Abstract
Objectives:
Hepatocellular carcinoma (HCC) surveillance with biannual ultrasound is currently recommended for all patients with cirrhosis. However, clinical implementation of this “one-size-fits-all” approach is challenging as evidenced by its low application rate. We aimed to evaluate the cost-effectiveness of risk-stratified HCC surveillance strategies in patients with cirrhosis.
Methods:
A Markov decision-analytic modeling was performed to simulate a cohort of 50-year-old subjects with compensated cirrhosis. Risk-stratified HCC surveillance strategies was implemented, in which patients were stratified into high-, intermediate-, or low-risk groups by HCC risk biomarker–based scores and assigned to surveillance modalities tailored to HCC risk (2 non-risk-stratified and 14 risk-stratified strategies) and compared with non-stratified biannual ultrasound.
Results:
Quality-adjusted life expectancy gains for biannual ultrasound in all patients and risk-stratified strategies compared with no surveillance were 1.3 and 0.9–2.1 years, respectively. Compared with the current standard of biannual ultrasound in all cirrhosis patients, risk-stratified strategies applying magnetic resonance imaging (MRI) and/or ultrasound only in high- and intermediate-risk patients, without screening in low-risk patients, were cost-effective. Abbreviated MRI (AMRI) for high- and intermediate-risk patients had the lowest incremental cost-effectiveness ratio (ICER) of $2,100 per quality-adjusted life year gained. AMRI in intermediate- and high-risk patients had ICERs <$3,000 across a wide range of HCC incidences.
Conclusions:
Risk-stratified HCC surveillance strategies targeting high- and intermediate-risk patients with cirrhosis are cost-effective and outperform the currently recommended non-stratified biannual ultrasound in all patients with cirrhosis.
Introduction
Hepatocellular carcinoma (HCC) is the leading cause of death in patients with cirrhosis, largely related to failed early detection. Liver disease societies worldwide recommend HCC screening in cirrhosis using biannual abdominal ultrasound,1, 2, 3, 4 which has been associated with early tumor detection, increased curative treatment receipt, and improved overall survival.5, 6 Although biannual ultrasound for all cirrhotic patients is cost-effective compared with no screening, ultrasound can miss one-third of early-stage HCC due to its suboptimal sensitivity.7, 8, 9 Other imaging modalities with higher sensitivity for early tumor detection, such as dynamic contrast-enhanced magnetic resonance imging (MRI), have not been recommended as alternatives given its higher cost.1
HCC screening is recommended when annual HCC incidence exceeds 1.5%, assuming uniform HCC incidence among patients’ subgroups with specific liver disease etiology and severity.1 However, studies have suggested that HCC risk is not uniform across all patients within a clinical risk group, and therefore the current one-size-fits-all approach likely results in overestimated or underestimated HCC risk for each individual.10 Furthermore, this approach has proved to be difficult to implement in actual clinical practice as evidenced by the low utilization rate <20% in the United States due to the sizable cirrhotic population.11, 12 Accurate HCC risk stratification and optimized allocation of medical resources could enable rational and practically feasible implementation of HCC screening.
Prior studies have demonstrated several clinical HCC risk factors in cirrhotics, including older age, male sex, viral etiology of liver disease, Child–Pugh B/C cirrhosis, diabetes, and obesity, although risk prediction based on these variables yielded insufficient accuracy.10, 13 Tissue-, blood-, or buccal swab–based molecular HCC risk biomarkers applicable to a wide range of liver disease etiologies, some of which are now clinically available as Laboratory Developed Test, combined with clinical variables have shown capability to predict 5- to 10-fold HCC risk differences using tissue or serum specimens.14 We aimed to evaluate the cost-effectiveness of risk-stratified HCC screening in cirrhosis based on molecular clinical HCC risk scores.
Methods
Model and patient population
A previously reported Markov model simulating HCC screening, diagnosis, and therapy based on a health system perspective was refined and updated (TreeAge Pro, Williamstown, MA)9 following the recommendations.15 The baseline population was a cohort of 50-year-old subjects with compensated cirrhosis (n=10,000) followed up with a 6-month cycle for 30 years (Figure 1). The setting of the study was based in the United States. Transition probabilities were derived from the published literature (Table 1, Supplementary Methods). Reporting followed the CHEERS guidelines (Supplementary Reporting Checklist).16
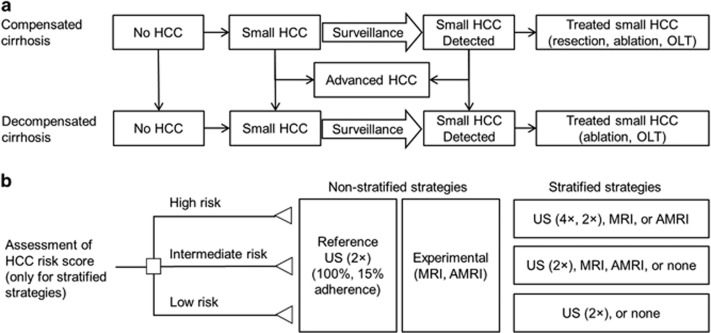
Figure 1.
Markov model and hepatocellular carcinoma (HCC) screening strategies. (a) Schematic of Markov states, starting from compensated cirrhosis without HCC and progressing throughout the model or die. (b) Risk stratification by a HCC risk score classifies subjects into high-, intermediate-, or low-risk groups. In non-stratified strategies, all subjects undergo biannual ultrasound (US; reference), abbreviated magnetic resonance imaging (AMRI), or MRI (experimental strategies), whereas, in risk-stratified strategies, each risk subgroup undergoes different modalities. OLT, orthotopic liver transplantation.
Table 1. Model variables (see Supplementary Table S6 for references).
| Variable | Baseline (range tested) |
|---|---|
| Disease progression | |
| Age (years) | 50 (40–60) |
| Cycle time | 6 months |
| Compensated cirrhosis prognosis | |
| Adjusted annual excess mortality of compensated cirrhosis | 4% (1.8–8%) |
| 10-year survival of compensated cirrhosis | 64% (43–80%) |
| Annual probability of transition from compensated to decompensated cirrhosis | 5% (3–8%) |
| Decompensated cirrhosis prognosis | |
| Annual mortality of decompensated cirrhosis | 28% (18–30%) |
| 2-year survival of decompensated cirrhosis | 52% (49–67%) |
| HCC prognosis | |
| Annual mortality of advanced HCC | 75% (30–95%) |
| HCC natural history | |
| Annual HCC probability | 2.9% (0.5–7.0%) |
| Annual probability of progression from small to advanced HCC | 40% (20–70%) |
| Probability of therapy | |
| Probability of HCC in compensated cirrhosis to be treated with surgical resection | 40% (20–60%) |
| Probability of liver transplantation for early HCC in compensated cirrhosis | 20% (0–50%) |
| Probability of local ablation for HCC in decompensated cirrhosis | 40% (20–100%) |
| Probability of treatment of early HCC after identification in compensated cirrhosis | 69% (50–100%) |
| Probability of treatment of early HCC after identification in decompensated cirrhosis | 30% (0–50%) |
| Probability of liver transplantation for early HCC in treatment-eligible decompensated cirrhosis | 40% (0–80%) |
| Probability of local ablation for early HCC in treatment-eligible decompensated cirrhosis | 60% (20–100%) |
| Prognosis after therapy | |
| 5-year survival after hepatic resection for HCC | 44% (38–51%) |
| Perioperative mortality of hepatic resection | 3.9% (3.7–4.5%) |
| 5-year survival after liver transplantation for HCC | 70% (65–80%) |
| Perioperative mortality of liver transplantation | 4.3% (2.3–6.3%) |
| 5-year survival after local ablation for HCC in compensated cirrhosis | 46% (32–77%) |
| 5-year survival after local ablation for HCC in decompensated cirrhosis | 31% (27–40%) |
| Perioperative mortality of local ablation | 0.3% (0–1.8%) |
| HCC risk score | |
| 186-gene-based risk score, proportion of each risk group | High: 36% (0–50%) Intermediate: 37% Low: 27% (10–50%) |
| 186-gene-based risk score, annual HCC incidence in each risk group | High: 4.9% (0.8–12%) Intermediate: 3.3% (0.6–8.0%) Low: 0.8% (0.1–1.9%) |
| EGF genotype-based risk score, proportion of each risk group | High: 14% (0–40%) Intermediate: 29% Low: 57% (0–60%) |
| EGF genotype-based risk score, annual HCC incidence in each risk group | High: 5% (2.5–10%) Intermediate: 1.8% (0.9–3.6%) Low: 0.4% (0.2–0.8%) |
| Screening and diagnosis test characteristics | |
| Probability of being screened for HCC | 100% (15–100%) |
| Reported probability of being screened for HCC | 15% (5–60%) |
| Probability of incidental early HCC in the non-screened group | 30% (0–50%) |
| Ultrasound sensitivity for early-stage HCC screening | 63% (35–78%) |
| Ultrasound specificity for early-stage HCC screening | 91% (70–95%) |
| Screening full MRI sensitivity for early-stage HCC screening | 96% (80–100%) |
| Screening full MRI specificity for early-stage HCC screening | 94% (85–98%) |
| Abbreviated MRI sensitivity for early-stage HCC screening | 83% (70–95%) |
| Abbreviated MRI specificity for early-stage HCC screening | 93% (86–96%) |
| Diagnostic MRI sensitivity for early-stage HCC | 88% (78–92%) |
| Diagnostic MRI specificity for early-stage HCC | 94% (85–98%) |
| HCC biopsy sensitivity | 62% (50–100%) |
| HCC biopsy specificity | 100% (80–100%) |
| Costs ($) | Medicare, National Impatient Sample |
| Annual cost of compensated cirrhosis | 1,220 (610–2,440) |
| Annual cost of decompensated cirrhosis | 15,000 (7,500–30,000) |
| Annual cost after liver transplantation | 14,600 (7,300–29,200) |
| Annual cost of advanced HCC | 41,320 (20,660–82,640) |
| Cost of hepatic resection | 42,540 (21,270–85,080) |
| Cost of liver transplantation | 177,000 (88,500–354,000) |
| Cost of local ablation | 3,650 (1,825–7,300) |
| Cost of imaging-guided HCC biopsy | 750 (375–1,500) |
| Cost of ultrasound | 143 (71–285) |
| Cost of screening full MRI | 528 (264–1,056) |
| Cost of screening abbreviated MRI | 313 (156–626) |
| Cost of diagnostic MRI | 528 (264–528) |
| Cost of risk score | 796 (500–4,000) |
| Rate of discounting costs | 3% |
| Quality-of-life weights | |
| Utility of compensated cirrhosis | 0.8 (0.6–1.0) |
| Utility of decompensated cirrhosis | 0.65 (0.5–0.8) |
| Utility after HCC diagnosis | 0.3 (0.1–0.4) |
| Utility after liver transplantation | 0.73 (0.5–0.8) |
AMRI, abbreviated MRI; EGF, epidermal growth factor; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging.
Screening-detected and undetected HCC were distinguished. Depending on the performance of screening modalities, HCC could be detected at an early stage or remain undetected until an advanced stage. HCC detected at an early stage in compensated cirrhosis were eligible for tumor resection, liver transplantation, or local ablative therapies; whereas patients with decompensated cirrhosis and early-stage HCC were eligible for liver transplantation or local ablative therapies. Patients with advanced tumors received palliative treatments, including chemoembolization, systemic therapy, or best supportive care, as recommended by guidelines.1 Our model assumptions include (1) positive screening tests (i.e., lesions ≥1 cm in diameter) were evaluated with diagnostic contrast-enhanced MRI; (2) patients with characteristic findings of HCC on the diagnostic MRI did not undergo further diagnostic evaluation prior to treatment; (3) patients with a positive screening test but negative diagnostic MRI underwent biopsy to evaluate the suspicious nodule; (4) patients with false positive screening tests and subsequent negative biopsy had a follow-up MRI examination and, if the MRI was negative, were returned to prior screening strategy; and (5) HCC risk was stable over time during the observation period.
HCC screening strategies
Based on our preceding cost-effectiveness analysis of HCC screening by different combinations of ultrasound, CT, MRI, and AFP, the most cost-effective strategy, biannual ultrasound with 100% utilization rate (US2 × -100%), was chosen as the reference strategy in the current study.9 The reference strategy was compared with two non-risk-stratified screening strategies: (1) biannual dynamic contrast-enhanced triple-phase MRI (full MRI) with 100% utilization rate (MRI2 × -100%); (2) biannual abbreviated contrast-enhanced MRI (AMRI)17, 18 with 100% utilization rate (AMRI2 × -100%), and 14 risk-stratified strategies with various combinations of screening modalities assigned for each risk subgroup (Table 2). In each of the risk-stratified strategies, patients were first stratified into high-, intermediate-, and low-risk groups by applying either of the two integrated molecular and clinical HCC risk scores discussed in the next section.19, 20, 21, 22 Subsequently, each risk group was subjected to different screening protocols according to the HCC risk level. The 16 experimental strategies were also compared with another alternative reference strategy, biannual ultrasound with 15% utilization rate (US2 × -15%), representing the current real-world usage of HCC screening in the United States.5
Table 2. Cost-effectiveness of non-stratified and risk-stratified HCC screening strategies.
| Strategy | QALE | Cost | ICER | ICER |
|---|---|---|---|---|
| (vs. US2 × -100%) | (vs. US2 × -15%) | |||
| No screening | 6.40 | $42,961 | ||
| Reference strategies | ||||
| Regular US screening (100% adherence; US2 × -100%) | 6.51 | $51,761 | Reference | |
| Regular US screening (15% adherence; US2 × -15%) | 6.39 | $44,078 | Reference | |
| Non-stratified experimental strategies | ||||
| MRI for all (MRI-100%) | 6.57 | $56,871 | 85,167 | 71,072 |
| AMRI for all (AMRI-100%) | 6.55 | $53,883 | 53,050 | 61,281 |
| Risk-stratified strategies (for high–intermediate–low risk groups) | ||||
| US4 × -US2 × -US2 × | 6.50 | $54,601 | Dominated | 95,664 |
| MRI-US2 × -US2 × | 6.54 | $54,442 | 89,367 | 69,093 |
| AMRI-US2 × -US2 × | 6.53 | $53,437 | 83,800 | 66,850 |
| US2 × -US2 × -none | 6.52 | $50,417 | Dominant | 48,762 |
| US4 × -US4 × -none | 6.51 | $54,391 | Dominated | 85,942 |
| MRI-MRI-none | 6.58 | $53,966 | 31,500 | 52,042 |
| AMRI-AMRI-none | 6.56 | $51,866 | 2,100 | 45,812 |
| US4 × -US2 × -none | 6.52 | $52,300 | 53,900 | 63,246 |
| MRI-US2 × -none | 6.55 | $52,140 | 9,475 | 50,388 |
| AMRI-US2 × -none | 6.54 | $51,136 | Dominant | 47,053 |
| US2 × -none-none | 6.48 | $47,086 | Less effective | 33,422 |
| US4 × -none-none | 6.47 | $48,969 | Less effective | 61,137 |
| MRI-none-none | 6.51 | $48,809 | Dominant | 39,425 |
| AMRI-none-none | 6.50 | $47,804 | Less effective | 33,873 |
AMRI, abbreviated magnetic resonance imaging; HCC, hepatocellular carcinoma; ICER, incremental cost-effectiveness ratio; QALE, quality-adjusted life expectancy; US, ultrasound. Dominant, improved QALE with lower cost; dominated, worse QALE with higher cost; less effective, reduced efficacy with lower cost; 2/4 ×, screening two/four times a year; MRI and AMRI are biannual. Risk-stratified strategies are named as screening modality in high-risk subjects–intermediate-risk subjects–low-risk subjects. For example, MRI-none-none corresponds to screening by MRI in high-risk subjects and no screening in intermediate- and low-risk subjects.
Baseline estimates of clinical parameters
Table 1 summarizes model parameters, base case values, and plausible ranges based on our previously published model,9 updated literature review,5 and expert input for sensitivity analyses.
Natural history of cirrhosis
The adjusted annual excess mortality of compensated cirrhosis was estimated as 4%, and 5% of compensated cirrhosis progress to decompensated cirrhosis each year.23 Range of annual HCC incidences tested was 0.5–7.0%, covering HCC incidence in global populations with a variety of HCC etiologies.24, 25, 26, 27, 28, 29, 30, 31 Annual progression from small to advanced HCC was 40%, and annual mortality of advanced HCC was 75%.32, 33
HCC risk-stratification strategies
A pan-etiology (hepatitis B, hepatitis C, hepatitis C after viral cure, alcohol, and non-alcoholic fatty liver disease) 186-gene signature-based HCC risk score, comprised of the liver gene signature, bilirubin, and platelet count, was used as the example of biomarker-based risk stratification.21, 34, 35 As an additional example, another HCC risk score based on epidermal growth factor single-nucleotide polymorphism, which can be measured using a buccal swab, was also tested.19 The score, awaiting external validation, comprises the epidermal growth factor single-nucleotide polymorphism, age, sex, smoking status, alkaline phosphatase level, and platelet count.
HCC screening, treatment, and prognosis
Sensitivity and specificity of screening ultrasound, screening full MRI, AMRI, diagnostic MRI, and biopsy were estimated based on published literature (Table 1,Supplementary Methods). Based on a meta-analysis of HCC treatment utilization, we estimated the probabilities of any treatment in compensated and decompensated cirrhosis patients.36, 37 The proportions of treatment-eligible patients and prognosis after treatment were defined based on population and cohort studies.9, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51
Costs and utility
Screening test costs were calculated based on 2015 Medicare Current Procedural Terminology reimbursement global costs. The AMRI cost was conservatively estimated by halving the technical cost of full MRI18 (Supplementary Table S1). Cost of the HCC risk biomarker test was calculated as median of multi-gene gapfill Current Procedural Terminology codes in Clinical Laboratory Fee Schedule (Supplementary Table S2). Other direct medical costs were derived from Medicare Current Procedural Terminology reimbursement, Nationwide Inpatient Sample, and published literature and adjusted for inflation to 2014 costs (Table 1). The cost incurred by incidental detection of non-liver-related diseases was outside the scope of this study and therefore not included. Literature-based estimates were used for the quality-of-life weights.
Study outcomes
Model outcomes included lifetime costs, quality-adjusted life expectancy (QALE), and incremental cost-effectiveness ratios (ICERs), defined as incremental cost in US dollars per quality-adjusted life year gained. An ICER<$50,000 was regarded as cost-effective. One- and two-way sensitivity analyses were performed on all model variables.
Patient involvement
Patients were not involved in this study.
Results
Model validity
Overall 1- and 2-year survival in our model (96% and 90%, respectively) were similar to those reported in a systematic review of Child–Pugh A cirrhosis (95% and 90%, respectively).23 Similarly, 3-year survival rates for screening-detected and non-screening HCC patients in our model (51% and 30%, respectively) recapitulated rates in a recent systematic review (51% and 28%, respectively).5 Finally, the proportion of screening-detected and non-screening HCC patients undergoing curative therapy in our model (57% and 21%, respectively) was comparable to probabilities in the systematic review (52% and 24%, respectively).5 These results collectively support the validity of our model.
Life expectancy
The mean QALE for 50-year-old patients with compensated cirrhosis was 6.4 years in the absence of screening. Although the current recommendation in practice guidelines (US2 × -100%) resulted in 1.3 months of QALE gain, the real-world ultrasound utilization (US2 × -15%) as well as substituting ultrasound with MRI (MRI-15%) or AMRI (AMRI-15%) did not extend QALE (Table 2). Non-stratified use of MRI (MRI-100%) and AMRI (AMRI-100%) yielded QALE gains of 2.0 and 1.8 months, respectively. Risk-stratified strategies yielded QALE gains ranging from 0.9 to 2.1 months compared with no screening (Table 2). Among patients who developed HCC, the mean survival after HCC development was 2.7 years in the absence of screening. The gain in QALE compared with no screening were 2.0 years for US2 × -100%, whereas the gain in the risk-stratified strategies was up to 2.2 years, indicating that survival of HCC patients is not sacrificed by the risk-stratified strategies.
Costs-effectiveness of risk-stratified HCC screening
In the absence of screening, the mean discounted lifetime cost for a 50-year-old patient with compensated cirrhosis was $42,961, which was increased by $8,322, $13,460, and $10,420 using US2 × -100%, MRI-100%, and AMRI-100% (indicating all patients are screened by biannual ultrasound, MRI, or AMRI), respectively, whereas risk-stratified strategies increased costs by $3,920–$10,900 compared with no screening (Table 2). Compared with the current HCC screening utilization (US2 × -15%), increasing ultrasound utilization to 100% (US2 × -100%), use of MRI (MRI-100%), and use of AMRI (AMRI-100%) resulted in ICERs of $64,072, $68,012, and $58,401 per quality-adjusted life year gained, respectively, which were all above the cost-effectiveness threshold of $50,000.
We next evaluated the stratified strategies. Each stratified HCC screening strategy is described by combining the screening modalities assigned to the HCC high-, intermediate-, and low-risk groups, respectively, separated by a hyphen. For example, an HCC screening strategy applying ultrasound (4 × per year) for high-risk group, ultrasound (2 × per year) for intermediate-risk group, and no screening for low-risk group is presented as “US4 × -US2 × -none”. We identified three risk-stratified screening strategies that were cost effective compared with US2 × -100%: MRI-MRI-none, AMRI-AMRI-none, and MRI-US2 × -none, among which AMRI-AMRI-none had the smallest ICER of $2,100, and three strategies dominating the reference: US2 × -US2 × -none, AMRI-US2 × -none, and MRI-none-none (Table 2). When compared with US2 × -15%, six risk-stratified strategies, US2 × -US2 × -none, AMRI-AMRI-none, AMRI-US2 × -none, US2x-none-none, MRI-none-none, and AMRI-none-none, were cost-effective with ICERs ranging from $33,422 to $48,762 per quality-adjusted life year gained. One common feature of the cost-effective risk-stratified strategies was omitted screening for low-risk patients, with minimal to no impact on QALE, which was also confirmed in subgroup analysis for each risk group (Supplementary Table S3). Screening only for high-risk patients was also cost-effective without substantially reducing QALE (Table 2) and may be an alternative screening strategy for resource-limited area that are only able to deliver HCC screening to a small fraction of the at-risk population. Risk-stratified strategies using another HCC risk score based on the epidermal growth factor single-nucleotide polymorphism19 was similarly cost-effective (Supplementary Table S4).
Factors affecting cost-effectiveness of risk-stratified HCC screening (sensitivity analyses)
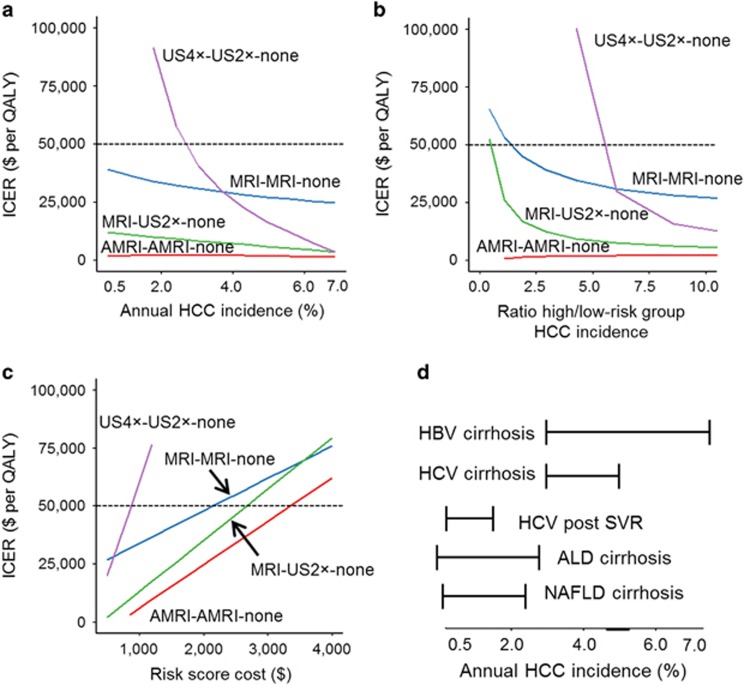
One-way sensitivity analyses on each model variable were performed to identify notable drivers of cost-effectiveness. We first assessed a range of annual HCC incidence rates to model a range of different cirrhosis etiologies24, 25, 26, 27, 28, 29 on cost-effectiveness of the four non-dominant, cost-effective (i.e., ICER<$50,000) risk-stratified screening strategies within the range of annual HCC incidence rate between 0.5% and 7%. As expected, all strategies were more cost-effective at higher annual HCC incidence (Figure 2a). MRI-MRI-none had the highest QALE and was cost-effective with an ICER consistently <$50,000. More frequent ultrasound among high-risk patients (US4 × -US2 × -none) was the only strategy that sharply became non-cost-effective when annual HCC incidence dropped <3%. In contrast, the ICER for AMRI-AMRI-none was consistently low, highlighting its robust performance across a wide range of annual HCC incidences.
Figure 2.
One-way sensitivity analysis of key model parameters and their effect on incremental cost-effectiveness ratio (ICER) compared with non-stratified ultrasound (US)-based screening in 100% of individuals for cost-effective risk-stratified strategies. Only the four non-dominant, risk-stratified strategies with an ICER <$50,000 per quality-adjusted life year (QALY) in the baseline model are shown for (a) global annual hepatocellular carcinoma (HCC) incidence, HCC risk score performance, (b) defined as ratio of annual HCC incidence in the high- over low-risk group, and (c) HCC risk biomarker cost. (d) Annual HCC incidence according to HCC etiology projected onto the range of annual HCC incidence tested in sensitivity analysis (Supplementary Table S5). 2/4 ×, screening two/four times a year; ALD, alcoholic liver disease; AMRI, abbreviated MRI; HBV, hepatitic B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; SVR, sustained virological response. Risk-stratified strategies are named as screening modality in high-risk subjects–intermediate-risk subjects–low-risk subjects. For example, MRI-none-none corresponds to screening by MRI in high-risk subjects and no screening in intermediate- and low-risk subjects.
We next evaluated HCC risk biomarker test performance (defined as fold difference in annual HCC incidence rates between the high- and low-risk groups with a range of 1.3–10), which showed that AMRI-AMRI-none remained cost-effective when compared with US2 × -100% even at the lowest-risk score performance with consistently low ICER, although MRI-MRI-none required approximately greater than twofold difference in HCC incidence between the high- and low-risk groups to be cost-effective (Figure 2b). The costs of the HCC risk biomarker test also affected cost-effectiveness (Figure 2c). MRI-MRI-none had an ICER<$50,000 up to a risk biomarker cost of $2,200, while AMRI-AMRI-none was cost-effective up to a cost of $3,400.
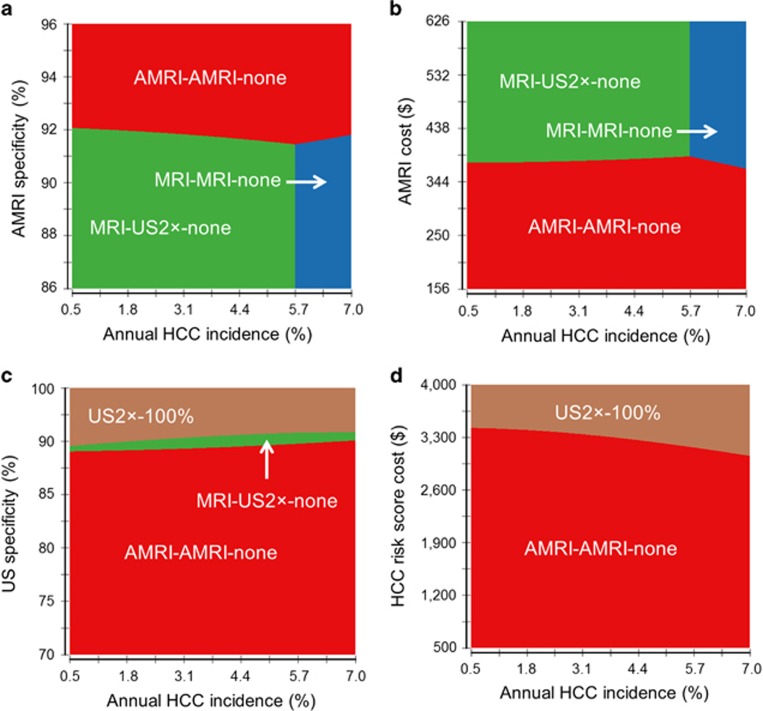
Subsequently, one-way sensitivity analyses were performed for the remaining model variables in a comparison between the reference strategy (US2 × -100%) and the non-dominant risk-stratified strategy with the smallest ICER, AMRI-AMRI-none. Ultrasound specificity, AMRI specificity and cost, and HCC risk biomarker cost were identified as the top influential variables (Supplementary Figure S1). AMRI-AMRI-none was not cost-effective when specificity of ultrasound >93%, specificity of AMRI was <89%, or cost of AMRI >$532.
Two-way sensitivity analysis of these variables against annual HCC incidence showed that AMRI-AMRI-none was the best strategy when AMRI specificity was >91%, replaced by MRI-US2 × -none when AMRI specificity dropped and annual HCC incidence was <5.7% or by MRI-MRI-none when >5.7% (Figure 3a). AMRI-AMRI-none was the most cost-effective strategy across a range of HCC incidences when its cost was <$360 (Figure 3b) and US specificity was <88% (Figure 3c). AMRI-AMRI-none remained the most cost-effective strategy irrespective of annual HCC incidence when the risk biomarker cost was <$2,900 (Figure 3d). The proportion of subjects in the low-risk group did not affect the superiority of AMRI-AMRI-none (Supplementary Figure S2). When varying AMRI cost and specificity, AMRI-AMRI-none remained superior when specificity was >90% or 96% when cost was <$156 or $532, respectively (Supplementary Figure S3).
Figure 3.
Two-way sensitivity analysis of annual hepatocellular carcinoma (HCC) incidence vs. variables affecting cost-effectiveness of risk-stratified HCC screening. Overall HCC incidence is varied along with model parameters, (a) abbreviated MRI (AMRI) specificity, (b) AMRI cost, (c) ultrasound (US) specificity, and (d) HCC risk biomarker cost, with the greatest effect on incremental cost-effectiveness ratio of non-dominant, cost-effective risk-stratified strategies.
Discussion
Cirrhosis is a prevalent condition with significant indirect costs approximating $10.2 billion in the United States in 2004.52 In addition, the human and economic burden of cirrhosis is expected to increase due to an aging population of hepatitis C virus–infected subjects who can develop HCC even a decade after viral cure and increases in prevalence of fatty liver disease.53 However, as evidenced by low-quality indicators, interventions are urgently needed to improve quality of care in this vulnerable and underserved population.54 Our study demonstrates for the first time the feasibility and cost-effectiveness of tailored HCC screening, in which cost for low-yield testing among low-risk patients is spared without significant drop in effectiveness. Imaging modalities, such as AMRI, with higher sensitivity while being affordable may, when applied to high-risk patients, improve early tumor detection in a cost-effective manner. Risk-based personalized screening has been increasingly adopted in multiple cancer types, e.g., MRI for BRCA-mutated breast cancer, with an intention to allocate costly but more sensitive modalities to those with the greatest need.55 With the recent development of more accurate HCC risk stratification tools, our study provides a blueprint of how HCC screening can be personalized and advanced into the era of precision medicine.
Of note, incorporation of MRI could become cost-effective if targeted to high- and intermediate-risk patients, with recent reductions in its costs. Compared with ultrasound, which may be affected by operator’s skill/experience as well as the presence of morbid obesity,8 MRI can yield more robust performance that will be critical considering the rapidly increasing and already highly prevalent obese patients with non-alcoholic fatty liver diseases. The emergence of simplified MRI protocols, such as AMRI, is expected to further lower the bar for introducing MRI-based screening by reducing examination costs and time.
Another issue limiting HCC screening effectiveness is low utilization rates due to providers overlooking at-risk patients and patient barriers to performing screening.56 Risk-stratified strategies may improve utilization rates as efforts and resources to maximize adherence can be concentrated on the highest-risk patients instead of being diffused among all cirrhotic patients, particularly in centers with limited radiological or subspecialty capacity.
Recent development of robust molecular assays has allowed clinical deployment of already reimbursable multi-gene biomarker tests.55 In addition, recent emergence of direct-to-consumer molecular diagnostics may drastically lower the current biomarker costs, which may further improve cost-effectiveness of the risk-stratified HCC screening. Our sensitivity analysis, evaluating a range of these dynamically changing variables, provides quantitative reference for further clinical development of HCC risk biomarker tests and AMRI protocols.
Study Highlights

Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantors of the article: Nicolas Goossens, MD, MSc and Yujin Hoshida, MD, PhD.
Specific author contributions: N.G., A.S., and Y.H, conceived, designed the study, acquired data, interpreted and analyzed the data, and obtained funding. N.G. and Y.H. performed the statistical analysis. All authors contributed to the conception, critically revised the manuscript for important intellectual content, approved the final version to be published, and L.K., K.A., B.F., C.B., B.T., and R.C. provided administrative, technical, or material support for this study. Y.H. supervised the overall study. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support: This work was supported by the FLAGS Foundation, the Nuovo-Soldati Cancer Research Foundation, and an advanced training grant from Geneva University Hospital; by grant numbers DK099558 and DK078772 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; the Irma T. Hirschl Trust; ERC-AdG-2014 HEPCIR from European Union; W81XWH-16-1-0363 from U.S. Department of Defense; and RP150587 from the Cancer Prevention Research Institute of Texas (CPRIT). The funders played no role in the collection, analysis and interpretation of the data, writing of the report, and the decision to submit the manuscript for publication. All authors are independent from funders.
Potential competing interests: None.
Supplementary Material
References
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer.. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943. [DOI] [PubMed] [Google Scholar]
- Omata M, Lesmana LA, Tateishi R et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010; 4: 439–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003; 52 (Suppl 3): iii1–iii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014; 11: e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer S, de Man RA, Coenraad MJ et al. Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in the Netherlands. J Hepatol 2015; 63: 1156–1163. [DOI] [PubMed] [Google Scholar]
- Singal A, Volk ML, Waljee A et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009; 30: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AG, Conjeevaram HS, Volk ML et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev 2012; 21: 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KL, Salomon JA, Goldie SJ et al. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008; 6: 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol 2015; 13: 2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila JA, Morgan RO, Richardson PA et al. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology 2010; 52: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AG, Yopp A, Skinner CS et al. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med 2012; 27: 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AG, Mukherjee A, Elmunzer BJ et al. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol 2013; 108: 1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens N, Nakagawa S, Hoshida Y. Molecular prognostic prediction in liver cirrhosis. World J Gastroenterol 2015; 21: 10262–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JE, Weinstein MC, Russell LB et al. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1339–1341. [DOI] [PubMed] [Google Scholar]
- Husereau D, Drummond M, Petrou S et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ 346: f1049.2013. [DOI] [PubMed] [Google Scholar]
- Marks RM, Ryan A, Heba ER et al. Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid–enhanced MRI for hepatocellular carcinoma surveillance. Am J Roentgenol 2015; 204: 527–535. [DOI] [PubMed] [Google Scholar]
- Besa C, Lewis S, Pandharipande PV et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY) 2016: 1–12. [DOI] [PubMed]
- Abu Dayyeh BK, Yang M, Fuchs BC et al. A functional polymorphism in the epidermal growth factor gene is associated with risk for hepatocellular carcinoma. Gastroenterology 2011; 141: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LY, Canasto-Chibuque C, Johnson KB et al. A genomic and clinical prognostic index for hepatitis C-related early-stage cirrhosis that predicts clinical deterioration. Gut 2014; 64: 1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshida Y, Villanueva A, Sangiovanni A et al. Prognostic gene expression signature for patients with hepatitis C-related early-stage cirrhosis. Gastroenterology 2013; 144: 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshida Y, Villanueva A, Kobayashi M et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359: 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44: 217–231. [DOI] [PubMed] [Google Scholar]
- Morgan RL, Baack B, Smith BD et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013; 158 (Pt 1): 329–337. [DOI] [PubMed] [Google Scholar]
- Ascha MS, Hanouneh IA, Lopez R et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010; 51: 1972–1978. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Banas C, Sargeant C et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006; 43: 682–689. [DOI] [PubMed] [Google Scholar]
- Bhala N, Angulo P, van der Poorten D et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011; 54: 1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Imai Y, Hiramatsu N et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med 2008; 148: 820–826. [DOI] [PubMed] [Google Scholar]
- Tsukuma H, Hiyama T, Tanaka S et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 1993; 328: 1797–1801. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Stroffolini T, Zagni I et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004; 127 (Suppl 1): S35–S50. [DOI] [PubMed] [Google Scholar]
- Mancebo A, González–Diéguez ML, Cadahía V et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol 2013; 11: 95–101. [DOI] [PubMed] [Google Scholar]
- Simonetti R, Liberati A, Angiolini C et al. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Annals of Oncology 1997; 8: 117–136. [DOI] [PubMed] [Google Scholar]
- Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer 2004; 40: 1474–1484. [DOI] [PubMed] [Google Scholar]
- King LY, Canasto-Chibuque C, Johnson KB et al. A genomic and clinical prognostic index for hepatitis C-related early-stage cirrhosis that predicts clinical deterioration. Gut 2015; 64: 1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Wei L, Song WM et al. Molecular liver cancer prevention in cirrhosis by organ transcriptome analysis and lysophosphatidic acid pathway inhibition. Cancer Cell 2016; 30: 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D, Yopp A, Beg M et al. Meta‐analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther 2013; 38: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal F, Befeler A, Chari RS et al. Potentially curative treatment in patients with hepatocellular cancer—results from the liver cancer research network. Aliment Pharmacol Ther 2012; 36: 257–265. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Siegel AB, Davila JA et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol 2006; 44: 158–166. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Fuster J, Bruix J. Intention‐to‐treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30: 1434–1440. [DOI] [PubMed] [Google Scholar]
- Fong Y, Sun RL, Jarnagin W et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229: 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon RT-P Fan ST, Lo CM et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002; 235: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco D, Capussotti L, Smadja C et al. Resection of hepatocellular carcinomas. Gastroenterology 1990; 98: 733–738. [PubMed] [Google Scholar]
- Mazzaferro V, Regalia E, Doci R et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–699. [DOI] [PubMed] [Google Scholar]
- Yao FY, Ferrell L, Bass NM et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33: 1394–1403. [DOI] [PubMed] [Google Scholar]
- Lim KC, Chow PH, Allen J et al. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg 2012; 99: 1622–1629. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Cioni D, Crocetti L et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005; 234: 961–967. [DOI] [PubMed] [Google Scholar]
- Shiina S, Tateishi R, Arano T et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012; 107: 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong L, Maddern G. Systematic review and meta‐analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg 2011; 98: 1210–1224. [DOI] [PubMed] [Google Scholar]
- Tateishi R, Shiina S, Teratani T et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. Cancer 2005; 103: 1201–1209. [DOI] [PubMed] [Google Scholar]
- Dhir M, Lyden ER, Smith LM et al. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: a meta-analysis. HPB (Oxford) 2012; 14: 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livraghi T, Solbiati L, Meloni MF et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003; 226: 441–451. [DOI] [PubMed] [Google Scholar]
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States. Part I: Overall and upper gastrointestinal diseases. Gastroenterology 2009; 136: 376–386. [DOI] [PubMed] [Google Scholar]
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13: 2031–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal F, El‐Serag H. Improving quality of care in patients with cirrhosis. Clin Liver Dis 2013; 2: 123–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens N, Nakagawa S, Sun X et al. Cancer biomarker discovery and validation. Transl Cancer Res 2015; 4: 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton-Fitzgerald E, Tiro J, Kandunoori P et al. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2015; 13: 791–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.