Abstract
Objectives:
Severe acute exacerbation (SAE) of chronic hepatitis B (CHB) may progress to liver failure with high potential mortality despite the prompt treatment with nucleos(t)ide analogs. This study aimed to evaluate the efficacy and safety of glycyrrhizin in the treatment of CHB with SAE.
Methods:
Sixty patients with SAE of CHB were randomly treated with tenofovir plus intravenous glycyrrhizin (group A, n=30) or with tenofovir alone (group B, n=30). Primary end points were the improvement of serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and model for end-stage liver disease (MELD) score. Secondary end point was overall mortality or receipt of liver transplantation by week 24.
Results:
Patients in group A had significant reductions of serum AST and ALT levels from baseline at days 3, 5, 8, and 15 than those in group B (all P<0.05). The MELD score significantly decreased since week 1 in the group A patients, whereas there were no changes relative to baseline in group B patients at weeks 1 and 2. By week 24, one (3.3%) of group A patients and four (13.3%) of group B patients died (n=3) or received liver transplantation (n=1) (P=0.177). Multivariate analysis identified baseline MELD score (P=0.021) as an independent factor for mortality or receipt of liver transplantation. There were no differences in the rates of grade 3 hypertension, hypokalemia and ascites between two groups.
Conclusions:
Early introduction of glycyrrhizin can be safe and helpful for patients with SAE of CHB.
Introduction
Chronic hepatitis B virus (CHB) infection is a major health problem leading to nearly one million deaths annually worldwide.1 A wide spectrum of clinical manifestations has been established for chronic hepatitis B virus (HBV) infection, ranging from inactive carrier, through persistent chronic hepatitis, and eventually to end-stage liver diseases including decompensated cirrhosis and hepatocellular carcinoma.2, 3 In some patients, severe acute exacerbation (SAE) of CHB characterized by high serum alanine aminotransferase (ALT) level and jaundice may occur spontaneously, during or after anti-viral therapy and in the setting of immunosuppression and/or chemotherapy.4, 5, 6 These exacerbations may progress to acute-on-chronic liver failure with significant mortality.6, 7, 8
Most guidelines recommend oral nucleos(t)ide analog (NUC) therapy in patients with SAE of CHB as soon as possible.9, 10, 11 Of the five NUCs approved for the treatment of CHB, tenofovir and entecavir are the first-line therapies,9, 10, 11 which produce similar treatment response and clinical outcomes in patients with SAE of CHB.12 However, a recent meta-analysis demonstrated that NUC therapy had no obvious impact on short-term survival in patients with SAE of CHB as compared with the untreated controls, despite better biochemical and virological responses.13 In particular, there were no benefits with respect to survival rate and prevention of disease deterioration if NUC therapy was given too late (after serum bilirubin level rise of >20 mg/dl or a model for end-stage liver disease (MELD) score of >30).7, 14 Liver transplantation is the last resort of treatment with a 5-year survival rate exceeding 90%.15 Moreover, this is neither readily available nor feasible in many parts of the world where HBV is highly endemic.5
Glycyrrhizin, an aqueous extract of licorice root, is hepatoprotective in vitro, probably by preventing changes in cell membrane permeability and providing membrane stabilization.16, 17 A recent European randomized controlled trial has demonstrated that glycyrrhizin reduces ALT and improves hepatic necroinflammation and fibrosis in chronic hepatitis C patients who did not respond to or tolerate previous interferon-based therapies.18 Glycyrrhizin has been reported to be safe and efficacious in lowering or normalizing ALT levels among Chinese patients with CHB.19 Recently, some reports have shown that glycyrrhizin can improve serum transaminases rapidly and may prevent the progression to severe and fulminant disease in patients with acute chronic hepatitis.20, 21 Whether glycyrrhizin is helpful for CHB with SAE is of great interest.
In this study, we determined the efficacy and safety of tenofovir monotherapy or the combination of tenofovir plus glycyrrhizin in patients with SAE of CHB to explore treatment options for this difficult-to-manage population.
Methods
Study patients
From April 2013 to June 2015, patients who were over 20 years, with reactive hepatitis B surface antigen for more than 6 months, elevation of serum aspartate aminotransferase (AST) and/or ALT level more than 10 times the upper limit of normal,10 and raised serum bilirubin level ≥2 mg/ml or prolonged prothrombin time (PT) ≥3 s at baseline, were prospectively enrolled.7, 12 Patients were excluded for coinfection with human immunodeficiency virus, hepatitis A virus, hepatitis C virus, hepatitis D virus or hepatitis E virus by serological assays, and hepatocellular carcinoma or biliary obstruction by imaging studies. Also, patients who had evidence of hepatic encephalopathy and ascites at baseline were excluded. Cirrhosis was diagnosed by ultrasound findings as coarse liver parenchyma with nodularity and small liver size and the presence of features of portal hypertension.22
Study design
This was a single-center, prospective, open-label, randomized, parallel-group trial. Patients who fulfilled the inclusion and exclusion criteria were screened for eligibility. After screening, all patients were recruited and randomized to one of the following treatment arms in a 1:1 ratio. One arm of patients received tenofovir 300 mg per day combined with 10 days of intravenous glycyrrhizin (Stronger Neo-Minophagen C; SNMC, Minophagen Pharmaceutical, Tokyo, Japan)) (group A) and the other arm received tenofovir monotherapy 300 mg per day (group B) (Supplementary Figure 1). All patients were followed for at least 24 weeks since treatment. The treatment dose in each group could be adjusted based on physician’s clinical judgment.
We consulted the liver transplantation team if any patients who had progressive evident symptoms/signs of hepatic failure showed willingness to receive liver transplantation. These patients were considered withdrawn from the study if they discontinued the observational treatment. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethical committees of Chang Gung Memorial Hospital (IRB no. 102-2700C). Written informed consent was obtained from all participants.
Conventional biochemical tests including serum ASL, ALT, total bilirubin, albumin, creatinine, lactate, and ammonia, and blood tests (complete blood count, PT, and international normalized ratio (INR)) were assessed at baseline (day 1), day 3, day 5, day 8, day 15, week 4, week 12, and week 24. Serum HBV-DNA levels were checked at baseline, week 4, week 12, and week 24.
Study objectives
The primary study objectives were to evaluate and compare the reduction of serum levels of AST and ALT and the improvement of MELD score between two groups. Secondary objective was overall mortality or receipt of liver transplantation by week 24. The intent-to treat population including all randomized patients who took at least one study medication and had at least one postbaseline measurement was conducted for the primary and secondary efficacy evaluation.
Laboratory assays
MELD score was calculated using the following formula: MELD=3.78 × ln [serum bilirubin (mg/dl)]+11.2 × ln [INR]+9.57 × ln [serum creatinine (mg/dl)]+6.43. Hepatitis B surface antigen, hepatitis B e antigen (HBeAg), anti-hepatitis C virus antibody, and anti-hepatitis D virus antibody were assessed using commercially available kits (hepatitis B surface antigen enzyme immunoassay (Abbott, North Chicago, IL); HBeAg enzyme immunoassay (Abbott); anti-hepatitis C virus, enzyme immunoassay 3.0 (Abbott); anti-hepatitis D virus, radioimmunoassay (Abbott)). Serum HBV-DNA levels were measured using the COBAS AmpliPrep-COBAS TaqMan HBV test (CAP-CTM; Roche Molecular Systems, Branchburg, NJ), with a detection limit of 15 international units (IU)/ml.
Statistical analysis
Continuous data are expressed as mean±s.d., and categorical data are expressed as number (percentage). Comparisons of differences in the categorical data between groups were performed using the χ2 test or Fisher's exact test. Distributions of continuous variables were analyzed by the Student’s t-test or Mann–Whitney U-test where appropriate. Paired t-test was performed to compare the MELD score in serial measurements. Cumulative incidences of mortality or receipt of liver transplantation were analyzed by the Kaplan–Meier method with a log-rank test. Univariate and multivariate analyses were carried out to identify independent factors using the Cox-proportional hazards regression models. All analyses were carried out using the SPSS Software, version 15.0 (SPSS, Chicago, IL). All statistical tests were two-tailed, and a P value <0.05 was considered statistically significant.
A linear relationship between the mean decrease in ALT and treatment was assumed in the power analysis of the randomized part of this study. This analysis was based on a two-tailed comparison with an α of 0.05 and a power of 0.80, showing that at least 23 patients per group were needed to detect a correlation of r≥ 0.40.
Results
Baseline characteristics
Sixty-two patients were screened (excluded 1 with acute hepatitis B and 1 with hepatocellular carcinoma) and 60 patients who fulfilled the inclusion criteria received tenofovir disoproxil fumarate plus intravenous glycyrrhizin (group A, n=30) or tenofovir monotherapy (group B, n=30). As shown in Table 1, the baseline demographic and virological as well as laboratory characteristics were similarly distributed between these two groups. The mean baseline MELD score was 16.7±6.0 vs. 17.4±6.1. A total of 13 patients had MELD score >20 at screening.
Table 1. Baseline demographics and disease characteristics.
| Group A (n=30), tenofovir+glycyrrhizin | Group B (n=30), tenofovir | |
| Age (years) | 49.1±12.8 | 45.3±13.3 |
| Male gender, n (%) | 23 (77) | 23 (77) |
| DM, n (%) | 2 (7) | 5 (17) |
| Hypertension, n (%) | 7 (23) | 5 (17) |
| NUC-naïve, n (%) | 24 (80) | 28 (93) |
| Cirrhosis, n (%) | 2 (7) | 4 (13) |
| Liver stiffness (kPa) | 28.7±20.4 | 30.6±15.5 |
| AST (U/l) | 1,033±932 | 848±588 |
| ALT (U/l) | 1,446±999 | 1,292±796 |
| Total bilirubin (mg/dl) | 7.8±6.0 | 9.4±8.3 |
| Creatinine (mg/dl) | 0.7±0.2 | 0.8±0.3 |
| Sodium (mmol/l) | 137.4±3.1 | 137.2±3.4 |
| Albumin (g/dl) | 3.6±0.6 | 3.6±0.5 |
| INR | 1.5±0.6 | 1.5±0.6 |
| MELD score | 16.7±6.0 | 17.4±6.1 |
| Platelet (109/l) | 160±91 | 162±68 |
| Lactate (mg/dl) | 13.7±6.2 | 21.5±33.3 |
| HBeAg positive, n (%) | 10 (33) | 12 (40) |
| Log HBV-DNA (IU/ml) | 5.9±1.7 | 6.2±1.5 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; INR, international normalized ratio of prothrombin time; MELD, model for end-stage liver disease; NUC, nucleos(t)ide analogs.
Data are expressed as mean±s.d. or number (percentage).
Biochemical response
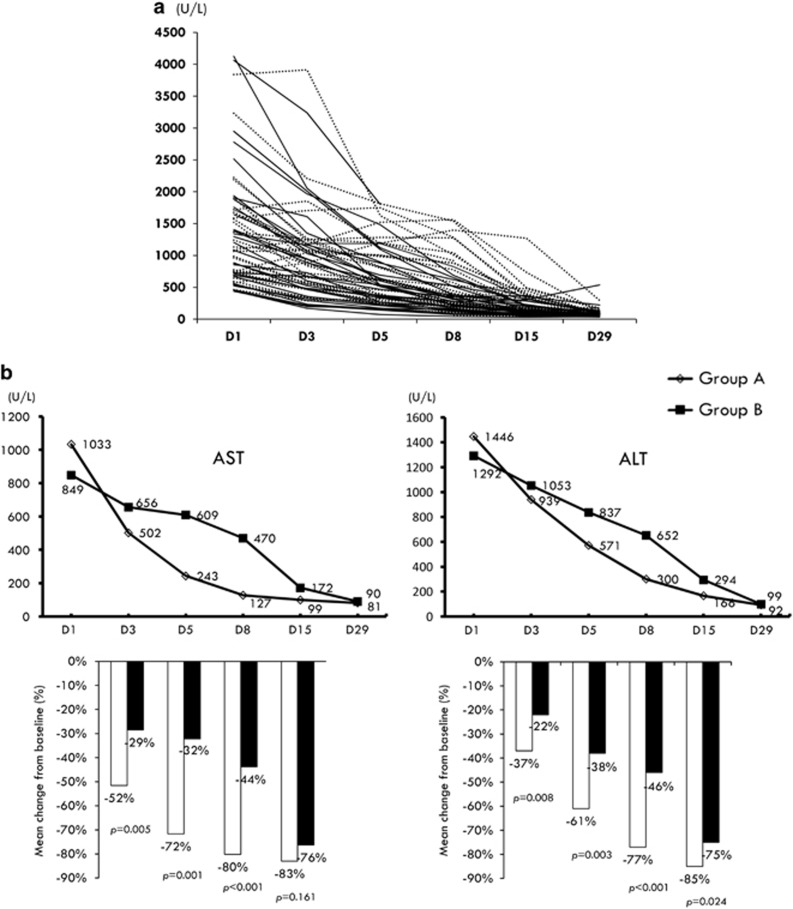
Figure 1a shows the serial serum ALT levels for the individual patients. For the first primary end point, serum ASL levels significantly lowered from baseline in the group A patients compared with the group B patients, especially within 1 week of therapy (Figure 1b). Similarly, the decline rates of serum ALT levels were significantly higher in the group A patients than those in the group B patients until day 15 (Figure 1b). Among patients who survived by week 24, 24 of 29 (82.8%) group A patients had ALT normalization, compared with 17 of 26 (65.4%) group B patients (P=0.215).
Figure 1.
(a) Serial serum alanine aminotransferase (ALT) levels for the individual patients (solid line: group A; dotted line: group B). (b) Serial mean levels and mean changes from baseline in serum aspartate aminotransferase (AST) and ALT during the first 4 week of treatment.
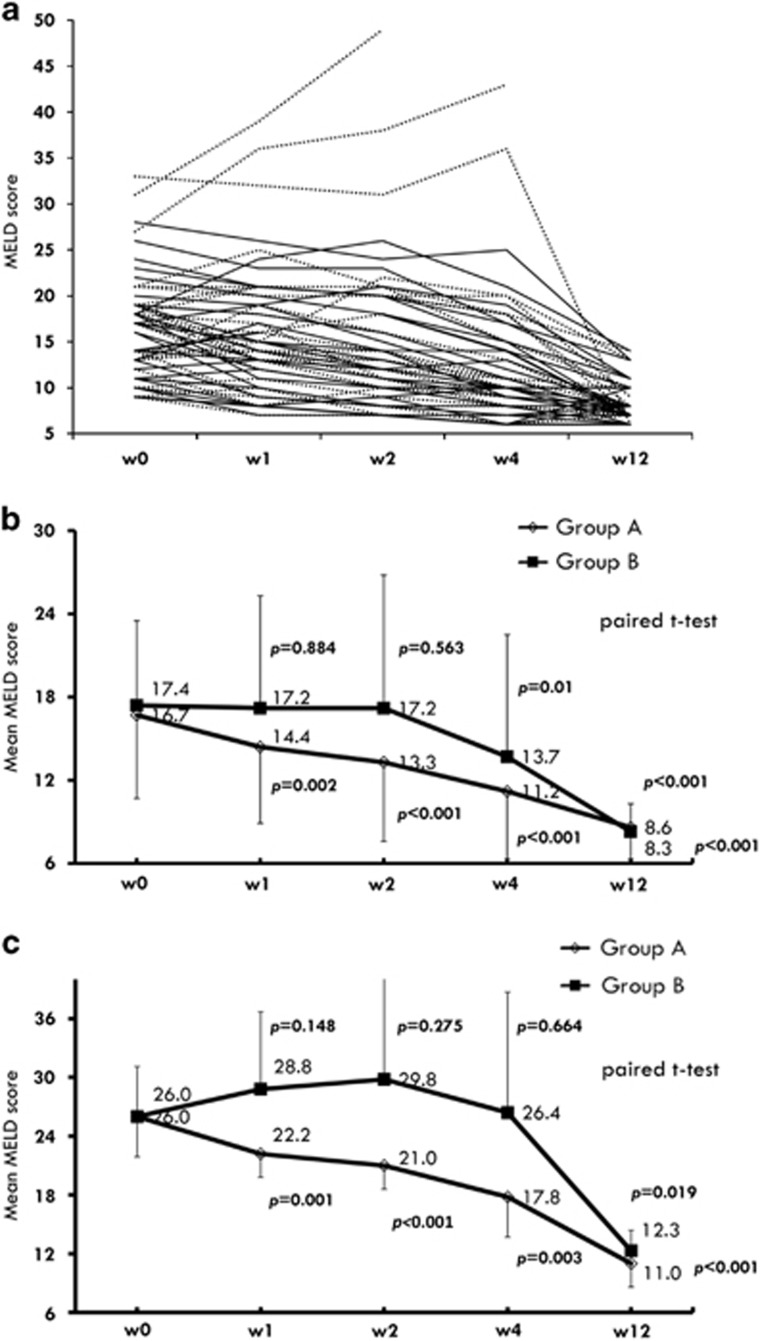
Figure 2 shows the comparison of serial changes in MELD score between these two groups. The mean of MELD score significantly decreased from baseline since week 1 in the group A patients, whereas there were no changes relative to baseline in group B patients at weeks 1 and 2 (Figure 2b). As limited to subjects with MELD score >20 at screening, MELD score still decreased significantly from baseline since week 1 in the group A patients. In contrast, there were no changes from baseline in MELD score until week 12 in group B patients (Figure 2c).
Figure 2.
(a) Serial model for end-stage liver disease (MELD) scores for the individual patients (solid line: group A; dotted line: group B). (b) Serial changes in mean MELD score at week 1, week 2, week 4 and week 12 in total cases. (c) Serial changes in mean MELD score in patients with baseline MELD score >20.
Virological response
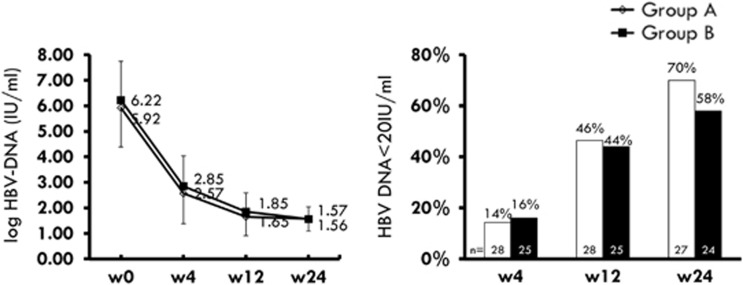
The HBV-DNA levels (mean±s.d.) in groups A and B were 2.57±0.79 and 2.85±1.19 log IU/ml at week 4 (P=0.301), 1.65±0.50 and 1.85±0.74 log IU/ml at week 12 (P=0.355), and 1.57±0.75 and 1.56±0.48 log IU/ml at week 24 (P=0.959), respectively (Figure 3). The number of patients with undetectable HBV-DNA was comparable between these two groups at week 4, week 12, and week 24 (Figure 3).
Figure 3.
Left: Serial mean hepatitis B virus (HBV) DNA levels at week 4, week 12, and week 24. Right: Virological response (HBV-DNA <20 IU/ml) at week 4, week 12, and week 24.
Six of 10 group A patients with reactive HBeAg at baseline had lost HBeAg compared with 7 of 12 group B patients at week 24 of therapy (P=0.730). None of them lost hepatitis B surface antigen by week 24.
Overall mortality or receipt of liver transplantation by week 24
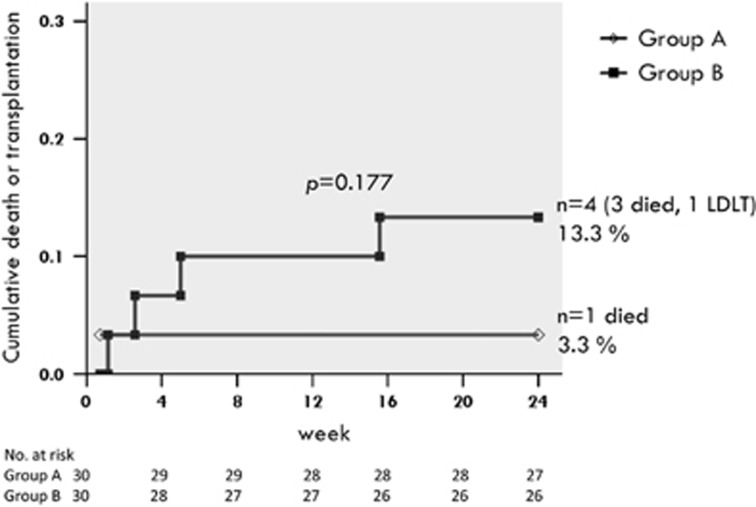
By week 24, 1 (3.3%) of group A patients and 4 (13.3%) of group B patients died (n=3) or received liver transplantation (n=1). All of the deaths were liver-related, and were considered unrelated to the study drug. The cumulative rate of overall mortality or receipt of liver transplantation was not significantly different between two groups (P=0.177) (Figure 4).
Figure 4.
Cumulative rate of overall mortality or receipt of liver transplantation by week 24.
Table 2 shows the factors associated with overall mortality or receipt of liver transplantation by week 24 of treatment. By univariate analyses, hypertension, cirrhosis, and higher levels of ASL, lactate, bilirubin, INR, creatinine, and MELD score were significant variables. Based on stepwise Cox-proportional hazard model of five significant variables in the univariate analysis (hypertension, cirrhosis, AST, lactate, and MELD score), baseline MELD score (hazard ratio (HR): 1.98, P=0.021) was the only independent factor.
Table 2. Univariate and multivariate analyses of factors associated with mortality or liver transplantation by week 24 of treatment.
| Comparison |
Univariate analyses |
Stepwise multivariate analyses |
|||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | per 1 year increase | 1.06 (0.99–1.13) | 0.105 | — | — |
| Sex | Male vs. female | 0.82 (0.09–7.28) | 0.853 | — | — |
| DM | Yes vs. no | 0.53 (0.06–4.77) | 0.574 | — | — |
| Hypertension | Yes vs. no | 6.91 (1.15–41.52) | 0.035 | — | — |
| Cirrhosis | Yes vs. no | 7.13 (1.19–42.96) | 0.032 | — | — |
| Glycyrrhizin | Yes vs. no | 0.25 (0.03–2.22) | 0.212 | — | — |
| NUC-naïve | Yes vs. no | 25.01 (0.00–1,810,741) | 0.572 | — | — |
| HBV-DNA | per log10 IU/ml increase | 1.45 (0.77–2.75) | 0.254 | — | — |
| AST | per 1 U/l increase | 1.00 (1.00–1.00) | 0.009 | — | — |
| ALT | per 1 U/l increase | 1.00 (1.00–1.00) | 0.115 | — | — |
| Albumin | per 1 g/dl increase | 0.27 (0.04–1.72) | 0.164 | — | — |
| Lactate | per 1 mg/dl increase | 1.02 (1.01–1.03) | 0.002 | — | — |
| MELD score | per score | 1.46 (1.18–1.80) | <0.001 | 1.98 (1.11–3.53) | 0.021 |
| Total bilirubin | per 1 mg/dl increase | 1.28 (1.09–1.49) | 0.002 | — | — |
| INR | Increase in ratio | 7.82 (3.01–20.29) | <0.001 | — | — |
| Creatinine | per 1 mg/dl increase | 9.18 (1.09–77.64) | 0.042 | — | — |
| Sodium | per 1 mmol/l increase | 0.89 (0.70–1.14) | 0.352 | — | — |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI; confidence interval; DM, diabetes mellitus; HBV, hepatitis B virus; INR, international normalized ratio of prothrombin time; MELD, model for end-stage liver disease; NUC, nucleos(t)ide analogs.
Safety
The expected side effects of intravenous glycyrrhizin treatment were pseudoaldosteronism with hypokalemia, sodium retention, and elevated blood pressure.18, 23, 24 Table 3 presents a summary of adverse effects possibly related to glycyrrhizin. There were no significant differences in hypertension and ascites or leg edema between these two groups. Patients in group A had a higher percentage of hypokalemia (<3.5 mmol/l) than those in group B (50 vs. 20%, P=0.029). However, most patients had a mild degree of hypokalemia (3–3.5 mmol/l) without symptoms. None of the patients dropped out early because of the side effects of glycyrrhizin.
Table 3. Summary of adverse effects.
| Group A (n=30), tenofovir+glycyrrhizin | Group B (n=30), tenofovir | P value | |
|---|---|---|---|
| Hypertension (grade 2/3)a | 16 (53%) | 14 (47%) | 0.797 |
| Grade 2 | 13 (43%) | 10 (33%) | 0.596 |
| Grade 3 | 3 (10%) | 4 (13%) | 1.000 |
| Hypokalemia (<3.5 mmol/l) | 15 (50%) | 6 (20%) | 0.029 |
| 3–3.5 mmol/l | 12 (40%) | 4 (13.3%) | 0.039 |
| <3 mmol/l | 3 (10%) | 2 (6.7%) | 1.000 |
| Ascites/leg edema | 2 (6.7) | 3 (10) | 1.000 |
CTCAE 4.03.
Discussion
These results provide the first report of the safety and efficacy of glycyrrhizin in the treatment of CHB with SAE. Combined glycyrrhizin and tenofovir therapy can significantly lower serum transaminases rapidly than tenofovir alone in first 2 weeks. Despite no statistical significance in mortality/transplantation-free rate, our data provide the first evidence that glycyrrhizin can significantly improve MELD score, even in more severe cases with higher baseline MELD score (>20). In general, glycyrrhizin was safe and well tolerated in these patients. Although glycyrrhizin could induce hypokalemia (<3.5 mmol/l), most patients had a mild degree without clinical symptoms.
HBV itself is non-cytopathic under the usual circumstances, with damage to the liver mainly caused by immune-mediated processes involving both innate and adaptive immune responses.6, 25, 26 NUCs, which have profound effect in suppression of HBV, can potentially calm down the immune activity and are recommended as the treatment of choice in CHB with SAE.9, 10, 11, 27 However, in up to 20% of patients, there were no benefits of NUC therapy on either improvement of short-term survival or protection against rapid progression of the disease because of liver failure.7, 8, 12 The high mortality rate despite NUC treatment could be partially related to the delayed beginning of NUCs and viral suppression.7, 14 More importantly, NUC therapy did not directly inhibit the ongoing necroinflammation or promote regeneration of decreased hepatic parenchyma after massive or submassive hepatic necrosis. In this study, although no additional anti-viral effects have been attributed to glycyrrhizin, we showed that glycyrrhizin could significantly lower serum levels of AST and ALT, which were markers of biochemical necroinflammatory activity of the liver. In particular, rapid decline of serum AST and ALT levels in the early course of CHB with SAE accompanied with significant decrease of MELD score, which was the most important factor predicting overall mortality or receipt of liver transplantation.
Recently, the protective effects of glycyrrhizin in acute liver disease have been well investigated in animal models.28, 29 It has been suggested that glycyrrhizin possibly acts as a radical scavenger in the cytoplasm of hepatocytes and decreases cell membrane permeability.17 A previous report has shown that intravenous glycyrrhizin can effectively protect liver against fulminant hepatic failure induced by lipopolysaccharide/d-galactosamine in mice, and that glycyrrhizin prevents hepatocytes' apoptosis by inhibiting inflammatory reaction and stabilizing mitochondria membrane to suppress the release of cytochrome C and sequential activation of caspase-3.28 In addition, Ikeda et al.29 have reported that glycyrrhizin inhibits the apoptosis of liver cells through the prevention of an interleukin-18- mediated inflammatory response in a caspase-independent manner in lipopolysaccharide/d-galactosamine-induced mouse liver injury.
There is some research being undertaken to improve the poor outcome of CHB with SAE. The use of bioartificial liver support system seems to be safe and well tolerated in patients with acute-on-chronic liver failure,30 but results of a randomized controlled study failed to identify benefit in survival.31 Another randomized placebo-controlled trial found the administration of granulocyte-colony-stimulating factor improved survival after 2 months.32 Since this study included a small portion of HBV patients, thus granulocyte-colony-stimulating factor cannot be recommended for routine clinical practice at this juncture. Corticosteroids, based on their anti-inflammatory activity, have been used in CHB with SAE. In a recent small study, corticosteroid treatment in combination with nucleotide analog has sufficient virological effect against SAE of CHB, and a rapid decline of HBV-DNA is conspicuous in survived patient.33 However, the increased risk of infectious complications after corticosteroid treatment should be a serious concern, particularly because sepsis is one of the most important causes of death in CHB with SAE.34 By contrast, our study medication “glycyrrhizin” appears to be safe and the expected side effects associated with pseudoaldosteronism are usually minor and reversible.
The present study has some limitations. First, the number of patients was relatively small, thus a larger sample size will be needed to reach statistical significance in mortality/transplantation-free rate. However, SAE of CHB is an uncommon but potentially life-threatening condition. Our study has been the largest trial of this condition to date. In addition, longer follow-up of patients will be necessary to investigate if the potential beneficial effects on disease activity have also a long-term impact on progression of liver disease.
In summary, our study demonstrated that the early introduction of glycyrrhizin therapy in combination with NUCs might be beneficial for patients with clinically SAE of CHB. Glycyrrhizin can be administered safely and be well tolerated in these patients. Further studies with more cases are worthy to investigate the survival benefit of glycyrrhizin in CHB with SAE based on our pilot findings.
Study Highlights
Acknowledgments
This study was funded, in part, by contract grants CMRPG8C0961 from Chang Gung Memorial Hospital, Taiwan. We thank Eisai Company for the grant of glycyrrhizin (SNMC).
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantor of the article: Chao-Hung Hung, MD.
Specific author contributions: Conception and design: Chao-Hung Hung and Sheng-Nan Lu; acquisition of data: Chao-Hung Hung, Kwong-Ming Kee, Chih-Hung Chen, Po-lin Tseng, Ming-Chao Tsai, Chien-Hung Chen, Jing-Houng Wang, Kuo-Chin Chang, Yuan-Hung Kuo, Yi-Hao Yen and Tsung-Hui Hu; data analysis and interpretation: Chao-Hung Hung and Sheng-Nan Lu; manuscript drafting and critical revising: Chao-Hung Hung, Chien-Hung Chen, Jing-Houng Wang, Tsung-Hui Hu and Sheng-Nan Lu.
Financial support: This work was supported by Eisai Company for the grant of glycyrrhizin (SNMC).
Potential competing interests: None.
Supplementary Material
References
- Locarnini S, Hatzakis A, Chen DS et al. Hepatitis B virus infection. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J Hepatol 2015; 62: S76–S86. [DOI] [PubMed] [Google Scholar]
- Chu CM, Liaw YF. Natural history of chronic hepatitis B virus infection: an immunopathological study. J Gastroenterol Hepatol 1997; 12: S218–S222. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Brunetto MR, Hadziyannis S. The natural history of chronic HBV infection and geographical differences. Antivir Ther 2010; 15: 25–33. [DOI] [PubMed] [Google Scholar]
- Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol 1990; 10: 29–34. [DOI] [PubMed] [Google Scholar]
- Wong VW, Chan HL. Severe acute exacerbation of chronic hepatitis B: a unique presentation of a common disease. J Gastroenterol Hepatol 2009; 24: 1179–1186. [DOI] [PubMed] [Google Scholar]
- Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J Hepatol 2014; 61: 1407–1417. [DOI] [PubMed] [Google Scholar]
- Chien RN, Lin CH, Liaw YF. The effect of lamivudine therapy in hepatic decompensation during acute exacerbation of chronic hepatitis B. J Hepatol 2003; 38: 322–327. [DOI] [PubMed] [Google Scholar]
- Wong VW, Wong GL, Yiu KK et al. Entecavir treatment in patients with severe acute exacerbation of chronic hepatitis B. J Hepatol 2011; 54: 236–242. [DOI] [PubMed] [Google Scholar]
- Terrault NA, Bzowej NH, Chang KM et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63: 261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw YF, Kao JH, Piratvisuth T et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012; 6: 531–561. [DOI] [PubMed] [Google Scholar]
- EASL Clinical Practice Guidelines.. Management of chronic hepatitis B virus infection. European Association for the Study of the Liver. J Hepatol 2012; 57: 167–185. [DOI] [PubMed] [Google Scholar]
- Hung CH, Hu TH, Lu SN et al. Tenofovir versus entecavir in treatment of chronic hepatitis B virus with severe acute exacerbation. Antimicrob Agents Chemother 2015; 59: 3168–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Zhao C, Shen C et al. The efficacy and safety of nucleos(t)ide analogues in patients with spontaneous acute exacerbation of chronic hepatitis B: a systematic review and meta-analysis. PLoS ONE 2013; 8: e65952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LJ, Yu JW, Zhao YH et al. Influential factors of prognosis in lamivudine treatment for patients with acute-on-chronic hepatitis B liver failure. J Gastroenterol Hepatol 2010; 25: 583–590. [DOI] [PubMed] [Google Scholar]
- Chan AC, Fan ST, Lo CM et al. Liver transplantation for acute-on-chronic liver failure. Hepatol Int 2009; 3: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crance JM, Lévêque F, Biziagos E et al. Studies on the mechanism of action of glycyrrhizin against hepatitis A virus replication in vitro. Antiviral Res 1994; 23: 63–76. [DOI] [PubMed] [Google Scholar]
- van Rossum TG, Vulto AG, de Man RA et al. Review article: glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther 1998; 12: 199–205. [DOI] [PubMed] [Google Scholar]
- Manns MP, Wedemeyer H, Singer A et alEuropean SNMC Study Group. Glycyrrhizin in patients who failed previous interferon alpha-based therapies: biochemical and histological effects after 52 weeks. J Viral Hepat 2012; 19: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang B. Randomized clinical trial with two doses (100 and 40ml) of Stronger Neo-Minophagen C in Chinese patients with chronic hepatitis B. Hepatol Res 2002; 24: 220. [DOI] [PubMed] [Google Scholar]
- Yasui S, Fujiwara K, Tawada A et al. Efficacy of intravenous glycyrrhizin in the early stage of acute onset autoimmune hepatitis. Dig Dis Sci 2011; 56: 3638–3647. [DOI] [PubMed] [Google Scholar]
- Lin CC, Wang PH. Intravenous glycyrrhizin improved serum transaminases rapidly in a chronic hepatitis B patient with acute exacerbation. J Formos Med Assoc 2015; 114: 188–189. [DOI] [PubMed] [Google Scholar]
- Hung CH, Lu SN, Wang JH et al. Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol 2003; 38: 153–157. [DOI] [PubMed] [Google Scholar]
- Conn JW, Rovner DR, Cohen EL. Licorice-induced pseudoaldosteronism. Hypertension, hypokalemia, aldosteronopenia, and suppressed plasma renin activity. JAMA 1968; 205: 492–496. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Waliacc AM, Valentino R et al. Mineralocorticoid activity of liquorice: II-beta-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 1987; 2: 821–824. [DOI] [PubMed] [Google Scholar]
- Tsai SL, Chen PJ, Lai MY et al. Acute exacerbations of chronic type B hepatitis are accompamed by increased T cell responses to hepatitis B core and e antigens. Implicabons for hepatitis B e atigen seroconversion. J Chin Invest 1992; 89: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 2001; 120: 1009–1022. [DOI] [PubMed] [Google Scholar]
- Tsai WL, Sun WC, Cheng JS. Chronic hepatitis B with spontaneous severe acute exacerbation. Int J Mol Sci 2015; 16: 28126–28145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BS, Ma YJ, Wang Y et al. Protective effect and mechanism of stronger neo-minophagen C against fulminant hepatic failure. World J Gastroenterol 2007; 13: 462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Abe K, Kuroda N et al. The inhibition of apoptosis by glycyrrhizin in hepatic injury induced by injection of lipopolysaccharide/d-galactosamine in mice. Arch Histol Cytol 2008; 71: 163–178. [DOI] [PubMed] [Google Scholar]
- Hassanein TI, Schade RR, Hepburn IS. Acute-on-chronic liver failure: extracorporeal liver assist devices. Curr Opin Crit Care 2011; 17: 195–203. [DOI] [PubMed] [Google Scholar]
- Kribben A, Gerken G, Haag S et al HELIOS Study Group. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 2012; 142: 782–789. [DOI] [PubMed] [Google Scholar]
- Garg V, Garg H, Khan A et al. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012; 142: 505–512. [DOI] [PubMed] [Google Scholar]
- Yasui S, Fujiwara K, Nakamura M et al. Virological efficacy of combination therapy with corticosteroid and nucleoside analogue for severe acute exacerbation of chronic hepatitis B. J Viral Hepat 2015; 22: 94–102. [DOI] [PubMed] [Google Scholar]
- Fan HL, Yang PS, Chen HW et al. Predictors of the outcomes of acute-on-chronic hepatitis B liver failure. World J Gastroenterol 2012; 18: 5078–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.