Summary
Growing evidence has established two major types of vulvar intraepithelial neoplasia (VIN), which correspond to two distinct oncogenic pathways to vulvar squamous cell carcinoma (VSCC). While the incidence of VSCC has remained relatively stable over the last three decades, the incidence of VIN has increased. VIN of usual type (uVIN) is human papillomavirus (HPV)-driven, affects younger women and is a multicentric disease. In contrast, VIN of differentiated type (dVIN) occurs in post-menopausal women and develops independent of HPV infection. dVIN often arises in a background of lichen sclerosus and chronic inflammatory dermatoses. Although isolated dVIN is significantly less common than uVIN, dVIN bears a greater risk for malignant transformation to VSCC and progresses over a shorter time interval. On histological examination, uVIN displays conspicuous architectural and cytological abnormalities, while the morphological features that characterise dVIN are much more subtle and raise a wide differential diagnosis. On the molecular level, dVIN is characterised by a higher number of somatic mutations, particularly in TP53. Here we review the classification, epidemiology, clinical features, histomorphology, ancillary markers and molecular genetics of both types of VIN, and discuss the morphological challenges faced by pathologists in interpreting these lesions.
Keywords: Diagnosis, pathology, precursor, squamous carcinoma in situ, squamous intraepithelial lesion, squamous dysplasia, vulva, vulvar intraepithelial neoplasia
INTRODUCTION
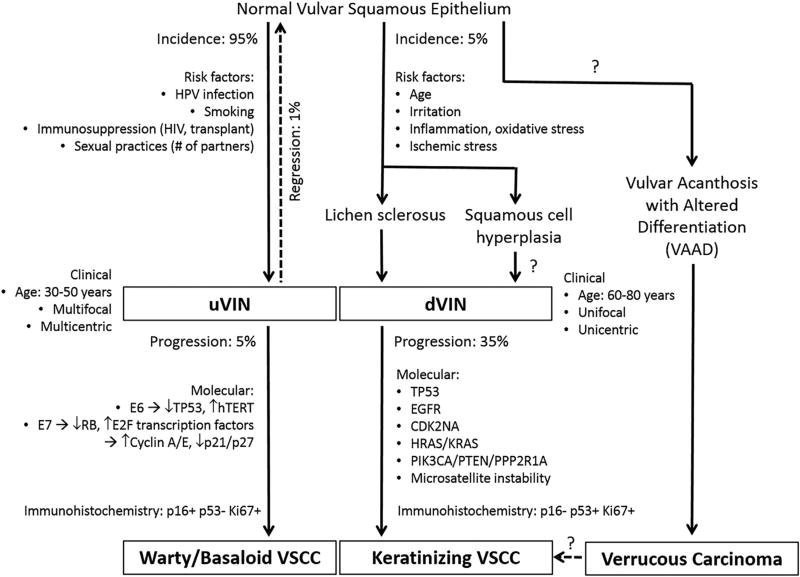
Squamous cell carcinomas account for 83% of all malignancies in the vulva.1 Although the incidence of vulvar squamous cell carcinoma (VSCC) has remained relatively stable over the last three decades, the incidence of vulvar intraepithelial neoplasia (VIN), the putative precursor lesion to VSCC, has increased over time.2,3 There are two distinct aetiopathogenic pathways leading to VSCC, associated with either: (1) VIN of usual type (uVIN) which is human papillomavirus (HPV)-driven, or (2) VIN of differentiated type (dVIN) which develops independently of HPV. The major features characterising these oncogenic pathways are summarised in Fig. 1.
Fig. 1.
Pathways of oncogenesis in vulvar squamous cell carcinoma. Modified from Nascimento et al.114
EVOLUTION OF NOMENCLATURE AND CURRENT CLASSIFICATION
Squamous precursor lesions of the vulva were first recognised a century ago, and since the initial description, numerous terms and classification schemes have been proposed (Table 1).4–6
Table 1.
Major classification schemes proposed for squamous precursor lesions of the vulva over time.
| 1958 | 1976 ISSVD | 1986 ISSVD | 2004 ISSVDa 2003 WHOa |
2005 Bethesda-like | 2012 LAST 2014 WHO 2015 ISSVD |
|---|---|---|---|---|---|
| CIS | Mild atypia | VIN I | –a | LG-VIL
|
LSIL
|
| Moderate atypia | VIN II | uVIN
|
HG-VIL
|
HSIL
|
|
| Severe atypia or CIS | VIN III, severe atypia or VIN III, CIS | ||||
| – | – | VIN III, differentiated type | dVIN | dVINb |
CIS, carcinoma in situ; dVIN, differentiated type VIN; HG-VIL, high-grade vulvar intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; ISSVD, International Society for the Study of Vulvovaginal Disease; LAST, Lower Anogenital Squamous Terminology; LG-VIL, low-grade vulvar intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; uVIN, usual type VIN; VIN, vulvar intraepithelial neoplasia; WHO, World Health Organization.
The 2004 ISSVD no longer recognised VIN 1 but the 2003 WHO retained the designation.
dVIN not included in the LAST guidelines.
Bowen’s disease was first described by the dermatologist J. T. Bowen in 1912. He noted extreme hyperplasia of the epidermis, absence of the stratum granulosum, and numerous mitoses as well as clumping and crowding of the nuclei. At the time, Bowen denied the features of ‘distinct carcinomatous formation’ due to the absence of dermal invasion, but did speculate on the premalignant nature of the lesions.7 In 1922, Hudelo et al. were the first to recognise the histological features of Bowen’s disease in the vulva and termed the disease ‘erythroplasiform dyskeratosis of the vulvar mucosa’.5,8 Twenty years later, Knight reported six cases of Bowen’s disease of the vulva, of which one was associated with VSCC. In a review of the literature, he identified an additional 26 cases.9
In 1958, Woodruff and Hildebrant recognised the variability in terminology used to describe squamous precursor lesions of the vulva and proposed a unifying term ‘carcinoma in situ’ (CIS).10 Several groups then noticed that a proportion of lesions that were morphologically identical to CIS demonstrated spontaneous regression, particularly in young, pregnant patients with multicentric disease.5,11,12 In order to distinguish these lesions from those which progressed to invasive carcinoma, Wade, Kopf and Ackerman in 1979 coined the term ‘Bowenoid papulosis’.13
In 1961, Abell and Gosling reviewed 150 VSCC and reported two types of squamous precursor lesions: (1) intraepithelial carcinoma of Bowen’s type, and (2) intraepithelial carcinoma of simplex type.14 In 1977, the term ‘differentiated’ was used to highlight the highly differentiated histological features of the simplex type.6
The 1976 International Society for the Study of Vulvovaginal Disease (ISSVD) endorsed the term ‘squamous cell carcinoma in situ’ and ‘hyperplastic dystrophy’. The latter was further qualified by mild, moderate or severe atypia. The initial appeal of this change in terminology was that it would replace the confusing array of terms in use at the time, including Bowen disease, erythroplasia of Queyrat, carcinoma simplex, squamous cell hyperplasia with atypia, atypical squamous dystrophy and leukoplakic vulvitis.15
The term ‘intraepithelial neoplasia’ was first proposed by Richart in 1967 and subsequently by Crum in 1982, initially for lesions of the cervix and later, the vulva.16,17 In 1986, the ISSVD adopted the term VIN which was graded as VIN I, II and III. By definition, the dysplasia was confined to the lower one-third of the epithelial thickness in VIN I, to the lower two-thirds in VIN II, and involved two-thirds of the epithelial thickness or more in VIN III. The additional category, ‘VIN III, differentiated type’ was also introduced.18
Over the ensuing years, evidence accrued showing that VIN 1, 2 and 3 did not exist on a biological continuum, as the classification implied. VIN 1 consisted almost entirely of condyloma acuminatum and was associated with low-risk HPV types 6 and 11. In contrast, VIN 2 and 3 were associated with high-risk HPV types and carried a risk of progression to VSCC.5,19 Recognising the aetiological and prognostic differences, the ISSVD in 2004 removed VIN 1 due to its negligible risk for cancer progression. It proposed a 2-tier classification scheme: (1) uVIN (including lesions previously classified as VIN 2 and VIN 3), and (2) dVIN. uVIN was subdivided into warty, basaloid, and mixed types. The 2003 World Health Organization (WHO) continued to use the VIN 1 designation, due to the small proportion of VIN 1 cases that were associated with high-risk HPV.4
In 2005, Medeiros et al. proposed a classification scheme similar to the Bethesda system for cervical precursor lesions. They proposed a low-grade vulvar intraepithelial lesion (LG-VIL) category which encompassed several variants of condyloma, and a high-grade VIL category (HG-VIL) which included uVIN and dVIN.20
After almost 100 years of evolution, the terminology has finally reached some consensus amongst multiple committees, all supporting the terminology ‘squamous intraepithelial lesion’. The College of American Pathologists (CAP) and American Society for Colposcopy and Cervical Pathology (ASCCP) jointly published the Lower Anogenital Squamous Terminology (LAST) guidelines in 2012, unifying the terminology applied to all HPV lesions involving the cervix, vulva, vagina, anus, perineum and penis, under two headings: (1) low-grade squamous intraepithelial lesion (LSIL), and (2) high-grade squamous intraepithelial lesion (HSIL). LSIL is equivalent to uVIN 1 and HSIL encompasses uVIN 2 and uVIN 3. The intraepithelial neoplasia (−IN) grade could be included in parentheses, if so desired. The two-tier system was advocated as being more reproducible and biologically meaningful than prior schemes. The 2014 WHO and 2015 ISSVD classifications also accept the SIL terminology, but in addition include dVIN as separate category.21,22
EPIDEMIOLOGY
The incidence of both uVIN and dVIN has increased over the last 30 years, while the incidence of VSCC has remained relatively unaltered.2,23 An analysis of 13,176 patients in the SEER (Surveillance Epidemiology and End Results) database between 1973 and 2000 found that VIN increased by 411% while VSCC only increased by 20%.2 A report of 2935 patients from the Netherlands nationwide database showed that the incidence of uVIN almost doubled from 1.2/100,000 in 1992 to 2.1/100,000 patients in 2005. The incidence of dVIN increased nine-fold, from 0.013/100,000 to 0.121/100,000 patients. Concurrently, the incidence of VSCC remained relatively unchanged.24 Trends of increasing VIN have also been reported in New Zealand, Norway, Austria and Greece.23 These trends may reflect a combination of increased detection of VIN and more effective treatment before the development of VSCC.
The recent introduction of universal HPV vaccination in multiple countries is expected to result in a significant reduction of HPV-associated neoplasms in the near future.25 Factors including the anticipated drop in the incidence of uVIN, growing age of the population and increased awareness of dVIN will likely lead to increased relative and absolute rates of dVIN. This emphasises the importance of accurate recognition of this entity.
AETIOLOGY
Prior to Harald zur Hausen’s proposal of HPV as the agent responsible for cervical carcinoma,26 multiple aetiological agents were suggested including herpes simplex virus (HSV), arsenic and even granulomas.9,10,26 Subsequently, HPV was found to be responsible for the vast majority of anogenital squamous carcinomas, and was also detected in VIN.26,27
HPV infection is strongly associated with uVIN, with the majority of studies reporting HPV positivity rates of >80%.19,24,28–34 In a study of 587 VIN from 39 countries, HPV was identified in 509 (86.7%) cases. HPV16 was the most common type (77.2%), followed by HPV33 (10.6%), and HPV18 (2.6%). Over 90% of LSIL were attributed to low-risk HPV types 6 and 11.35 Maniar et al. investigated 11 patients with concurrent LSIL and HSIL. All patients had a history of immunosuppression. All HSIL harboured high-risk HPV types, while all LSIL were positive for low-risk HPV 6 or 11.36 Hence, the presence of concurrent LSIL and HSIL was due to concurrent infections with low-risk and high-risk HPV. The findings reiterate that LSIL and HSIL are biologically distinct entities.
Although the majority of uVIN are associated with high-risk HPV, the rate of HPV positivity in VSCC is considerably lower. In a study of 1709 VSCC, only 28.6% of cases harboured HPV,37 and the reported rates in the literature vary from 15% to 79%.34 This discrepancy led investigators to explore alternative HPV independent pathways to VSCC, leading to the identification of dVIN as a separate oncogenic pathway to VSCC. A cumulative 134 cases of dVIN have been tested for HPV in the literature, of which only two (1.5%) were positive.28,29,38–41
CLINICAL FEATURES
VIN, usual type
uVIN tends to occur in young women, in the third to fifth decades of life.28,37,42,43 Multiple studies have also shown a trend towards younger ages at first diagnosis.28,43–45 Risk factors include smoking, number of sexual partners and immunosuppression.38,41,43,46–48 HPV infection is strongly associated with uVIN.19,24,28–34 Concurrent infection with HSV has been reported in up to 30%.30,48
Most patients complain of pruritus or dysuria. Twenty percent of patients are asymptomatic and present with an abnormal self-exam.9,49 uVIN presents as white or erythematous macules or papules, which can coalesce to create verrucous plaques. Approximately 10% of lesions are pigmented.41 Over half of patients have multifocal lesions in the vulva,28,41,49–52 and 18–52% have squamous dysplasia at other anogenital sites, particularly the cervix.28,32,43,49,51–53 Thus the finding of VIN on clinical examination should prompt thorough examination of the anogenital region and performance of cervicovaginal pap smears.
VIN, differentiated type
dVIN typically occurs in post-menopausal women in the sixth to eighth decades of life, but can occur in younger patients.6,37 In a survey of 21 VSCC occurring in women <40 years of age, three patients had associated dVIN.44 dVIN is often associated with adjacent lichen sclerosus (LS) and/or chronic inflammatory dermatoses.6 In comparison to uVIN, dVIN tends to be unicentric at presentation and produces less bulky lesions. Clinically, the lesions may appear as focal grey-white discolourations with a rough surface, vaguely defined thick white plaques, or elevated nodules.6
CLINICAL BEHAVIOUR
VIN, usual type
The rate of progression of uVIN to VSCC has been reported to be less than 5%.43,45,48,49,52 In an early study, seven of eight (87.5%) patients with untreated VIN 3 progressed to invasive VSCC within 8 years while only four of 105 (3.8%) treated patients developed VSCC after 7–18 years.43 Another large study reported that eight of 88 (9%) of untreated VIN patients and 108 of 3322 (3.3%) of treated VIN patients developed VSCC.52 Risk factors for malignant progression included advanced age, radiotherapy and immunocompromised status.45,50
VIN recurs in approximately 13–36% of patients.42,43,54,55 It is unclear whether the lesions represent true recurrences or acquisition of new lesions, given the ‘field effect’ of HPV infection. The relationship between margin status and recurrence risk is yet to be established unequivocally.45,49,52
A small proportion of patients exhibit spontaneous regression of disease. A systematic review of 3322 patients with VIN 3 found that spontaneous regression occurred in 1.2%. All patients were <35 years of age and 40% were related to pregnancy. Most lesions regressed 10 months after diagnosis.52
VIN, differentiated type
Differentiated VIN comprises 2–29% of all VIN,3,19,28,56 but studies characterising in situ lesions adjacent to VSCC found that dVIN was present in approximately 40%. These findings implied that dVIN was more likely to progress to VSCC than uVIN.57 This was confirmed by a study of 1826 uVIN and 67 dVIN which found that dVIN had a higher risk of progression to VSCC (32.8% versus 5.7%) and over a shortened timeframe (22.8 months versus 41.4 months) than uVIN.3 dVIN has also been shown to be more often associated with a history of prior, synchronous or subsequent VSCC (85.7%, versus 25.7% for uVIN).56
TREATMENT
Aggressive full or deep vulvectomies were performed for VIN until the mid 1960s. Based on the fact that not all VIN progressed to VSCC and on the recognition of the psychological and sexual morbidity of vulvectomies, less aggressive therapies became available by the late 1970s.58 Treatment options include local excision, topical imiquimod, cidofovir or 5-fluorouracil, photodynamic therapy and laser ablation.59
HISTOLOGY
VIN, usual type
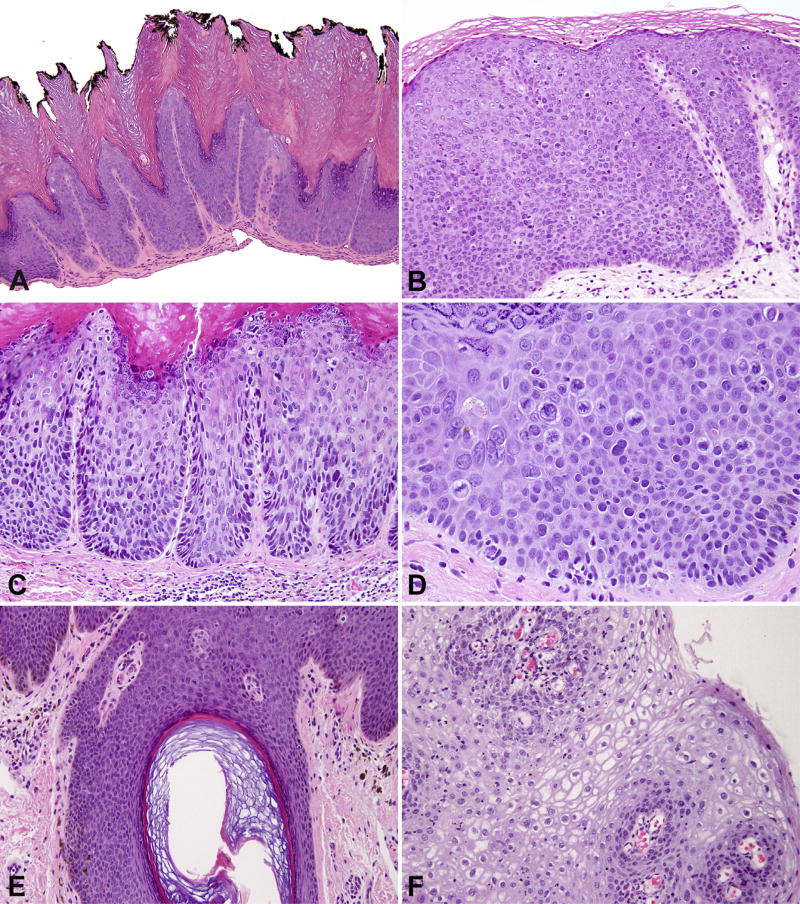
Due to its conspicuous architectural and cytological abnormalities, uVIN is often appreciated on low power examination. Knight’s description in 1943 highlights the major features of uVIN, which have stood the test of time: (1) hyperkeratosis and parakeratosis; (2) acanthosis with clubshaped rete ridges; (3) disorientation of the individual cells commencing above the basal cell layer with variable extension to the surface; (4) nuclear clumping with mitotic figures; and (5) an intact basement membrane.9 The architectural disarray has been referred to as a ‘wind-blown’ pattern. High nuclear-to-cytoplasmic ratios, hyperchromasia, pleomorphism, mitoses and apoptotic bodies are also common (Fig. 2). In one-third of cases, uVIN extends into the follicular epithelium or sebaceous glands, but seldom involves the acrosyringium.6,60,61 Rare cases are associated with dermal amyloid deposition.6
Fig. 2.
Vulvar intraepithelial neoplasia, usual type (uVIN). (A) Warty type; (B) basaloid type; (C) expansion of the rete ridges; (D) loss of nuclear organisation, hyperchromasia and mid-level mitoses; (E) VIN extending into hair follicle; (F) condyloma acuminatum or low-grade intraepithelial lesion with abundant koilocytes.
uVIN has been subdivided into warty and basaloid types, although many cases exhibit mixed morphologies. The warty (condylomatous) type has a spiked or papillary surface with deep and wide rete ridges. Koilocytes, dyskeratotic cells and multinucleated cells are conspicuous. The basaloid (or undifferentiated) type of uVIN is flat and shows basaloid cells typically replacing the full thickness epithelium. Some studies suggest that basaloid uVIN has a worse prognosis than the warty type, but this has been variable in the literature.4,6
VIN, differentiated type
The recognition of dVIN is a challenge, even for experienced gynaecological pathologists. On low power examination, there is acanthosis, occasional parakeratosis, and irregular elongation and anastomoses of the rete ridges (Fig. 3). The architectural disarray seen in uVIN is not seen in dVIN. On high power, nuclear atypia is often confined to the basal and parabasal layers. The nuclei are enlarged, uniform in size, contain coarse chromatin or open vesicular nuclei, prominent nucleoli and scattered mitoses.6,41 One of the most helpful features relates to the phenomenon of ‘premature differentiation or keratinisation’. The cells have ample eosinophilic cytoplasm due to the accumulation of intracellular keratin, and the eosinophilic cells can be juxtaposed to the epidermal-dermal junction, which imparts a hypereosinophilic appearance to the lesion. Dyskeratosis, extracellular keratin and abortive squamous pearls may be seen within the lower layers of the epidermis.62 Prominent intercellular bridges are seen in the absence of inflammation, a feature which is thought to be due to loss of cellular cohesion rather than spongiosis.6 Extension into the skin appendages, in contrast to uVIN, is rare.41
Fig. 3.
Vulvar intraepithelial neoplasia, differentiated type (dVIN). (A) Partial thickness dysplasia with retention of keratohyaline granules; (B) basal atypia, nuclei with prominent nucleoli and intercellular bridges; (C) hypereosinophilia and premature keratinisation; (D) irregular branching and anastomoses of rete ridges; (E) pseudoinvasion, regular spacing of nests with rounded contours (arrows); (F) paradoxical maturation suggestive of early invasion.
dVIN often lacks full thickness atypia, with normal maturation in the superficial layers and retention of keratohyaline granules. Chronic inflammation can also complicate the diagnosis, as the subtle atypia seen in dVIN becomes very difficult to distinguish from reactive atypia. The optimal biopsy should include the interface between the lesion and normal skin because dVIN often has an abrupt edge.63 Approximately 83% of cases have adjacent squamous cell hyperplasia (SCH) or LS.41
Vulvar squamous cell carcinoma
Kurman et al. made the observation that VSCC arising from uVIN displayed warty or basaloid morphology compared to VSCC arising from dVIN which was more likely to be keratinising VSCC.54,64,65 Subsequent studies have revealed that this distinction is not always clear-cut. Approximately 9–21% of cases have shown a discrepancy between histology and HPV or p16 detection.37,66,67 Santos et al. found that 37.5% of HPV-positive tumours were keratinising VSCC and 9.2% of HPV-negative carcinomas had basaloid or warty features.40 Therefore, the presence of keratinisation, basaloid or warty features alone do not necessarily indicate the HPV status of VSCC.
IMMUNOHISTOCHEMISTRY
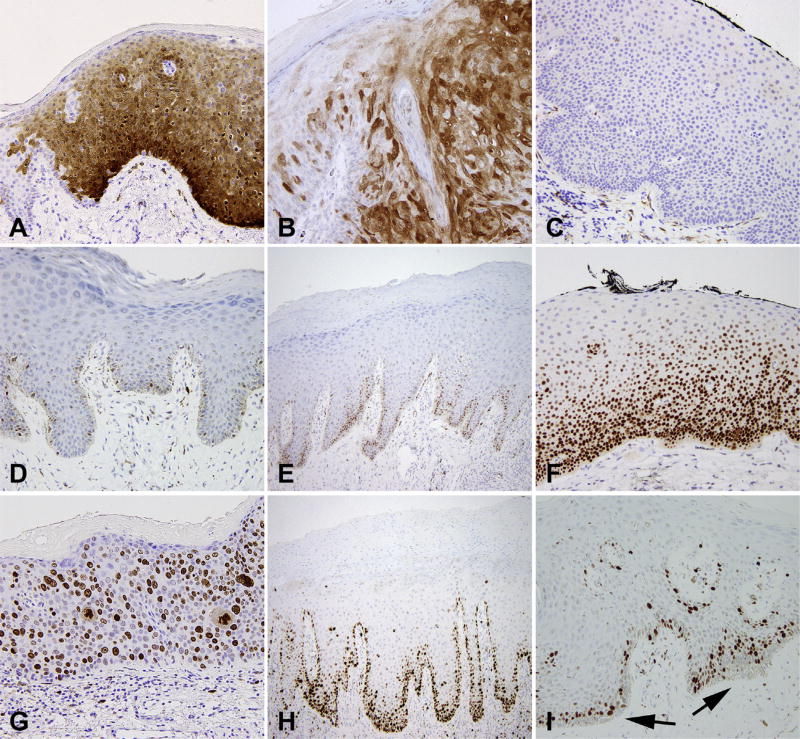
The major immunochemical patterns seen in VIN are depicted in Fig. 4.
Fig. 4.
Vulvar intraepithelial neoplasia (VIN), immunohistochemical features. p16 with (A) diffuse strong block-like staining in VIN 3; (B) patchy staining in condyloma acuminatum; (C) negative in dVIN. p53 shows (D) weak patchy staining in normal skin, (E) increased basal staining in squamous cell hyperplasia, and (F) increased basal and parabasal staining in dVIN. Ki-67 shows (G) full thickness staining in VIN 3, and (H) increased basal and parabasal staining in dVIN. (I) normal skin shows no Ki-67 staining in the basal layer (arrows).
p16
Immunohistochemical positivity for p16 correlates extremely well with high-risk HPV status (>90%) and is commonly used as a surrogate marker for high-risk HPV infection.39,40,66,68 The staining pattern should be diffuse, strong and continuous (nuclear and cytoplasmic), referred to as a ‘block-like’ pattern. Staining should be present in the basal layer with extension upwards to involve at least one-third of the epithelial thickness.58
Almost 100% of HSIL are strongly p16 positive, LSIL is less intense and patchy, and only 0–17% of dVIN show p16 staining, which is generally weak.39,40,68–71 LS and SCH tend to be negative for p16.40,68,71
p53
Yang and Hart reported p53 positivity in 10 of 12 (83%) cases of dVIN with positive staining in the basal layer and suprabasilar extension (ranging from lower one-third to full thickness), with staining in >90% of basal cells. The adjacent normal epidermis showed patchy positivity in <10% of basal cells with no suprabasilar extension.41 Subsequent studies have reiterated these findings, reporting positive p53 in 66–100% of dVIN.39,40,69,71,72 Occasional cases of dVIN display strong suprabasilar staining and minimal basal staining (unpublished observations).
However, as a diagnostic adjunct to help distinguish dVIN from SCH and reactive changes, p53 has its limitations. Increased p53 staining can be seen in 5–61% of LS and up to 40% of SCH, and is thought to be due to increased oxidative stress.71–75
There are two patterns of aberrant immunostaining due to p53 mutations: strong and diffuse staining due to missense mutations, and completely negative staining (null-pattern) due to nonsense mutations. In a survey by Singh et al., six of 22 (27%) dVIN had null-pattern staining.63 The null-pattern presents another diagnostic challenge, because normal epithelium can show weak focal p53 making the distinction between the two very difficult.
Ki-67/MIB-1
Ki-67 can be another helpful marker to distinguish dVIN from SCH and normal epithelium. A Ki-67-negative basal cell layer is a distinct feature of normal epithelium; uVIN and dVIN show positive staining for Ki-67 in the basal and suprabasilar layers. In dVIN, Ki-67 staining is positive in the basal layer and a thin parabasal layer38 which is in contrast to the basal expression seen in LS, which can be a helpful distinguishing feature.76 Other studies have reiterated these findings.39,69,77 The staining for Ki-67 in uVIN is much more conspicuous, and usually stains the full thickness of the epithelium.38,39,77
Other markers
Increased immunohistochemical expression of ProEx C, telomerase, β-catenin and osteopontin as well as abnormal loss of E-cadherin have been reported in uVIN in a limited number of studies, but their diagnostic utility does not exceed that already offered by p16.78–81
Single studies have suggested SOX2, phosphorylated-S6 and cyclin-D1 are helpful in diagnosing dVIN, but further studies with larger numbers are needed to confirm the utility of these markers.74,77,82–84
MOLECULAR PATHOGENESIS
The pathogenesis of HPV-induced malignancy has been well described. The HPV protein E6 degrades the tumour suppressor p53, resulting in deregulation of cell cycle arrest. E7 inactivates the tumour suppressor RB and releases E2F transcription factors, causing cellular hyperproliferation. The physiological function of RB is to inhibit the transcription of cyclin-dependent kinase inhibitors p16 and p14. Therefore HPV-associated neoplasms typically show increased p16 expression and minimal to no expression of p53.34
The clonality of uVIN has been demonstrated by X-chromosome inactivation and loss of heterozygosity.85–88
The pathogenesis of HPV-independent VSCC, on the other hand, is not well understood. When dVIN was first described, many pathologists remained skeptical of the entity. They debated whether dVIN was a true precursor to HPV-negative VSCC or merely a reactive squamous change adjacent to a growing tumour. Others likened dVIN to well-differentiated squamous lesions seen in the oral cavity.84 Pinto et al. studied 11 cases of dVIN with six associated VSCC and sequenced exons 2–11 of TP53. Six of 10 cases harboured at least one TP53 mutation. Two cases had identical TP53 mutations in the VSCC and adjacent dVIN, confirming their clonal relationship. In addition, disparate foci of dVIN showed different TP53 mutations, highlighting the presence of multiple neoplastic clones.89 Some studies have demonstrated clonality and allelic imbalances in LS and SCH, while others have not.85,87,88,90
A review by Trietsch et al. stated that TP53 mutations were found in two of 66 (3%) HPV-positive VIN and 10 of 47 (21%) HPV-negative VIN.91 It is clear that not all HPV-independent VSCC follow the TP53 pathway, and other pathways of oncogenesis remain elusive. HPV-negative VSCC have mutations in CDKN2A (14.8%), HRAS (11.2%), PIK3CA (7.9%), PPP2R1A (3.3%) and EGFR, but these mutations have not been investigated or confirmed in VIN.67,91 Additional molecular alterations in HPV-negative VSCC include gains in chromosome 3q26 and hyper-methylation of RASSF2A, MGMT and TSP1.91,92
DIAGNOSTIC CHALLENGES AND DIFFERENTIAL DIAGNOSIS
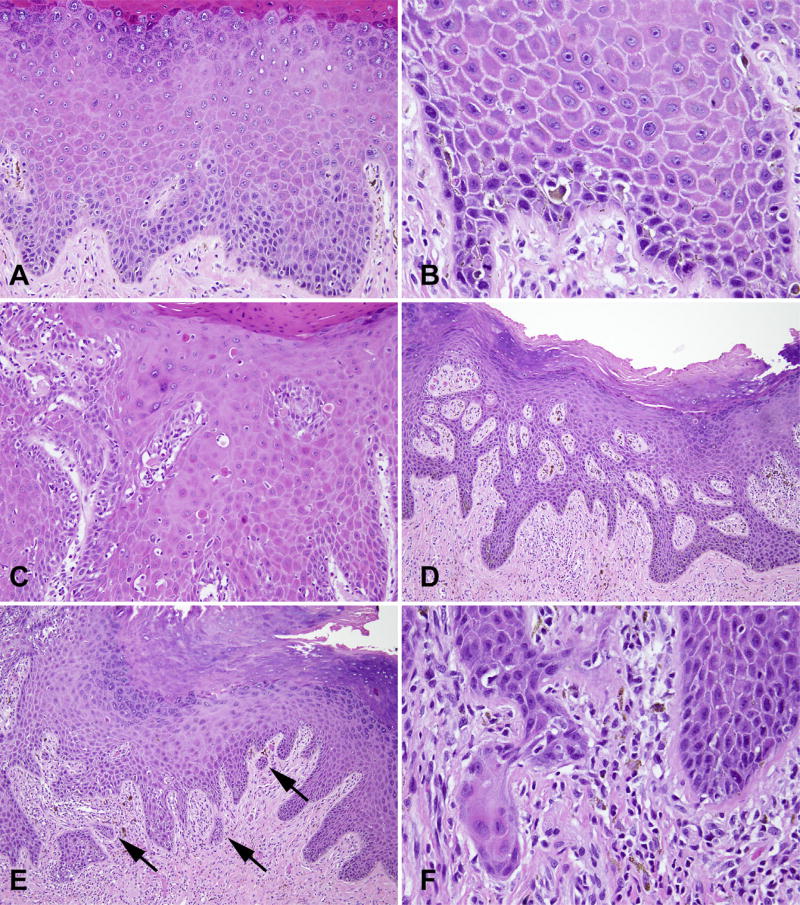
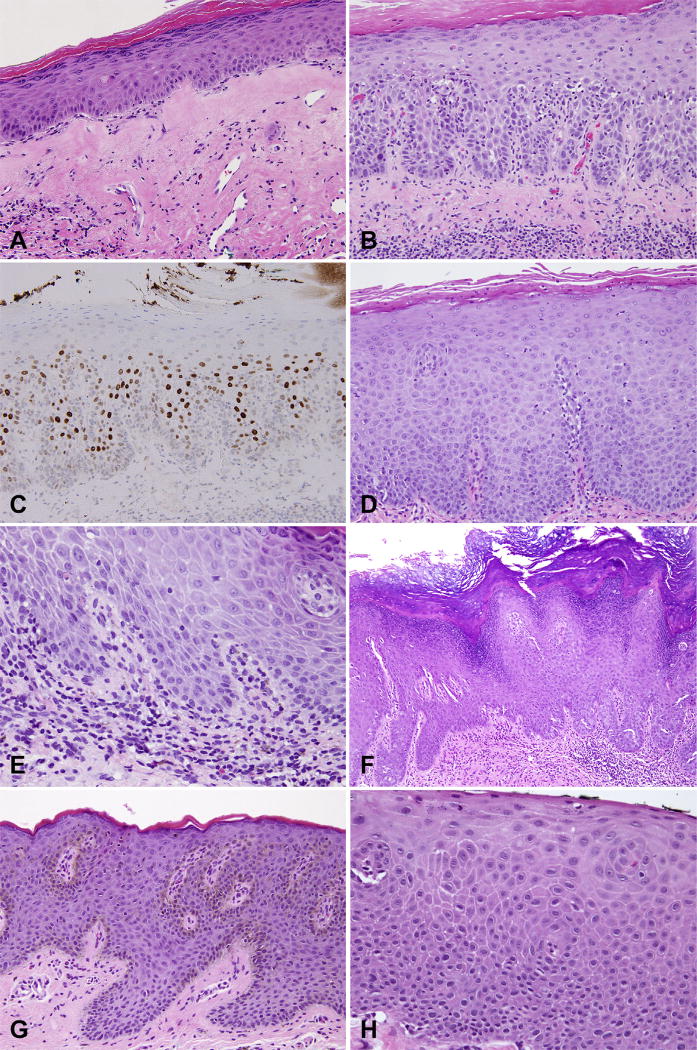
There are several diagnostic and differential diagnostic considerations that come into play in the pathological evaluation of vulvar lesions in general, and VIN in particular (Fig. 5). Some of these issues are summarised below.
Fig. 5.
Differential diagnosis for vulvar intraepithelial neoplasia (VIN). (A) Lichen sclerosus; (B) hypertrophic lichen sclerosus with (C) increased p53 staining; (D) squamous hyperplasia due to candida; (E) spongiotic dermatitis with rare eosinophils; (F) pseudoepitheliomatous hyperplasia due to extramammary Paget’s disease; (G) squamous hyperplasia due to melanoma in situ; (H) basaloid variant of dVIN mimicking uVIN.
Assessment of coexistent invasion
Thorough sampling of VIN lesions is important because 3.2–18.8% of biopsies can have unsuspected stromal invasion.50,52 Early invasion usually presents as single cells or nests of eosinophilic keratinocytes with irregular or angulated contours, invading from the basilar epidermis or from the elongated rete ridges.34 A desmoplastic stromal reaction is a helpful feature, if present. Tangential sectioning of the rete ridges can mimic invasion; in this situation, the nests are evenly spaced, have rounded or bulbous contours and are not associated with stromal desmoplasia.
The depth of invasion is measured from the epithelialstromal junction of the adjacent most superficial dermal papilla to the deepest point of the tumour. The International Federation of Gynecology and Obstetrics (FIGO) defines a stage IA vulvar carcinoma as having a diameter ≤2 cm and stromal invasion ≤ 1 mm. A stage IA tumour has <1% risk of lymph node metastases, compared to tumours invading 1.1–5.0 mm having lymph node metastasis in up to 20%.93 Lymphadenectomy is recommended for any tumour beyond stage IA in most institutions and hence accurate assessment of invasion is critically important for guiding patient care. Caution should be exercised when assessing invasion in hair-bearing skin, as one-third of cases can involve the skin appendages as deep as 2.7 mm.5 However, the interobserver variability amongst pathologists for assessing invasion is suboptimal. Abdel-Mesih et al. reports a kappa of 0.24 for diagnosing invasion and 0.51 for measuring depth of invasion.94 van den Einden et al. suggested an alternative method of measuring invasion, from the basement membrane of the adjacent tumour-free rete ridge to the deepest point of invasion. This allowed for 19% of patients with stage IB disease to be downstaged to stage IA. All these patients had fewer recurrences and higher disease-specific survival than the remaining stage IB tumours.95
Lichen sclerosus
LS is frequently seen in association with dVIN. The classic appearance of LS is a thinned epidermis with loss of the rete ridges, basal vacuolar change and a wide band of homogenised collagen within the dermis. A band-like lymphocytic infiltrate and variable oedema may also be present, especially in the early phase of development.96 Long term studies have shown that LS has a very low risk (1–3%) of progression to VSCC, and is not considered a premalignant lesion by most authors.4,97,98
The finding of basal nuclear atypia in otherwise ordinary LS has been referred to as atypical LS. Atypical LS can show increased p53 staining, and some authors suspect this may represent a very early form of dVIN.57,97 LS with hyperplasia, dyskeratosis and parakeratosis (usually as columns above the dermal papillae), has also been referred to as hypertrophic LS. There is minimal basal cytological atypia, no crowding and minimal to no mitoses. While some authors have found an increased progression of hypertrophic LS to VSCC, others have not.99,100 Further studies with long-term follow-up are needed to clarify the natural history of LS, atypical LS and hypertrophic LS.
Squamous cell hyperplasia
SCH is considered a diagnosis of exclusion, where the cause of hyperplasia is not attributable to a more specific dermatological condition. SCH lacks atypia and the 1976 ISSVD termed this lesion ‘hyperplastic dystrophy without atypia’6. SCH has an organised proliferation of mildly enlarged but non-atypical keratinocytes and absent or minimal mitoses restricted to the basal layer. Unlike dVIN, SCH does not exhibit features of premature keratinisation, expanded rete ridges nor parakeratosis.6
Pseudoepitheliomatous hyperplasia
Pseudoepitheliomatous hyperplasia is a well-documented phenomenon in the skin outside of the vulva. It is a benign proliferative reaction, usually incited by adjacent ulceration, infections and neoplasms. It is characterised by acanthosis, papillomatosis, dyskeratotic cells, and may show some nuclear pleomorphism. Atypical mitotic figures and increased p53 expression are not seen.96 Patients with HIV have been found to have striking pseudoepitheliomatous hyperplasia due to HSV infection in the vulva, mimicking squamous cell carcinoma.101–103
Inflammatory, infectious and other dermatological disorders
The vulva can be affected by a range of dermatological disorders including seborrheic keratosis, psoriasis, lichen planus, eczematous/spongiotic dermatitis, Zoon’s vulvitis, hidradenitis suppurativa, Behçet’s disease, radiation dermatitis and infections.104 Lichen simplex chronicus is the most common chronic inflammatory disorder affecting the vulva. It manifests as acanthosis, hyperkeratosis and inflammation. The cells have open chromatin and lack interface atypia.6
dVIN with basaloid pattern
Ordi et al. described four cases of dVIN with a basaloid pattern, mimicking uVIN. All four cases had conspicuous architectural disorganisation and homogeneous populations of basaloid undifferentiated keratinocytes. They were negative for p16 and HPV, and positive for p53.105
Extramammary Paget’s disease
Extramammary Paget’s disease should always be considered in the differential diagnosis of VIN. Classically, extramammary Paget’s disease is positive for CK7, CAM5.2, CEA, PAS, mucin stains, GCDFP-15 and HER2, whereas VIN is positive for CK5/6 and p63. Sah and McCluggage report two cases of Paget’s disease where the cells were confluent and sheet-like, rather than exhibiting the usual nested pattern. Interestingly, both cases were strongly positive for p16. They were also positive for CK7 and CEA. The authors report this lesion as potential mimic of uVIN.106
Pagetoid VIN
An estimated 5% of vulvar HSIL has a nested pattern, also known as pagetoid squamous cell carcinoma in situ or pagetoid Bowen’s disease.107,108 A challenge in distinguishing it from Paget’s disease is that pagetoid VIN is positive for cytokeratin 7. However, it is also positive for cytokeratin 5/6, p63, and negative for CEA, mucin and GCDFP-15, which are helpful in arriving at the correct diagnosis.107–110
VIN with mucinous differentiation
Two cases of VIN with mucinous differentiation have been reported. The mucinous cells were positive for CK7, CAM5.2, CEA, EMA, p16 and tested positive for HPV. The authors suggest the mucinous cells were metaplastic and due to aberrant differentiation.111
Melanoma
Melanoma should always be in the differential diagnosis of VIN. Neoplastic melanocytes may colonise the epidermis in the form of single cells, nests or more confluent groups, potentially mimicking VIN. Unlike VIN, melanoma is positive for S100, SOX10, HMB45 and MelanA. SOX2 is responsible for self-renewal in neural crest melanocytes and has also been used to confirm a diagnosis of melanoma.112 A recent study showed that SOX2 was positive in five of 16 (31%) uVIN and eight of 18 (44%) dVIN. Thus, caution should be taken with the use of SOX2 in the differential diagnosis with melanoma.83
Verrucous carcinoma
Verrucous carcinoma (VC) is well-known in the oral cavity and is less common in the lower anogenital tract. VC is a non-HPV-related and well-differentiated form of VSCC, displaying acanthosis, parakeratosis, orthokeratosis, organised keratinocytes, minimal cellular atypia and most characteristically bulbous rete ridges termed ‘baggy trousers’ by Scurry and Wilkinson.4,113,114 They may be associated with local recurrences but rarely exhibit distant metastases or fatal outcomes.113,115
Vulvar acanthosis with altered differentiation (VAAD)
This entity was first described by Nascimento and Crum in a study of nine VC in 2004. Seven cases had a distinct lesion adjacent to VC, termed VAAD. The triad of features that characterised VAAD included: (1) acanthosis with variable verruciform architecture, (2) loss of the granular layer with superficial epithelial pallor, and (3) multilayered plaque-like parakeratosis. All cases of VAAD were HPV negative. VAAD was proposed as the precursor lesion to VC. It was thought to be distinct from dVIN due to its presence of plaque-like parakeratosis, lack of atypia and lack of premature keratinisation as seen in dVIN.114 Only two other reports of VAAD have been published in the literature since 2004, and both question whether VAAD is truly a distinct entity or whether it represents a morphological variant of hypertrophic LS or dVIN.116,117 Further studies are needed to answer these questions.
CONCLUSIONS
Vulvar squamous precursor lesions, uVIN and dVIN, are distinct entities. The histological features of uVIN are more readily recognised than dVIN, which is a particularly challenging diagnosis. Further ancillary markers with greater specificity (likely based on studies that better characterise the genetic and epigenetic alterations in VSCC carcinogenesis) are needed to help pathologists more accurately diagnose these entities.
Footnotes
Conflicts of interest and sources of funding: The authors state that there are no conflicts of interest to disclose.
References
- 1.Kosary CL. National Cancer Institute, SEER Program, 1988–2001. Bethesda: National Cancer Institute; 2007. Cancer of the Vulva; pp. 147–154. [Google Scholar]
- 2.Judson PL, Habermann EB, Baxter NN, et al. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107:1018–22. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- 3.van de Nieuwenhof HP, Massuger LF, van der Avoort IA, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer. 2009;45:851–6. doi: 10.1016/j.ejca.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Scurry J, Wilkinson EJ. Review of terminology of precursors of vulvar squamous cell carcinoma. J Low Genit Tract Dis. 2006;10:161–9. doi: 10.1097/00128360-200607000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson EJ, Cox JT, Selim MA, et al. Evolution of terminology for human-papillomavirus-infection-related vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2015;19:81–7. doi: 10.1097/LGT.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 6.Hart WR. Vulvar intraepithelial neoplasia: historical aspects and current status. Int J Gynecol Pathol. 2001;20:16–30. doi: 10.1097/00004347-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bowen JT. Centennial paper. May 1912 (J Cutan Dis Syph 1912;30:241–255). Precancerous dermatoses: a study of two cases of chronic atypical epithelial proliferation. By John T. Bowen, M.D., Boston. Arch Dermatol. 1983;119:243–60. [PubMed] [Google Scholar]
- 8.Hudelo ML, Cailliau Oury. Dyskeratose erythroplasiforme de la muqueuse vulvaire. Bull Soc Franc Dermatol Syph. 1922;29:139–42. [Google Scholar]
- 9.Knight RVD. Bowen’s disease of the vulva. Am J Obstet Gynecol. 1943:514–24. [Google Scholar]
- 10.Woodruff JD, Hildebrandt EE. Carcinoma in situ of the vulva. Obstet Gynecol. 1958;12:414–24. [PubMed] [Google Scholar]
- 11.Berger BW, Hori Y. Multicentric Bowen’s disease of the genitalia: spontaneous regression of lesions. Arch Dermatol. 1978;114:1698–9. [PubMed] [Google Scholar]
- 12.Friedrich EG. Reversible vulvar atypia. A case report. Obstet Gynecol. 1972;39:173–81. [PubMed] [Google Scholar]
- 13.Wade TR, Kopf AW, Ackerman AB. Bowenoid papulosis of the genitalia. Arch Dermatol. 1979;115:306–8. [PubMed] [Google Scholar]
- 14.Abell MR, Gosling JR. Intraepithelial and infiltrative carcinoma of vulva: Bowen’s type. Cancer. 1961;14:318–29. doi: 10.1002/1097-0142(196103/04)14:2<318::aid-cncr2820140212>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman R, DiPaola G, Friedrich E, et al. New nomenclature for vulvar disease. Obstet Gynecol. 1976;47:122–4. [PubMed] [Google Scholar]
- 16.Richart RM. Natural history of cervical intraepithelial neoplasia. Clin Obstet Gynecol. 1967;10:748–84. [Google Scholar]
- 17.Crum CP, Fu YS, Levine RU, et al. Intraepithelial squamous lesions of the vulva: biologic and histologic criteria for the distinction of condylomas from vulvar intraepithelial neoplasia. Am J Obstet Gynecol. 1982;144:77–83. doi: 10.1016/0002-9378(82)90398-2. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson EJ, Kneale B, Lynch PJ. Report of the ISSVD Terminology Committee. J Reprod Med. 1986;31:973–4. [Google Scholar]
- 19.Scurry J, Campion M, Scurry B, et al. Pathologic audit of 164 consecutive cases of vulvar intraepithelial neoplasia. Int J Gynecol Pathol. 2006;25:176–81. doi: 10.1097/01.pgp.0000189238.19027.df. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros F, Nascimento AF, Crum CP. Early vulvar squamous neoplasia: advances in classification, diagnosis, and differential diagnosis. Adv Anat Pathol. 2005;12:20–6. doi: 10.1097/01.pqp.0000151268.72556.f3. [DOI] [PubMed] [Google Scholar]
- 21.Bornstein J, Bogliatto F, Haefner HK, et al. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. J Low Genit Tract Dis. 2016;20:11–4. doi: 10.1097/LGT.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 22.Kurman RJ, Carcangiu ML, Herrington CS, et al., editors. WHO Classification of Tumours of Female Reproductive Organs. 4. Lyon: International Agency for Research on Cancer; 2014. pp. 229–253. [Google Scholar]
- 23.Joura EA, Lösch A, Haider-Angeler MG, et al. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J Reprod Med. 2000;45:613–5. [PubMed] [Google Scholar]
- 24.van de Nieuwenhof HP, van der Avoort IAM, de Hullu JA. Review of squamous premalignant vulvar lesions. Crit Rev Oncol Hematol. 2008;68:131–56. doi: 10.1016/j.critrevonc.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Castle PE, Maza M. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect. 2016;144:449–68. doi: 10.1017/S0950268815002198. [DOI] [PubMed] [Google Scholar]
- 26.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Zachow KR, Ostrow RS, Bender M, et al. Detection of human papillomavirus DNA in anogenital neoplasias. Nature. 1982;300:771–3. doi: 10.1038/300771a0. [DOI] [PubMed] [Google Scholar]
- 28.van Beurden M, ten Kate FJ, Smits HL, et al. Multifocal vulvar intraepithelial neoplasia grade III and multicentric lower genital tract neoplasia is associated with transcriptionally active human papillomavirus. Cancer. 1995;75:2879–84. doi: 10.1002/1097-0142(19950615)75:12<2879::aid-cncr2820751214>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Bonvicini F, Venturoli S, Ambretti S, et al. Presence and type of oncogenic human papillomavirus in classic and in differentiated vulvar intraepithelial neoplasia and keratinizing vulvar squamous cell carcinoma. J Med Virol. 2005;77:102–6. doi: 10.1002/jmv.20420. [DOI] [PubMed] [Google Scholar]
- 30.Bornstein J, Kaufman RH, Adam E, et al. Multicentric intraepithelial neoplasia involving the vulva. Clinical features and association with human papillomavirus and herpes simplex virus. Cancer. 1988;62:1601–4. doi: 10.1002/1097-0142(19881015)62:8<1601::aid-cncr2820620824>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Buscema J, Naghashfar Z, Sawada E, et al. The predominance of human papillomavirus type 16 in vulvar neoplasia. Obstet Gynecol. 1988;71:601–6. [PubMed] [Google Scholar]
- 32.Haefner HK, Tate JE, McLachlin CM, et al. Vulvar intraepithelial neoplasia: age, morphological phenotype, papillomavirus DNA, and coexisting invasive carcinoma. Hum Pathol. 1995;26:147–54. doi: 10.1016/0046-8177(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 33.Park JS, Jones RW, McLean MR, et al. Possible etiologic heterogeneity of vulvar intraepithelial neoplasia. A correlation of pathologic characteristics with human papillomavirus detection by in situ hybridization and polymerase chain reaction. Cancer. 1991;67:1599–607. doi: 10.1002/1097-0142(19910315)67:6<1599::aid-cncr2820670622>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 34.del Pino M, Rodriguez-Carunchio L, Ordi J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology. 2013;62:161–75. doi: 10.1111/his.12034. [DOI] [PubMed] [Google Scholar]
- 35.Léonard B, Kridelka F, Delbecque K, et al. A clinical and pathological overview of vulvar condyloma acuminatum, intraepithelial neoplasia, and squamous cell carcinoma. BioMed Res Int. 2014;2014:480573. doi: 10.1155/2014/480573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maniar KP, Ronnett BM, Vang R, et al. Coexisting high-grade vulvar intraepithelial neoplasia (VIN) and condyloma acuminatum: independent lesions due to different HPV types occurring in immunocompromised patients. Am J Surg Pathol. 2013;37:53–60. doi: 10.1097/PAS.0b013e318263cda6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Sanjosé S, Alemany L, Ordi J, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 1990;49:3450–61. doi: 10.1016/j.ejca.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 38.van der Avoort IAM, Shirango H, Hoevenaars BM, et al. Vulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathways. Int J Gynecol Pathol. 2006;25:22–9. doi: 10.1097/01.pgp.0000177646.38266.6a. [DOI] [PubMed] [Google Scholar]
- 39.Hoevenaars BM, van der Avoort IA, de Wilde PC, et al. A panel of p16(INK4A), MIB1 and p53 proteins can distinguish between the 2 pathways leading to vulvar squamous cell carcinoma. Int J Cancer. 2008;123:2767–73. doi: 10.1002/ijc.23857. [DOI] [PubMed] [Google Scholar]
- 40.Santos M, Landolfi S, Olivella A, et al. p16 overexpression identifies HPV-positive vulvar squamous cell carcinomas. Am J Surg Pathol. 2006;30:1347–56. doi: 10.1097/01.pas.0000213251.82940.bf. [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Hart WR. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: a clinicopathologic study including analysis of HPV and p53 expression. Am J Surg Pathol. 2000;24:429–41. doi: 10.1097/00000478-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Crum CP, Liskow A, Petras P, et al. Vulvar intraepithelial neoplasia (severe atypia and carcinoma in situ). A clinicopathologic analysis of 41 cases. Cancer. 1984;54:1429–34. doi: 10.1002/1097-0142(19841001)54:7<1429::aid-cncr2820540733>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 43.Jones RW, Rowan DM. Vulvar intraepithelial neoplasia III: a clinical study of the outcome in 113 cases with relation to the later development of invasive vulvar carcinoma. Obstet Gynecol. 1994;84:741–5. [PubMed] [Google Scholar]
- 44.Al-Ghamdi A, Freedman D, Miller D, et al. Vulvar squamous cell carcinoma in young women: a clinicopathologic study of 21 cases. Gynecol Oncol. 2002;84:94–101. doi: 10.1006/gyno.2001.6466. [DOI] [PubMed] [Google Scholar]
- 45.Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106:1319–26. doi: 10.1097/01.AOG.0000187301.76283.7f. [DOI] [PubMed] [Google Scholar]
- 46.Di Luca D, Rotola A, Pilotti S, et al. Simultaneous presence of herpes simplex and human papilloma virus sequences in human genital tumors. Int J Cancer. 1987;40:763–8. doi: 10.1002/ijc.2910400609. [DOI] [PubMed] [Google Scholar]
- 47.Goffin F, Mayrand MH, Gauthier P, et al. High-risk human papillomavirus infection of the genital tract of women with a previous history or current high-grade vulvar intraepithelial neoplasia. J Med Virol. 2006;78:814–9. doi: 10.1002/jmv.20628. [DOI] [PubMed] [Google Scholar]
- 48.Sykes P, Smith N, McCormick P, et al. High-grade vulval intraepithelial neoplasia (VIN 3): a retrospective analysis of patient characteristics, management, outcome and relationship to squamous cell carcinoma of the vulva 1989–1999. J Obstet Gynaecol. 2002;42:69–74. doi: 10.1111/j.0004-8666.2002.00075.x. [DOI] [PubMed] [Google Scholar]
- 49.McNally OM, Mulvany NJ, Pagano R, et al. VIN 3: a clinicopathologic review. Int J Gynecol Cancer. 2002;12:490–5. doi: 10.1046/j.1525-1438.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- 50.Chafe W, Richards A, Morgan L, et al. Unrecognized invasive carcinoma in vulvar intraepithelial neoplasia (VIN) Gynecol Oncol. 1988;31:154–65. doi: 10.1016/0090-8258(88)90284-3. [DOI] [PubMed] [Google Scholar]
- 51.Lara-Torre E, Perlman SE. Vulvar intraepithelial neoplasia in adolescents with abnormal Pap smear results: a series report. J Pediatr Adolesc Gynecol. 2004;17:45–8. doi: 10.1016/j.jpag.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 52.van Seters M, van Beurden M, de Craen AJM. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97:645–51. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Buscema J, Woodruff JD, Parmley TH, et al. Carcinoma in situ of the vulva. Obstet Gynecol. 1980;55:225–30. [PubMed] [Google Scholar]
- 54.Hørding U, Junge J, Poulsen H, et al. Vulvar intraepithelial neoplasia III: a viral disease of undetermined progressive potential. Gynecol Oncol. 1995;56:276–9. doi: 10.1006/gyno.1995.1046. [DOI] [PubMed] [Google Scholar]
- 55.Powell LC, Dinh TV, Rajaraman S, et al. Carcinoma in situ of the vulva. A clinicopathologic study of 50 cases. J Reprod Med. 1986;31:808–14. [PubMed] [Google Scholar]
- 56.Eva LJ, Ganesan R, Chan KK, et al. Differentiated-type vulval intraepithelial neoplasia has a high-risk association with vulval squamous cell carcinoma. Int J Gynecol Cancer. 2009;19:741–4. doi: 10.1111/IGC.0b013e3181a12fa2. [DOI] [PubMed] [Google Scholar]
- 57.Chiesa-Vottero A, Dvoretsky PM, Hart WR. Histopathologic study of thin vulvar squamous cell carcinomas and associated cutaneous lesions: a correlative study of 48 tumors in 44 patients with analysis of adjacent vulvar intraepithelial neoplasia types and lichen sclerosus. Am J Surg Pathol. 2006;30:310–8. doi: 10.1097/01.pas.0000180444.71775.1a. [DOI] [PubMed] [Google Scholar]
- 58.Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16:205–42. doi: 10.1097/LGT.0b013e31825c31dd. [DOI] [PubMed] [Google Scholar]
- 59.Committee on Gynecologic Practice of American College Obstetricians and Gynecologists. ACOG Committee Opinion No. 509: Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011;118:1192–4. doi: 10.1097/AOG.0b013e31823b17c2. [DOI] [PubMed] [Google Scholar]
- 60.Baggish MS, Sze EH, Adelson MD, et al. Quantitative evaluation of the skin and accessory appendages in vulvar carcinoma in situ. Obstet Gynecol. 1989;74:169–74. [PubMed] [Google Scholar]
- 61.Shatz P, Bergeron C, Wilkinson EJ, et al. Vulvar intraepithelial neoplasia and skin appendage involvement. Obstet Gynecol. 1989;74:769–74. [PubMed] [Google Scholar]
- 62.Friedrich EG, Wilkinson EJ, Fu YS. Carcinoma in situ of the vulva: a continuing challenge. Am J Obstet Gynecol. 1980;136:830–43. doi: 10.1016/0002-9378(80)91039-x. [DOI] [PubMed] [Google Scholar]
- 63.Singh N, Leen SL, Han G, et al. Expanding the morphologic spectrum of differentiated VIN (dVIN) through detailed mapping of cases with p53 loss. Am J Surg Pathol. 2015;39:52–60. doi: 10.1097/PAS.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 64.Kurman RJ, Toki T, Schiffman MH. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. 1993;17:133–45. doi: 10.1097/00000478-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Nuovo GJ, Delvenne P, MacConnell P, et al. Correlation of histology and detection of human papillomavirus DNA in vulvar cancers. Gynecol Oncol. 1991;43:275–80. doi: 10.1016/0090-8258(91)90035-4. [DOI] [PubMed] [Google Scholar]
- 66.Cheng AS, Karnezis AN, Jordan S, et al. p16 Immunostaining allows for accurate subclassification of vulvar squamous cell carcinoma into HPV-associated and HPV-independent cases. Int J Gynecol Pathol. 2015 Dec;:1. doi: 10.1097/PGP.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 67.Dong F, Kojiro S, Borger DR, et al. Squamous cell carcinoma of the vulva: a subclassification of 97 cases by clinicopathologic, immunohistochemical, and molecular features (p16, p53, and EGFR) Am J Surg Pathol. 2015;39:1045–53. doi: 10.1097/PAS.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 68.Riethdorf S, Neffen EF, Cviko A, et al. p16INK4A expression as biomarker for HPV 16-related vulvar neoplasias. Hum Pathol. 2004;35:1477–83. doi: 10.1016/j.humpath.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Mulvany NJ, Allen DG. Differentiated intraepithelial neoplasia of the vulva. Int J Gynecol Pathol. 2008;27:125–35. doi: 10.1097/pgp.0b0318134ea34. [DOI] [PubMed] [Google Scholar]
- 70.Saglam O, Samayoa E, Somasekar S, et al. No viral association found in a set of differentiated vulvar intraepithelial neoplasia cases by human papillomavirus and panviral microarray testing. PloS One. 2015;10:e0125292. doi: 10.1371/journal.pone.0125292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santos M, Montagut C, Mellado B, et al. Immunohistochemical staining for p16 and p53 in premalignant and malignant epithelial lesions of the vulva. Int J Gynecol Pathol. 2004;23:206–14. doi: 10.1097/01.pgp.0000130108.03231.89. [DOI] [PubMed] [Google Scholar]
- 72.Hantschmann P, Sterzer S, Jeschke U, et al. P53 expression in vulvar carcinoma, vulvar intraepithelial neoplasia, squamous cell hyperplasia and lichen sclerosus. Anticancer Res. 2005;25:1739–45. [PubMed] [Google Scholar]
- 73.Gambichler T, Kammann S, Tigges C, et al. Cell cycle regulation and proliferation in lichen sclerosus. Regul Pept. 2011;167:209–14. doi: 10.1016/j.regpep.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Rolfe KJ, Eva LJ, MacLean AB, et al. Cell cycle proteins as molecular markers of malignant change in vulvar lichen sclerosus. Int J Gynecol Cancer. 2001;11:113–8. doi: 10.1046/j.1525-1438.2001.011002113.x. [DOI] [PubMed] [Google Scholar]
- 75.Liegl B, Regauer S. p53 immunostaining in lichen sclerosus is related to ischaemic stress and is not a marker of differentiated vulvar intraepithelial neoplasia (d-VIN) Histopathology. 2006;48:268–74. doi: 10.1111/j.1365-2559.2005.02321.x. [DOI] [PubMed] [Google Scholar]
- 76.Scurry J, Beshay V, Cohen C, et al. Ki67 expression in lichen sclerosus of vulva in patients with and without associated squamous cell carcinoma. Histopathology. 1998;32:399–404. doi: 10.1046/j.1365-2559.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- 77.Stewart CJR, Crook ML. Fascin and cyclin D1 immunoreactivity in non-neoplastic vulvar squamous epithelium, vulvar intraepithelial neoplasia and invasive squamous carcinoma: correlation with Ki67 and p16 protein expression. J Clin Pathol. 2014;67:319–25. doi: 10.1136/jclinpath-2013-201920. [DOI] [PubMed] [Google Scholar]
- 78.Chen H, Gonzalez JL, Brennick JB, et al. Immunohistochemical patterns of ProEx C in vulvar squamous lesions: detection of overexpression of MCM2 and TOP2A. Am J Surg Pathol. 2010;34:1250–7. doi: 10.1097/PAS.0b013e3181ecf829. [DOI] [PubMed] [Google Scholar]
- 79.Li B, Zhang Q, Ouyang L, et al. Aberrant staining patterns of E-cadherin and β-catenin: a potential diagnostic value for distinguishing vulvar intraepithelial neoplasia from non-neoplastic vulvar lesions. Int J Clin Exp Pathol. 2013;6:1362–6. [PMC free article] [PubMed] [Google Scholar]
- 80.Wellenhofer A, Brustmann H. Expression of human telomerase reverse transcriptase in vulvar intraepithelial neoplasia and squamous cell carcinoma: an immunohistochemical study with survivin and p53. Arch Pathol Lab Med. 2012;136:1359–65. doi: 10.5858/arpa.2011-0440-OA. [DOI] [PubMed] [Google Scholar]
- 81.Wu Z, Shen Y, Gong K, et al. Increased osteopontin expression is associated with progression from vulvar precancerous lesions to vulvar squamous cell carcinoma. Arch Gynecol Obstet. 2014;289:637–44. doi: 10.1007/s00404-013-3009-3. [DOI] [PubMed] [Google Scholar]
- 82.Carlson BC, Hofer MD, Ballek N, et al. Protein markers of malignant potential in penile and vulvar lichen sclerosus. J Urol. 2013;190:399–406. doi: 10.1016/j.juro.2013.01.102. [DOI] [PubMed] [Google Scholar]
- 83.Brustmann H, Brunner A. Immunohistochemical expression of SOX2 in vulvar intraepithelial neoplasia and squamous cell carcinoma. Int J Gynecol Pathol. 2013;32:323–8. doi: 10.1097/PGP.0b013e31825d820e. [DOI] [PubMed] [Google Scholar]
- 84.Pinto AP, Degen M, Barron P, et al. Phosphorylated S6 as an immunohistochemical biomarker of vulvar intraepithelial neoplasia. Mod Pathol. 2013;26:1498–507. doi: 10.1038/modpathol.2013.85. [DOI] [PubMed] [Google Scholar]
- 85.Pinto AP, Lin MC, Sheets EE, et al. Allelic imbalance in lichen sclerosus, hyperplasia, and intraepithelial neoplasia of the vulva. Gynecol Oncol. 2000;77:171–6. doi: 10.1006/gyno.2000.5739. [DOI] [PubMed] [Google Scholar]
- 86.Rosenthal AN, Ryan A, Hopster D, et al. Molecular evidence of a common clonal origin and subsequent divergent clonal evolution in vulval intraepithelial neoplasia, vulval squamous cell carcinoma and lymph node metastases. Int J Cancer. 2002;99:549–54. doi: 10.1002/ijc.10362. [DOI] [PubMed] [Google Scholar]
- 87.Tate JE, Mutter GL, Boynton KA, et al. Monoclonal origin of vulvar intraepithelial neoplasia and some vulvar hyperplasias. Am J Pathol. 1997;150:315–22. [PMC free article] [PubMed] [Google Scholar]
- 88.Ueda Y, Enomoto T, Miyatake T, et al. Analysis of clonality and HPV infection in benign, hyperplastic, premalignant, and malignant lesions of the vulvar mucosa. Am J Clin Pathol. 2004;122:266–74. doi: 10.1309/65MK-PQT3-E2BD-M67E. [DOI] [PubMed] [Google Scholar]
- 89.Pinto AP, Miron A, Yassin Y, et al. Differentiated vulvar intraepithelial neoplasia contains Tp53 mutations and is genetically linked to vulvar squamous cell carcinoma. Mod Pathol. 2010;23:404–12. doi: 10.1038/modpathol.2009.179. [DOI] [PubMed] [Google Scholar]
- 90.Kim YT, Thomas NF, Kessis TD, et al. p53 mutations and clonality in vulvar carcinomas and squamous hyperplasias: evidence suggesting that squamous hyperplasias do not serve as direct precursors of human papillomavirus-negative vulvar carcinomas. Hum Pathol. 1996;27:389–95. doi: 10.1016/s0046-8177(96)90113-6. [DOI] [PubMed] [Google Scholar]
- 91.Trietsch MD, Nooij LS, Gaarenstroom KN, et al. Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursor lesions: a review of the current literature. Gynecol Oncol. 2015;136:143–57. doi: 10.1016/j.ygyno.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 92.Aulmann S, Schleibaum J, Penzel R, et al. Gains of chromosome region 3q26 in intraepithelial neoplasia and invasive squamous cell carcinoma of the vulva are frequent and independent of HPV status. J Clin Pathol. 2008;61:1034–7. doi: 10.1136/jcp.2008.056275. [DOI] [PubMed] [Google Scholar]
- 93.Yoder BJ, Rufforny I, Massoll NA, et al. Stage IA vulvar squamous cell carcinoma: an analysis of tumor invasive characteristics and risk. Am J Surg Pathol. 2008;32:765–72. doi: 10.1097/PAS.0b013e318159a2cb. [DOI] [PubMed] [Google Scholar]
- 94.Abdel-Mesih A, Daya D, Onuma K, et al. Interobserver agreement for assessing invasion in stage IA vulvar squamous cell carcinoma. Am J Surg Pathol. 2013;37:1336–41. doi: 10.1097/PAS.0b013e31829f306a. [DOI] [PubMed] [Google Scholar]
- 95.van den Einden LCG, Massuger LF, Jonkman JK, et al. An alternative way to measure the depth of invasion of vulvar squamous cell carcinoma in relation to prognosis. Mod Pathol. 2015;28:295–302. doi: 10.1038/modpathol.2014.103. [DOI] [PubMed] [Google Scholar]
- 96.Reyes MC, Cooper K. An update on vulvar intraepithelial neoplasia: terminology and a practical approach to diagnosis. J Clin Pathol. 2014;67:290–4. doi: 10.1136/jclinpath-2013-202117. [DOI] [PubMed] [Google Scholar]
- 97.Hart WR, Norris HJ, Helwig EB. Relation of lichen sclerosus et atrophicus of the vulva to development of carcinoma. Obstet Gynecol. 1975;45:369–77. [PubMed] [Google Scholar]
- 98.Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: A prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061–7. doi: 10.1001/jamadermatol.2015.0643. [DOI] [PubMed] [Google Scholar]
- 99.van de Nieuwenhof HP, Bulten J, Hollema H, et al. Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod Pathol. 2011;24:297–305. doi: 10.1038/modpathol.2010.192. [DOI] [PubMed] [Google Scholar]
- 100.Weyers W. Hypertrophic lichen sclerosus with dyskeratosis and parakeratosis—a common presentation of vulvar lichen sclerosus not associated with a significant risk of malignancy. Am J Dermatopathol. 2013;35:713–21. doi: 10.1097/DAD.0b013e31827e7ea9. [DOI] [PubMed] [Google Scholar]
- 101.Frimer M, Chudnoff S, Hebert T, et al. Pseudoepitheliomatous hyperplasia mimicking vulvar cancer in a patient with AIDS. J Low Genit Tract Dis. 2011;15:66–8. doi: 10.1097/LGT.0b013e3181f0b8f5. [DOI] [PubMed] [Google Scholar]
- 102.Strehl JD, Mehlhorn G, Koch MC, et al. HIV-associated hypertrophic herpes simplex genitalis with concomitant early invasive squamous cell carcinoma mimicking advanced genital cancer: case report and literature review. Int J Gynecol Pathol. 2012;31:286–93. doi: 10.1097/PGP.0b013e318237d581. [DOI] [PubMed] [Google Scholar]
- 103.Tangjitgamol S, Loharamtaweethong K, Thawaramara T, et al. Vulvar pseudoepitheliomatous hyperplasia associated with herpes simplex virus type II mimicking cancer in an immunocompromised patient. J Obstet Gynaecol Res. 2014;40:255–8. doi: 10.1111/jog.12129. [DOI] [PubMed] [Google Scholar]
- 104.Chan MP, Zimarowski MJ. Vulvar dermatoses: a histopathologic review and classification of 183 cases. J Cutan Pathol. 2015;42:510–8. doi: 10.1111/cup.12541. [DOI] [PubMed] [Google Scholar]
- 105.Ordi J, Alejo M, Fusté V, et al. HPV-negative vulvar intraepithelial neoplasia (VIN) with basaloid histologic pattern: an unrecognized variant of simplex (differentiated) VIN. Am J Surg Pathol. 2009;33:1659–65. doi: 10.1097/PAS.0b013e3181b40081. [DOI] [PubMed] [Google Scholar]
- 106.Sah SP, McCluggage WG. Florid vulval Paget disease exhibiting p16 immunoreactivity and mimicking classic VIN. Int J Gynecol Pathol. 2013;32:221–7. doi: 10.1097/PGP.0b013e31825909f6. [DOI] [PubMed] [Google Scholar]
- 107.Armes JE, Lourie R, Bowlay G, et al. Pagetoid squamous cell carcinoma in situ of the vulva: comparison with extramammary paget disease and nonpagetoid squamous cell neoplasia. Int J Gynecol Pathol. 2008;27:118–24. doi: 10.1097/pgp.0b013e318142acf0. [DOI] [PubMed] [Google Scholar]
- 108.Raju RR, Goldblum JR, Hart WR. Pagetoid squamous cell carcinoma in situ (pagetoid Bowen’s disease) of the external genitalia. Int J Gynecol Pathol. 2003;22:127–35. doi: 10.1097/00004347-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 109.Memezawa A, Okuyama R, Tagami H, et al. p63 constitutes a useful histochemical marker for differentiation of pagetoid Bowen’s disease from extramammary Paget’s disease. Acta Derm Venereol. 2008;88:619–20. doi: 10.2340/00015555-0512. [DOI] [PubMed] [Google Scholar]
- 110.Sah SP, Kelly PJ, McManus DT, et al. Diffuse CK7, CAM5.2 and BerEP4 positivity in pagetoid squamous cell carcinoma in situ (pagetoid Bowen’s disease) of the perianal region: a mimic of extramammary Paget’s disease. Histopathology. 2013;62:511–4. doi: 10.1111/his.12003. [DOI] [PubMed] [Google Scholar]
- 111.McCluggage WG, Jamison J, Boyde A, et al. Vulval intraepithelial neoplasia with mucinous differentiation: report of 2 cases of a hitherto undescribed phenomenon. Am J Surg Pathol. 2009;33:945–9. doi: 10.1097/PAS.0b013e3181966f2d. [DOI] [PubMed] [Google Scholar]
- 112.Santini R, Pietrobono S, Pandolfi S, et al. SOX2 regulates self-renewal and tumorigenicity of human melanoma-initiating cells. Oncogene. 2014;33:4697–708. doi: 10.1038/onc.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gualco M, Bonin S, Foglia G, et al. Morphologic and biologic studies on ten cases of verrucous carcinoma of the vulva supporting the theory of a discrete clinico-pathologic entity. Int J Gynecol Cancer. 2003;13:317–24. doi: 10.1046/j.1525-1438.2003.13200.x. [DOI] [PubMed] [Google Scholar]
- 114.Nascimento AF, Granter SR, Cviko A, et al. Vulvar acanthosis with altered differentiation: a precursor to verrucous carcinoma? Am J Surg Pathol. 2004;28:638–43. doi: 10.1097/00000478-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 115.Brisigotti M, Moreno A, Murcia C, et al. Verrucous carcinoma of the vulva. A clinicopathologic and immunohistochemical study of five cases. Int J Gynecol Pathol. 1989;8:1–7. doi: 10.1097/00004347-198903000-00001. [DOI] [PubMed] [Google Scholar]
- 116.Al-Bannai R, Miller D, Sadownik L, et al. Vulvar acanthosis with altered differentiation (VAAD): report of a case with progression to poorly differentiated carcinoma over a 5-yr period. Int J Gynecol Pathol. 2015;34:385–9. doi: 10.1097/PGP.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 117.Walton DB, Stearns L, Fillman EP, et al. Vulvar acanthosis with altered differentiation: is this entity a variant of hypertrophic lichen sclerosus? J Cutan Pathol. 2015 Aug 12; doi: 10.1111/cup.12557. http://dx.doi.org/10.1111/cup.12557; PubMed ID 26267856. [DOI] [PubMed]