Abstract
Throughout the twenty-first century, robotic surgery has been used in multiple oral surgical procedures for the treatment of head and neck tumors and non-malignant diseases. With the assistance of robotic surgical systems, maxillofacial surgery is performed with less blood loss, fewer complications, shorter hospitalization and better cosmetic results than standard open surgery. However, the application of robotic surgery techniques to the treatment of head and neck diseases remains in an experimental stage, and the long-lasting effects on surgical morbidity, oncologic control and quality of life are yet to be established. More well-designed studies are needed before this approach can be recommended as a standard treatment paradigm. Nonetheless, robotic surgical systems will inevitably be extended to maxillofacial surgery. This article reviews the current clinical applications of robotic surgery in the head and neck region and highlights the benefits and limitations of current robotic surgical systems.
Keywords: head and neck, maxillofacial surgery, oral surgical procedures, robotic surgery
Introduction
Maxillofacial surgeries have conventionally been performed with large incisions, either via a transmandibular or a transpharyngeal approach, because of the complicated anatomy and limited surgical space. These procedures typically result in significant surgical morbidity, speech dysfunction and dyspepsia from the dissection of large amounts of normal tissue. However, minimally invasive surgical technologies have evolved dramatically over the past two decades since Mouret1 completed the first laparoscopic cholecystectomy in 1987. This technique allows surgeons to access tissue through a few small incisions instead of a large incision. The focus of these procedures is now on preserving function, reducing postoperative morbidity and improving quality of life.
Nevertheless, the use of minimally invasive surgery (MIS) in maxillofacial surgery has posed challenges related to neurovascular control, illumination of the surgical field and protection of the surrounding structures. In 2000, Steinier2 advocated transoral laser microsurgery, which demonstrated superior results. Unfortunately, this approach obstructs the line of sight, as visualization is provided by merely a microscope. With this approach, sufficient exposure of the surgical field cannot be obtained, and resection is not possible in the cranial and axial axes. To overcome these limitations, robotic surgical systems were innovated and introduced into surgical practice. Transoral robotic surgery (TORS) was proposed and first applied clinically in maxillofacial surgery by McLeod and Melder3 to excise a vallecular cyst. This procedure was approved by the US Food and Drug Administration (FDA) in 2009 for use in stage T1 and T2 oropharyngeal cancer. Since that time, robot-assisted maxillofacial surgery has been growing steadily in popularity. Taking inspiration from its use in other surgical fields, the benefits to surgeons include a three-dimensional magnified view, precise movements, bimanual operation with articulated arms and suppression of tremor, which enhances the surgeon's physical capabilities. Thus, procedures with robotic assistance can be performed with less blood loss, fewer complications, shorter hospital stays and better cosmetic results than standard open techniques.4
Hence, robotic surgery may hold promise in the treatment of craniofacial conditions, such as head and neck neoplasms, cleft palate and craniofacial asymmetry, among others. In this review, we summarize the current applications of robot-assisted maxillofacial surgery.
History of robotic surgical systems
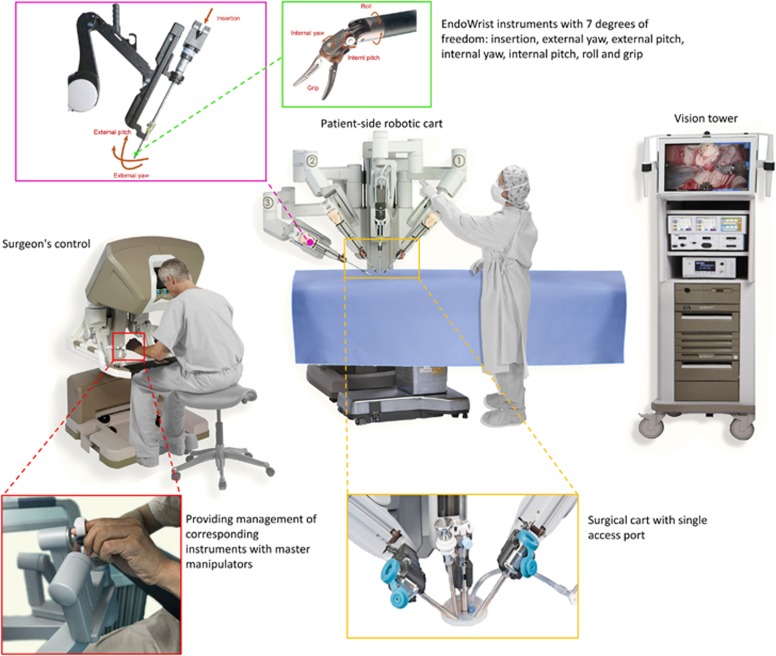
For decades, robots and surgery have been developing along two independent paths. During the late 1980s and early 1990s, endoscopic techniques were booming, and limitations were being reached as well. Subsequently, the potential capability of telerobotics in MIS was well recognized. However, robots and surgery only reached a safe enough stage for their combination via telemanipulation for surgical innovation in the last few years. The robotic surgical system is truly an information system rather than a machine, and it can be simply divided into input, analysis and output. A human is interposed between the input and output instead of a computer in case there are any unexpected events or anatomy during surgery, and these components serve as a teleoperation system.5 The input side consists of several chemical and biologic sensors and imagers, and there are various devices on the output side, such as manipulators and lasers, to contact organs and tissues. The robotic surgical system was manufactured to overcome the limitations of laparoscopic surgery, including tremor, fatigue, 2D imaging and a limited range of freedom. Additionally, robotic surgery can also be described as an ability to enable surgical interventions via the application of telecommunications and robotic systems, where the patient and surgeon are separated. Since Puma 560,6 the first robotic surgical system was introduced in the mid-1980s to orient a needle for brain biopsy, three generations of systems have followed. Generation I: CMI’s Automated Endoscopic System for Optimal Positioning (AESOP). AESOP, a voice-controlled robot, was developed to serve as a stable camera platform and not multi-arm units. AESOP eliminates the need for an extra surgical assistant, and AESOP 1000 was approved by the FDA for use in surgery in 1995. Even though AESOP was widely applied in various surgical settings, including cardiology, urology and gynecology, until 1999,7 there were several deficiencies. In addition, the robotic system required a few alterations to cooperate with surgeon’s style of operation. Generation II: Telerobot Zeus. Zeus was a kind of master-slave teleoperator between the surgeon and the patient-side manipulator. Zeus was introduced in 1995 to provide improved precision for the laparoscopic surgeon, and it was approved by the FDA in 2000. Zeus consists of an AESOP robotic scope and two additional manipulators to hold the operating instruments, and the three arms are mounted to an operating table. It had the advantages of remote control, three-dimensional visualization and tremor suppression. In addition, this telemanipulator allowed a surgeon to perform surgical procedures from a remote region, such as hospital-to-hospital settings. However, it was no longer technically supported once the da Vinci surgical system began being used worldwide. Generation III: da Vinci surgical system. Comparatively, the da Vinci system aimed at recreating the feeling of open surgery and was preferred by the open surgeon, while the Zeus system was primarily adopted by the laparoscopic surgeon. The initial da Vinci robot was invented in 1999 by Intuitive Surgical, and it consists of three major parts: a surgeon’s console, a robotic cart on the patient’s side and a high-definition 3-dimensional vision tower.8 The surgeon’s console enables management of the corresponding instruments with master controls, and it was derived from part of the M7 system developed by Stanford Research Institute (SRI)—a surgical robot for open surgery.5 The surgeon can operate from a comfortably seated position while having a high-definition real-time view inside the patient. The patient-side surgical cart consists of three or four arms that were originally developed from the Black Falcon system: one arm handles the endoscopic camera (passes through a 12-mm trocar), while the other two or three arms hold the EndoWrist instruments (pass through 8-mm trocars), which provide enhanced degrees of freedom and excellent 3D imaging. This permits large-scale movement in surgery, such as the movements needed for dissecting and suturing. Moreover, the camera used in the system provides a true-to-life stereoscopic image of the patient’s anatomy, which is transmitted to both the surgeon’s console and the vision tower beside the surgical assistant.8 The vision tower provides a broad perspective and visualization of the procedure to the surgical assistant at the patient’s side (Figure 1). Recently, several developments have been made. First, the da Vinci Si system was manufactured to support two consoles operating in concert with one patient-side robot; thus, an instrument “give-and-take” was made available. Second, 5-mm-diameter instruments are now available. Third, in the da Vinci Xi robot, the laser targeting system can simply point the scope at the target anatomy, and a smaller robotic arm and footprint along with improved articulation provide increased flexibility and decreased arm collisions. Fourth, a single port robotic technique, which is less invasive than procedures with several access ports, has already been launched and is on the market, but it has unfortunately not been applied in maxillofacial surgery. Apart from those mentioned above, there are several other robotic surgical systems, including ROBODOC, Computer-Assisted Surgical Planning and Robotics (CASPAR), Robotic Arm Interactive Orthopedic System (MAKO Surgical Corp RIO) and so forth, that have been generally applied in orthopedic surgery, such as arthroplasty.5
Figure 1.
Robotic surgery operating room schematic.
Overall, the da Vinci surgical system is currently considered the most successful robotic surgery system; it has been widely utilized in multiple anatomic regions since Pasticier et al.9 first utilized it in radical prostatectomy. This system was first used in maxillofacial surgery in 2005, and it was approved by the FDA in 2009. Currently, the da Vinci robot is used for almost all surgical procedures performed in the head and neck region.3
Clinical applications of robotic surgery in the head and neck
Search methods
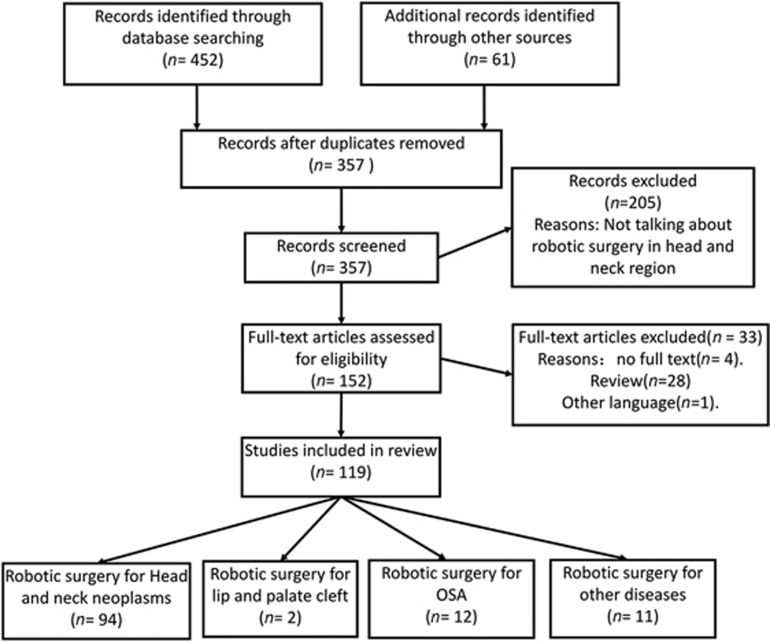
The literature search was performed using the Cochrane Central Register of Controlled Trials (CENTRAL; 2016), MEDLINE (via PubMed, 1948 to September 2016), Embase (1974 to September 2016), the China National Knowledge Infrastructure (CNKI; 1979 to September 2016) and China Biology Medicine (CBM; 1978 to September 2016). Gray databases, such as OpenGrey and Sciencepaper Online, were also searched. Manual searches were also conducted in relevant Chinese journals, and reference lists of relevant articles were reviewed. To find ongoing clinical trials, the World Health Organization International Clinical Trials Registry Platform was searched. MeSH heading words and free text words were combined. They included “Robotics,” “Operation, Remote,” “Oral Surgical Procedures,” “Oral Surgery” and “Head and Neck Neoplasms.” Language was restricted to Chinese and English. As a result, a total of 503 studies were identified; of these, 119 that were associated with the application of robotic surgery in the head and neck region were included in this review (Figure 2).
Figure 2.
Diagram of article retrieval.
Clinical applications
The development of a robotic surgical system for maxillofacial surgery has been relatively delayed because of the limited surgical field and compact surrounding anatomy. The first application of a robotic surgical system in maxillofacial tumors was reported by Haus et al.10 for resection of the submandibular gland in animal models. Since that time, the use of robotic surgery for head and neck diseases has been gradually increasing. Currently, the chief indications for robotic surgery in the head and neck region are (1) removal of head and neck neoplasms or cysts that can be sufficiently exposed via a robotic approach; (2) therapeutic and selective neck dissection; and (3) obstructive sleep apnea syndrome (OSAS). Meanwhile, tumors with jaw or internal carotid artery invasion are not currently suitable for robot-assisted resection.10
Head and neck neoplasms
Head and neck neoplasm is a group of neoplasms that arise from the oral cavity, pharynx, larynx, sinuses or salivary glands, among others. Head and neck cancers are regarded as the sixth most common malignancy and ninth most frequent cause of death worldwide; ~529 500 new patients are diagnosed annually, and head and neck cancers are responsible for 3.6% of cancer-specific deaths.11 In high-risk countries (that is, India, Sri Lanka, Bangladesh and Pakistan), oral cavity cancer has the highest incidence of the head and neck cancers and is increasing in incidence.12 The average 5-year survival rate of head and neck cancer following diagnosis in the developed world is 42–64%, and the 1-year survival rate of advanced oral cavity cancer is <50%.13 Currently, surgery is frequently applied as a treatment in most head and neck cancers. However, surgery can be particularly difficult if the tumor is near the larynx, which might result in dysphasia. Of these surgeries, robotic surgery allows the surgeon to remove tumors with minimal damage to normal tissues, and it gives patients as much speech and swallowing function as possible postoperatively. Specific clinical applications of robotic surgery in head and neck neoplasms are presented below.
Oral cavity, oropharynx, nasopharynx and laryngopharynx
On the basis of preclinical experiments, robot-assisted surgery for the excision of a vallecular cyst was first performed by McLeod and Melder3 in 2005, with no complications experienced. Later, O’Malley and colleagues14 reported the technical feasibility of robot-assisted surgery for base of tongue (BOT) neoplasm resection; Weinstein and colleagues15 successfully performed a robot-assisted radical tonsillectomy in 2007 after cadaveric robotic surgery. With this much groundwork completed, several studies subsequently focused on the application of TORS in various types of neoplasms, including squamous cell carcinoma,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 mucoepidermoid carcinoma,16, 35, 43, 50, 60, 61 malignant melanoma,62 synoviosarcoma,33, 63 adenoid cystic carcinoma,33, 35, 43, 50, 60, 64 pleomorphic adenoma,32, 35, 47, 65 lipoma33 and neurilemmoma.64
Several studies have demonstrated that robotic surgery for primary or recurrent neoplasms in the oral cavity, oropharynx, nasopharynx and laryngopharynx has superior functional recovery; higher rates of negative margin, recurrence-free survival, disease-free survival and overall survival; and a lower risk of hemorrhage, gastrostomy tube and tracheostomy tube dependence, and other intraoperative or postoperative complications than conventional open surgery or radiochemical therapy.38, 52, 66, 67, 68 However, it is also worth noting that Blanco et al.47 reported an application of TORS in the treatment of recurrent oropharynx squamous cell carcinoma, in which three of four patients experienced postoperative regional or distal transference. Furthermore, TORS appeared to be more effective in the detection and diagnosis of unknown primary tumors than conventional methods, including computed tomography, positron-emission tomography and directed biopsies, especially for human papillomavirus (HPV)-positive patients.51, 55, 56, 57, 58, 59
In addition to the factors mentioned above, other aspects of robotic surgery were assessed. For instance, HPV is one of the most important known risk factors for oropharynx cancer. It is widely accepted that HPV-positive patients with head and neck cancers may have a better prognosis than patients who are HPV-negative. Cohen et al.69 found that TORS may provide similar surgical and oncologic outcomes to HPV-negative patients, such as negative resection margin; local, regional and distant disease recurrence rates; and disease-free and overall survival rates that are comparable to those of HPV-positive patients; however, other surgeons24, 42, 43 held different opinions. Blanco et al.47 and Olsen et al.28 determined that the 2-year disease-free survival rate of HPV-positive patients was higher than that of HPV-negative patients, and Quon et al.46 study showed that HPV-positive patients have a higher positive margin rate. Regarding postoperative quality of life, swallowing and speech functions decreased significantly 3–6 months after TORS and recovered to the preoperative state 1 year later.23, 70 Furthermore, the study by Park et al.38 showed that robotic surgery resulted in significantly decreased postoperative pain and anxiety and a better appetite compared to open surgery. Moreover, the time to functional recovery seemed to be associated with preoperative T stage, tumor location, tumor size, status of tumor (primary or recurrent) and pretreatment M.D. Anderson Dysphagia Inventory (MDADI) score.19 Robotic surgery allows surgical instruments to be mounted on the robotic arms; some studies showed that dissection with a laser may provide better surgical outcomes in terms of hemorrhage, intraoperative pharyngotomy, postoperative pain and operation time compared to electrocautery.34, 49 Abel et al.34 proposed that this difference might be related to decreased collateral thermal damage using the laser.
Parapharyngeal space.
The parapharyngeal space is a potentially deep and anatomically compact space in the head and neck that contains important structures, including the internal carotid artery and cranial nerves IX, X and XI. Traditionally, the extended facial recess approach, transcochlear approach and transtemporal–infratemporal fossa approach were associated with tumors in this area.71 However, these approaches seemed to be associated with significant degrees of morbidity as well as visible scars. O’Malley and Weinstein72 first performed robot-assisted resection of a benign neoplasm in the parapharyngeal space based on cadaveric and animal robotic surgery. Several subsequent reports showed favorable results, such as short hospital stays, quick functional recovery and a lack of significant complications, when parapharyngeal neoplasms (squamous cell carcinoma, lipoma, pleomorphic adenoma, adenoid cystic carcinoma, cartilaginous tumor and neurilemmoma) were removed using the robot.36, 61, 73, 74, 75 Chan et al.76 reported that 24% of patients with pleomorphic adenoma experienced unexpected capsule breakage or neoplasm fracture during surgery, potentially resulting from an inability to safely grasp the tumor, sharp instruments and a lack of tactile and haptic feedback.
Thyroid gland and mediastinal parathyroid.
Bodner et al.77 described the first use of a robotic surgical system for mediastinal parathyroid resection via a transaxillary incision in 2004 and showed that transaxillary robotic surgery is a minimally invasive, effective and safe procedure. Later, Lewis et al.78 and Miyano et al.79 demonstrated the feasibility of transaxillary robotic thyroidectomy. No significant bleeding or edema occurred intraoperatively or postoperatively. Recently, Byeon et al.80 performed robotic retroauricular thyroidectomy for clinically suspicious papillary thyroid carcinoma. Other previous studies found that robotic thyroidectomy via a retroauricular incision is a safe, technically feasible approach with satisfactory cosmetic results.81, 82, 83, 84, 85, 86 However, their results indicated that this approach required a longer operative time, longer hospitalization and longer postoperative drainage than endoscopic surgery and open surgery because of the remote access.
In addition, a lingual thyroglossal duct cyst was also excised using a robotic surgery system via a transoral approach or a retroauricular approach without complications or recurrence.87, 88, 89 A lingual thyroglossal duct cyst is a congenital fibrous cyst that forms from a persistent thyroglossal duct, which was conventionally dissected via a transcervical approach. However, the traditional surgery was always associated with an undesirable scar in the neck and a high relapse rate. In Kim et al.89 opinion, the 3-dimensional, magnified visualization of the robot resulted in less damage to the surrounding normal tissues, reduced intraoperative bleeding and infection, and the ability to ligate the tract after carefully tracing it.
Salivary glands.
Submandibular gland tumors were traditionally excised via a transcervical approach, which always left a visible scar, and possibly even hypertrophic scarring in the neck. In comparison, on the basis of its guaranteed curative effect, robotic resection of the submandibular gland through a retroauricular approach or modified face-lift approach can produce an invisible scar, making it more acceptable to patients.90, 91, 92, 93 The study by Yang et al.93 showed that gland-preserving robotic surgery has a potentially lower risk of intraoperative hemorrhage, positive margins and postoperative functional nerve deficit than conventional transcervical surgery. However, it is worth noting that postoperative hospitalization and the duration of drainage are much longer in robotic surgery than open surgery because of the extent of the flap. Moreover, the use of TORS for oropharyngeal minor salivary gland tumors, parotid gland tumors and sublingual gland ranulas was also reported by several surgeons, and the results showed favorable oncologic, surgical and functional outcomes, including no apparent neurovascular damage, a low positive margin rate and quick functional recovery, with excellent cosmetic results.35, 36, 94, 95
Neck dissection.
Neck dissection followed by head and neck tumor removal is always necessary to reduce locoregional recurrence. Kang et al.96 first applied a robotic surgical system in a radical neck dissection via a transaxillary track for the staged treatment of thyroid carcinoma to avoid a long visible incision scar and muscle deformities in the neck area as well as to strengthen deep and corner dissections. However, the region of level I is hard to completely dissect via this approach. Therefore, to overcome the limitations mentioned above, robot-assisted radical or selective neck dissections via a retroauricular approach or a modified face-lift approach have been reported.97, 98, 99, 100, 101, 102, 103, 104, 105, 106 The results suggested that the robot-assisted surgery lasted longer than conventional surgery, but the intraoperative bleeding, lymph node retrieval, volume of drainage, hospitalization and related complications of robot-assisted neck dissection (RAND) were similar to those of open neck dissection. Furthermore, the patients who underwent robotic surgery were much more satisfied with the postsurgical aesthetics than those who underwent open surgery. Additionally, the study of Kim et al.100 and Tae et al.105 demonstrated that RAND may have a lower risk of lymphedema and lymph node recurrence than conventional neck dissection.
Post-ablative defect reconstruction.
An extensive mucosal defect and, in some cases, direct orocervical fistula or pharyngocervical communication and exposure of the great vessels can result from en bloc resection of a head and neck neoplasm and subsequent or simultaneous neck dissection. Consequently, it is important to achieve a reliable reconstruction for these patients. The first use of a robotic surgical system in post-ablative defect reconstruction was reported by Genden et al.,17 in which a mucosal advancement flap, two pyriform mucosal flaps and three posterior pharyngeal wall flaps were performed. Since then, the robotic surgical system has been increasingly employed in head and neck defect reconstruction. Various flaps, including a mucosal muscle flap, radial forearm flap and free anterolateral femoral skin flap, were applied for reconstruction.40, 60, 62, 107, 108 All flaps survived, except for four mucosal muscle flaps in Genden et al.107 study. Moreover, the studies mentioned above also showed that robotic reconstruction surgery has a shorter operative time, better functional recovery and more satisfactory aesthetics than conventional surgery. Kim109 performed a mandibular reconstruction with a fibular flap using a robotic surgical system combined with simultaneous virtual surgical planning (VSP). His results indicated that robotic surgery with VSP may have a higher flap survival rate than conventional surgery, with less time and effort.
Cleft lip and palate
Currently, the use of robotic surgical systems in the treatment of cleft lip and palate is still in an early stage of development. Khan et al.110 first reported the theoretical feasibility of robotic intra-oral cleft surgery and Hynes pharyngoplasty in a pediatric airway manikin and human cadaver in 2015. In the same year, Nadjmi111 demonstrated the technical feasibility and safety of robot-assisted soft palate muscle reconstruction in 10 consecutive patients (mean age: 9.5 months) with palatal clefts after cadaveric TORS. The results showed that the surgical duration of TORS is much longer than conventional surgery; however, the hospital stays and functional recovery for the robotic approach were significantly shorter than for the manual approach. Nadjmi111 believed that this was because of the precise dissection provided by the robotic surgical system, which might reduce damage to the vascularization and related innervation of surrounding muscles.
Maxillofacial fracture
The management of bone fracture, similar to the robotic surgical system for fracture treatment, mainly consists of two procedures: reduction and fixation. However, the development of robotics for the treatment of fractures is much more difficult than in other regions for two main reasons. First, the position of fracture segments changes before and after reduction, making it difficult to provide precise navigation. Second, it is impossible to provide appropriate resistance during the fixation period because of the lack of tactile and haptic feedback. Therefore, improvements in the identification capability and mechanical properties of the surgical robot are anxiously awaited. Currently, several robotic surgical systems with an integrated force sensor were applied for arthroplasty, such as ROBODOC, Active Constraint Robot (ACROBOT) and Bone Resection Instrument Guidance by Intelligent Telemanipulator (BRIGIT). However, robotic fracture reduction and fixation are only used for long bone and pelvic fractures.112, 113 The clinical application of robotic surgical systems in maxillofacial fractures has not been reported.
Craniofacial asymmetry
The theoretical feasibility of robot-assisted orthognathic surgery was proposed in 2010 by Chen et al.,114 who suggested a method using the six degrees of freedom robot MOTOMAN to perform bone cutting and drilling based on the navigation system that they programmed. Later, Peking University developed a robotic surgical system for the design of orthognathic surgery, bone reconstruction and intraoperative navigation. However, the clinical application of robotic orthognathic surgery has not been reported, and the robotic surgical system mentioned above remains in an experimental stage.
OSAS
OSAS is the most common type of sleep apnea, resulting from complete or partial obstruction of the upper airway. It can be caused by decreased muscle tone, thickened soft tissue around the airway, such as nasal polyps or adenoid hypertrophy, and structural features, such as nasal septum deviation, which result in a narrowed airway. Continuous positive airway pressure (CPAP) was often used as a standard treatment for OSAS.115 For those OSAS sufferers unwilling or unable to comply with CPAP, a properly selected surgical treatment would be an alternative option, based on the patient’s-specific anatomy.116 Such treatments include tonsillectomy, uvulopalatopharyngoplasty (UPPP), reduction of the tongue base, maxillomandibular advancement and hyoid suspension. However, the BOT has important physiologic functions and has close contacts to surrounding muscles, vessels and nerves, and the conventional reduction of the BOT usually results in severe adverse postoperative reactions. Therefore, the robotic surgical system has emerged as a potential solution to this dilemma.
Vicini et al.117 reported the first application of TORS in the resection of the BOT, combined with conventional septoplasty, UPPP or supraglottoplasty, for OSAS patients in 2010 without any intraoperative and postoperative complications. The result showed a similar surgical duration to open surgery. No tracheotomy was required during surgery, and all patients had an excellent functional recovery. The postoperative Apnea–Hypopnea Index (AHI) and Epworth Sleepiness Scale (ESS) were significantly decreased from their preoperative values, and 90% of patients were satisfied with the results. Subsequently, TORS became widely applied for OSA sufferers for tonsillectomy, supraglottoplasty and glossectomy.118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128 Most of the studies demonstrated that TORS has a similar therapeutic efficacy and decreased postoperative pain, hospital stay and incidence of dysphagia compared with conventional surgery. Although almost all of the studies showed that the postoperative AHI, EES and snoring intensity are significantly improved by TORS, the cure rate still varies from 45 to 90%. Hoff et al.122 found that preoperative body mass index (BMI) may help the clinician predict the success of TORS; specifically, the cure rate is significantly higher in patients with BMI<30 than those with BMI>30. Moreover, when compared to submucosal minimally invasive lingual excision and radiofrequency BOT reduction, Friedman et al.120, 121 study indicated that robot-assisted partial glossectomy resulted in a greater AHI reduction, but longer functional recovery.
However, there are some specific adverse events that have been reported with TORS. A 12.5% transient dysgeusia rate was reported by Lee et al.124 in robotic lingual tonsillectomy; 3 of 12 patients complained of taste disturbance after robotic BOT resection in the study by Lin et al.,125 while 18.3% of patients experienced transient hypogeusia in Crawford et al.126 study after robot-assisted BOT resection. Toh et al.127 study showed that all patients experienced temporary anterior tongue numbness and temporary tongue soreness, while 35% of patients reported a temporary postoperative change in taste. Muderris et al.128 reported six cases of robotic lingual tonsillectomy, all of which had lingual edema. Lin and Crawford proposed that these complications might have resulted from the pressure of the tongue blade or mouth gag.
Others
Laryngeal clefts and laryngocele
Rahbar et al.129 described the application of TORS in five pediatric patients with laryngeal cleft after cadaver experiments. As a result, one patient with a type I laryngeal cleft and one with a type II cleft who underwent TORS for closure of the laryngeal cleft achieved great success without any intraoperative or postoperative complications. However, the surgical duration was much longer than conventional surgery because of the restriction of the surgical space; the surgical procedure failed to be completed in three patients because of limited transoral access. Ciabatti et al.130 used TORS for the excision of a large mixed laryngocele with short operative time and satisfactory aesthetics. No complications were observed, and an oral diet was started 1 day postoperatively and the patient was discharged 2 days after TORS.
Ectopic lingual thyroid
In May 2011, robot-assisted dissection of a lingual thyroid gland in three patients with minimal morbidity and excellent functional outcomes was successfully performed.131 Recently, an increasing number of ectopic lingual thyroids have been excised via a robotic surgical system.43, 132, 133 The results showed that patients undergoing TORS could start oral feeding on the first postoperative day, and no recurrence was observed within 2 months of follow-up. In Prisman et al.133 opinion, TORS should be regarded as a valid option for the treatment of ectopic lingual thyroid.
Ptyalolithiasis
Walvekar et al.134 first reported the successful removal of a 20-mm submandibular megalith and the subsequent repair of the salivary duct using a robotic surgical system. The total time involved was 120 min, and no complications were noted. Recently, Razavi et al.135 facilitated large submandibular gland stone removal using TORS in 22 patients. Procedural success was 100%, and no symptoms of recurrence or lingual nerve damage were recorded at follow-up. Meanwhile, they studied 135 patients who underwent TORS for removal of submandibular gland stones and showed that procedural success was reported in 75% of these patients; the lingual nerve damage rate was 2%.
Vascular lesions
Recently, the excision of BOT vascular lesions via a robotic surgical approach was described by Dziegielewski et al.,136 who found that it could be used in a safe manner to dissect BOT vascular lesions with maximum preservation of the surrounding vessels, nerves and muscles. Consequently, the postoperative damage to swallowing and speech function is minimal.
Discussion
Superiority and limitations
Robot-assisted surgery has been increasingly applied in the head and neck region and has ushered in a new era of MIS. Compared with conventional or endoscopic surgery, robotic surgery has several distinctive advantages and limitations (Table 1 and 2).
Table 1. Current application and future development of robotic surgery in head and neck neoplasms.
| Patients | Superiority | Limitations | Future development |
|---|---|---|---|
| Head and neck neoplasms resection | |||
| Upper aerodigestive tract tumor16–65 | In common: decreased damage to surrounding tissues; superior function recovery, better oncologic control and lower morbidity than conventional open surgery as well as radiochemical therapy; excellent aesthetics | In common: long surgical duration; lack of specific instruments (sharp instrumentation); lack of haptic feedback, and expensive | In common: realization of haptic feedback; bimanual operation and improvement of sharp instruments |
| Parapharyngeal spcae tumor36, 61, 73–75 Thyroid gland tumor and mediastinal parathyroid77–89 | Upper aerodigestive tract tumor: high effectiveness in detection of unknown primary tumors | Thyroidectomy: long hospitalization and considerable duration of drainage | Thyroidectomy: modified surgical approach to reduce the extent of the flap |
| Salivary glands tumor90–95 | |||
| Neck dissection96–106 | Thyroidectomy: easy to ligate the tract after carefully tracing it | Flap reconstruction: combination of robotic surgery and virtual surgical planning | |
| Post-ablative defect reconstruction17, 40, 60, 62, 107–109 | Neck dissection: low risk of lymph-edema and lymph node recurrence | ||
| Flap reconstruction: high survive rate | |||
Table 2. Current application and future development of robotic surgery in head and neck non-malignant diseases.
| Patients | Superiority | Limitations | Future development |
|---|---|---|---|
| Lip and palate cleft110–111 | Low damage to the vascularization and related innervation of surrounding muscles, quick function recovery | Long surgical duration | More high-quality clinical investigation |
| Maxillofacail fracture | Insufficient data | Insufficient data | Specific design of related robotic surgical system |
| Craniofacial asymmetry114–115 | Insufficient data | Insufficient data | Transition from theoretical feasibility to clinical application |
| OSAS117–128 | Low intropetative bleeding and tracheotomy, decreased postoperative pain, hospital stay as well as incidence of dysphagia | Unstable cure rate varies from 45% to 90%, significant postoperative lingual oedema and transient hypogeusia | Combination of robotic resection of BOT and conventional surgery like uvulopalatopharyngoplasty or sphincter pharyngoplasty |
| Others | |||
| Laryngeal clefts129 | In common; minimal damage to surrounding normal tissues as well as speech and swallow function; excellent aesthetics | Laryngeal lefts: unsatisfactory cure rate | Laryngeal lefts: application of specific miniaturized instruments to obtain enough surgical space |
| Laryngocele130 | Laryngocele: short operative time | ||
| Ectopic lingual thyroid131–133 | Ectopic lingual thyroid: short operative time and low recurrence | ||
| Ptyalolithiasis134–135 | Ptyalolithiasis: high cure rate and low lingual nerve damage rate | ||
| Vascular lesion136 |
OSAS, obstructive sleep apnea syndrome.
Superiority of robotic surgery
Magnified 3-dimensional visualization. The surgical space can be stereoscopic and 10–15 times magnified via two or more integrated cameras that are used in the system, which can enhance the surgeon’s capability to distinguish normal tissues from tumors and to preserve normal tissues to the highest extent. Thus, the tumor can be removed en bloc, with minimal morbidity and accelerated functional recovery.
Breaking the limit of human hands. The robotic arms are equipped with articulating surgical instruments, which provide increased degrees of freedom and extend the range of motion. As a result, the stability and accuracy of surgical procedures are improved.
Minimally invasive. A transcervical approach is often applied for the resection of head and neck neoplasms with or without mandibulotomy or a lip-splitting incision to obtain sufficient surgical space; this is accompanied by high morbidity and poor postoperative swallowing and speech functions. In contrast, robotic surgery could remove tumors via a minimally invasive approach, such as a transoral and a retroauricular approach, to decrease surgical complications and functional damage to a large extent. The average blood loss was minimal, and no patient required blood transfusions intra- or postoperatively.
Excellent manipulability. Remote operation and real-time shared surgery can be available via Internet and satellite technology.
Economizing medical staff. The robotic surgical system is highly automated; thus, only one surgeon, one anesthesiologist and one or two nurses are required, even for a difficult surgical operation. This could overcome the restrictions of operating room capacity and the shortage of medical resources.
Limitations of robotic surgery
Lack of tactile perception and proprioception. It is impossible, through a robotic surgical system, to feel the strength and resiliency of tissues or the radial pulse. Therefore, it is difficult to control bleeding in a timely fashion once exsanguinating hemorrhage occurs.
Lack of haptic feedback. For some fine motions, such as tying, suture breakage can occur as a result of excess tension. Additionally, several studies found that the postoperative rate of lingual edema is significantly higher with robotic surgery than with the conventional approach, as mentioned above, which may be due to long-term excess pressure. However, Hans et al.32 and several other researchers found that 3D visualization would compensate for the lack of haptic feedback, to some extent, with increased experience.
Complicated. The robotic surgical procedure is complicated and the operative duration is much longer than with open surgery. This is because the robot needs to be docked in an appropriate position before surgery, which requires additional time, especially in this early stage. With additional robotic surgery experience, the operative duration would be similar to open surgery.
Expensive. Cost is a major problem that limits its wide application. The primary expense of a single robotic surgical system, including installation, is ~1.5 million dollars, in addition to ~$100 000 for annual maintenance and ~$200 in disposable instruments per patient, which results in increased costs of surgery.9 In the short term, the robotic surgical system will not have a positive impact on cost because of several costs associated with systems, telecommunication, training personnel and infrastructure.5 However, several studies found that the reduction of related morbidity and hospitalization, and the decreased need for tracheotomy partially offset the additional cost engendered by robotic surgical systems.33, 50, 52
Large size. Robotic surgical systems are unwieldy and require considerable space. The bulky size of the instruments limits its application in the treatment of laryngeal carcinoma patients, who have limited mouth opening or mandibular retraction, and in transnasal surgeries or otology.
Lack of specific instruments for maxillofacial surgery. For instance, electric bone saws and drills. This problem will need to be resolved in the near future.
Prospective of robotics in the head and neck region
The robotic surgical system is a novel, minimally invasive procedure with promising impact, and the development of robotic surgery is still in an early stage. There are several challenges and barriers to broader application and adoption of this technique. Further refinements are necessary before its wide application in maxillofacial surgery for head and neck neoplasms and in non-malignant diseases.
From a clinical perspective, the widespread use of robotic surgical systems in head and neck surgery is an inevitable development. The available research indicated excellent outcomes in terms of surgical morbidity, oncologic control and functional recovery for head and neck tumor patients treated by robotic surgical systems. However, there are several problems and uncertainties associated with robotic surgery. The incidence of capsule breakage or neoplasm fracture during robotic surgery is relatively high. Robotic surgery typically requires a long surgical duration or large storage of drainage, especially via a retroauricular approach or a modified face-lift approach, because of the extended flap. It remains unclear whether robotic surgery would improve the prognosis of HPV-negative patients. The regional or distant metastasis rate for robot-assisted resection of recurrent tumors is quite variable. However, because the robotic surgical system has been used for a relatively short time in the treatment of head and neck neoplasms, the problems mentioned above as well as the long-term effects and cost-effect analysis of this approach will require further study prior to it becoming a standard treatment paradigm. Particularly, specialization of robotic instruments for head and neck therapy, progressive miniaturization of its components, realization of haptic feedback, multisurgeon capability and flexible multiport access devices are anticipated for the future development of robotic surgery. Furthermore, VSP was reported to provide good guidance for robotic surgery, which will potentially enhance the accuracy and efficiency of robotic surgical systems. Therefore, a shorter surgical duration and superior reconstruction might be achieved when combining robotic surgery and VSP; this approach is another anticipated trend in robotic surgery in the future (Table 1).
Regarding other applications in the head and neck, robotic surgery has been widely used in OSAS patients, and it is undoubtedly a promising approach for those who cannot tolerate CPAP. However, the success rate remains unsatisfactory, possibly because of the nature of the multiple risk factors for OSAS. Therefore, robotic surgery for OSAS should only be used after careful patient selection regarding severity, age, BMI and related soft tissue structures. Furthermore, the combination of robotic resection of the BOT and conventional UPPP or sphincter pharyngoplasty might be a rational operation in the future. Moreover, it is almost impossible to use a robotic surgical system in the treatment of maxillofacial fractures and craniofacial asymmetry owing to the current lack of tactile and haptic feedback. Specifically, an appropriate resistance is not provided by current robotic surgical technology to prevent additional damage when performing a fracture reduction or an osteotomy. More work needs to be done, from theoretical feasibility to the clinical application of robotic surgical systems, in the management of maxillofacial fractures and craniofacial asymmetry. Additionally, the available studies that used robotic surgery in the treatment of lip and palate patients are quite limited. Although the only clinical research demonstrated significantly shorter hospital stays and better functional recovery than conventional surgery because of the precise dissection and reconstruction in robotic surgery, further studies with larger samples are still of paramount importance to ensure the safety and feasibility of robot-assisted surgery for cleft lip and palate patients. Similarly, the long-term effectiveness and safety of robotic surgery applied in other conditions, such as ectopic lingual thyroid and ptyalolithiasis, also require further study. Furthermore, selection of surgical procedures appropriate for the system is a challenge as well, except for the requirement of more well-designed studies. Standard surgical procedures permit the application of surgical robots. The diversity of maxillofacial surgery (that is, cleft lip surgery) set the development of robotic surgery back to a certain extent, and these procedures should be standardized before surgical robots are widely applied. In addition, although oscillating and surgical drills were applied in robotic arthroplasty, a similar application suitable for maxillofacial surgery has not been pursued. To summarize, instrument specialization, the realization of more precise intraoperative navigation, and further applications with large samples in various maxillofacial surgeries will all further the development of robotic surgery in the treatment of non-malignant craniofacial conditions (Table 2).
From a technical perspective, the considerable operative duration is currently one of the main deficiencies of robotic surgery because of extended times for robot docking, changing tools and inserting supplies. To address this deficiency, two technical projects were recently proposed.5 One is “Robotic systems,” which integrates multiple surgical robots into a single “robotic cell.” A robotic tool changer or a robotic supply dispenser may perform the function instead of nurses when a different tool is needed during an operation in the future. The other is “automatic or autonomous surgery.” To perform a pre-programmed task under an unstructured environment in a living system is difficult because of the greater variability, but it is theoretically realizable by collecting large amounts of previously “rehearsed” and “saved” surgical procedures. In addition, a lack of tactile and haptic feedback is an important deficiency of a robotic surgical system as well. Haptic feedback provides an operator with both sense and interaction with an interface. Haptic feedback can help prevent inadvertent damage to normal tissues and distinguish specific tissues features, such as cardiac arteries. Today’s operating instruments in robotic systems are all simple mechanical devices; the surgeon could only proceed to dissect depending on the subjective sense of touch via visualization. There is no suitable haptic sensor that is incorporated with current robotic surgical system, although several related mechanical sensors have been investigated. Tsang137 determined that VerroTouch, an early add-on, including a sensor placed on the robotic instrument and a vibration actuator fixed on the handle to provide haptic feedback, is capable of solving this problem, but none found it essential. In orthopedic surgery, several robotic systems, such as ACROBOT and MAKO RIO, were reported to have the ability to realize haptic feedback during the execution phase of arthroplasty by constraining the surgeon to operate within a predefined safe region. Once the surgeon attempts to operate outside the boundary, the control systems and drive systems inside the manipulator apply resistance to the motion to keep the effector within the predefined surgical plan.5 With the development of Computer-Aided Manufacturing/Computer-Aided Design in maxillofacial surgery, a similar technique to MAKO RIO could be applied for head and neck disease soon. Additionally, there are a number of other engineering barriers have to overcome, including: (1) ease of use: the current robotic surgical systems always have a high level of complexity and require advanced training, which may cause some highly specialized surgeons to shy away from these procedures; (2) reliability of telecommunication: low packet loss and limited latency are of great importance for consistently and safely operating at a distance.
Conclusion
The primary outcomes of robotic surgery in the head and neck region demonstrate good disease control, quick postoperative functional recovery and low surgical morbidity. However, definitive recommendations for the application of robotic surgical systems in the treatment of head and neck tumors, cleft lip and palate, OSAS and other conditions will require more well-designed studies and technical modifications in current surgical robots and in the future.
Acknowledgments
This work is supported by grants from the NSFC 81621062. This clinical report was supported by grants from the NSFC 81621062.
References
- Mouret P. How I developed laparoscopic cholecystectomy. Ann Acad Med Singapore 1996; 25 (5): 744–747. [PubMed] [Google Scholar]
- Steiner W. Endoscopic laser surgery of the upper aerodigestive tract. New York: Georg Thieme. 2000. [Google Scholar]
- Mcleod IK, Melder PC. Da Vinci robot-assisted excision of a vallecular cyst: a case report. Ear Nose Throat J 2005; 84 (3): 170–172. [PubMed] [Google Scholar]
- Dean NR, Rosenthal EL, Carroll WR et al. Robotic-assisted surgery for primary or recurrent oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2010; 136 (4): 380–384. [DOI] [PubMed] [Google Scholar]
- Rosen J, Hannaford B, Satava et al. Surgical robotics. Philadelphia: Springer US. 2011. [Google Scholar]
- Kwoh YS, Hou J, Jonckheere EA et al.A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng 1988; 35(2): 153–160. [DOI] [PubMed] [Google Scholar]
- Butner SEA Real-Time System for Telesurgery. Mesa: Proc. IEEE Int. Conf. Distributed Computing Systems, 2001: pp 236-243.
- Talamini M, Campbell K, Stanfield C. Robotic gastrointestinal surgery: early experience and system description. J Laparoendosc Adv Surg Tech A 2002; 12 (4): 225–232. [DOI] [PubMed] [Google Scholar]
- Pasticier G, Rietbergen JB, Guillonneau B et al. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol 2001; 40 (1): 70–74. [DOI] [PubMed] [Google Scholar]
- Haus BM, Kambham N, Le D et al. Surgical robotic applications in otolaryngology. Laryngoscope 2003; 113 (7): 1139–1144. [DOI] [PubMed] [Google Scholar]
- Shield KD, Ferlay J, Jemal A et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin 2017; 67 (1): 51–64. [DOI] [PubMed] [Google Scholar]
- Hashim D, Sartori S, Brennan P et al. The role of oral hygiene in head and neck cancer: results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann Oncol 2016; 27 (8): 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warram JM, De BE, van Dam GM et al. Fluorescence imaging to localize head and neck squamous cell carcinoma for enhanced pathological assessment. J Pathol Clin Res 2016; 2 (2): 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Jr GS, O'Malley BW, Snyder W et al. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg 2007; 133 (12): 1220–1226. [DOI] [PubMed] [Google Scholar]
- Jr OMB, Weinstein GS, Snyder W et al. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 2006; 116 (8): 1465–1472. [DOI] [PubMed] [Google Scholar]
- Boudreaux B, Rosenthal E, Magnuson JS et al. Robot-assisted surgery for upper aerodigestive track neoplasms. Arch Otolaryngol Head Neck Surg 2009; 135 (4): 397–401. [DOI] [PubMed] [Google Scholar]
- Genden E, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck 2009; 31 (3): 283–289. [DOI] [PubMed] [Google Scholar]
- Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope 2009; 119 (11): 2156–2164. [DOI] [PubMed] [Google Scholar]
- Iseli TA, Kulbersh BD, Iseli CE et al. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg 2009; 141 (2): 166–171. [DOI] [PubMed] [Google Scholar]
- Mardirossian VA, Zoccoli MC, Alphi Elackattu MD et al. A pilot study to evaluate the use of the da Vinci surgical robotic system in transoral surgery for lesions of the oral cavity and pharynx. Laryngoscope 2009; 119 (S1): 7. [Google Scholar]
- Park YM, Kim WS, Byeon HK et al. A novel technique for the resection of the symptomatic lingual thyroid: transoral robotic surgery. Thyroid 2013; 23 (4): 466–471. [DOI] [PubMed] [Google Scholar]
- White HN, Moore EJ, Rosenthal EL et al. Transoral robotic-assisted surgery for head and neck squamous cell carcinoma: one- and 2-year survival analysis. Arch Otolaryngol Head Neck Surg 2010; 136 (12): 1248–1252. [DOI] [PubMed] [Google Scholar]
- Leonhardt FD, Quon H, Abrahão M et al. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient‐reported quality of life and function. Head Neck 2012; 34 (2): 146–154. [DOI] [PubMed] [Google Scholar]
- Remacle M, Matar N, Lawson G et al. Combining a new CO2 laser wave guide with transoral robotic surgery: a feasibility study on four patients with malignant tumors. Eur Arch Otorhinolaryngol 2012; 269 (7): 1833–1837. [DOI] [PubMed] [Google Scholar]
- Moore EJ, Olsen KD, Martin EJ. Concurrent neck dissection and transoral robotic surgery. Laryngoscope 2011; 121 (3): 541–544. [DOI] [PubMed] [Google Scholar]
- Sinclair CF, Mccolloch NL, Carroll WR et al. Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2011; 137 (11): 1112–1116. [DOI] [PubMed] [Google Scholar]
- Aubry K, Yachine M, Perez AF et al. Transoral robotic surgery for head and neck cancer: a series of 17 cases. Eur Ann Otorhinolaryngol Head Neck Dis 2011; 128 (6): 290–296. [DOI] [PubMed] [Google Scholar]
- Olsen SM, Moore EJ, Koch CA et al. Transoral robotic surgery for supraglottic squamous cell carcinoma. Am J Otolaryngol 2012; 33 (4): 379–384. [DOI] [PubMed] [Google Scholar]
- Vergez S, Lallemant B, Ceruse P et al. Initial multi-institutional experience with transoral robotic surgery. Otolaryngol Head Neck Surg 2012; 147 (3): 475–481. [DOI] [PubMed] [Google Scholar]
- Moore EJ, Olsen SM, Laborde RR et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc 2012; 87 (87): 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtuk AM, Marcinow A, Agrawal A et al. Quality-of-life outcomes in transoral robotic surgery. Otolaryngol Head Neck Surg 2012; 146 (1): 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Badoual C, Gorphe P et al. Transoral robotic surgery for head and neck carcinomas. Eur Arch Otorhinolaryngol 2012; 269 (8): 1979–1984. [DOI] [PubMed] [Google Scholar]
- Aubry K, Yachine M, Lerat J et al. Transoral robotic surgery for the treatment of head and neck cancer of various localizations. Surg Innov 2011; 19 (1): 60–66. [DOI] [PubMed] [Google Scholar]
- Van Abel KM, Moore EJ, Carlson ML et al. Transoral robotic surgery using the thulium:YAG laser: a prospective study. Arch Otolaryngol Head Neck Surg 2012; 138(2): 158–166. [DOI] [PubMed] [Google Scholar]
- Weinstein Jr GS, O'Malley BW, Magnuson JS et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 2012; 122 (8): 1701–1707. [DOI] [PubMed] [Google Scholar]
- Park YM, Kim WS, Byeon HK et al. Clinical outcomes of transoral robotic surgery for head and neck tumors. Ann Otol Rhinol Laryngol 2013; 122 (2): 73–84. [DOI] [PubMed] [Google Scholar]
- Park YM, Byeon HK, Chung HP et al. Comparison of treatment outcomes after transoral robotic surgery and supraglottic partial laryngectomy: our experience with seventeen and seventeen patients respectively. Clin Otolaryngol 2013; 38 (3): 270–274. [DOI] [PubMed] [Google Scholar]
- Park YM, Byeon HK, Chung HP et al. Comparison study of transoral robotic surgery and radical open surgery for hypopharyngeal cancer. Acta Otolaryngol 2013; 133 (6): 641–648. [DOI] [PubMed] [Google Scholar]
- Oysu C, Sahin-Yilmaz A. En bloc resection of epiglottic tumors with transoral robotic approach—preliminary results. Int J Med Robot 2013; 9 (4): 477–479. [DOI] [PubMed] [Google Scholar]
- Park YM, Lee WJ, Yun IS et al. Free flap reconstruction after robot-assisted neck dissection via a modified face-lift or retroauricular approach. Ann Surg Oncol 2013; 20 (3): 891–898. [DOI] [PubMed] [Google Scholar]
- Park YM, Kim WS, De VA et al. Transoral robotic surgery for hypopharyngeal squamous cell carcinoma: 3-year oncologic and functional analysis. Oral Oncol 2012; 48 (6): 560–566. [DOI] [PubMed] [Google Scholar]
- White HN, Frederick J, Zimmerman T et al. Learning curve for transoral robotic surgery: a 4-year analysis. JAMA Otolaryngol Head Neck Surg 2013; 139 (6): 1–4. [DOI] [PubMed] [Google Scholar]
- Park YM, Kim WS, Byeon HK et al. Oncological and functional outcomes of transoral robotic surgery for oropharyngeal cancer. Br J Oral Maxillofac Surg 2013; 51 (5): 408–412. [DOI] [PubMed] [Google Scholar]
- Mendelsohn AH, Remacle M, Van DVS et al. Outcomes following transoral robotic surgery: supraglottic laryngectomy. Laryngoscope 2013; 123 (1): 208–214. [DOI] [PubMed] [Google Scholar]
- White H, Ford S, Bush B et al. Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches. JAMA Otolaryngol Head Neck Surg 2013; 139 (8): 773–778. [DOI] [PubMed] [Google Scholar]
- Quon H, Cohen MA, Montone KT et al. Transoral robotic surgery and adjuvant therapy for oropharyngeal carcinomas and the influence of p16 INK4a on treatment outcomes. Laryngoscope 2013; 123 (3): 635–640. [DOI] [PubMed] [Google Scholar]
- Blanco RG, Fakhry C, Ha PK et al. Transoral robotic surgery experience in 44 cases. J Laparoendosc Adv Surg Tech A 2013; 23 (11): 900–907. [DOI] [PubMed] [Google Scholar]
- Lallemant B, Chambon G, Garrel R et al. Transoral robotic surgery for the treatment of T1-T2 carcinoma of the larynx: preliminary study. Laryngoscope 2013; 123 (10): 2485–2490. [DOI] [PubMed] [Google Scholar]
- Ansarin M, Zorzi S, Massaro MA et al. Transoral robotic surgery vs transoral laser microsurgery for resection of supraglottic cancer: a pilot surgery. Int J Med Robot 2014; 10 (1): 107–112. [DOI] [PubMed] [Google Scholar]
- Richmon JD, Quon H, Gourin CG. The effect of transoral robotic surgery on short-term outcomes and cost of care after oropharyngeal cancer surgery. Laryngoscope 2014; 124 (1): 165–171. [DOI] [PubMed] [Google Scholar]
- Kasim Durmus MD, Patwa HS, Gokozan HN et al. Functional and quality-of-life outcomes of transoral robotic surgery for carcinoma of unknown primary. Laryngoscope 2014; 124 (9): 2089–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim Hammoudi MD, Eric Pinlong MD, Soo Kim MD et al. Transoral robotic surgery versus conventional surgery in treatment for squamous cell carcinoma of the upper aerodigestive tract. Head Neck 2015; 37 (9): 1304–1309. [DOI] [PubMed] [Google Scholar]
- Lorincz BB. TORS for head and neck squamous cell carcinoma (HNSCC): the Hamburg experience. B-ENT 2015; 11 (24): 33–36. [PubMed] [Google Scholar]
- Dziegielewski PT, Kang SY, Ozer E. Transoral robotic surgery (TORS) for laryngeal and hypopharyngeal cancers. J Surg Oncol 2015; 112 (7): 702–706. [DOI] [PubMed] [Google Scholar]
- Mehta V, Johnson P, Tassler A et al. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope 2013; 123 (1): 315–322. [DOI] [PubMed] [Google Scholar]
- Abuzeid WM, Bradford CR, Divi V. Transoral robotic biopsy of the tongue base: a novel paradigm in the evaluation of unknown primary tumors of the head and neck. Head Neck 2013; 35 (4): 126–130. [DOI] [PubMed] [Google Scholar]
- Channir HI, Rubek N, Nielsen HU et al. Transoral robotic surgery for the management of head and neck squamous cell carcinoma of unknown primary. Acta Otolaryngol 2015; 135 (10): 1051–1057. [DOI] [PubMed] [Google Scholar]
- Patel SA, Magnuson JS, Holsinger FC et al. Robotic surgery for primary head and neck squamous cell carcinoma of unknown site. JAMA Otolaryngol Head Neck Surg 2013; 139 (11): 376–381. [DOI] [PubMed] [Google Scholar]
- Byrd JK, Smith KJ, de Almeida JR et al. Transoral robotic surgery and the unknown primary: a cost-effectiveness analysis. Otolaryngol Head Neck Surg 2014; 150 (6): 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhija VK, Sung CK, Desai SC et al. Transoral robotic assisted free flap reconstruction. Otolaryngol Head Neck Surg 2009; 140 (140): 124–125. [DOI] [PubMed] [Google Scholar]
- Cognetti DM, Luginbuhl AJ, Nguyen AL et al. Early adoption of transoral robotic surgical program: preliminary outcomes. Otolaryngol Head Neck Surg 2012; 147 (3): 482–488. [DOI] [PubMed] [Google Scholar]
- Bonawitz SC, Duvvuri U. Robot-assisted oropharyngeal reconstruction with free tissue transfer. J Reconstr Microsurg 2012; 28 (7): 485–490. [DOI] [PubMed] [Google Scholar]
- Wine TM, Duvvuri U, Maurer SH et al. Pediatric transoral robotic surgery for oropharyngeal malignancy: a case report. Int J Pediatr Otorhinolaryngol 2013; 77 (7): 1222–1226. [DOI] [PubMed] [Google Scholar]
- Asher SA, White HN, Kejner AE et al. Hemorrhage after transoral robotic-assisted surgery. Otolaryngol Head Neck Surg 2013; 149 (1): 112–117. [DOI] [PubMed] [Google Scholar]
- Chan JY, Richmon JD. Transoral robotic surgery (TORS) for benign pharyngeal lesions. Otolaryngol Clin North Am 2014; 47 (3): 407–413. [DOI] [PubMed] [Google Scholar]
- Kelly K, Johnson-Obaseki S, Lumingu J et al. Oncologic, functional and surgical outcomes of primary transoral robotic surgery for early squamous cell cancer of the oropharynx: a systematic review. Oral Oncol 2014; 50 (8): 696–703. [DOI] [PubMed] [Google Scholar]
- de Almeida JR, Byrd JK, Wu R et al. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer. Laryngoscope 2014; 124 (9): 2096–2102. [DOI] [PubMed] [Google Scholar]
- Wei WI, Ho WK. Transoral robotic resection of recurrent nasopharyngeal carcinoma. Laryngoscope 2010; 120 (10): 2011–2014. [DOI] [PubMed] [Google Scholar]
- Cohen MA, Weinstein GS, O'Malley BW et al. Transoral robotic surgery and human papillomavirus status: oncologic results. Head Neck 2011; 33 (4): 573–580. [DOI] [PubMed] [Google Scholar]
- More YI, Tsue TT, Girod DA et al. Functional swallowing outcomes following transoral robotic surgery vs primary chemoradiotherapy in patients with advanced-stage oropharynx and supraglottis cancers. Arch Otolaryngol Head Neck Surg 2013; 139 (1): 1–6. [DOI] [PubMed] [Google Scholar]
- Catalano P, David C. Variations and modifications of the infratemporal fossa approaches. Oper Tech Neurosurg 2005; 8 (1): 31–34. [Google Scholar]
- O'Malley Jr BW, Weinstein GS. Robotic skull base surgery: preclinical investigations to human clinical application. Arch Otolaryngol Head Neck Surg 2007; 133 (12): 1215–1219. [DOI] [PubMed] [Google Scholar]
- Arshad H, Durmus K, Ozer E. Transoral robotic resection of selected parapharyngeal space tumors. Eur Arch Otorhinolaryngol 2013; 270 (5): 1737–1740. [DOI] [PubMed] [Google Scholar]
- De VA, Park YM, Kim WS et al. Transoral robotic surgery for the resection of parapharyngeal tumour: our experience in ten patients. Clin Otolaryngol 2012; 37 (6): 483–488. [DOI] [PubMed] [Google Scholar]
- Park YM, De VA, Kim WS et al. Parapharyngeal space surgery via a transoral approach using a robotic surgical system: transoral robotic surgery. J Laparoendosc Adv Surg Tech 2013; 23 (3): 231–236. [DOI] [PubMed] [Google Scholar]
- Chan JY, Tsang RK, Eisele DW et al. Transoral robotic surgery of the parapharyngeal space: a case series and systematic review. Head Neck 2015; 37 (2): 293–298. [DOI] [PubMed] [Google Scholar]
- Bodner J, Prommegger R, Profanter C et al. Thoracoscopic resection of mediastinal parathyroids: current status and future perspectives. Minim Invasive Ther Allied Technol 2004; 13 (3): 199–204. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Chung WY, Holsinger FC. Feasibility and surgical approach of transaxillary robotic thyroidectomy without CO 2 insufflation. Head Neck 2010; 32 (1): 121–126. [DOI] [PubMed] [Google Scholar]
- Miyano G, Lobe TE, Wright SK. Bilateral transaxillary endoscopic total thyroidectomy. J Pediatr Surg 2008; 43 (2): 299–303. [DOI] [PubMed] [Google Scholar]
- Byeon HK, Kim da H, Chang JW et al. Comprehensive application of robotic retroauricular thyroidectomy: the evolution of robotic thyroidectomy. Laryngoscope 2015; 126 (8): 1952–1957. [DOI] [PubMed] [Google Scholar]
- Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech 2011; 21 (4): 237–242. [DOI] [PubMed] [Google Scholar]
- Terris DJ, Singer MC. Qualitative and quantitative differences between 2 robotic thyroidectomy techniques. Otolaryngol Head Neck Surg 2012; 147 (1): 20–25. [DOI] [PubMed] [Google Scholar]
- Terris DJ, Singer MC. Robotic facelift thyroidectomy: facilitating remote access surgery. Head Neck 2012; 34 (5): 746–747. [DOI] [PubMed] [Google Scholar]
- Singer MC, Terris DJ. Robotic facelift thyroidectomy. Otolaryngol Clin North Am 2014; 47 (3): 425–431. [DOI] [PubMed] [Google Scholar]
- Kandil E, Saeed A, Mohamed SE et al. Modified robotic-assisted thyroidectomy: an initial experience with the retroauricular approach. Laryngoscope 2015; 125 (3): 767–771. [DOI] [PubMed] [Google Scholar]
- Mohamed SE, Saeed A, Moulthrop T et al. Retroauricular robotic thyroidectomy with concomitant neck-lift surgery. Ann Surg Oncol 2015; 22 (1): 1. [DOI] [PubMed] [Google Scholar]
- Kayhan FT, Kaya KH, Koc AK et al. Transoral surgery for an infant thyroglossal duct cyst. Int J Pediatr Otorhinolaryngol 2013; 77 (9): 1620–1623. [DOI] [PubMed] [Google Scholar]
- Kimple AJ, Eliades SJ, Richmon JD. Transoral robotic resection of a lingual thyroglossal duct cyst. J Robot Surg 2012; 6 (4): 367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Byeon HK, Shin YS et al. Robot-assisted Sistrunk operation via a retroauricular approach for thyroglossal duct cyst. Head Neck 2014; 36 (3): 456–458. [DOI] [PubMed] [Google Scholar]
- De VA, Park YM, Kim WS et al. Robotic sialoadenectomy of the submandibular gland via a modified face-lift approach. Int J Oral Maxillofac Surg 2012; 41 (11): 1325–1329. [DOI] [PubMed] [Google Scholar]
- Lee HS, Park DY, Hwang CS et al. Feasibility of robot-assisted submandibular gland resection via retroauricular approach: preliminary results. Laryngoscope 2013; 123 (2): 369–373. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim D, Lee SY et al. Robot-assisted versus endoscopic submandibular gland resection via retroauricular approach: a prospective nonrandomized study. Br J Oral Maxillofac Surg 2014; 52 (2): 179–184. [DOI] [PubMed] [Google Scholar]
- Yang TL, Ko JY, Lou PJ et al. Gland-preserving robotic surgery for benign submandibular gland tumours: a comparison between robotic and open techniques. Br J Oral Maxillofac Surg 2014; 52 (5): 420–424. [DOI] [PubMed] [Google Scholar]
- Walvekar RR, Peters G, Hardy E et al. Robotic-assisted transoral removal of a bilateral floor of mouth ranulas. World J Surg Oncol 2011; 9 (1): 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva NL, de Almeida JR, Sikora AG et al. Transoral robotic surgery for the management of oropharyngeal minor salivary gland tumors. Head Neck 2014; 36 (1): 28–33. [DOI] [PubMed] [Google Scholar]
- Kang SW, Lee SH, Ryu HR et al. Initial experience with robot-assisted modified radical neck dissection for the management of thyroid carcinoma with lateral neck node metastasis. Surgery 2010; 148 (6): 1214–1221. [DOI] [PubMed] [Google Scholar]
- Koh YW, Chung WY, Hong HJ et al. Robot-assisted selective neck dissection via modified face-lift approach for early oral tongue cancer: a video demonstration. Ann Surg Oncol 2012; 19 (4): 1334–1335. [DOI] [PubMed] [Google Scholar]
- Koh YW, Choi EC. Robotic approaches to the neck. Otolaryngol Clin North Am 2014; 47 (3): 433–454. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim WS, Hong HJ et al. Robot-assisted supraomohyoid neck dissection via a modified face-lift or retroauricular approach in early-stage cN0 squamous cell carcinoma of the oral cavity: a comparative study with conventional technique. Ann Surg Oncol 2012; 19 (12): 3871–3878. [DOI] [PubMed] [Google Scholar]
- Kim WS, Byeon HK, Park YM et al. Therapeutic robot-assisted neck dissection via a retroauricular or modified facelift approach in head and neck cancer: a comparative study with conventional transcervical neck dissection. Head Neck 2015; 37 (2): 249–254. [DOI] [PubMed] [Google Scholar]
- Kim CH, Koh YW, Kim D et al. Robotic-assisted neck dissection in submandibular gland cancer: preliminary report. J Oral Maxillofac Surg 2013; 71 (8): 1450–1457. [DOI] [PubMed] [Google Scholar]
- Kim WS, Koh YW, Byeon HK et al. Robot-assisted neck dissection via a transaxillary and retroauricular approach versus a conventional transcervical approach in papillary thyroid cancer with cervical lymph node metastases. J Laparoendosc Adv Surg Tech 2014; 24 (6): 367–372. [DOI] [PubMed] [Google Scholar]
- Kim WS, Lee HS, Kang SM et al. Feasibility of robot-assisted neck dissections via a transaxillary and retroauricular (“TARA”) approach in head and neck cancer: preliminary results. Ann Surg Oncol 2012; 19 (3): 1009–1017. [DOI] [PubMed] [Google Scholar]
- Tae K, Ji YB, Song CM et al. Robotic selective neck dissection using a gasless postauricular facelift approach for early head and neck cancer: technical feasibility and safety. J Laparoendosc Adv Surg Tech 2013; 23 (3): 240–245. [DOI] [PubMed] [Google Scholar]
- Tae K, Ji YB, Song CM et al. Robotic selective neck dissection by a postauricular facelift approach: comparison with conventional neck dissection. Otolaryngol Head Neck Surg 2014; 150 (3): 394–400. [DOI] [PubMed] [Google Scholar]
- Greer AW, Kenneth BJ, De Almeida JR et al. Robot-assisted level II-IV neck dissection through a modified facelift incision: initial North American experience. Int J Med Robot 2014; 10 (4): 391–396. [DOI] [PubMed] [Google Scholar]
- Genden EM, Park R, Smith C et al. The role of reconstruction for transoral robotic pharyngectomy and concomitant neck dissection. Arch Otolaryngol Head Neck Surg 2011; 137 (2): 151–156. [DOI] [PubMed] [Google Scholar]
- Selber JC. Transoral robotic reconstruction of oropharyngeal defects: a case series. Plast Reconstr Surg 2010; 126 (6): 1978–1987. [DOI] [PubMed] [Google Scholar]
- Jae-Young K, Shik KW, Chang CE et al. The role of virtual surgical planning in the era of robotic surgery. Yonsei Med J 2016; 57 (1): 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Dobbs T, Swan MC et al. Trans-oral robotic cleft surgery (TORCS) for palate and posterior pharyngeal wall reconstruction: a feasibility study. J Plast Reconstr Aesthet Surg 2015; 69 (1): 97–100. [DOI] [PubMed] [Google Scholar]
- Nadjmi N. Transoral robotic cleft palate surgery. Cleft Palate Craniofac J 2015; 44: e114–e115. [DOI] [PubMed] [Google Scholar]
- Du H, Hu L, Li C et al. Advancing computer-assisted orthopaedic surgery using a hexapod device for closed diaphyseal fracture reduction. Int J Med Robot 2014; 11 (3): 348–359. [DOI] [PubMed] [Google Scholar]
- Lefaivre KA, Starr AJ, Barker BP et al. Early experience with reduction of displaced disruption of the pelvic ring using a pelvic reduction frame. Bone Joint J 2009; 91 (9): 1201–1207. [DOI] [PubMed] [Google Scholar]
- Chen LM, Luan N, Zhang SL et al. Research on the application of multi DOF robot on orthog nathic navigation surgery. Mach Electron 2010: (4): 57–60.
- Giles TL, Lasserson TJ, Smith BH et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2002; 6 (2): CD001106-CD. [DOI] [PubMed] [Google Scholar]
- Fracs JC, Michael F. Sleep apnea and snoring—surgical and non-surgical therapy. Philadelphia: Saunders. 2009. [Google Scholar]
- Vicini C, Dallan I, Canzi P et al. Transoral robotic tongue base resection in obstructive sleep apnoea-hypopnoea syndrome: a preliminary report. ORL J Otorhinolaryngol Relat Spec 2010; 72 (1): 22–27. [DOI] [PubMed] [Google Scholar]
- Vicini C, Montevecchi F, Tenti G et al. Transoral robotic surgery: tongue base reduction and supraglottoplasty for obstructive sleep apnea. Oper Tech Otolayngol Head Neck Surg 2012; 23 (1): 45–47. [Google Scholar]
- Claudio Vicini MD, Filippo Montevecchi MD, Kenny Pang MD et al. Combined transoral robotic tongue base surgery and palate surgery in obstructive sleep apnea-hypopnea syndrome: expansion sphincter pharyngoplasty versus uvulopalatopharyngoplasty. Head Neck 2014; 36 (1): 77–83. [DOI] [PubMed] [Google Scholar]
- Friedman M, Hamilton C, Samuelson CG et al. Transoral robotic glossectomy for the treatment of obstructive sleep apnea-hypopnea syndrome. Otolaryngol Head Neck Surg 2012; 146 (5): 854–862. [DOI] [PubMed] [Google Scholar]
- Friedman M, Kelley K, Maley A. Robotic glossectomy for obstructive sleep apnea technique. Oper Tech Otolayngol Head Neck Surg 2013; 24 (24): 106–110. [Google Scholar]
- Hoff PT, Glazer TA, Spector ME. Body mass index predicts success in patients undergoing transoral robotic surgery for obstructive sleep apnea. ORL J Otorhinolaryngol Relat Spec 2014; 76 (5): 266–272. [DOI] [PubMed] [Google Scholar]
- PTHM MD, D'Agostino MA, Thaler ER. Transoral robotic surgery in benign diseases including obstructive sleep apnea: safety and feasibility. Laryngoscope 2014; 125 (5): 1249–1253. [DOI] [PubMed] [Google Scholar]
- Lee JM, Weinstein Jr GS, O'Malley BW et al. Transoral robot-assisted lingual tonsillectomy and uvulopalatopharyngoplasty for obstructive sleep apnea. Ann Otol Rhinol Laryngol 2012; 121 (10): 635–639. [DOI] [PubMed] [Google Scholar]
- Lin HS, Rowley JA, Badr MS et al. Transoral robotic surgery for treatment of obstructive sleep apnea-hypopnea syndrome. Laryngoscope 2013; 123 (7): 1811–1816. [DOI] [PubMed] [Google Scholar]
- Crawford JA, Montevechi F, Vicini C et al. Transoral robotic sleep surgery: the obstructive sleep apnea-hypopnea syndrome. Otolaryngol Clin North Am 2014; 47 (3): 397–406. [DOI] [PubMed] [Google Scholar]
- Toh ST, Han HJ, Tay HN et al. Transoral robotic surgery for obstructive sleep apnea in Asian patients: a Singapore sleep centre experience. JAMA Otolaryngol Head Neck Surg 2014; 140 (7): 624–629. [DOI] [PubMed] [Google Scholar]
- Muderris T, Sevil E, Bercin S et al. Transoral robotic lingual tonsillectomy in adults: preliminary results. Acta Otolaryngol 2015; 135 (1): 64–69. [DOI] [PubMed] [Google Scholar]
- Rahbar R, Ferrari LR, Borer JG et al. Robotic surgery in the pediatric airway: application and safety. Arch Otolaryngol Head Neck Surg 2007; 133 (1): 46–50. [DOI] [PubMed] [Google Scholar]
- Ciabatti PG, Burali G, D'Ascanio L. Transoral robotic surgery for large mixed laryngocoele. J Laryngol Otol 2013; 127 (4): 1–3. [DOI] [PubMed] [Google Scholar]
- Iv JTM, Newman JG, Padhya TA. Transoral robot-assisted excision of a lingual thyroid gland. J Robot Surg 2011; 5 (3): 217–220. [DOI] [PubMed] [Google Scholar]
- Dallan I, Montevecchi F, Seccia V et al. Transoral robotic resection of an ectopic tongue-base thyroid gland. J Robot Surg 2013; 7 (1): 83–86. [DOI] [PubMed] [Google Scholar]
- Prisman E, Patsias A, Genden EM. Transoral robotic excision of ectopic lingual thyroid: case series and literature review. Head Neck 2014; 37 (8): E88–E91. [DOI] [PubMed] [Google Scholar]
- Walvekar RR, Ba PDT, Neelima Tammareddi MD et al. Robotic-assisted transoral removal of a submandibular megalith. Laryngoscope 2011; 121 (3): 534–537. [DOI] [PubMed] [Google Scholar]
- Razavi C, Pascheles C, Samara G et al. Robot-assisted sialolithotomy with sialendoscopy for the management of large submandibular gland stones. Laryngoscope 2015; 126 (2): 345–351. [DOI] [PubMed] [Google Scholar]
- Dziegielewski PT, Durmus K, Ozer E. Transoral robotic surgery for the excision of base of tongue vascular lesions. Head Neck 2015; 37 (8): 1211–1212. [DOI] [PubMed] [Google Scholar]
- Tsang RK. Transoral robotic surgery: development and challenges. Robot Surg Res Rev 2015; 2 (1): 1–10. [Google Scholar]