Abstract
Background:
A progressive trajectory toward renal failure is common in patients with Alport syndrome. Genotype-phenotype correlations have been well described; however, the natural history of the trajectory toward renal failure is not well described.
Objective:
The objective of this study is to describe the natural history of renal function decline in a cohort of Alport syndrome patients.
Design:
Retrospective observational cohort study.
Setting:
British Columbia, Canada, chronic renal disease registry 1995-2012.
Patients:
37 biopsy proven Alport syndrome or hematuria with family history of Alport syndrome.
Measurements:
Serial estimated glomerular filtration rate (eGFR) Trajectory of renal decline described graphically by fitting a cubic smoothing spline to patient’s eGFR measures. Various time points within a trajectory were indexed, randomly sampled, and followed for 2 years to estimate portion of progressors (>5 mL/min/1.73 m2 /y decline), stable state (0-2 mL/min/1.73 m2 /y decline), and regressors (>2 mL/min/1.73 m2 /y incline).
Methods:
In this retrospective observational cohort study, participants were identified through a chronic renal disease registry in British Columbia, Canada, from 1995 to 2012. Inclusion criteria were biopsy proven or hematuria with a family history of Alport syndrome. Individual patients and family group members were studied. Trajectory of renal decline described graphically by fitting a cubic smoothing spline to patient’s serial estimated glomerular filtration rate (eGFR) measures. Various time points within a trajectory were indexed, randomly sampled, and followed for 2 years to estimate portion of progressors (>5 mL/min/1.73 m2/y decline), stable state (0-2 mL/min/1.73 m2/y decline), and regressors (>2 mL/min/1.73 m2/y incline).
Limitations:
Histological or genetic evidence of Alport syndrome is not available in all patients.
Results:
Median follow-up time was 48.2 months of 37 patients (78% male), with a median age of 36 (interquartile range [IQR], 18-47) and a median age of renal replacement therapy commencement (n = 23) of 38 (IQR = 20-52). Renal function changes were found to be heterogeneous overall, intra-individual and within families. Portion of progressors in eGFR 45-60 mL/min/1.73 m2 was 73.7% (SD, 10.3), whereas 23.6% (SD, 11.0) remained stable. Within eGFR 30-45 mL/min/1.73 m2, 45.6% (SD, 7.0) were progressors, whereas 53.4% (SD, 7.4) remained stable. A large portion of eGFR 15-30 mL/min/1.73 m2 patients were stable (54.8%; SD, 8.4), whereas 25.7% (SD, 7.1) progressed and 19.5% (SD, 5.6) regressed.
Conclusions:
The renal decline in Alport syndrome patients is heterogeneous which has implications for designing clinical trials of interventions.
Keywords: Alport syndrome, clinical trials, kidney failure, chronic renal failure, renal failure
Abrégé
Mise en contexte:
Les patients atteints du syndrome d’Alport voient souvent leur état de santé évoluer vers l’insuffisance rénale. La corrélation entre le génotype et le phénotype est bien établie. Par contre, l’histoire naturelle de la trajectoire menant à l’insuffisance rénale demeure mal connue.
Objectifs de l’étude:
Le principal objectif de cette étude était de caractériser l’histoire naturelle du déclin de la fonction rénale chez une cohorte de patients atteints du syndrome d’Alport.
Cadre et type d’étude:
Une étude de cohorte observationnelle rétrospective effectuée entre 1995 et 2012 à partir du registre provincial des maladies rénales chroniques de la Colombie-Britannique, au Canada.
Patients:
Une cohorte de 37 patients dont le diagnostic avait été confirmé par biopsie ou qui présentaient une hématurie conjointement à un historique familial de syndrome d’Alport.
Mesures:
Le débit de filtration glomérulaire estimé (DFGe) a été mesuré à plusieurs reprises. La trajectoire du déclin de la fonction rénale a été représentée graphiquement en ajustant une méthode d’interpolation cubique par splines de lissage à la série de mesures du débit de filtration glomérulaire estimé (eGFR) du patient. Plusieurs périodes suivant une trajectoire déterminée ont été indexées, échantillonnées aléatoirement et suivies pendant deux ans afin d’évaluer la proportion de cas de progression de la maladie (>5 mL/min/173m2/année de déclin), de patients stationnaires (0 à 2 mL/min/1,73 m2/année de déclin) et de cas de régression (>2 mL/min/1,73 m2/année de remontée).
Méthodologie:
Les patients qui ont participé à cette étude de cohorte observationnelle et rétrospective ont été recrutés entre 1995 et 2012 au sein d’un registre des patients souffrant d’insuffisance rénale chronique de la Colombie-Britannique, au Canada. La sélection des participants s’est effectuée sur la base d’un diagnostic posé par biopsie OU d’une hématurie en présence d’un historique familial de syndrome d’Alport. Les patients eux-mêmes, ainsi que des membres de leurs familles respectives ont été observés. La trajectoire du déclin de la fonction rénale a été représentée graphiquement en ajustant une méthode d’interpolation cubique par splines de lissage à la série de mesures du débit de filtration glomérulaire estimé (eGFR) du patient. Plusieurs périodes suivant une trajectoire déterminée ont été indexées, échantillonnées aléatoirement et suivies pendant deux ans afin d’évaluer la proportion de cas de progression de la maladie (>5 mL/min/173m2/année de déclin), de patients stationnaires (0 à 2 mL/min/1,73m2/année de déclin) et de cas de régression (>2 mL/min/1,73m2/année de remontée).
Limites de l’étude:
Les données génétiques ou histologiques nécessaires pour la confirmation du syndrome d’Alport étaient absentes pour certains patients.
Résultats:
Le suivi s’est effectué auprès de 37 patients dont 78% étaient des hommes et s’est échelonné sur une période moyenne de 48,2 mois. L’âge médian des participants était de 36 ans (Ecart Interquartile [EI] 18, 47) et l’âge médian où ceux-ci avaient commencé une thérapie de remplacement de la fonction rénale était de 38 ans (EI 20, 52). Les changements dans la fonction rénale se sont avérés hétérogènes tant de façon globale pour l’étude que chez les individus ou au sein de leurs familles. La proportion de progression de la maladie pour les patients présentant des valeurs de eGFR entre 45 et 60 mL/min/1,73m2 était de 73,7% (Écart-type [ÉT]: 10,3) tandis que 23,6% (ÉT: 11,0) sont demeurés stables. Dans le groupe de patients dont les valeurs de eGFR se situaient entre 30 et 45 mL/min/1,73m2, une proportion de 45,6% (ÉT: 7,0) était en progression alors que 53,4% (ÉT: 7,4) sont demeurés stables. Une forte proportion des patients présentant des valeurs de eGFR entre 15 et 30 mL/min/1,73m2 soit 54,8% (ÉT: 8,4) sont demeurés stables, tandis que 25,7% (ÉT: 7,1) étaient en progression et 19,5% (ÉT: 5,6) étaient en régression.
Conclusions:
Le déclin de la fonction rénale chez les patients atteints du syndrome d’Alport est hétérogène ce qui entraîne des répercussions sur la façon de concevoir les essais d’intervention cliniques.
What Was Known Before
There is progressive loss of renal function in Alport syndrome. This is more pronounced in males with X-linked Alport syndrome, in whom renal failure is almost universal.
What This Adds
We describe the longitudinal trajectory of loss of renal function. We show that over extended periods of time, the loss of renal function is heterogeneous when considered from the perspective of both between and within individual. Such findings are important when planning interventional clinical trials that are often limited to shorter time frames.
Introduction
Alport syndrome is a rare genetic disorder that is the primary renal diagnosis in 0.5% of all patients undergoing renal replacement therapy.1 It is predominantly due to an X-linked inheritance of a COL4A5 mutation2,3; however, autosomal recessive4-6 and rarely autosomal dominant mutations with more clinical variability in outcomes7,8 affecting COL4A3 and COL4A4 have been described. Progression to renal failure is inexorable, particularly in males with X-linked Alport syndrome.3,9 Females also develop renal failure, although the incidence of end-stage kidney disease due to Alport syndrome in females is less compared with males.2,10 In addition, those with proteinuria and extrarenal pathology associated with earlier renal failure.2,9 Earlier onset organ failure and the severity of extrarenal pathology have been related to different and more severe genetic mutations.3,9,11
While there has been considerable progress in understanding the genetics of Alport syndrome, the natural history of Alport syndrome in the community setting is not well described. As renal decline may occur over more than 20 to 30 years, Alport syndrome is a difficult disease to study prospectively and outcomes can be affected by awareness, detection, attempts at management as well as other environmental exposures. Nonetheless, given the progressive organ failure trajectory, there is increasing interest in therapies for Alport syndrome.12-14 Currently, expert opinion recommends the use of renin-angiotensin blockade,15,16 although there is still discussion concerning the timing of introduction of such agents, particularly while awaiting the results of randomized control trials.13 In addition, although there is much interest in newer therapies, it is recognized that a large sample size is required to demonstrate a moderate positive effect.15 However, both sample size and follow-up time required will be affected if the assumption that recognized genotypic-phenotypic associations imply a homogeneous and predictable organ function decline does not hold true. As such, this analysis aims to describe the degree of heterogeneity of renal function decline in a cohort of community-dwelling Alport syndrome patients managed according to prevalent principles or care within the Canadian universal access health care system.
Materials and Methods
The study population included patients with biopsy-proven Alport syndrome or hematuria with a family history of Alport syndrome, identified through the chronic kidney disease clinical information system known as PROMIS (Patient Records and Outcome Management Information System) in the province of British Columbia in Canada from 1995 to 2012. PROMIS contains data on provincial-wide chronic kidney disease (CKD) patients who have been referred to nephrologists. Approval was obtained through University of British Columbia Providence Health Care Research Ethics Board (H14-01195). Entry criteria for the database include estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or diagnosis of kidney disease presumed to be chronic on the basis of biopsy diagnosis, ultrasound, or clinical history of deterioration. Data collected in this study population included demographic data such as age at first available eGFR in the system, sex, and ethnicity. Baseline clinical data at first available eGFR included blood pressure, hemoglobin, albumin, serum calcium, serum phosphate, intact parathyroid hormone level, proteinuria, and use of renin-angiotensin system blockade agents. The primary data of interest were the serial eGFR measures over time, collected from first available in the system until the commencement of renal replacement therapy, death, or the end of study period April 30, 2014, whichever came first. The Modification of Diet in Renal Disease equation was used for eGFR calculation during the study period of interest. Patients with fewer than 4 eGFR data points recorded or having less than 8 months of follow-up duration were excluded (see Figure 1). The primary outcome of interest was disease progression based on the trajectory of eGFR over time.
Figure 1.

Cohort selection.
Note. eGFR = estimated glomerular filtration rate calculated by MDRD; CKD = chronic kidney disease.
Statistical Methods
Demographic and baseline data were summarized for the overall study cohort and by the categories of first available eGFR. Continuous variables were presented by mean with standard deviation or median with interquartile range (IQR) as appropriate. Categorical variables were presented by frequencies and percentages, where percentages were calculated with respect to nonmissing data.
First, eGFR trajectory over time was described graphically based on fitting a cubic smoothing spline to the given data using the smooth.spline function in the R software. A preselected set of trajectories were selected to demonstrate the heterogeneity in the disease progression in the entire cohort of patients, as well as between members within the same families.
Second, we followed individuals starting at various time points within their trajectory, stratified by different eGFR levels (irrespective of the duration of the disease at the time of enrollment) for 2 years. This was to demonstrate the degree of variability in the annual rate of change in eGFR within and across eGFR levels. Three eGFR levels considered were: 45-60 mL/min/1.73 m2, 30-45 mL/min/1.73 m2, and 15-30 mL/min/1.73 m2. The annual rate of change was grouped into 3 categories: (1) >5 mL/min decline in eGFR (progressor), (2) 0 to 2 mL/min decline/incline in eGFR (stable state), and (3) >2 mL/min incline in eGFR (regressor).
We identified all eligible entry point indexes by eGFR levels: One set contained all entry indexes with eGFR 45-60 mL/min/1.73 m2, one for 30-45 mL/min/1.73 m2, and another one for 15-30 mL/min/1.73 m2. For illustration purposes, the 45-60 mL/min data set is used as an example to explain how this data set was derived:
We identified all corresponding time indexes where a patient’s eGFR was between 45 and 60 mL/min. These time points were referred to as the potential indexes.
- Each potential index needed to satisfy the following conditions to be included in the data set:
- Must have at least 2 eGFR measures within 6 months prior to the potential index;
- The potential index must be “stabilized” for at least 6 months as defined by eGFR at 6 months prior being within ±5 mL/min of the eGFR at the potential index;
- Must have a minimum of 3 eGFR measures during the 24-month follow-up.
We then generate random samples to estimate the proportions of progressors, regressors, and patients in stable state by eGFR levels at the time of enrollment. We generated 1000 samples each for the 3 eGFR levels. The algorithm used was described below:
Generate a random number for each index within the eligible study entry data set as aforementioned.
Keep only the eligible entry index with the smallest random number for each patient to mimic the point of enrollment to the study. Each random sample consisted of unique patients.
Obtain all eGFR measures within 2 years from the point of enrollment identified in the previous step. Calculate the annual rate of change in eGFR for each patient using a simple linear regression model with eGFR as the dependent variable and time as the independent variable. Group the annual rate of change into 3 categories and compute the proportions.
Repeat steps 1 to 3 to get 1000 random samples.
All analyses were performed in SAS version 9.3 (Cary, North Carolina) and R software version 2.15.2.
Results
The total cohort consisted of 37 patients with a median follow-up time of 48.2 months and a range of 8.5 to 222.6 months. Table 1 shows characteristics of the cohort at registration into the clinical database. Seven-eight percent were males. The ages of the study cohort ranged from 6 to 71 years, with a median age of 36 (IQR = 18-47). Renal replacement therapy (dialysis or transplantation) was required in 23 (64%) patients with median age of 38 (IQR = 20-52) at the commencement of renal replacement therapy. Only 1 patient died before requiring renal replacement therapy at the age of 81. Of the total cohort, 49% were treated with renin-angiotensin blockade agents during the period of time of analysis.
Table 1.
Characteristics of Patients at Entry Into Registry.
| First reported GFR (mL/min) |
|||||
|---|---|---|---|---|---|
| Overall (n = 37) | ≥90 (n = 4) | 60-90 (n = 7) | 30-60 (n = 14) | <30 (n = 12) | |
| eGFR (mL/min) | 49.9 (25.6-71.7) | 113.2 (100.5-132.7) | 82.3 (71.7-86.5) | 50 (39.0-53.4) | 23.1 (19.8-25.1) |
| Age (y) | 36 (18-47) | 13 (10-19.5) | 18 (13-39) | 39 (21-55) | 42 (26.5-54.5) |
| Male | 29 (78%) | 4 (100%) | 7 (100%) | 9 (64%) | 9 (75%) |
| Caucasian | 29 (81%) | 4 (100%) | 4 (57%) | 11 (85%) | 10 (83%) |
| Systolic BP (mm Hg) | 126 (107-139) | 100 (98-102) | 104 (102-122) | 133 (119-140) | 142 (134-144) |
| Diastolic BP (mm Hg) | 74 (63-88) | 64 (57-71) | 68 (62-70) | 80 (68-96) | 86 (80-90) |
| Hemoglobin (g/L) | 127 (117-139) | 128 (128-128) | 130 (112-144) | 130 (126-142) | 119 (93-128) |
| Albumin (g/L) | 36.5 (32.5-38.5) | 31 (22-40) | 33.5 (32.0-34.0) | 37.5 (36-38) | 37.5 (33-39) |
| Calcium (mmol/L) | 2.27 (2.15-2.32) | 2.32 (2.08-2.54) | 2.19 (2.09-2.25) | 2.28 (2.26-2.37) | 2.22 (2.00-2.34) |
| Phosphate (mmol/L) | 1.2 (1.06-1.40) | 1.34 (1.17-1.40) | 1.19 (0.93-1.38) | 1.19 (0.95-1.31) | 1.28 (1.17-2.02) |
| PTH (pg/mL) | 7 (4.8-15.26) | — | 4.7 (2.20-5.70) | 6.24 (4.8-10.3) | 14.6 (6.9-18) |
| Proteinuria | |||||
| Normal | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (8%) |
| Moderate (1+) | 7 (19%) | 1 (25%) | 1 (14%) | 2 (14%) | 3 (25%) |
| Severe (3+) | 13 (35%) | 2 (50%) | 3 (43%) | 5 (36%) | 3 (25%) |
| Data not available | 16 (43%) | 1 (25%) | 3 (43%) | 7 (50%) | 5 (42%) |
| ACEi/ARB | 18 (49%) | 1 (25%) | 5 (71%) | 7 (50%) | 5 (42%) |
Note. eGFR = estimated glomerular filtration rate; BP = blood pressure; PTH = parathryoid hormone; ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker.
eGFR Trajectory Over Time
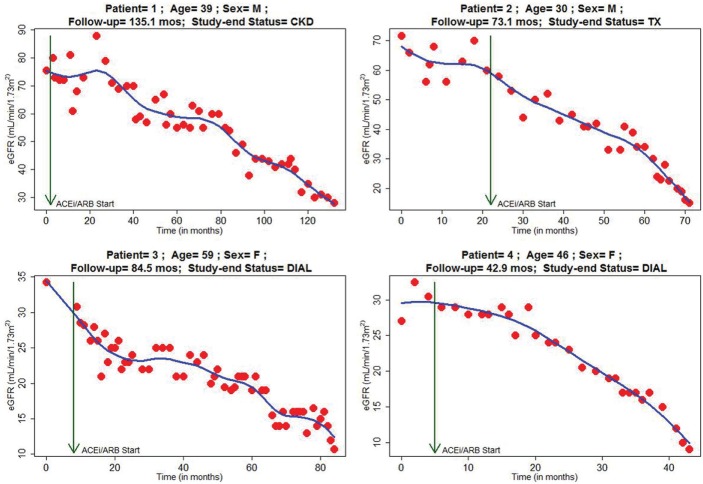
Figure 2 shows characteristic plots of eGFR trajectory with age, sex, and initiation of renin-angiotensin blockade identified. Different patterns are discernible: linear and nonlinear changes over time, as well as periods of progression, stability, and regression of disease state as measured by eGFR. Figure 2 shows patients with progressive renal decline. Patients 1 and 2, both male, have discernible linear and progressive renal function decline. However, patient 3 (female), although having progressive renal decline, progresses in a nonlinear manner, and patient 4 (female) appears to have accelerated decline toward need for renal replacement. Figure 3 displays eGFR plots of patient representative of those with considerable intra-individual variability. Patients 1 and 2 (both male) have lengthy durations of both regression and progression of disease state. Patient 3 (female) suffered a reasonably linear loss of renal function before having a prolonged period of stability of renal disease, whereas patient 4 (female) can be seen to have nonlinear progression of disease state.
Figure 2.
eGFR trajectory over entire follow-up on selected patient representatives of linear progressive renal function loss.
Note. eGFR = estimated glomerular filtration rate; CKD = chronic renal disease; DIAL = dialysis dependent; TX = transplant recipient.
Figure 3.
eGFR trajectory over entire follow-up on selected patient representatives of intra-individual variability of progression, regression, or stability of renal function.
Note. eGFR = estimated glomerular filtration rate; CKD = chronic renal disease; DIAL = dialysis dependent; TX = transplant recipient.
Figure 4 describes that the variability found in the entire cohort is also found within family groups. There are 4 family groups included in our cohort. The trajectories of family members in within the same family are variable: Some members of the same family share a similar eGFR trajectory (Figure 4), whereas other members of the same family have different trajectories that can be described as progressors or nonprogressors (Figure 5). We note that family 2 consisted of one male and one female; however, families 1 and 3 consisted of males only who had different trajectories compared with their siblings.
Figure 4.
Family member with similar eGFR trajectory.
Note. eGFR = estimated glomerular filtration rate; CKD = chronic renal disease; DIAL = dialysis dependent.
Figure 5.
Family member with heterogeneous eGFR trajectory.
Note. Members of family 2 and 3 demonstrate heterogeneous eGFR trajectories; eGFR = estimated glomerular filtration rate; CKD = chronic renal disease; DIAL = dialysis dependent; TX = transplant recipient.
Study on Disease Progression Over 2 Years
Observations were divided into 3 eGFR entry ranges: The 45-60 mL/min/1.73 m2 set consisted of 56 eligible study entries contributed from 11 patients (9 males and 2 females), the 30-45 mL/min/1.73 m2 had 81 eligible study entries from 12 patients (8 males and 4 females), and the 15-30 mL/min/1.73 m2 data set had 86 eligible study entries from 10 patients (7 males and 3 females). Figure 6 displays the distribution of within-individual variabilities of a 2-year eGFR progression, stratified by progression, stability, or regression. The simulation results show that, for those who started at eGFR 45-60 mL/min/1.73 m2, on average 73.7% (SD, 10.3) patients had more than 5 mL/min per year decline in eGFR, and 23.6% (SD, 11.0) had a stable annual rate of change. For those who were followed at eGFR 30-45 mL/min/1.73 m2, 45.6% (SD, 7.0) patients experienced disease progression and 53.4% (SD, 7.4) remained in stable state. As for those who followed at eGFR 15-30 mL/min/1.73 m2, 54.8% (SD, 8.4) patients were in stable state, 25.7% (SD, 7.1) had progression of disease, and 19.5% (SD, 5.6) had an improvement in the rate of change in eGFR.
Figure 6.

Distribution of within-individual variability of eGFR over randomly selected 2-year follow-up period.
Note. eGFR = estimated glomerular filtration rate.
Discussion
To the best of our knowledge, this is the first description of the variability of the natural history of Alport syndrome in community-dwelling patients with access to universal health care coverage. Other publications have noted heterogeneity in the outcome of dialysis dependence and described relationships with different mutations and the presence of extrarenal lesions.2,3,9,11 However, there has not been a comprehensive longitudinal description of a cohort of patients who have been exposed to numerous factors and management strategies over a longtime period. Our analysis permits the description of different trajectories within and between individuals, including those from the same family.
In our cohort of community-dwelling Alport syndrome patients, the documented renal function changes are considerably heterogeneous, both across the entire cohort and also within individuals. While the expected progressive renal function loss is described, in some cases there is stability and even improvement as measured by serial eGFR. Furthermore, some patients do not have inexorably progressive disease but appear to have considerable periods of stability and periods of improvement of eGFR in combination with periods of progression. Our data demonstrate that those who lose kidney function over time do not necessarily have linear decline. That is, there is heterogeneity within the cohort and within individuals with respect to direction of change of renal function, and in regard to rates of change.
We examined the trajectories within families, with presumed similar genetic mutations, and also describe that the course of Alport syndrome is variable. Other analyses within families have found a variability of ages of onset of end-stage kidney disease.2,3,6,12 This may have been due to a number of factors, including sex, earlier recognition and management of Alport syndrome in younger siblings, exposures to medications, or episodes of acute kidney injury, or differences in phenotype which impact outcomes, such as nephron mass at birth. We have previously described monozygotic twins with Alport syndrome in whom the trajectory of renal decline was significantly different, despite similar management, with the only factor differentiating them was different birth weights.17 In the present analysis, members in 3 out of the 4 families experienced progression that was markedly different from other family members, even after accounting for different first eGFR at registration, and performing analyses anchoring at similar eGFR values. This may reflect recognized differences in outcomes according to sex, altered genetic mutation penetration, or different attempts to manage the disease in particular family members. It may also reflect the impact of various environmental exposures that impact upon the progression of any genetic disorder. Aside from sex, these are factors that cannot be predicted in any observational or interventional study.
The heterogeneity over an extended period of time that we have described here poses challenges for the conduct of clinical interventional studies in Alport syndrome, especially those of limited duration (eg, 2 years), using eGFR as the outcome. In examining the individual trajectory plots of our cohort data, the reader will recognize the significant challenge of understanding and interpreting results from an intervention trial. In Figure 2, regardless of the starting point eGFR, over a 2-year duration, the trajectories are mostly uniform. However, when we consider the representative patients of Figure 3, selecting different time points over an extended period of time with 2 years of follow-up, the result is very different discordant intra-individual trajectories. The trajectory of patient 2 in Figure 3 illustrates 2 periods of approximately 2-year duration that are markedly different: one a progressive decline from month 20 and later a period of stability from month 80. Similar distinct differences in trajectories over approximately 2-year duration can be seen in each of the representative patients in Figure 3. These cases exemplify the complexity of enrolling patients into clinical trials if variability to this extent can occur based on the different time points chosen for follow-up. Our analysis also shows that the heterogeneity demonstrated in individual trajectory plots persists across a cohort-wide analysis. When analyzed according to 3 levels of initial eGFR (45-60, 30-45, and 15-30 mL/min). A patient with moderately advanced CKD (eGFR 30-45 mL/min) had an equal chance of stable renal function, which was slightly greater chance than progressive loss of renal function over any randomly selected 2-year follow-up period. In comparison, a patient with less severe CKD (eGFR 45-60 mL/min) was more likely to have progressive renal function loss albeit with a moderate chance of experiencing stable renal function over a randomly selected 2-year period. On the contrary, a patient with more severe CKD (eGFR 15-30 mL/min) had a high chance of experiencing stable renal function, and only a moderate chance of progressive loss of renal function and still a small chance of improvement in renal function in a randomly selected 2-year follow-up period. This set of analyses suggest that there is considerable difficulty in predicting the course of progression of Alport syndrome, which will ultimately impact on calculation of appropriate sample sizes and duration of follow-up of intervention groups in any interventional trial.
The strength of our analysis is that we present a longitudinal analysis of renal function decline, as measured by repeated eGFR results, of Alport syndrome community-dwelling patients. Such a cohort is consistent with a cohort that may be expected to be encountered in the recruitment for clinical trials. We have granular and complete laboratory data and complete set of kidney function trajectories. This includes data on the currently recommended therapy of renin-angiotensin system blockade, which we note was not universal in our cohort. While larger cross-sectional analyses have been documented,1-3,10 our analysis more uniquely considers the heterogeneity of renal function decline toward the end points of renal replacement therapy. Our cohort characteristics are similar to those previously reported with respect to median age 38 (IQR = 20-52) of commencement of renal replacement therapy (dialysis or preemptive transplantation).1-5,11,15,18 In cohorts of individuals from similar health care environments to ours, the median age of commencement is comparable: for example, 141 Alport syndrome patients (62% male) in Australia and New Zealand between 1997 and 2010 who had a mean age of commencement of renal replacement therapy of 37.7.1
The major limitation of our findings is the observational nature of the data, and its derivation from clinical records. As a result, the ascertainment of creatinine values was not standardized but driven by clinical indication. In addition, we are not able to account for all comorbidities. We also acknowledge limitations borne out of collecting data on “real-world” trajectories of Alport syndrome patients managed individually by nephrologists across a time period in which our understanding of pathology and treatment has developed. We acknowledge that we have not obtained histological or genetic evidence of Alport syndrome in all patients. However, we note that there is ongoing discussion as to the most clinically useful definition of Alport syndrome to ensure timely diagnosis. Note has been made that heterozygote patients may be underdiagnosed and undertreated19 whilst labeled as familial benign hematuria and that international discussion as to appropriate classification continues.15,20 As such, including all patients with persistent hematuria and family history and no other obvious cause of renal injury, in the absence of histological or genetic evidence, is only a relative limitation.
Alport syndrome is a rare disease in which the progression toward renal failure can appear inexorable, particularly in the most common form of disease, X-linked Alport syndrome. Over the time period of these data, there has been documented improvement in outcomes with the use of rennin-angiotensin blockade12 and general changes in care of patients with chronic kidney disease. Nonetheless, novel treatments that may alter morbidity and mortality outcomes are clearly required and are being developed. This multifaceted descriptive analysis of trajectories of individuals and families over extended time periods describes wide variability in the natural history of community-dwelling patients with Alport syndrome. This information is important to consider in the design of studies of interventions in this disease. It may be that interventional trials will need to use markers other than, or in addition to, those based on eGFR, such as serial biopsies or molecular analyses to determine impact. Detailed analyses such as described here should assist in designing such studies.
Footnotes
List of abbreviations: eGFR = estimated glomerular filtration rate; CKD = chronic kidney disease.
Ethics Approval and Consent to Participate: Ethics approval for the study was obtained from Providence Health Care Ethics Board.
Availability of data and materials: Upon request.
Author Contributions: Research idea and study design: DL, AL, OD, LE, MT; statistical analysis: LE; interpretation: LE, DL, AL; mentorship: AL. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Regulus Therapeutics.
References
- 1. Mallett A, Tang W, Clayton PA, et al. End-stage kidney disease due to Alport syndrome: outcomes in 296 consecutive Australia and New Zealand Dialysis and Transplant Registry cases. Nephrol Dial Transplant. 2014;29(12):2277-2286. [DOI] [PubMed] [Google Scholar]
- 2. Jais JP, Knebelmann B, Giatras I, et al. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol. 2003;14(10):2603-2610. [DOI] [PubMed] [Google Scholar]
- 3. Jais JP, Knebelmann B, Giatras I, et al. X-linked Alport syndrome: natural history in 195 families and genotype-phenotype correlations in males. J Am Soc Nephrol. 2000;11:649-657. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Sivakumar V, Mohammad M, et al. Clinical and genetic features in autosomal recessive and X-linked Alport syndrome. Pediatr Nephrol. 2014;29(3):391-396. [DOI] [PubMed] [Google Scholar]
- 5. Storey H, Savige J, Sivakumar V, Abbs S, Flinter FA. COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J Am Soc Nephrol. 2013;24(12):1945-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oka M, Nozu K, Kaito H, et al. Natural history of genetically proven autosomal recessive Alport syndrome. Pediatr Nephrol. 2014;29:1535-1544. doi: 10.1007/s00467-014-2797-4. [DOI] [PubMed] [Google Scholar]
- 7. Pescucci C, Mari F, Longo I, et al. Autosomal-dominant Alport syndrome: natural history of a disease due to COL4A3 or COL4A4 gene. Kidney Int. 2004;65(5):1598-1603. [DOI] [PubMed] [Google Scholar]
- 8. Marcocci E, Uliana V, Bruttini M, et al. Autosomal dominant Alport syndrome: molecular analysis of the COL4A4 gene and clinical outcome. Nephrol Dial Transplant. 2009;24(5):1464-1471. [DOI] [PubMed] [Google Scholar]
- 9. Gross O, Netzer KO, Lambrecht R, Seibold S, Weber M. Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol Dial Transplant. 2002;17(7):1218-1227. [DOI] [PubMed] [Google Scholar]
- 10. Savige J, Colville D, Rheault M, et al. Alport syndrome in women and girls. Clin J Am Soc Nephrol. 2016;11:1713-1720. doi: 10.2215/CJN.00580116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bekheirnia MR, Reed B, Gregory MC, et al. Genotype-phenotype correlation in X-linked Alport syndrome. J Am Soc Nephrol. 2010;21(5):876-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gross O, Licht C, Anders HJ, et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012;81(5):494-501. [DOI] [PubMed] [Google Scholar]
- 13. Gross O, Friede T, Hilgers R, et al. Safety and efficacy of the ACE-inhibitor ramipril in Alport syndrome: the double-blind, randomized, placebo-controlled, multicenter phase III EARLY PRO-TECT Alport trial in pediatric patients. ISRN Pediatr. 2012;2012:436046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gross O, Perin L, Deltas C. Alport syndrome from bench to bedside: the potential of current treatment beyond RAAS blockade and the horizon of future therapies. Nephrol Dial Transplant. 2014;29(suppl 4):124-130. [DOI] [PubMed] [Google Scholar]
- 15. Savige J, Gregory M, Gross O, Kashtan C, Ding J, Flinter F. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J Am Soc Nephrol. 2013;24(3):364-375. [DOI] [PubMed] [Google Scholar]
- 16. Kashtan CE, Ding J, Gregory M, et al. Clinical practice recommendations for the treatment of Alport syndrome: a statement of the Alport syndrome research collaborative. Pediatr Nephrol. 2013;28(1):5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajan T, Barbour SJ, White CT, Levin A. Low birth weight and nephron mass and their role in the progression of chronic kidney disease: a case report on identical twins with Alport disease. Nephrol Dial Transplant. 2011;26(12):4136-4139. [DOI] [PubMed] [Google Scholar]
- 18. Demosthenous P, Voskarides K, Stylianou K, et al. X-linked Alport syndrome in Hellenic families: phenotypic heterogeneity and mutations near interruptions of the collagen domain in COL4A5. Clin Genet. 2012;81(3):240-248. [DOI] [PubMed] [Google Scholar]
- 19. Temme J, Peters F, Lange K, et al. Incidence of renal failure and nephroprotection by RAAS inhibition in heterozygous carriers of X-chromosomal and autosomal recessive Alport mutations. Kidney Int. 2012;81(8):779-783. [DOI] [PubMed] [Google Scholar]
- 20. Gross O, Kashtan CE, Rheault MN, et al. Advances and unmet needs in genetic, basic and clinical science in Alport syndrome: report from the 2015 international workshop on Alport syndrome [published online ahead of print May 10, 2016]. Nephrol Dial Transplant. doi: 10.1093/ndt/gfw095. [DOI] [PMC free article] [PubMed] [Google Scholar]