Abstract
Objective:
The operating procedure of a resternotomy in open-heart surgery is a complicated procedure with potentially problematic outcomes partly due to potential adhesions in the pericardial cavity and retrosternal space. Use of a collagen membrane has shown encouraging results in adhesion prevention in several regions of the body. This study was designed to evaluate the effectiveness of the use of this collagen membrane in the prevention of pericardial adhesions.
Materials and methods:
A total of 12 pigs were divided randomly into 2 groups: an experimental group in which collagen membranes were used and a control group. After sternotomy and an anterior pericardiectomy, the epicardial surface was exposed to room air and irrigated with saline, and an epicardial abrasion was performed using a sponge. The pericardial defect was repaired using a collagen membrane in the experimental group or left uncovered in the control group. After 8 to 12 weeks, the pigs were killed, and a resternotomy was performed by a single-blinded surgeon enabling the evaluation of adhesions. The heart was then removed and sent for microscopic assessment conducted by a single-blinded pathologist.
Results:
The resternotomy operations performed using a collagen membrane demonstrated a nonstatistically significant trend of fewer macroscopic and microscopic adhesions in all regions (P > .05), particularly in the retrosternal and defect regions.
Conclusions:
This study showed nonstatistically significant differences between the outcomes in the collagen membrane group and the control group in both macroscopic and microscopic adhesion prevention. Due to the many limitations in animal study design, further studies in human models will be needed before the true value of this procedure can be evaluated.
Keywords: Mediastinum, experimental cardiac surgery, pericardium
Introduction
The incidence of congenital heart disease is 8 in 1000 live births.1,2 The principal treatment for congenital heart disease is surgery. Some patients need several operations to correct the anomalies. Subsequent operations account for 32% of all congenital anomaly operations each year in Thailand as well as for 10% to 20% of cases in adult cardiac surgery.3,4 The most common problem occurring in a reoperation situation is adhesion resulting from the wound-healing process. These adhesions, specifically adherence between the heart, sternum, and surrounding organs, cause many problems such as decreased right ventricle contraction, reduced diastolic left ventricle filling time, increased operative time, and an increased risk of cardiac and great vessel injury during any subsequent sternotomy.5–7
The pericardium cannot be completely closed in most cardiac operations because of pericardial contraction, the swelling of cardiac tissue, or inadequate pericardium tissue after pericardial excision for grafting. A too tight pericardial closure may result in cardiac tamponade.4,7 There are some studies which have shown the efficacy of the Gore-Tex polytetrafluoroethylene (PTFE) membrane for the prevention of adhesions. However, a PTFE membrane may enhance the inflammatory process leading to the obscurity of the exact anatomy of the heart and hence increase the risk of iatrogenic cardiac injury. This is described in the publication by Kuschel et al.8 From the outcomes of this previous study, the PTFE membrane was found to have the greatest extension of fibrosis from microscopic findings and denser adhesions from macroscopic findings in all regions except that of the coronary artery. Moreover, an inappropriate size of a PTFE graft used in the case of a pediatric patient may contribute to constrictive pericarditis as the child matures.8 To date, most cardiac surgeons in Thailand do not use any patches for pericardial substitution in cases where complete pericardial closure is impossible.
CollaGUARD (Innocoll, Inc., Monksland, Athlone, County Roscommon, Ireland) is made from type I collagen fibers, a tissue commonly found in skin, tendons, bones, and vascular structures and can be absorbed within 8 to 12 weeks. It was initially introduced as a postoperative intra-abdominal adhesion-preventive material. The biochemical advantages of collagen are that it is fully biodegradable and bioresorbable via natural pathways and plays an integral role in the repair and replacement of both soft and hard tissues by providing an extracellular scaffold, stimulating certain growth factors, and propagating tissue granulation which can stimulate the natural process of wound healing. A previous study reported that use of a collagen membrane was able to reduce postoperative intra-abdominal adhesions in laboratory animals. The data from this study were statistically significant.9–11 Use of collagen membranes has been approved by the Food and Drug Administration of the United States and Thailand. Nevertheless, to date, there have been no published reports of the use of collagen membranes in the prevention of postoperative intrathoracic adhesions in humans. There was, however, an initial study in 2013 into using collagen membranes for preventing postoperative cardiac surgery adhesion in pediatric patients. This study reported positive outcomes regarding the reduction in adhesions and was deemed safe to use as there were no complications.12
According to the properties and the good outcomes to date of the use of collagen membranes, it may be claimed that using this technique is reasonable for use in cardiac surgery especially in the case of repeat operations. It may reduce adhesions, decrease risk of cardiac and great vessel injury, minimize operative time, and also be cost-effective. However, there have been no studies in humans yet, so this study intends to extend the knowledge surrounding the use of this potentially exciting product using a large animal model.
This study was designed to determine the effectiveness of use of the collagen membrane in the prevention of postoperative pericardial adhesions and evaluate any side effects of use of the collagen membrane in an animal model.
Materials and Methods
Study design
This study is a single-blinded, prospective pilot study in laboratory animals. A total of 12 Landrace-Large White-Duroc pigs aged between 28 and 40 days were randomly divided by a simple randomization method into 2 groups: a collagen membrane and a control group. This study was approved by the ethics committee of Maharaj Nakorn Chiang Mai Hospital and was registered with the Thai Clinical Trials Registry (www.clinicaltrials.in.th), registration number TCTR20160416002. This study was initially considered as a pilot study, and therefore, the size of the population was selected by the ethical committee. Every operation was conducted in the animal laboratory unit of Maharaj Nakorn Chiangmai Hospital in the period May 1, 2015 to October 31, 2015. This article was prepared according to the CONSORT checklist and CONSORT flow diagram.
Initial operation
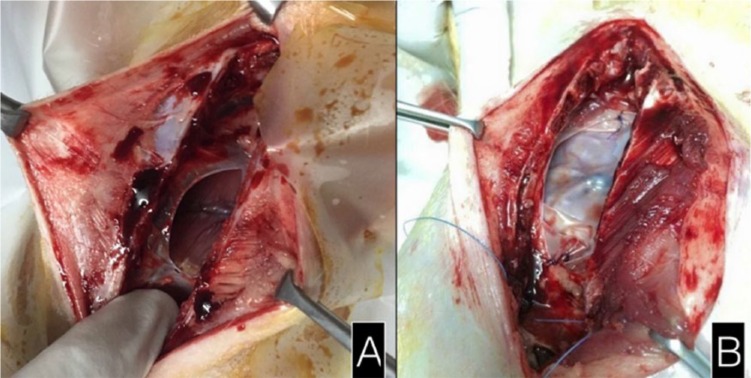
An intramuscular injection of intramuscular tiletamine/zolazepam (zolitil: 10 mg/kg) and xylazine (1 mg/kg) was used to induce anesthesia. A prophylactic antibiotic marbofloxacin (2 mg/kg) was given by intramuscular injection. After a routine sternotomy, a pericardiectomy was performed to create a pericardial defect. The epicardial surface was exposed to the aggravating effects of room air, warm saline irrigation, and gauze abrasion. In the control group, the pericardial defect was left open without any tissue coverage. In the collagen membrane group, the pericardial defect was covered with the collagen membrane which was sutured to the pericardial edge with 3/0 polyglactin before closure of the sternum (Figure 1). Then, in both groups, 2-0 polyglactin sutures were used to re-approximate the sternum. The wound was closed in layers.
Figure 1.
Initial sternotomy: the pericardial defect (A) in the control group and (B) covered by the collagen membrane.
Resternotomy
After 8 to 12 weeks, a resternotomy was performed to compare the severity of adhesion formation between the 2 groups. The time of the resternotomy was scheduled according to the expected size of the pigs, a size which was appropriate for laboratory space and not too big for management. The pigs were killed with an intramuscular xylazine (1 mg/kg) and intravenous pentobarbital sodium (>80 mg/kg) injection before the resternotomy was performed. The reason for this euthanasia before the operation was due to limited access to resources to fully anesthetize the pigs for the operation. The severity of adhesion formation was macroscopically evaluated by a single-blinded surgeon according to the grading score devised by Lopes et al13 (Table 1), which was found to significantly correlate with dissection time and the amount of sharp dissection required for the takedown of pericardial adhesions. The 6 regions of interest included the retrosternal region, defect region, right ventricle, aorta, right atrium, and the regions bordering the pericardial defect. Then, the hearts were removed and sent to the pathologist to enable the evaluation and recording of any microscopic findings. The grading score developed by Joon Hwa Hong and colleagues14,15 (Table 2) was modified for the assessment of the severity of the microscopic adhesions in terms of fibrosis, inflammation, and adhesion by the single-blinded pathologist. A flow chart summary of the study method is shown in Figure 2.
Table 1.
The grading score devised and tested by Lopes et al used for assessment of the severity of macroscopic adhesions.13
| Score | Description |
|---|---|
| 0 | Without adhesions |
| 1 | Filmy, light, with a foamy dissection plane without bleeding |
| 2 | Required some sharp dissection but most were lysed by digital manipulation. This process resulted in moderate bleeding |
| 3 | Dense, easy bleeding, with marked obliteration of tissue plane and required exclusive sharp dissection |
Table 2.
| Score | Fibrosis grade | Inflammation grade | Adhesion to sternum grade |
|---|---|---|---|
| 0 | None | None | None |
| 1 | Minimal, loose | Giant cells, lymphocytes, plasma cells | Minimal fibrous adhesion to the sternum |
| 2 | Moderate | Giant cells, eosinophils, neutrophils | Fibrous adhesion to the sternum within the defect region |
| 3 | Florid, dense | Many inflammatory cells, microabscess | Extended dense fibrous adhesion to the sternum |
Figure 2.
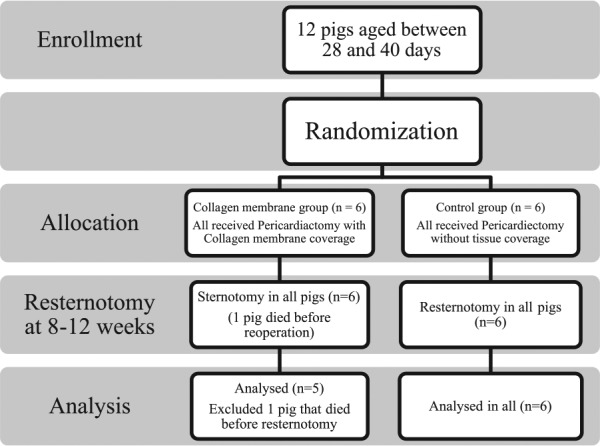
Flow chart summary of the research method.
All data were recorded on research record forms. The Student t test was used to compare differences in the mean values of demographic data and adhesion score between the 2 groups. Statistical results were calculated using the Stata statistical package (Release 12, 2013: Stata Corporation, College Station, TX, USA). P value of less than .05 was considered to be statistically significant.
Results
This study was conducted between May 1, 2015 and October 31, 2015. All sternotomy wounds had healed with granulation tissue. One pig from the collagen membrane group had a sternal dehiscence with mediastinitis. One pig from the collagen membrane group died 8 hours before the resternotomy procedure from an unknown cause, but a resternotomy was still performed and the investigation was completed on the day of death. There were no statistically significant differences in demographic data between the 2 groups in terms of sex, body weight at both first and second operations, and duration between first and second operations (Table 3).
Table 3.
Demographic data.
| Control group (n = 6) | Collagen membrane group (n = 6) | P value | |
|---|---|---|---|
| Sex (male: female) | 2:4 | 2:4 | 1.0 |
| Weight at first operation: kg, mean (minimum-maximum) | 9.67 (4-16) | 9.33 (5-19) | .91 |
| Weight at second operation: kg, mean (minimum-maximum) | 35.17 (21-55) | 32.50 (21-50) | .69 |
| Duration between first and second surgeries: days, mean (minimum-maximum) | 65.67 (44-90) | 63.67 (56-85) | .88 |
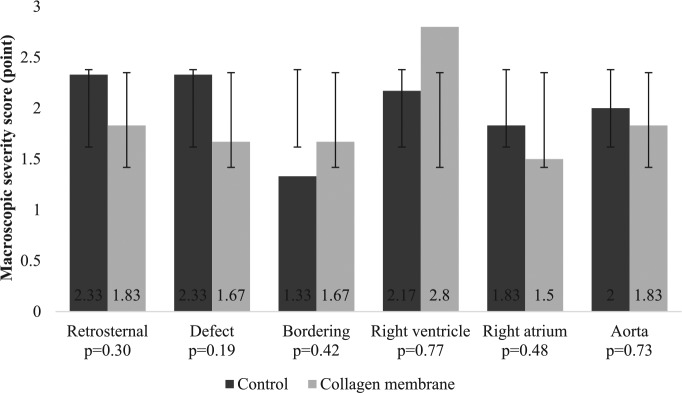
There was a nonsignificant trend between the use of a collagen membrane and fewer macroscopic adhesions in almost all regions (P > .05) especially in the retrosternal and defect regions (Figure 3). The bordering regions in the control group appeared to have fewer adhesions; however, that area was difficult to identify due to there being no marks on the heart which led to possible misinterpretation and so was a limitation of the study.
Figure 3.
A comparison of the severity of the macroscopic adhesions.
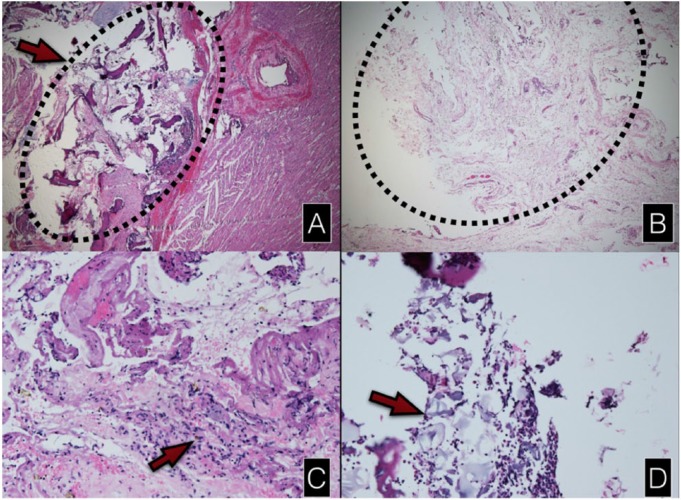
One specimen from the pig that died before resternotomy was excluded from the microscopic study because the specimen was lost during the transfer process. Hence, there were 5 specimens from the collagen membrane group compared with 6 specimens from the control group. No extensive inflammatory reaction was apparent in the specimens in either of the groups. A minimal chronic inflammatory reaction with accumulation of lymphocytes was seen in all specimens. The residual collagen membranes surrounded by inflammatory cells were identified in 2 specimens from the collagen membrane group (40%). In most of the control group specimens, many bony fragments could be identified near the epicardium of the heart in contrast to the collagen membrane group where overgrowth granulation tissue separated the bony fragments from the epicardium in 2 cases where the bony fragments could be seen (Figure 4).
Figure 4.
Light microscopic findings. A,B: hematoxylin and eosin staining H&E, original magnification ×25. (A) Bony fragments which adhered to the epicardium in the control group. (B) The overgrowth of granulation tissue from the defect area in the collagen membrane group. (C) Giant cells demonstrated in only 1 specimen of the collagen membrane group (H&E stain, ×100). (D) The residual collagen membrane surrounded by inflammatory cells (H&E stain, ×100).
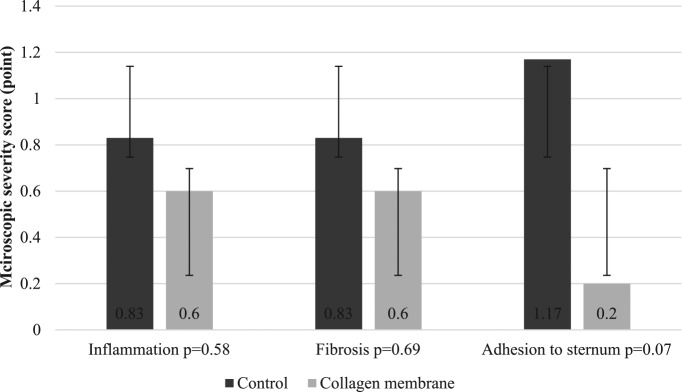
All microscopic adhesion scores were lower in the collagen membrane group in particular the adhesions to sternum score (Figure 5). However, statistically, the results were not significant at P > .05.
Figure 5.
A comparison of the severity of the microscopic adhesions.
Discussion
Improvements involving staged approaches for the treatment of congenital heart disease have led to an increasing number of reoperations performed in cases of congenital cardiac surgery. Patients may undergo multiple reoperations throughout their lifetimes to enable the complete correction of their congenital anomaly.16,17 They may also face acquired coronary disease or valvular defects unrelated to the primary congenital anomaly which may need surgical intervention in the future.18 Thus, the prevention of pericardial adhesions which can make reoperation a problem is becoming more and more important.
According to Armoiry et al,12 the collagen membrane was used with good tolerability in their first human clinical study including 36 pediatric patients. The use of collagen membranes has been proven to be successful in the prevention of intra-abdominal and gynecologic adhesions. In this study by Armoiry, there were no adverse events from using the collagen membrane.
In common with this study, the reoperations performed on the pigs showed a nonsignificant trend of fewer adhesions both macroscopically and microscopically in the collagen membrane group. One of the factors which may be responsible for these inconclusive results is the small sample size limiting the power of the data to exhibit a significant difference. Originally, it was planned to use 8 pigs in both groups, but the number was decreased to 6 in accordance with a suggestion made by the animal research committee.
Previous animal studies concerning pericardial adhesion formation prevention covered a time frame of a few weeks to months between the initial and subsequent operation. Kuschel and colleagues found a significant reduction in pericardial adhesion formation in rabbits when using TachoSil, a bioabsorbable collagen sponge coated on one side with the human coagulation factors fibrinogen and thrombin. The study by Kuschel involved a 6-month period before resternotomy.8 In this study, however, an 8- to 12-week period lapse in time before performing a resternotomy to see whether there was any residual collagen membrane was aimed for and achieved. This was done to investigate the manufacturer’s claim of full resorption within 8 to 12 weeks. A longer duration between the first and second surgeries, which would be more comparative with the typical interval between operations in staged repairs for the correction of congenital defects, may demonstrate clearer differences in adhesion formation between the 2 groups in future trials.
There were several limitations in this study. As previously intimated, the sample size is too small to achieve a level of statistical significance. Further limitations are the short duration between the first and second operations and that all pigs had to be killed before the resternotomy because they were all much bigger than they were at the first operation and were not able to be fully anesthetized and intubated, causing difficulties in performing the resternotomy. It would have been preferable to perform the resternotomy on a beating heart as it could affect the grading score in macroscopic adhesion formation. There were no markers at the pericardial edge which meant that the surgeon could not easily identify the defect regions. This study did not include the performance of pre-resternotomy echocardiography which would show the impact of the adhesions on the hearts, cardiac function, and pericardial or pleural effusions. This should be included in future studies especially in a study involving the human model.
Conclusions
This study showed nonstatistically significant differences between the collagen membrane group and the control group in both macroscopic and microscopic adhesion prevention. Due to the many limitations to study designs involving animal models due to extensive variation, further studies in human models may be needed to conclude whether the use of a collagen membrane has significant potential in the prevention of pericardial adhesions.
Acknowledgments
The authors would like to thank Apichat Tantraworasin, PhD, Department of Surgery, Faculty of Medicine, Chiang Mai University, for assistance with the statistical analysis and extend particular gratitude to Joan Peagam, MSc (London) and NPQH, from Medical and Scientific English Language Consultancy, Chiang Mai University for her gracious assistance with the English editing of the final manuscript.
Footnotes
Peer review:Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 444 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by the funding from the Faculty of Medicine, Chiang Mai University (grant number: 19/2558). The collagen membrane used in this study was donated free of charge by the CollaGUARD company.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Conceived and designed the experiments: MT and NT. Parenting animal experiment and perioperative care: AK. Pathological analysis: NL. Analyzed the data: NT, MK. Wrote the first draft of the manuscript: MK. Contributed to the writing of the manuscript: NT, MK. Made critical revisions and approved final version: NT. All authors reviewed and approved of the final manuscript.
References
- 1. Dolk H, Loane M, Garne E; European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–849. [DOI] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elahi MM, Kirke R, Lee D, Dhannapuneni RR, Hickey MS. The complications of repeat median sternotomy in paediatrics: six-months follow-up of consecutive cases. Interact Cardiovasc Thorac Surg. 2005;4:356. [DOI] [PubMed] [Google Scholar]
- 4. Nkere UU, Whawell SA, Sarraf CE, Schofield JB, Thompson JN, Taylor KM. Pericardial trauma and adhesions in relation to reoperative cardiac surgery. Thorac Cardiovasc Surg. 1995;43:338–346. [DOI] [PubMed] [Google Scholar]
- 5. Bailey LL, Ze-jian L, Schulz E, Roost H, Yahiku P. A cause of right ventricular dysfunction after cardiac operations. J Thorac Cardiovasc Surg. 1984;87:539–542. [PubMed] [Google Scholar]
- 6. Jiamsripong P, Alharthi MS, Calleja AM, et al. Impact of pericardial adhesions on diastolic function as assessed by vortex formation time, a parameter of transmitral flow efficiency. Cardiovasc Ultrasound. 2010;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gabbay S. The need for intensive study of pericardial substitution after open heart surgery. ASAIO Trans. 1990;36:789–791. [DOI] [PubMed] [Google Scholar]
- 8. Kuschel TJ, Gruszka A, Hermanns-Sachweh B, et al. Prevention of postoperative pericardial adhesions with TachoSil. Ann Thorac Surg. 2013;95:183–188. [DOI] [PubMed] [Google Scholar]
- 9. Arnold PB, Green CW, Foresman PA. Evaluation of resorbable barriers for preventing surgical adhesions. Fertil Steril. 2000;73:157–161. [DOI] [PubMed] [Google Scholar]
- 10. Cervantes-Sanchez CR, Olaya E, Testas M, et al. Collagen-PVP, a collagen synthesis modulator, decreases intraperitoneal adhesions. J Surg Res. 2003;110:207–210. [DOI] [PubMed] [Google Scholar]
- 11. Atsushi I, Noriko S. Topical non-barrier agents for postoperative adhesion prevention in animal models. Eur J Obstet Gynecol Reprod Biol. 2010;149:131–135. [DOI] [PubMed] [Google Scholar]
- 12. Armoiry X, Viprey M, Constant H, et al. Potential interest of a new absorbable collagen membrane in the prevention of adhesions in paediatric cardiac surgery: a feasibility study. Arch Cardiovasc Dis. 2013;106:433–439. [DOI] [PubMed] [Google Scholar]
- 13. Lopes JB, Dallan LA, Moreira LF, et al. New quantitative variables to measure postoperative pericardial adhesions (Useful tools in experimental research). Acta Cir Bras. 2009;24:82–86. [DOI] [PubMed] [Google Scholar]
- 14. Hong JH, Choe JW, Kwon GY, et al. The effects of barrier materials on reduction of pericardial adhesion formation in rabbits: a comprehensive study of hyaluronan-based solution and a temperature sensitive poloxamer solution/gel method. J Surg Res. 2011;166:206–213. [DOI] [PubMed] [Google Scholar]
- 15. Connors RC, Munir JJ, Liu Y, et al. Postoperative pericardial adhesion prevention using Carbylan-SX in a rabbit model. J Surg Res. 2007;140:237–242. [DOI] [PubMed] [Google Scholar]
- 16. Warnes CA. The adult with congenital heart disease: born to be bad? J Am Coll Cardiol. 2005;46:1–8. [DOI] [PubMed] [Google Scholar]
- 17. Lodge AJ, Wells WJ, Backer CL, et al. A novel bioresorbable film reduces postoperative adhesions after infant cardiac surgery. Ann Thorac Surg. 2008;86:614–621. [DOI] [PubMed] [Google Scholar]
- 18. Van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nat Rev Cardiol. 2011;8:50–60. [DOI] [PubMed] [Google Scholar]