Abstract
Background:
The use of an incremental peritoneal dialysis (PD) strategy in a large contemporary patient population has not been described.
Objective:
We report the use of this strategy in clinical practice, the prescriptions required, and the clearances achieved in a large center which has routinely used this approach for more than 10 years.
Design:
This is a cross-sectional observational study.
Setting:
A single large Canadian academic center.
Patients:
This study collected data on 124 prevalent PD patients at a single Canadian academic center.
Methods and Measurements:
The proportion of patients who achieve the clearance target on a low clearance or incremental PD prescription; the actual PD prescriptions and consequent total, peritoneal, and renal urea clearances [Kt/V] achieved; and patient and technique survival and peritonitis rate in comparison with national and international reports.
Results:
Of the 124 prevalent PD patients in this PD unit, 106 (86%) were achieving the Kt/V target, and of these, 54 (44% of all patients) were doing so using incremental PD prescriptions. Fifty of these incremental PD patients were using automated PD (APD) with either no day dwell (68%) or less than 7 days a week treatment (12%) or both (20%). Patient survival in our PD unit was not different from that reported in Canada as a whole. Peritonitis rates were better than internationally recommended standards.
Limitations:
This is an observational study with no randomized control group.
Conclusions:
Incremental PD is feasible in a contemporary PD population treated mainly with APD. Almost half of the patients were able to achieve clearance targets while receiving less onerous and less costly low clearance prescriptions. We suggest that incremental PD should be widely used as a cost-effective strategy in PD.
Keywords: incremental peritoneal dialysis, Kt/V, peritoneal clearance, automated peritoneal dialysis
Abstract
Mise en contexte:
L’utilisation de stratégies de dialyse péritonéale (DP) incrémentale au sein d’une grande population contemporaine n’a pas encore été bien documentée.
Objectifs de l’étude:
Cette étude est un compte rendu de l’utilisation de telles stratégies en pratique clinique, des prescriptions exigées ainsi que des clairances atteintes au sein des grands centres hospitaliers ayant intégré cette approche dans leur programme de soins depuis plus de dix ans.
Cadre et type d’étude:
Une étude observationnelle transversale qui s’est tenue dans un seul grand centre hospitalier universitaire au Canada.
Patients:
L’étude a porté sur un total de 124 patients prévalents pour la dialyse péritonéale dans un centre hospitalier universitaire canadien.
Méthodologie:
On a mesuré la proportion de patients ayant atteint les valeurs cibles de clairance rénale à la suite d’une ordonnance pour une dialyse péritonéale supplémentaire. On a également répertorié le nombre de prescriptions de dialyse péritonéales et conséquemment, les valeurs de clairance d’urée totale, péritonéale et rénale atteintes (Kt/V). Les taux de survie des patients, les taux de péritonites ainsi que les taux de succès de la procédure ont été comparés aux valeurs rapportées au niveau national ainsi qu’à l’international.
Résultats:
De la cohorte de 124 patients prévalents pour la dialyse péritonéale recensés dans l’unité de dialyse étudiée, 106 (86%) ont atteint la cible de Kt/V et de ceux-ci, 54 patients (44%) y sont parvenus par la prescription d’une dialyse péritonéale incrémentale. De ces 54 patients sous DP incrémentale, 50 étaient traités par dialyse péritonéale automatisée (DPA) tous les jours (68%), quelques jours par semaine (12%) ou les deux (20%). Les taux de survie des patients dans l’unité de dialyse étudiée ne présentaient aucune différence significative lorsque comparés au taux rapporté dans tout le Canada. Les taux de péritonites se sont avérés meilleurs que les standards recommandés à l’international.
Limites de l’étude:
Le fait que cette étude observationnelle n’ait pas été contrôlée de façon aléatoire par un groupe témoin constitue une limite.
Conclusions:
La dialyse péritonéale incrémentale est possible dans une population contemporaine de patients traités principalement par DPA. Près de la moitié des patients ont pu atteindre les valeurs cibles de clairance tout en recevant des prescriptions de faible clairance moins complexes et moins coûteuses. Nous suggérons que la DP incrémentale devrait être plus largement utilisée comme stratégie économique de dialyse péritonéale.
What Was Known Before
The concept of incremental peritoneal dialysis (PD) has been discussed for almost 2 decades, but there is no literature describing its use in a large contemporary PD program.
What This Adds
This study shows that it is feasible to practice incremental PD in a large contemporary patient population and that target clearances can be achieved with less onerous and less expensive PD prescriptions.
Introduction
Incremental dialysis is the practice of initiating chronic dialysis with low clearance prescriptions, relying on the presence of significant residual renal function to ensure clearance targets are achieved. It was first defined in the 1997 National Kidney Foundation Dialysis Outcome Quality Initiative Guideline on Peritoneal Dialysis Adequacy and was initially discussed more as a concept than as an actual practice, with the idea being that it would be associated with earlier initiation of chronic PD.1-4 The underlying aim is to make the initial dialysis prescription less onerous for the patient. Typical initial incremental dialysis prescriptions include twice weekly hemodialysis or cycler PD without a day dwell. The strategy is attractive because it reduces costs and decreases workload for patients and their caregivers. Implicit in the practice is the idea that, as patients lose their residual renal function, their dialysis dose will be raised in order that they stay at or slightly above clearance targets.1
Incremental PD has been discussed in the literature for many years. A number of articles, mainly from Italy, have described its use in small numbers of patients, mostly treated with continuous ambulatory PD (CAPD).5-9 A survey of North American PD centers in 1999 suggested it was being used by a significant number of PD practitioners.10 Its potential relevance increased with the worldwide tendency in the 1990s and 2000s to initiate chronic dialysis at higher levels of renal function as this provides greater margin for its use.11,12 However, no large-scale experience with the approach has been reported and what it actually means to apply it in clinical practice is not well described or widely understood.
Our center has practiced incremental PD routinely in all patients for the past decade. We review here, in a cross-sectional manner, our experience in a large contemporary patient population treated predominantly with automated PD (APD). Our aim is to describe what incremental PD means in terms of the types of PD prescriptions required and the clearances achieved.
Methods
Setting
The London Health Sciences Centre PD Program is based at an academic medical center in South Western Ontario, Canada. It serves a population of approximately 1.2 million people and is part of a comprehensive renal replacement treatment program that also includes in-center, satellite, and home hemodialysis, as well as kidney transplantation.
Study Population
PD patients are trained in CAPD and then APD and can choose which modality they prefer. The program uses Baxter Twin Bag (Baxter Deerfield, Illinois) for CAPD and Home Choice Pro Cycler (Baxter Deerfield) for APD.
In mid-2013, we collected data on all 124 patients who were on PD at that time. We recorded basic demographic data, their exact PD prescription, and their most recent peritoneal, renal, and total urea clearances, normalized to total body water (fractional urea clearance [Kt/V]). The practice at the time was to measure peritoneal, renal, and total Kt/V at least once every 3 months. In accordance with Canadian Society of Nephrology guidelines, the target total Kt/V is 1.7 per week, although we also use clinical judgment to avoid excessively onerous PD prescriptions, especially in elderly, frail patients where the disadvantages of a rigid approach might outweigh the potential benefits.13
Definitions
For the purposes of the study, a patient was defined as doing “Incremental PD” if they had a peritoneal Kt/V less than 1.7 per week and a total Kt/V of 1.7 per week or greater and any one of the following: (1) a CAPD with less than 8-L PD solution daily, (2) a CAPD for less than 7 days a week, (3) APD without any day dwell, or (4) APD for less than 7 days a week. This definition is derived from the 1997 National Kidney Foundation Dialysis Outcome Quality Initiative Guideline on Peritoneal Dialysis Adequacy although the Kt/V target is now lower than it was then.1,13 “Full-Dose PD” was defined as CAPD with at least 8 L daily or APD with a cycler treatment every night and with at least 1 day dwell each day. This was also based on the 1997 Guideline and on widely used PD practices in Canada in previous surveys.14 It is recognized that standard PD prescriptions may differ between countries.15 These “Full-Dose PD” patients can be subdivided into those who achieve the Kt/V target with peritoneal Kt/V alone and those who achieve it through a combination of both peritoneal and residual renal clearance.
Outcome Measures
The outcome of interest here was the proportion of patients who achieved the target clearance with an incremental PD strategy. Clearances are measured in the conventional manner.13 Renal urea clearance is calculated by collecting a 24-hour urine, measuring its urea content and dividing by the daytime blood urea. This value is normalized to total body water, estimated using the Watson formula, based on age, sex, and weight, to give a renal Kt/V. In this calculation, we use the standardized or “nonobese” weight, rather than the actual weight, as suggested by Canadian Society of Nephrology guidelines.13 This is based on the rationale that body fat has low water content and does not require the same amount of clearance as lean body mass. Residual renal clearance is also estimated in the standard manner using the mean of renal urea and renal creatinine clearance with normalization to body surface area calculated using the DuBois formula.13
Peritoneal urea clearance is calculated in CAPD patients by performing a 24-hour effluent collection and by measuring its urea content. In APD patients, a 24-hour effluent collection is not practical and cycler effluent volume is estimated by adding the known prescribed volume of PD solution to the ultrafiltration volume as recorded by the cycler. A 10-mL sample of the cycler effluent taken by the patient after careful mixing of the effluent in a single container is sent for measurement of urea concentration, and total effluent urea content can thus be estimated. Any additional effluent volume from “manually” performed day dwells is collected and its urea content added to give to the total 24-hour effluent urea content. In both CAPD and APD, the effluent urea content is divided by the daytime blood urea to give peritoneal urea clearance and again normalization to total body water gives a peritoneal Kt/V. While both renal and peritoneal Kt/V are measured over 1 day, they are expressed per week, as is the convention for Kt/V in PD.13 The actual calculations are carried out using Baxter PD Adequest (Baxter Deerfield).
Program outcomes were assessed by looking at peritonitis rates, patient survival, and by proportion of patients meeting the Kt/V target of 1.7 per week. Peritonitis rates are calculated annually using POET software (Baxter Deerfield).16 Patient survival is calculated for each center in Canada by the Canadian Organ Replacement Register and compared with the rates for the country as a whole.
Statistical Analysis
Among those who were achieving the target Kt/V, we compared the characteristics of patients using incremental PD prescriptions with those using full-dose PD. We also compared both groups with those not achieving the target. The normality of continuous variables was tested with the Shapiro-Wilk test, and verified with a Quantile-Quantile Plot (QQ plot). Normal continuous variables were compared using a t test. Nonnormal continuous variables were compared using the nonparametric Mann-Whitney U test. Binary variables were compared using the Pearson chi-square test.
Results
Data were collected on all 124 patients on the PD program in June and July 2013. Demographic data are shown in Table 1. Residual renal function was present in 105 (85%) patients with the renal Kt/V ranging from 0.05 to 2.82 per week. APD was used by 114 patients (92%) and CAPD by 10 (8%).
Table 1.
Basic Demographic Details on 124 Patients.
| Number of patients | 124 |
| Age ± SD (y) | 63 ± 15 |
| Male:female (%) | 72:52 (58%:42%) |
| Diabetic (%) | 47 (38%) |
| Weight ± SD (kg) | 78.9 ± 19 |
| Anuric (%) | 19 (15%) |
| Previous hemodialysis | 41 (33%) |
| Time on PD ± SD (mo) | 22 ± 22 |
Note. PD = peritoneal dialysis.
Of the 124 patients, 106 (86%) had Kt/V >1.7 per week and 18 (14%) did not achieve this target. Of the 106 patients with Kt/V >1.7 per week, 54 (51%) were on incremental PD and 52 (49%) were on full-dose PD prescriptions. These 52 patients comprised 29 (56%) who were dependent on residual renal function to achieve the target Kt/V and 23 (44%) who achieved the target independent of renal function (Figure 1). Of the 18 not achieving a Kt/V of 1.7 per week, 14 were on full-dose prescriptions and 4 on low-dose prescriptions. The patients achieving target Kt/V using incremental PD were compared with those achieving it on standard prescriptions. Compared with those on full-dose prescriptions, the incremental patients had of course significantly more residual renal function and less duration of time on PD. They did not differ significantly in age, weight, sex, previous exposure to hemodialysis, or prevalence of diabetes (Table 2). It should be emphasized that these 2 populations are not being managed by 2 different strategies. The patients using incremental PD will be switched to full-dose PD once they lose residual renal function and the full-dose PD patients were almost all previously on incremental PD prescriptions. The patients not achieving the Kt/V target had similar characteristics to the full-dose PD patients, but both their peritoneal and renal clearances were significantly lower (Table 2).
Figure 1.
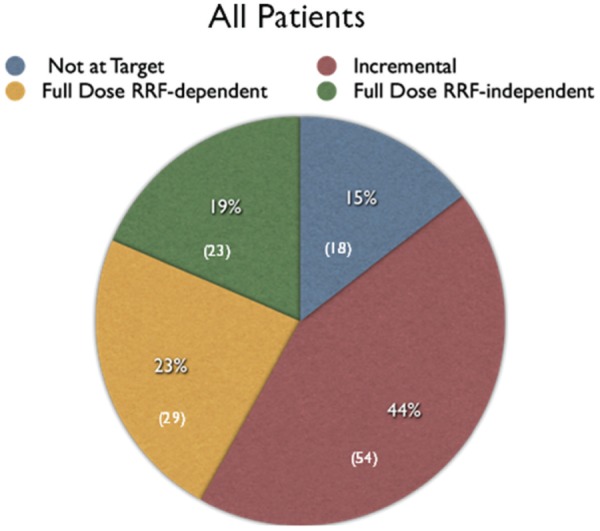
Pie chart showing percentage of patients on incremental PD, on full-dose PD and achieving target clearance independent of (RRF), and on full-dose PD but requiring residual function to reach target and not achieving target.
Note. Number of patients in parentheses. PD = peritoneal dialysis.
Table 2.
Comparison Between Patients Achieving Target Kt/V 1.7 per Week Using Incremental PD, Those Achieving It Using Full-Dose Prescriptions, and Those Not at Target.
| Incremental PD with Kt/V >1.7 | Full-dose PD with Kt/V >1.7 | Not at target Kt/V | |
|---|---|---|---|
| No. of patients (%) | 54 | 52 | 18 |
| Age ± SD | 64 ± 13 | 62 ± 16 | 62 ± 16 |
| No. of males (%) | 32 (59%) | 29 (56%) | 11 (62%) |
| Time on PD ± SD (mo) | 15 ± 14 | 27 ± 26* | 28 ± 30** |
| Weight ± SD (kg) | 77 ± 20 | 80 ± 19 | 81 ± 18 |
| No. with diabetes (%) | 21 (38%) | 19 (37%) | 7 (38%) |
| No. with previous HD (%) | 13 (24%) | 21 (40%) | 7 (38%) |
| Serum Cr ± SD (µmol/L) | 625 ± 225 | 840 ± 247† | 830 ± 299‡ |
| Renal Cr clearance ± SD (mL/min) | 6.2 ± 3.4 | 2.7 ± 2.4† | 1.9 ± 3.0† |
| Renal Kt/V per week ± SD | 1.2 ± 0.6 | 0.6 ± 0.5† | 0.2 ± 0.3†§ |
| Peritoneal Kt/V per week ± SD | 1.15 ± 0.3 | 1.62 ± 0.4† | 1.36 ± 0.3** |
Note. PD = peritoneal dialysis; Cr = creatinine; HD = hemodialysis.
p < 0.001 compared to incremental PD.
p < 0.05 compared to incremental PD.
p < 0.0001 compared to incremental PD.
p < 0.05 compared to full dose PD.
p < 0.005 compared to full dose PD.
Of the 114 APD patients, 50 (43.8%) were on Incremental PD and their prescriptions comprised being “day dry” in 34 (68%), having at least 1 night off cycling each week in 6 (12%) and being both “day dry” and having at least 1 night off in 10 (20%) (Figure 2). “Day dry” APD typically comprised 10 L of PD solution delivered over 8 to 9 hours each night with an initial dwell volumes of 1.5 to 2.0 L and with a mix of tidal and nontidal prescriptions.
Figure 2.
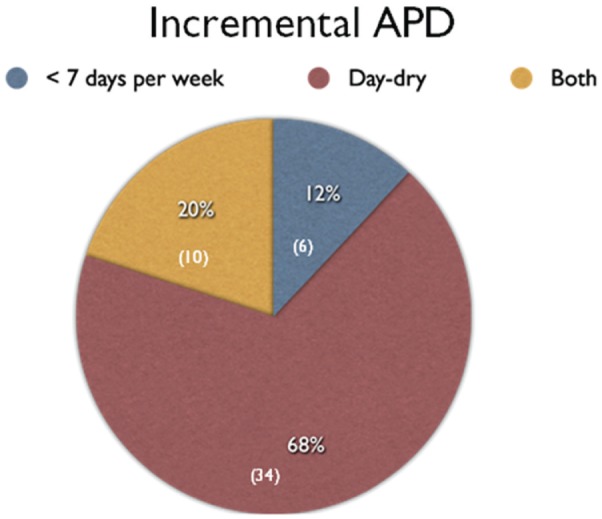
Pie chart showing distribution of incremental APD prescriptions.
Note. Number of patients in parentheses. APD = automated peritoneal dialysis.
Of the 10 CAPD patients, 4 (40%) were on Incremental PD and their prescriptions comprised 3 exchanges a day in 3 patients and 2 exchanges a day in 1 patient; one of the patients on 3 exchanges a day used 1.5-L dwell volumes.
The mean and median total Kt/V in the 124 patients was 2.15 (±0.5) and 2.05, respectively. The mean and median renal Kt/V was 0.78 (± 0.6) and 0.75, respectively, with a range from 0 to 2.82. The mean and median residual renal clearances were 41 ± 35 and 34 L/week per 1.73 meter squared body surface area (range, 0-141). These are equivalent to 4.1 ± 3.5 and 3.4 mL/min per 1.73 meter squared body surface area, respectively.
In the Incremental PD patients, the mean and median total Kt/V was 2.30 (±0.5) and 2.23, respectively. The mean and median peritoneal Kt/V was 1.15 (±0.30) and 1.10, respectively. The mean and median renal Kt/V was 1.2 (±0.6) and 1.00, respectively, with a range from 0.21 to 2.82.
Peritonitis rates for the 3 years prior to the study (2010, 2011, and 2012), during which incremental PD was being practiced, were 1 per 35, 25, and 40 months, respectively, and for 2013, the year of the study, the peritonitis rate was 1 per 44 months. One- and 3-year patient survivals for PD patients initiating PD at our center between 2007 and 2011 were 91% and 67%, respectively, and were not significantly different from expected survivals or from those in Canada as a whole (Table 3).14
Table 3.
Patient Survival for Patients Initiating PD 2007-2011 With Follow-Up Until End of 2011 in LHSC Compared With Province of Ontario and With Canada as a Whole.
| 1 year | 3 years | |
|---|---|---|
| LHSC | 91% | 67% |
| LHSC expected | 93%a (P = .83) | 70%a (P = .73) |
| Ontario | 91% | 69% |
| Ontario expected | 92%a (P = .91) | 70%a (P = .89) |
| Canada | 92% | 70% |
Note. PD = peritoneal dialysis; LHSC = London Health Sciences Center.
Differences nonsignificant.
Discussion
The concept of incremental PD has been discussed in the literature for almost 2 decades. It is referenced as an option in a number of prominent clinical guideline and practice recommendations.1,13 However, most of the reports of its use involve small numbers of patients, ranging from 5 to 25, mainly involve CAPD, and occur in the context of deliberate early initiation of dialysis, a practice which has been called into question after a recent large randomized controlled trial showing no benefit.5-9,17 There are no descriptions of its systematic use in a large contemporary PD population or in a population predominantly treated with APD. Our center has routinely practiced an incremental strategy in all PD patients for over a decade in a large, primarily APD-based program which manages more than 100 patients. We show that in our center, incremental PD is feasible and associated with rates of peritonitis that compare favorably with the International Society of Peritoneal Dialysis target of no more than 1 episode every 18 months18 and with patient survival similar to those seen in other Canadian PD centers.19 The impact of incremental PD on clinical practice and the patient experience is apparent in that more than 40% of the patients were able to achieve clearance targets while using a less onerous low peritoneal clearance prescription.
A comment should be made about the 14% of patients not reaching the Kt/V target. In any cross-sectional review, there will always be patients who are below the clearance target and who then have their prescription adjusted and clearances remeasured. This is more likely to occur in patients with less or no residual renal function as was the case in this study. There are other cases where a decision is made in consultation with the patient to accept a lower clearance because the extra volumes of solution or procedures required to deliver higher clearance are not tolerated by, or acceptable to, the patient. This is especially so in older frailer patients where the potential disadvantages of increasing the prescription may outweigh the potential benefits.
Incremental PD has become increasingly relevant with the worldwide trend to initiate chronic dialysis at gradually greater levels of renal function over the past 2 decades.11,12 The median estimated glomerular filtration rate initiating dialysis in North America has been reported to be greater than 10 mL/min.11 However, a recent randomized controlled trial suggests there is no benefit to elective early initiation, and this trend may reverse.17 Our center did not routinely practice early initiation of dialysis. The mean renal Kt/V in the patient population described here was 0.75 per week, equivalent to a urea clearance of about 3 mL/min and the mean residual renal clearance measured as the average of renal urea and creatinine clearance was just 4.1 mL/min. In those on incremental PD, the mean residual renal clearance was 6 mL/min. The patient population described did not therefore have notably high levels of residual renal function. Despite this, 44% of patients were able to receive an incremental PD prescription.
There are some clear advantages to the use of incremental PD. First, the PD prescription is less onerous with specifically less need for day dwells in those on APD and less need for large dwell volumes for those on both CAPD and APD. The result is greater simplicity and less workload for the patient or their caregivers. It is also likely that with less use of day dwells and of large dwell volumes, there will be a lower likelihood of the symptoms and complications associated with raised intraperitoneal volume and intra-abdominal pressure. Higher dwell volumes have been associated with more adverse mechanical symptoms in a randomized controlled trial.20
For many patients, the benefit of incremental PD is not transient because the time course of residual renal function loss is very variable.21-23 Incremental PD can be practiced for years, often until the patient is transplanted or switched to hemodialysis or dies from an unrelated cause. In this study, the patients on incremental PD prescriptions had already been on PD for a mean of 15 months and some had been on for more than 2 years. The impaired quality of life associated with chronic dialysis is well recognized, and so the benefit of making chronic PD easier for patients should not be underestimated. This is particularly the case in the elderly where transplant is less likely to be an option. In an era in which patient centered care is being emphasized, these considerations are central.24
In addition, there are potential metabolic benefits to incremental PD. There is less exposure of the peritoneal membrane to glucose and glucose degradation products when less solution is used and there is less absorption of glucose systemically.25 Recent randomized trials suggest that glucose-sparing PD solutions are associated with less hyperglycemia and hyperlipidemia in diabetic PD patients.25,26 One might also speculate that less glucose exposure will decrease the risk of progressive obesity in PD patients.25
Another advantage is that incremental PD prescriptions are less costly than standard ones, because less solution is required. In programs such as ours where relatively more expensive icodextrin solution is commonly used for day dwells in APD patients, the cost savings are considerable. In Canada, prices of PD solutions are not published and vary between centers, but the addition of a 2-L icodextrin day dwell to a standard 10-L glucose solution APD prescription typically raises the cost about 15% to 20%. Similarly, CAPD with 3 dwells costs 25% less than CAPD with 4 dwells daily.
There are some challenges associated with the use of incremental PD. First, it requires regular monitoring of residual renal function as its rate of loss is unpredictable. Our center measures renal function every 3 months in patients on incremental prescriptions. An alternative might be to measure it every 6 months and also if there is a change in the patient’s health or an intercurrent illness likely to lead to a fall in (RRF). Second, patients who start PD with an incremental prescription may find it challenging to transition to a more onerous full prescription. This is hardly an argument against doing incremental PD, but it is appropriate to warn the patient in advance that such a transition may eventually be required. In practice, many patients never have to make this transition because of transplant, switch to hemodialysis, or death.
It should be noted that of the 52 patients achieving target Kt/V on full prescriptions, more than half were still dependent on residual renal function to do so. These patients are not doing incremental PD by our definition in that they receive a “full” PD prescription. Without residual renal function, these patients would have required a more aggressive prescription with higher dwell volumes or more day dwells or more frequent cycles. These patients also receive some of the same benefits of those on incremental PD—a less onerous prescription, less glucose exposure, less cost—although to a lesser degree. They also need regular monitoring of their residual renal clearance.
It is unlikely that an incremental PD strategy will ever be compared with an approach that uses full-dose PD from the start in a randomized controlled trial. The failure of previous randomized trials to show benefit for higher peritoneal clearance regimens makes such a study improbable. Rather, the rationale for incremental PD rests on deductions from a number of well-proven premises. First, it has been well shown that, within the usual therapeutic range, higher peritoneal clearances are not associated with improved outcomes.17,27 Second, renal clearance is associated with superior outcomes in a way that equivalent amounts of peritoneal clearance are not.15,28 It is a reasonable conclusion from this that if 2 patients have the same total clearance but one derives it from a mix of renal and peritoneal clearance while the other has it from peritoneal clearance alone, then the former scenario is associated with better survival. A patient receiving incremental PD is therefore unlikely to be disadvantaged relative to the same patient receiving a higher dose of peritoneal clearance. Notwithstanding this, we have presented here our PD patient survival rates, compared with nationwide results, and shown that there is no difference.
Conclusion
In an era in which patient-centered care is being strongly emphasized, changes in clinical practice that make dialysis easier for patients without having an adverse effect on outcomes and with lower cost deserve attention.24 Incremental PD is an example of such a strategy and should be widely considered.
Footnotes
List of Abbreviations: APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; Kt/V, fractional urea clearance; PD, peritoneal dialysis.
Ethics Approval and Consent to Participate: The study was approved by the Western University Ethics Review Committee.
Consent for Publication: Not Applicable.
Availability of Data and Materials: Not Applicable.
Author Contributions: GAA collected the data, analyzed them, prepared the data tables, and wrote the first draft of the manuscript. NIW collected the clearance data, analyzed them, and provided critical review of the data presentation. AKJ and AXG provided feedback on study design, data presentation, and critical review of the manuscript. AXG supervised the statistical analysis. PGB conceived the project and edited the successive drafts of the manuscripts. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs Peter G. Blake and Arsh K. Jain have received speaking honoraria and research support from Baxter Healthcare.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Golper T, Churchill D, Burkart J, et al. National Kidney Foundation Dialysis Outcomes Quality Initiative: Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. 1997;30(suppl 2):S67-S136. [DOI] [PubMed] [Google Scholar]
- 2. Mehrotra R, Nolph KD, Gotch F. Early initiation of chronic dialysis: role of incremental dialysis. Perit Dial Int. 1997;17:426-430. [PubMed] [Google Scholar]
- 3. Nolph KD. Rationale for early incremental dialysis with continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 1998;13(suppl 6):117-119. [DOI] [PubMed] [Google Scholar]
- 4. Golper T. Incremental dialysis. J Am Soc Nephrol. 1998;9:S107-S111. [PubMed] [Google Scholar]
- 5. Burkart JM, Satko SG. Incremental initiation of dialysis: one center’s experience over a two-year period. Perit Dial Int. 2000;20:418-422. [PubMed] [Google Scholar]
- 6. De Vecchi AF, Scalamogna A, Finazzi S, Colucci P, Ponticelli C. Preliminary evaluation of incremental peritoneal dialysis in 25 patients. Perit Dial Int. 2000;20:412-417. [PubMed] [Google Scholar]
- 7. Neri L, Viglino G, Cappelletti A, Gandolfo C, Barbieri S. Incremental dialysis with automated peritoneal dialysis. Adv Perit Dial. 2003;19:93-96. [PubMed] [Google Scholar]
- 8. Viglino G, Neri L, Barbieri S. Incremental peritoneal dialysis: effects on the choice of peritoneal dialysis, residual renal function and adequacy. Kidney Int. 2008;73:S52-S55. [DOI] [PubMed] [Google Scholar]
- 9. Domenici A, Comunian MC, Fazzari L, et al. Incremental peritoneal dialysis favourably compares with Hemodialysis as a bridge to renal transplantation. Int J Nephrol. 2011;2011:204216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tzamaloukas A. Incremental initiation of peritoneal dialysis. Adv Perit Dial. 1999;15:175-178. [PubMed] [Google Scholar]
- 11. Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009;76:257-261. [DOI] [PubMed] [Google Scholar]
- 12. Jain AK, Sontrop JM, Perl J, Blake PG, Clark WF, Moist LM. Timing of peritoneal dialysis initiation and mortality: analysis of the Canadian Organ Replacement Register. Am J Kidney Dis. 2014;63:798-805. [DOI] [PubMed] [Google Scholar]
- 13. Blake PG, Bargman JM, Brimble KS, et al. Canadian Society of Nephrology Work Group on peritoneal dialysis. Clinical practice guidelines and recommendations on peritoneal dialysis adequacy. Perit Dial Int. 2011;31:218-239. [DOI] [PubMed] [Google Scholar]
- 14. Perez RA, Blake PG, Jindal KA, Badovinac K, Trpeski L, Fenton SS. Changes in peritoneal dialysis in Canada 1996-1999. Perit Dial Int. 2003;23:53-57. [PubMed] [Google Scholar]
- 15. Lo WK, Jiang Y, Cheng SW, Cheng IK. Survival of CAPD patients in a center using three two-liter exchanges as standard regime. Perit Dial Int. 1996;16(suppl 1):S163-S169. [PubMed] [Google Scholar]
- 16. Nessim ST, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large prospective Canadian database. Clin J Am Soc Nephrol. 2009;4:1195-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper BA, Branley P, Bulfone L, et al. A randomized controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609-619. [DOI] [PubMed] [Google Scholar]
- 18. Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393-423. [DOI] [PubMed] [Google Scholar]
- 19. Canadian Institute for Health Information. Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2003-2012. Ottawa, Ontario: Canadian Institute for Health Information; 2014. [Google Scholar]
- 20. Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307-1320. [DOI] [PubMed] [Google Scholar]
- 21. Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046-1053. [DOI] [PubMed] [Google Scholar]
- 22. Liao CT, Shiao CC, Huang JW, et al. Predictors of faster decline in residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int. 2008;28(suppl 3):S191-S195. [PubMed] [Google Scholar]
- 23. Szeto CC, Kwan BC, Chow KM, et al. Predictors of residual renal function decline in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 2015;35:180-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Hare AM, Armistead N, Schraq WL, Diamond L, Moss AH. Patient-centered care: an opportunity to accomplish the “Three Aims” of the National Quality Strategy in the Medicare ESRD Program. Clin J Am Soc Nephrol. 2014;9:2189-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paniagua R, Ventura MD, Avila-Díaz M, et al. Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int. 2009;29:422-432. [PubMed] [Google Scholar]
- 26. Johnson DW, Brown FG, Clark M, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol. 2012;23:1097-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lo WK, Ho YW, Li CS, et al. Effect of Kt/V on survival and clinical outcome in CAPD patients in a randomized prospective study. Kidney Int. 2003;64:649-656. [DOI] [PubMed] [Google Scholar]
- 28. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158-2162. [DOI] [PubMed] [Google Scholar]


