpartum
Includes Chlamydia trachomatis, Neisseria gonorrhoeae and syphilis
T. vaginalis prevalence and incidence
At enrolment, 81 (6%) of women had prevalent T. vaginalis; 16 (20%) of whom were co-infected with another curable STI. During 1079 total person-years of follow-up (median 0.9 years, IQR 0.8–1.1), 112 incident infections of T. vaginalis were detected (44 in pregnancy and 68 postpartum), resulting in an overall incidence of 10.4 per 100 person-years (Figure 1). Women with either prevalent or incident T. vaginalis infection were 3 times as likely to report genital symptoms at any time during follow up compared to women that never had T. vaginalis (Odds Ratio [OR] 3.08, 95% CI 1.92–4.94, p<0.001). However, symptoms were only reported by 41 (51%) women with prevalent and 9 (8%) women with incident T. vaginalis infections at the time of detection.
Figure 1.
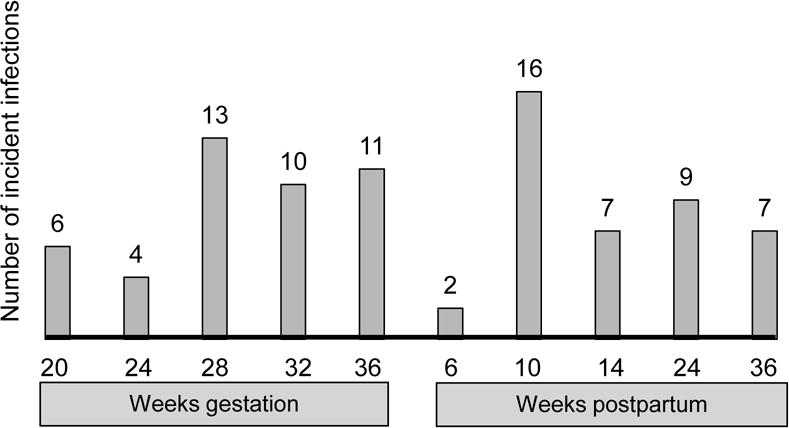
Timing of detection of incident TV infection during pregnancy and postpartum
Correlates of T. vaginalis incidence
Incidence of T. vaginalis was higher in pregnancy versus postpartum (22.3 vs 7.7 per 100 person-years, respectively, p<0.001, Table 2). In unadjusted models, male circumcision (hazard ratio [HR] 0.48, 95% CI 0.29–0.81), pregnancy (HR 3.08, 95% CI 1.93–4.91), and detection of other curable STIs (HR 2.03, 95% CI 1.14–3.65) were associated with incident T. vaginalis. Among postpartum women, we did not detect an association between those using implant, injectable, or oral, hormonal contraception and incident T. vaginalis infection compared to non-users (HR 0.86, 95% CI 0.27–2.72, p=0.795; HR 0.84, 95% CI 0.34–2.09, p=0.713; HR 0.92, 95% CI 0.28–2.97, p=0.886, respectively).
Table 2.
Correlates of time-to-first incident Trichomonas vaginalis1
| Characteristic | T. vaginalis infections/Person-years | Incidence (per 100 person-years) | Univariate Analysis
|
Multivariate Analysis
|
||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | Adj HR (95% CI) | p-value2 | |||
| Overall | 112/1078.9 | 10.4 (8.6–12.5) | ||||
|
| ||||||
| Demographic | ||||||
|
| ||||||
| Age (years) | ||||||
| ≤21 | 58/497.7 | 11.7 (9.0–15.1) | 1.28 (0.89–1.86) | 0.189 | ||
| >21 | 54/581.2 | 9.3 (7.1–12.1) | ref | |||
| Level of education (years) | ||||||
| ≤8 | 77/666.0 | 11.6 (9.2–14.5) | 1.36 (0.91–2.01) | 0.133 | ||
| >8 | 35/412.9 | 8.5 (6.1–11.8) | ref | |||
| Current marital status | ||||||
| Married | 87/857.5 | 10.1 (8.2–12.5) | 0.88 (0.56–1.38) | 0.577 | ||
| Unmarried | 25/221.3 | 11.3 (7.6–16.7) | ref | |||
| Duration of partnership (years)3 | ||||||
| ≤1 | 30/270.1 | 11.1 (7.7–15.9) | 1.14 (0.74–1.75) | 0.544 | ||
| >1 | 67/686.2 | 9.8 (7.7–12.4) | ref | |||
| Partner age3 (years older) | ||||||
| ≥10 | 16/149.0 | 10.7 (6.6–17.5) | 1.02 (0.59–1.75) | 0.954 | ||
| <10 | 76/732.7 | 10.4 (8.3–13.0) | ref | |||
| Employment status | ||||||
| Employed | 40/483.2 | 8.3 (6.1–11.3) | 0.69 (0.47–1.02) | 0.060 | 0.49 (0.31–0.79) | 0.003 |
| Unemployed | 72/595.7 | 12.1 (9.6–15.2) | ref | |||
| Crowding living conditions (people/room) | ||||||
| ≥3 | 41/335.7 | 12.2 (9.0–16.6) | 1.26 (0.86–1.824) | 0.243 | ||
| <3 | 71/742.0 | 9.6 (7.6–12.1) | ref | |||
|
| ||||||
| Sexual behavior | ||||||
|
| ||||||
| Reported condomless sex (last month) | ||||||
| Any | 52/507.8 | 10.2 (7.8–13.4) | 1.14 (0.77–1.68) | 0.517 | ||
| None | 60/565.4 | 10.6 (8.2–13.7) | ref | |||
| Number of sexual partners (last month) | ||||||
| >1 | 1/3.2 | 31.2 (4.4–221.4) | 1.29 (0.52–22.98) | 0.196 | ||
| ≤1 | 111/1075.3 | 10.3 (8.6–12.4) | ref | |||
| Male partner circumcision status4 | ||||||
| Circumcised | 18/316.4 | 5.7 (3.6–9.0) | 0.48 (0.29–0.81) | 0.006 | 0.42 (0.23–0.76) | 0.004* |
| Uncircumcised | 80/657.6 | 12.2 (9.8–15.1) | ref | |||
| Male partner HIV status4 | ||||||
| HIV-infected | 1/13.7 | 7.3 (1.0–51.9) | 0.82 (0.12–5.61) | 0.840 | ||
| HIV-uninfected | 65/718.6 | 9.0 (7.1–11.5) | ref | |||
|
| ||||||
| Gynaecological history | ||||||
|
| ||||||
| Pregnancy status | ||||||
| Pregnant | 44/197.5 | 22.3 (16.6–29.9) | 3.08 (1.93–4.91) | <0.001 | 3.68 (1.90–7.15) | <0.001* |
| Postpartum | 68/881.3 | 7.7 (6.1–9.8) | ref | |||
| Number of living children | ||||||
| ≥3 | 24/243.8 | 9.8 (6.6–14.7) | 1.04 (0.64–1.70) | 0.873 | ||
| <3 | 47/504.5 | 9.3 (7.0–12.4) | ref | |||
| Time since previous birth (years)5 | ||||||
| <2 | 13/137.2 | 9.5 (5.5–16.3) | 1.10 (0.60–2.03) | 0.749 | ||
| ≥2 | 56/583.9 | 9.6 (7.4–12.4) | ref | |||
| Reported vaginal washing (last week) | ||||||
| Any | 65/641.9 | 10.1 (7.9–12.9) | 0.90 (0.62–1.31) | 0.595 | ||
| None | 47/435.3 | 10.8 (8.1–14.4) | ref | |||
| Reported vaginal drying (last week) | ||||||
| Any | 15/106.5 | 14.0 (8.5–23.4) | 1.22 (0.70–2.12) | 0.482 | ||
| None | 97/972.1 | 100 (8.2–12.2) | ref | |||
| Self-reported history of STIs | ||||||
| Any | 9/68.7 | 13.1 (6.8–25.2) | 1.30 (0.66–2.59) | 0.448 | ||
| None | 103/1010.2 | 10.2 (8.4–12.4) | ref | |||
| Hormonal contraceptive use6 | ||||||
| Implant | 3/50.8 | 5.9 (1.9–18.3) | 0.86 (0.27–2.72) | 0.795 | ||
| Injectable | 5/74.9 | 6.7 (2.8–16.0) | 0.84 (0.34–2.09) | 0.713 | ||
| Oral | 3/38.2 | 7.9 (2.5–24.4) | 0.92 (0.28–2.97) | 0.886 | ||
| None | 57/717.4 | 7.9 (6.1–10.3) | ref | |||
|
| ||||||
| STI diagnoses7 | ||||||
|
| ||||||
| Non-TV curable STI 8 | ||||||
| Yes | 13/63.3 | 20.5 (11.9–35.3) | 2.03 (1.14–3.65) | 0.017 | 2.10 (1.16–3.79) | 0.014* |
| No | 78/778.0 | 10.0 (8.0–12.5) | ref | |||
| Chlamydia trachomatis | ||||||
| Yes | 9/54.4 | 16.5 (8.6–31.8) | 1.70 (0.87–3.30) | 0.119 | ||
| No | 103/1022.9 | 10.1 (8.3–12.2) | ref | |||
| Neisseria gonorrhoeae | ||||||
| Yes | 7/23.0 | 30.4 (14.5–63.8) | 2.90 (1.38–6.09) | 0.005 | ||
| No | 105/1054.3 | 9.96 (8.2–12.1) | ref | |||
| Syphilis | ||||||
| Yes | 2/6.6 | 30.3 (7.6–121.0) | 2.68 (0.64–11.3) | 0.179 | ||
| No | 89/834.8 | 10.6 (8.7–13.1) | ref | |||
| Current bacterial vaginosis | ||||||
| Yes | 36/235.7 | 15.3 (11.0–21.2) | 1.21 (0.82–1.79) | 0.339 | ||
| No | 75/572.0 | 13.1 (10.5–16.4) | ref | |||
| Current candidiasis | ||||||
| Yes | 15/101.5 | 14.8 (8.9–24.5) | 0.95 (0.55–1.64) | 0.864 | ||
| No | 97/711.3 | 13.6 (11.2–16.6) | ref | |||
HR = hazards ratio; Adj HR = adjusted hazards ratio; 95% CI = 95% confidence interval
Missing data not shown, all correlates assessed at enrolment unless indicated
Cox proportional hazards p-value adjusted for employment, male partner circumcision status, pregnancy status and other non-TV curable STIs detected at enrolment
Among women reporting current relationship
Male circumcision and HIV status of male partners reported by female partner
Among women with ≥1 children
Among postpartum women only
Chlamydia trachomatis, Neisseria gonorrhoeae and syphilis were assessed enrolment only. Bacterial vaginosis and candidiasis were assessed at enrolment and at follow up visits during pregnancy
Includes Chlamydia trachomatis, Neisseria gonorrhoeae and syphilis
In the multivariate model, risk of incident T. vaginalis was 3-fold higher during pregnancy compared to postpartum (adjusted hazard ratio [aHR]=3.68, 95% CI 1.90–7.15, p=<0.001) after adjusting for employment, male partner circumcision, and infection with other STIs during pregnancy. Women with circumcised male partners had a 58% lower risk of incident T. vaginalis compared to women with uncircumcised partners (aHR=0.42, 95% CI 0.23–0.76, p=0.004). Risk of incident T. vaginalis was also lower among women who were employed versus unemployed (aHR=0.49, 95% CI 0.31–0.79, p=0.003), and higher among women with other curable STIs (aHR=2.10, 95% CI 1.16–3.79, p=0.014). When non-TV STIs were individually assessed, only N. gonorrhoeae was significantly associated with incident T. vaginalis infection (aHR=2.97, 95% CI 1.49–5.94, p=0.002), though infection with C. trachomatis trended towards an association (aHR=1.82, 95% CI 0.90–3.69, p=0.097); syphilis was not associated with incident T. vaginalis (aHR=2.56, 95% CI 0.62–10.46, p=0.192).
Sensitivity analyses
In sensitivity analyses, we excluded women with T. vaginalis detected at enrolment and/or at first post-enrolment visit. Overall T. vaginalis incidence was comparable to the primary results (6.8 per 100 person-years), though incidence during pregnancy was lower (9.5 per 100 person-years) than in the primary analysis. Pregnancy and STIs remained associated with increased risk of incident T. vaginalis (aHR=4.97, 95% CI 2.41–10.24, p≤0.001 and aHR=2.09, 95% CI 1.04–4.22, p=0.040, respectively). Male circumcision also remained associated with reduced risk (aHR=0.42, 95% CI 0.21–0.87, p=0.019). Other cofactors were not significantly associated with incident T. vaginalis (data not shown).
DISCUSSION
In this prospective study of HIV-uninfected pregnant and postpartum Kenyan women, T. vaginalis infection was relatively common. Incidence of T. vaginalis during follow-up was high, with increased T. vaginalis incidence during pregnancy compared to the postpartum period. Infection with other STIs at enrolment was associated with increased risk of incident T. vaginalis. In multivariate analyses, employment and male partner circumcision were associated with a lower risk of incident T. vaginalis. These data suggest that there is persistent risk of T. vaginalis during pregnancy and postpartum, and that male circumcision is a potential modifiable factor that may decrease risk of T. vaginalis in this period.
Male circumcision has been associated with decreased risk of human papillomavirus, genital ulcers, herpes simplex virus 2, BV, syphilis and T. vaginalis in women in randomized clinical trials (RCT).27 w7-w13 One RCT from Uganda reported a 48% reduction of T. vaginalis associated with male partner circumcision, similar to our findings.27 In contrast, an observational study of HIV-uninfected women from Zimbabwe, Uganda and Thailand reported no association between male partner circumcision and incident T. vaginalis.w11 Male circumcision may decrease men’s risk of T. vaginalis infection, and, in turn, reduce the probability that female partners are exposed to T. vaginalis. Additionally, the moist subpreputial space in uncircumcised men may enhance survival of trichomonads; thus, foreskin removal may reduce male-to-female transmissions of T. vaginalis.27 w11 w14 We are not aware of other studies examining STI acquisition and male circumcision specifically during or after pregnancy, a critical period of potential vulnerability with implications for pregnancy outcomes. Male circumcision promotion may confer benefits in preventing T. vaginalis. Thus, additional benefits of expanding male circumcision as a strategy to prevent HIV acquisition may include prevention of transmission of other STIs, such as T. vaginalis.
Our finding that other curable STIs increased risk of acquiring T. vaginalis infection is consistent with other studies in non-pregnant women that have found recent infection with STIs increases risk of acquiring other STIs.w15 w16 Exposure to sexual networks where multiple STIs are prevalent could explain this relationship. Alternatively, there could be biological factors that concurrently increase susceptibility to multiple STIs. Similar to other studies, we found sociodemographic and relationship characteristics, including partner characteristics, were associated with T. vaginalis.24,25 w17 Women with lower socioeconomic status may have less access to STI preventative information and care or may have less power in relationships to negotiate safer sexual practices due to economic dependence on male partners.w18 Maximal cure rates in infected women are achieved with concurrent treatment of their sexual partners.w19 Treatment of male partners is critical to preventing reinfection with T. vaginalis, especially during pregnancy when prevention has immediate benefits for mothers and infants. We have previously reported results from a pilot study of patient-delivered partner treatment (PDPT) for management of T. vaginalis, which demonstrated acceptability and feasibility among pregnant and postpartum Kenyan women.w3. Further evaluation of this model will be useful in settings with high T. vaginalis prevalence.
Prevalence of T. vaginalis infection at enrolment was lower in our HIV-uninfected cohort than some previous studies in African pregnant and postpartum women (prevalence 15–32%)24,25,28 w20. Previous studies did not exclude HIV-infected women and used a variety of T. vaginalis diagnostics, including more sensitive assays which may account for differences with our results. However, a recent study of HIV-uninfected, non-pregnant women from Malawi, South Africa, Uganda, and Zimbabwe reported a T. vaginalis prevalence of 7%, similar to our findings.w21 Our estimate of incidence of T. vaginalis infection during pregnancy of 22.8 per 100 person-years was similar to another study of HIV-uninfected women in South Africa, which reported an incidence of 17.6 per 100 person-years.28 We found increased incidence of T. vaginalis in pregnancy compared to postpartum. Hormonal alterations unique to pregnancy may alter susceptibility to T. vaginalis, similar to other infectious diseases.2,22 w22 Among pregnant and postpartum women in other studies, 72–88% of T. vaginalis infections were asymptomatic, similar to our cohort in which over half of prevalent infections were asymptomatic.24,28 We also found that incident infections were less frequently symptomatic which could be due to early detection as symptoms can arise up to 28 days after initial infection in women. Thus, many early T. vaginalis infections may go undetected and untreated in settings with exclusively syndromic management.
Our findings raise important questions about the diagnosis and management of T. vaginalis infection in pregnant and postpartum women in high prevalence settings particularly in areas of high HIV risk. Meta-analyses have found associations of T. vaginalis infection in pregnancy with premature birth, SGA, and premature rupture of membranes.20 However, it has not been clear that treatment of T. vaginalis will decrease this risk. One systematic review suggested that treatment of T. vaginalis during pregnancy may increase incidence of preterm birth21; perhaps due to use of higher dosages of metronidazole than typically recommended in included studies or coinfection with other STIs.20,25 More recent studies, including a RCT, noted no association of T. vaginalis infection or treatment in pregnancy with preterm birth.25 w23 w24 In our study, women were regularly screened for T. vaginalis and women with confirmed T. vaginalis were treated with metronidazole according to national guidelines. The benefit and cost-effectiveness of routine screening for T. vaginalis in asymptomatic pregnant women has not been established. However, prenatal T. vaginalis screening and prompt treatment are recommended for HIV-infected pregnant women because T. vaginalis infection is a risk factor for vertical transmission of HIV. Our results suggest that T. vaginalis incidence is high during pregnancy and often asymptomatic, and will thus requires alternative approaches to syndromic management for adequate detection and treatment.
Our study has some limitations. We used microscopy for T. vaginalis detection, which is less sensitive for T. vaginalis detection than polymerase chain reaction (PCR) or other types of NAAT.24,25 w17 w4-w6 However, this approach is similar to other studies in Africa, and is easy to contextualize given the same approach.24,25 w17 While some studies from Africa have demonstrated comparable specificity of wet mount to PCR and other diagnostic methods for T. vaginalis,w25 lower sensitivity would result in underestimates of prevalence and incidence in our cohort. We did conduct exploratory analyses to estimate the potential impact of misclassifying undetected prevalent infections, and results were similar in both analyses. We relied on women’s report of male partner circumcision status, which may have introduced exposure misclassification based on the accuracy of women’s report. While studies from Zambia and Swaziland suggest that women overestimate male partner circumcision,w26 recent data from Uganda, a setting with male circumcision sensitization programs more similar to Western Kenya, suggest <10% of women misreport male partner circumcision status.w27 Therefore, misreporting likely did not substantially influence our results. Finally, other curable STIs were only assessed at baseline and we were unable to evaluate the relationship of incident STIs on T. vaginalis.
In summary, our results suggest that pregnant and postpartum women frequently acquire T. vaginalis and most infections are asymptomatic, supporting scale-up of improved diagnostic techniques for T. vaginalis infections in this group.28 We found male circumcision was associated with reduced T. vaginalis incidence, adding to the body of evidence that male circumcision prevents STIs in female partners. Our results suggest that male circumcision could reduce T. vaginalis incidence and related sequelae among pregnant and postpartum female partners. Improved T. vaginalis control through detection of asymptomatic infections and reduced male-to-female transmissions could have important implications, particularly in settings of high STI morbidity and where poor birth outcomes persist as a public health problem.
KEY MESSAGES.
Pregnant and postpartum women frequently acquire T. vaginalis and most infections are asymptomatic
Male circumcision could reduce T. vaginalis incidence among pregnant and postpartum female partners.
Acknowledgments
FUNDING
This study was funded through National Institutes of Health grant (P01 HSD 064915; T32 T32AI07140 to J.P; K01 AI116298 to A.L.D; K24 HD054314 to GJS) and received assistance from the University of Washington Center for AIDS Research (P30 AI27757). The Mama Salama Study Team was supported by the University of Washington’s Global Center for Integrated Health of Women Adolescents and Children (Global WACh). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in STI and any other BMJPGL products and sub-licences such use and exploit all subsidiary rights, as set out in our licence http://group.bmj.com/products/journals/instructions-for-authors/licence-forms
COMPETING INTERESTS
The authors declare that no competing interests exist.
AUTHORS’ CONTRIBUTIONS
J.P., A.L.D., J.K., J.A.U., D.M., R.S.M., and G.J-S. conceived the question and designed the study. G.J-S. obtained funding for the study. A.L.D., J.K., J.A.U., D.M., R.S.M., and G.J-S. participated in data collection. J.P, A.L.D., and G.J-S. conducted the data analyses. All authors participated in preparation of the manuscript and approved the final draft for submission.
DATA SHARING
Detailed information on study data is available from the last author (Grace John-Stewart) upon request at gjohn@uw.edi
References
- 1.Lewis DA, Latif AS, Ndowa F. WHO global strategy for the prevention and control of sexually transmitted infections: time for action. Sex Transm Infect England. 2007:508–9. doi: 10.1136/sti.2007.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poole DN, McClelland RS. Global epidemiology of Trichomonas vaginalis. Sex Transm Infect. 2013;89(6):418–22. doi: 10.1136/sextrans-2013-051075. [DOI] [PubMed] [Google Scholar]
- 3.Allsworth JE, Ratner JA, Peipert JF. Trichomoniasis and other sexually transmitted infections: results from the 2001–2004 National Health and Nutrition Examination Surveys. Sex Transm Dis. 2009;36(12):738–44. doi: 10.1097/OLQ.0b013e3181b38a4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton M, Sternberg M, Koumans EH, et al. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis. 2007;45(10):1319–26. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 5.Schmid G, Samuelson J, Rowley J. Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates. Vol. 2011. Geneva, Switzerland: World Health Organization; p. 5. [Google Scholar]
- 6.Walker G. In: Interventions for Trichomoniasis in Pregnancy: RHL Commentary. Library TWRH, editor. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 7.Mullick S, Watson-Jones D, Beksinska M, et al. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect. 2005;81(4):294–302. doi: 10.1136/sti.2002.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis. 2002;34(4):519–22. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]
- 9.Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. Aids. 1993;7(1):95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 10.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195(5):698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 11.Van Der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis. 2008;197(4):548–54. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 12.Mavedzenge SN, Pol BV, Cheng H, et al. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sex Transm Dis. 2010;37(7):460–6. doi: 10.1097/OLQ.0b013e3181cfcc4b. [DOI] [PubMed] [Google Scholar]
- 13.Cotch MF, Pastorek JG, 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24(6):353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Hardy PH, Hardy JB, Nell EE, et al. Prevalence of six sexually transmitted disease agents among pregnant inner-city adolescents and pregnancy outcome. Lancet. 1984;2(8398):333–7. doi: 10.1016/s0140-6736(84)92698-9. [DOI] [PubMed] [Google Scholar]
- 15.Minkoff H, Grunebaum AN, Schwarz RH, et al. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am J Obstet Gynecol. 1984;150(8):965–72. doi: 10.1016/0002-9378(84)90392-2. [DOI] [PubMed] [Google Scholar]
- 16.Johnson HL, Ghanem KG, Zenilman JM, et al. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex Transm Dis. 2011;38(3):167–71. doi: 10.1097/OLQ.0b013e3181f2e85f. [DOI] [PubMed] [Google Scholar]
- 17.Azargoon A, Darvishzadeh S. Association of bacterial vaginosis, trichomonas vaginalis, and vaginal acidity with outcome of pregnancy. Arch Iran Med. 2006;9(3):213–7. [PubMed] [Google Scholar]
- 18.Mathai E, Muthaiah A, Mathai M, et al. Prevalence and effects of trichomoniasis in pregnancy. Natl Med J India. 1998;11(3):151. [PubMed] [Google Scholar]
- 19.Buchmayer S, Sparen P, Cnattingius S. Signs of infection in Pap smears and risk of adverse pregnancy outcome. Paediatr Perinat Epidemiol. 2003;17(4):340–6. doi: 10.1046/j.1365-3016.2003.00508.x. [DOI] [PubMed] [Google Scholar]
- 20.Silver BJ, Guy RJ, Kaldor JM, et al. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis. 2014;41(6):369–76. doi: 10.1097/OLQ.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 21.Okun N, Gronau KA, Hannah ME. Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: a systematic review. Obstet Gynecol. 2005;105(4):857–68. doi: 10.1097/01.AOG.0000157108.32059.8f. [DOI] [PubMed] [Google Scholar]
- 22.Brabin L. Interactions of the female hormonal environment, susceptibility to viral infections, and disease progression. AIDS Patient Care STDS. 2002;16(5):211–21. doi: 10.1089/10872910252972267. [DOI] [PubMed] [Google Scholar]
- 23.Abdelaziz ZA, Ibrahim ME, Bilal NE, et al. Vaginal infections among pregnant women at Omdurman Maternity Hospital in Khartoum, Sudan. J Infect Dev Ctries. 2014;8(4):490–7. doi: 10.3855/jidc.3197. [DOI] [PubMed] [Google Scholar]
- 24.Kurewa N, Mapingure M, Munjoma M, et al. The burden and risk factors of Sexually Transmitted Infections and … - PubMed - NCBI. BMC Infect Dis. 2010;10(127) doi: 10.1186/1471-2334-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stringer E, Read S, Hoffman I, et al. Treatment of trichomoniasis in pregnancy in sub-Saharan Africa does…- PubMed - NCBI. South African Medical Journal. 2010;100(1):58–64. [PMC free article] [PubMed] [Google Scholar]
- 26.Fonck K, Kidula N, Jaoko W, et al. Validity of the vaginal discharge algorithm among pregnant and non-pregnant women in Nairobi, Kenya. Sex Transm Infect. 2000;76(1):33–8. doi: 10.1136/sti.76.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray RH, Kigozi G, Serwadda D, et al. The effects of male circumcision on female partners’ genital tract symptoms and vaginal infections in a randomized trial in Rakai, Uganda. Am J Obstet Gynecol. 2009;200(1):42.e1–7. doi: 10.1016/j.ajog.2008.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moodley D, Moodley P, Sebitloane M, et al. High prevalence and incidence of asymptomatic sexually transmitted … - PubMed - NCBI. Sexually Transmitted Diseases. 2015;42(1) doi: 10.1097/OLQ.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 29.Gray RH, Wabwire-Mangen F, Kigozi G, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am J Obstet Gynecol. 2001;185(5):1209–17. doi: 10.1067/mob.2001.118158. [DOI] [PubMed] [Google Scholar]
- 30.Kinuthia J, Drake AL, Matemo D, et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. Aids. 2015;29(15):2025–33. doi: 10.1097/QAD.0000000000000793. [DOI] [PMC free article] [PubMed] [Google Scholar]


