Abstract
We previously demonstrated two statistically distinct factors of degeneration in Alzheimer’s disease (AD): one strongly related to white matter damage and age interpreted as ‘age- and vascular-related’, and the other related to cortical atrophy thought to represent ‘neurodegenerative changes associated with AD’. Those factors are now replicated in a distinct cross-sectional dataset of 364 participants from the Alzheimer’s Disease Neuroimaging Initiative and their interpretation is improved using correlations with CSF biomarkers. Furthermore, we now show that changes in both factors over two years are independently associated with decline in Mini-Mental State Examination (MMSE) score in a longitudinal subset of 116 individuals with mild cognitive impairment. Progression in the ‘age- and vascular-related’ factor was greater for individuals with two APOE ε 4 alleles and linked to a greater attributable change in MMSE than the ‘neurodegenerative’ factor. These results suggest benefits of targeting white matter and vascular health to complement interventions focused on the neurodegenerative aspect of the disease, even in individuals with little discernable vascular comorbidity.
Keywords: White matter, mild cognitive impairment, longitudinal cohort study, cognitive decline, Alzheimer’s disease, small-vessel disease, CSF biomarkers
1 Introduction
The conclusive diagnosis of Alzheimer’s disease (AD)3 is currently determined based on the presence of AD pathology, such as beta-amyloid plaques and neurofibrillary tangles, which guides models of the pathophysiology of the disease (Hyman et al., 2012; Jack Jr et al., 2010). However, there is a diversity of additional changes that occur in the brain throughout the course of the disease which are typically highly prevalent across patients yet considered either secondary or independent to the primary diagnostic pathologies. In a recent study (Coutu et al., 2016), we found two statistically distinct classes of imaging markers (factors) indicative of degenerative processes that were affected by AD. One factor was strongly linked to imaging measures of cortical atrophy that are presumed to be related to the neurodegenerative changes in AD that are linked to plaque and tangle accumulation. This factor was therefore interpreted to be ‘neurodegenerative’ (neurodegenerative factor: NDF). The other factor was statistically independent from the NDF, was highly weighted by white matter lesions of presumed vascular origin (Gottesman et al., 2010; Gouw et al., 2011; Jeerakathil et al., 2004; Pantoni, 2010; Rostrup et al., 2012; Wardlaw et al., 2013), and was strongly associated with age. This factor was therefore interpreted to represent ‘age- and vascular-related’ tissue damage (age- and vascular-related factor: AVF). Of particular interest, both factors were independently weighted by hippocampal volume demonstrating the multiple sources of variance contributing to this often used imaging marker of AD neurodegeneration (Atiya et al., 2003). Both factors were also related to Mini Mental State Examination (MMSE) scores cross-sectionally. The main goals of this follow-up work were to replicate our previous factor analysis in a distinct dataset and determine the longitudinal associations between these degenerative factors and cognitive decline in older adults with MCI. Secondary exploratory goals included investigating associations between those classes of degenerative change and CSF biomarkers, and distinguishing converters from older adults with MCI who have not converted.
2 Materials and Methods
2.1 Participants and MRI acquisition
The cross-sectional dataset used to replicate the factor analysis came from the Alzheimer’s Disease Neuroimaging Initiative GO/2 (ADNI, http://adni.loni.usc.edu) and included 113 controls, 159 participants with MCI and 92 participants with AD who underwent whole-brain MRI scanning on a 3-Tesla Siemens scanner as described in ADNI Core MRI protocols (Jack Jr et al., 2008) and had sagittal T1-weighted images and pulsed arterial spin labeling images available at the time of download. The specific requirement of arterial spin labeling data availability was to obtain a dataset that is similar but independent from the one used in our previous study which was also from ADNI GO/2 (Coutu et al., 2016), as participants who had arterial spin labeling data available were not scanned with diffusion-weighted imaging by design in ADNI GO/2. The arterial spin labeling data was not used in this study, except to obtain estimates of participant motion. One participant did overlap both datasets, but data for that participant were obtained four years apart on different scanners. Cerebrospinal fluid (CSF) biomarkers (Aβ1–42, t-tau and p-tau181) were available in 319 participants, but only data from the 251 participants who had their CSF drawn within one year of scanning were used (on average drawn 145 days before scan). The longitudinal dataset included 122 individuals with MCI from the cross-sectional dataset who underwent whole-brain MRI scanning twice approximately two years apart and had data for these two visits. Five individuals were excluded from the analysis due to outlier, improbable longitudinal segmentation data (i.e. large expansion of the volume of tissue and/or shrinking of the ventricles). One individual was excluded because of missing clinical information.
Clinical profiles and diagnostic information were obtained from the assessment closest in time to the MRI acquisition. This included the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), the Alzheimer’s Disease Assessment Scale (ADAS-Cog 13-item scale) (Mohs et al., 1997) and the Clinical Dementia Rating – Sum of Boxes (CDR-SB) (Morris, 1993). Group designation of control, MCI and probable AD was determined by ADNI based on the standard criteria (McKhann et al., 1984) (see ADNI 2 Procedures Manual on www.adni-info.org for more information). Among other exclusion criteria, individuals with vascular comorbidities, such as a history of stroke, were excluded if they had a Modified Hachinski score greater than 4 (Rosen et al., 1980). Written informed consent was obtained from all participants or their representatives through ADNI. The study procedures were approved by institutional review boards of all participating institutions.
2.2 Automated subcortical and white matter lesion segmentation
Automated subcortical and white matter segmentation were obtained from the T1-weighted images using the longitudinal processing stream of FreeSurfer version 5.3 (https://surfer.nmr.mgh.harvard.edu) (Fischl et al., 2002; Reuter et al., 2012). The automated segmentation also included a white matter lesion segmentation, and FreeSurfer mri_relabel_hypointensities was used to refine the white matter lesion segmentation using the surface reconstruction described below. These methods are the same as those used in our previous study (Coutu et al., 2016) to ensure proper replication. The FreeSurfer segmentation method is highly correlated with T2-weighted and FLAIR MRI as demonstrated in our previous study (Coutu et al., 2016). Furthermore, the concordance correlation coefficient between the publicly-available FreeSurfer and FLAIR-based white matter lesion volume estimates is high (r = 0.84, p < 0.001, n = 854 unique participants, data not shown). The Bayesian approach for the FLAIR MRI segmentation is fully described online: http://adni.bitbucket.org/docs/UCD_ADNI2_WMH/UCD%20ADNI%20II%204%20tissue%20segmentation%20Method.pdf.
2.3 Cortical surface reconstruction and extraction of thickness measures
The same version of FreeSurfer was used for cortical surface reconstruction and to extract the average thickness weighted by the surface area of each cortical surface labels representing the regions that undergo thinning in early AD (Bakkour et al., 2009; Dickerson et al., 2011, 2009), as per our previous study (Coutu et al., 2016) to ensure proper replication. Those regions are described as the cortical signature of AD given the reliability of this effect across samples (Bakkour et al., 2009; Dickerson et al., 2011, 2009) and include the angular gyrus, the superior frontal gyrus, the inferior frontal sulcus, the superior parietal lobule, the precuneus, the inferior temporal gyrus, the supramarginal gyrus, the medial temporal cortex and the temporal pole. Those regions are clearly represented in (Dickerson et al., 2009).
2.4 Computation of factor scores
The same factor analysis (with VARIMAX) as performed in (Coutu et al., 2016) was independently replicated in this distinct dataset using JMP 10 statistical software (SAS Institute Inc., Cary, NC, U.S.A.). As described previously (Coutu et al., 2016), the factor analysis was performed on the normalized measures of white matter lesion volume, total white matter volume, hippocampal volume, ventricular volume and AD signature cortical thickness. Briefly, the total white matter volume, ventricular volume (lateral ventricles) and hippocampal volume were divided by the estimated total intracranial volume in each individual. The natural logarithm of the volume of white matter lesions divided by total white matter volume was used instead of the lesion volume divided by estimated intracranial volume to obtain a more normalized distribution of this typically skewed measure and to represent a more accurate measure of neural compromise. The average cortical thickness in AD regions was not normalized. Factor scores for each participant were also obtained by standardizing the normalized measures and multiplying them by the standard score coefficients extracted from the factor analysis described previously (Coutu et al., 2016). The factor analysis was also replicated using white matter lesion volume estimates based on FLAIR MRI available for 326 individuals of our cross-sectional sample.
2.5 Statistical analyses
Statistical analyses were performed using JMP 10 (SAS Institute Inc., Cary, NC, U.S.A.). In the cross-sectional dataset, the factor scores were used as independent variables in a standard least squares, forced introduction general linear model of the CSF biomarkers using diagnostic group (control, MCI or AD), age, sex, education and number of APOE ε4 alleles as covariates. In the longitudinal dataset, the factors at baseline and their progression over time (factor at follow-up minus factor at baseline) were used as independent variables in a standard least squares, forced introduction general linear model of the change in MMSE score using age, time between scans, sex, education and number of APOE ε4 alleles. Motion measures from arterial spin labeling data, which allow for an indirect yet quantitative estimation of the propensity of an individual to move during a scan, were omitted from the final models as they were never significant when added to the models and had no impact on the results. Secondary forced introduction general linear models using the change in ADAS-Cog 13-item scale and in CDR-SB were also tested with the same covariates. Additional forced introduction general linear models of the progression of each factor score as the dependent variable were performed with age, time between scans, sex, education and number of APOE ε4 alleles as the independent variables. The general linear models were also replicated with only the significant variables included to confirm that the results remain unchanged. All continuous variables were standardized for easier comparison of parameter estimates (β) in the models. Nominal variables included sex and the number of APOE ε4 alleles. All variables included have generally been shown to have some influence on the five neuroimaging markers used in the factor analysis and were therefore all included in the models to prevent any potential omitted-variable bias.χ2, F and t tests were also used to characterize sample demographics and compare with the previous dataset used in (Coutu et al., 2016), as well as to distinguish converters from individuals who have not converted in the longitudinal sample, for which a Bonferroni correction for multiple comparisons was applied.
2.6 Alzheimer’s Disease Neuroimaging Initiative
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For up-to-date information, see www.adni-info.org.
3 Results
3.1 Replication of the factor analysis in a distinct cross-sectional dataset
The cross-sectional dataset used for replication was completely separate from the dataset used in the original factor analysis (Coutu et al., 2016), but had comparable demographics with no significant differences within or across groups (Supplementary Table 1). The replication of the factor analysis yielded two significant factors (AVF′ and NDF′) with very similar loadings as AVF and NDF from our previous study (Coutu et al., 2016) (Supplementary Table 2). Both factors showed a high loading from hippocampal volume. AVF and AVF′ also had high loadings (≥ 0.4) from volume of white matter lesions, total WM volume and ventricular volume, while NDF and NDF′ also had a high loading from AD signature cortical thickness. Similar results were obtained from the factor analysis using the FLAIR MRI-based WML volume estimates (AVF″ and NDF″), though lower loadings were observed for both WML volume and hippocampal volume. Scatterplots of the relationship between AVF and AVF′ and between NDF and NDF′ (Supplementary Figure 1) show a very high correlation between factor scores of both factor analyses in the same individuals, demonstrating the robustness of the two-factor construct of degeneration in AD. All further analyses use the factor scores derived from the factor analysis of our previous study (Coutu et al., 2016), though we have confirmed that all results and conclusions are the same using both sets of factors (not shown).
3.2 Cross-sectional associations between CSF biomarkers and factor scores
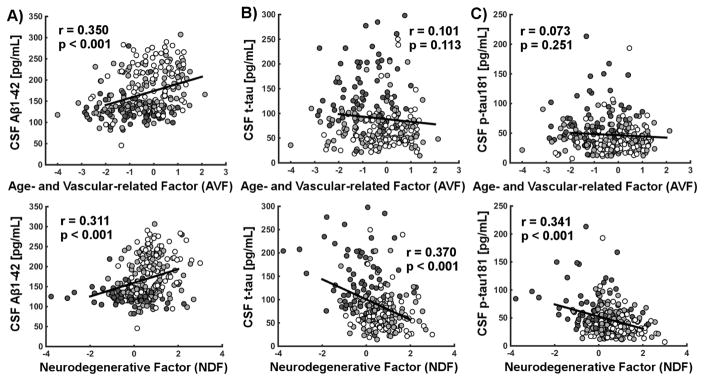
Both AVF and NDF were independently related to the level of CSF Aβ1–42 (p < 0.001 and p < 0.01 respectively), while only NDF was related to both t-tau (p < 0.05) and p-tau181 (p < 0.01), when accounting for all covariates as demonstrated in our general linear model (Supplementary Table 3). Of note, group, sex and having two APOE ε4 alleles were also associated with the level of CSF biomarkers independently of AVF and NDF. Associations between factor scores and CSF Aβ1–42, t-tau and p-tau181 are presented in Figure 1A, 1B and 1C respectively.
Figure 1.
Scatterplots of the associations between factor scores and CSF A) Aβ1–42, B) t-tau and C) p-tau181 are presented in the cross-sectional dataset. Pearson’s correlation coefficients and associated p- values are shown. Controls, individuals with mild cognitive impairment and Alzheimer’s disease are shown respectively in white, light gray and dark gray. All significant relationships remain significant when correcting for all covariates as detailed in the models, but uncorrected data is presented here to further support the hypothesis that CSF biomarkers are related to the factor scores.
3.3 Demographics of the longitudinal dataset
Demographics of the longitudinal dataset of participants with MCI are provided (Table 1), with 17 participants converting to AD during the two-year follow-up. The individuals with MCI who converted to AD had a greater decrease in MMSE than individuals with MCI who did not convert. Only progression in AVF was significantly different between the participants with MCI who converted to AD and those who did not after correcting for multiple comparisons, though both AVF and NDF at baseline and their progression were significant when uncorrected.
Table 1.
Demographics for all participants with MCI who had longitudinal data
| Converted to AD | Other participants | p-value | |
|---|---|---|---|
| Participants (female) | 17 (9) | 99 (42) | 1.0000 |
| Age at baseline [years] | 72.05 (1.97) | 70.86 (0.69) | 1.0000 |
| Time between scans [years] | 2.04 (0.02) | 2.01 (0.01) | 1.0000 |
| Education [years] | 16.94 (0.57) | 16.44 (0.27) | 1.0000 |
| APOE ε4 [# alleles] | 1.06 (0.18) | 0.45 (0.06) | 0.1060* |
| MMSE at baseline [−] | 27.06 (0.50) | 28.35 (0.16) | 0.4672* |
| MMSE difference [−] | −3.65 (0.62) | −0.28 (0.20) | 0.0010 |
| ADAS13 at baseline [−] | 21.94 (1.37) | 12.11 (0.54) | <0.0001 |
| ADAS13 difference [−] | 8.06 (1.43) | −0.20 (0.43) | 0.0005 |
| CDR-SB at baseline [−] | 2.50 (0.20) | 1.23 (0.11) | <0.0001 |
| CDR-SB difference [−] | 2.68 (0.33) | −0.04 (0.12) | <0.0001 |
| Global thickness diff. [mm/year] | −0.033 (0.008) | −0.009 (0.002) | 0.1455* |
| AD regional thick. diff. [mm/year] | −0.041 (0.009) | −0.013 (0.002) | 0.1656* |
| WM Lesion vol. diff. [mm3/year] | 1199 (291) | 413 (57) | 0.3522* |
| Hippocampal vol. diff. [mm3/year] | −264 (31) | −58 (10) | <0.0001 |
| Ventricular vol. diff. [mm3/year] | 3949 (496) | 1439 (140) | 0.0024 |
| Total WM vol. diff. [mm3/year] | −5922 (979) | −3331 (346) | 0.4487* |
| AVF (baseline) | −0.594 (0.230) | −0.008 (0.106) | 0.6326* |
| AVF (difference) | −0.381 (0.053) | −0.146 (0.014) | 0.0089 |
| NDF (baseline) | 0.286 (0.248) | 0.872 (0.069) | 0.7300* |
| NDF (difference) | −0.434 (0.115) | −0.129 (0.028) | 0.3915* |
Standard errors of the mean are shown in parentheses, except for the first row where number of female participants is displayed. χ2 and two-tailed t tests were used to obtain the p-values. Difference defined as value at follow-up minus value at baseline. All tests were corrected using a Bonferroni correction for 21 comparisons (bolded represents significant when corrected, while * represents significant when uncorrected). An average negative AVF value at baseline indicates that the average individual of the replication sample had slightly higher white matter hyperintensities and ventricular volume when compared to the distribution of the original sample. An average positive NDF value at baseline in turn means that individuals of the replication sample had slightly higher hippocampal volume and cortical thickness when compared to the distribution of the original sample. The scale is largely arbitrary and is generally useful to compare across individuals. (MMSE: Mini-Mental State Exam; ADAS-Cog 13: Alzheimer’s Disease Assessment Scale; CDR-SB: Clinical Dementia Rating – Sum of Boxes; MCI: mild cognitive impairment; AVF: age- and vascular-related factor; NDF: neurodegenerative factor)
3.4 Associations between MMSE decline and progression of factors scores
Both the progression of AVF and NDF were significantly related to the decline in MMSE over two years independently of each other in our general linear model (Table 2). These independent relationships also held true when MMSE was replaced by other clinical tests such as the Alzheimer’s Disease Assessment Scale (ADAS-Cog 13-item scale) and the Clinical Dementia Rating – Sum of Boxes (CDR-SB). In addition to the progression of factors scores, NDF and MMSE at baseline each significantly and independently predicted decline in MMSE over two years. Based on our general linear model, we estimated that the progression of AVF was linked to an average loss of 2.25 MMSE units over two years while the progression of NDF was linked to an average loss of 0.64 MMSE units over two years in individuals with MCI who converted to AD.
Table 2.
Model of the longitudinal decline in MMSE and other clinical tests using both sets of factors in participants with MCI
| Parameters | Diff. MMSE (β p-value) | Diff. ADAS-Cog 13 (β p-value) | Diff. CDR-SB (β p-value) |
|---|---|---|---|
| Age (baseline) | 0.15; 0.1728 | 0.03; 0.7905 | −0.17; 0.0909 |
| Time between scans | 0.00; 0.9753 | −0.02; 0.8235 | −0.00; 0.9920 |
| Sex (female) | −0.10; 0.2353 | −0.08; 0.3238 | −0.04; 0.6238 |
| Education | 0.14; 0.1105 | −0.10; 0.2001 | 0.00; 0.9904 |
| APOE ε4 (1 allele) | −0.08; 0.5334 | 0.12; 0.3677 | −0.06; 0.5992 |
| APOE ε4 (2 alleles) | −0.08; 0.6726 | 0.09; 0.6171 | 0.32; 0.0715 |
| AVF (baseline) | 0.12; 0.3324 | 0.12; 0.2749 | −0.09; 0.4031 |
| AVF (difference) | ***0.58; 0.0001 | ***−0.56; 0.0001 | ***−0.48; 0.0001 |
| NDF (baseline) | *0.21; 0.0259 | 0.05; 0.5403 | −0.05; 0.5897 |
| NDF (difference) | *0.22; 0.0186 | ***−0.41; 0.0001 | ***−0.36; 0.0001 |
| Cognition (baseline) | **−0.26; 0.0026 | **−0.21; 0.0064 | −0.13; 0.0890 |
All continuous variables were standardized prior to applying the model for easier comparison of parameter estimates (β). Significant associations with p < 0.05 are bolded (*, ** and *** for p < 0.05, 0.01 and 0.001 respectively). Cognition at baseline represents the score on the test being modeled at baseline. Difference defined as value at follow-up minus value at baseline. (MMSE: Mini-Mental State Exam; ADAS-Cog 13: Alzheimer’s Disease Assessment Scale; CDR-SB: Clinical Dementia Rating – Sum of Boxes; AVF: age- and vascular-related factor; NDF: neurodegenerative factor).
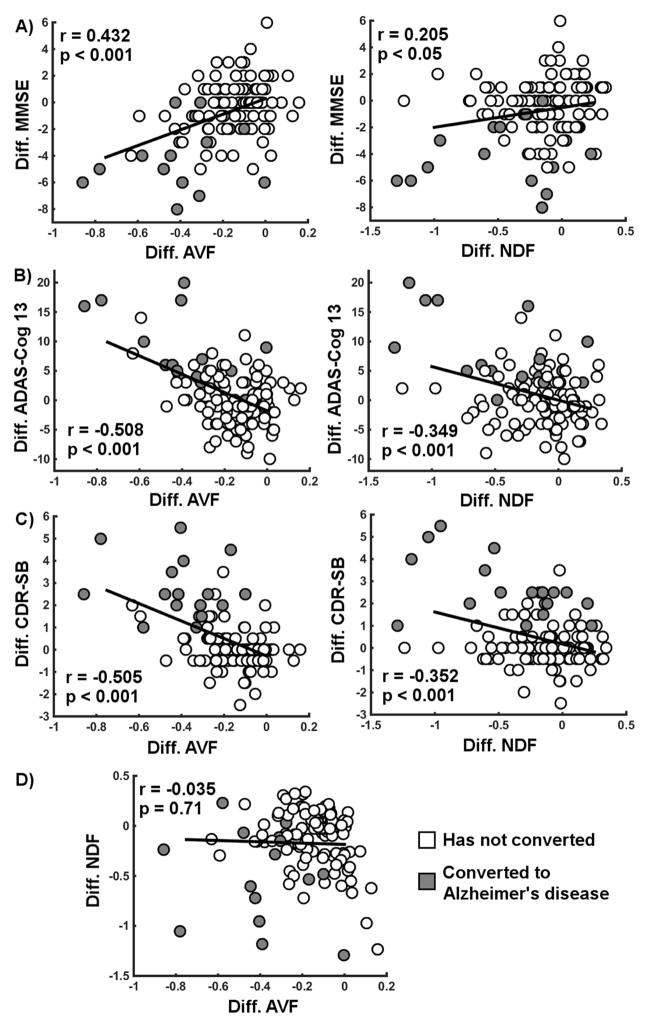
Scatterplots of the relationships between progression of factor scores and decline in MMSE score are shown without any covariates (Figure 2A). Similar scatterplots are presented for ADAS-Cog 13 (Figure 2B) and CDR-SB (Figure 2C). The lack of relationship between the progression of AVF and the progression of NDF is also shown (Figure 2D). These findings highlight the unique statistical properties of each factor relative to progression of impairment.
Figure 2.
Longitudinal difference in clinical scales over two years related to the change in factor scores. Clinical scales included the A) Mini-Mental State Examination (MMSE), the B) Alzheimer’s Disease Assessment Scale (ADAS-Cog 13-item scale) and the C) Clinical Dementia Rating – Sum of Boxes (CDR-SB). D) The correlation between the difference in ‘age- and vascular-related’ factor (AVF) and the difference in ‘neurodegenerative’ factor (NDF) is also shown. Pearson’s correlation coefficients and associated p-values are shown. Individuals with MCI who converted to AD during the two-year follow-up are shown in gray while those who did not convert are shown in white. Difference defined as value at follow-up minus value at baseline. All significant relationships remain significant when correcting for all covariates as detailed in the models, but uncorrected data is presented here to further support the hypothesis that longitudinal cognitive decline is related to a change in the factor scores.
3.5 Determinants of the progression of factor scores
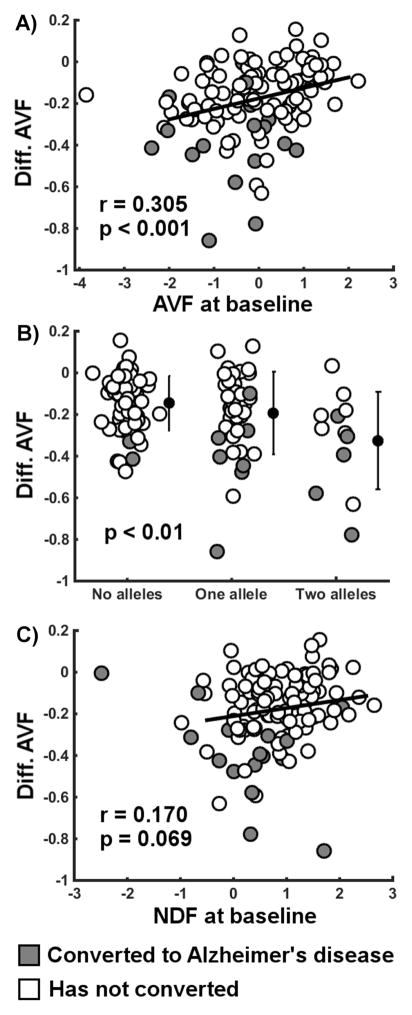
Having two APOE ε4 alleles and lower score at baseline for AVF were both strongly associated with a greater longitudinal reduction in AVF (Table 3). Lower score at baseline for NDF was also related to greater longitudinal reduction in AVF, though to a much lower extent. Scatterplots of these relationships are presented without any covariates (Figure 3). Those bivariate relationships were significant, except for the relationship between score at baseline for NDF and progression of AVF. The scatterplot also highlights that converters have a disproportionate progression of AVF compared to what is expected from their factor score at baseline. No variables used in the models predicted the progression of NDF.
Table 3.
Model of the longitudinal change in factor scores in participants with MCI
| Parameters | Diff. AVF (β p-value) | Diff. NDF (β p-value) |
|---|---|---|
| Age (baseline) | 0.08; 0.4055 | 0.17; 0.1456 |
| Time between scans | −0.07; 0.3736 | −0.00; 0.9860 |
| Sex (female) | −0.12; 0.1275 | −0.09; 0.3143 |
| Education | −0.12; 0.1078 | −0.14; 0.1061 |
| APOE ε4 (1 allele) | 0.18; 0.1384 | −0.00; 0.9990 |
| APOE ε4 (2 alleles) | **−0.59; 0.0005 | −0.13; 0.4880 |
| AVF (baseline) | ***0.41; 0.0001 | 0.18; 0.1229 |
| NDF (baseline) | *0.18; 0.0240 | 0.08; 0.3631 |
All continuous variables were standardized prior to applying the model for easier comparison of parameter estimates (β). Significant associations with p < 0.05 are bolded (*, ** and *** for p < 0.05, 0.01 and 0.001 respectively). Difference defined as value at follow-up minus value at baseline. (AVF: age- and vascular-related factor; NDF: neurodegenerative factor).
Figure 3.
Longitudinal difference in ‘age- and vascular-related’ factor (AVF) over two years related to its determinants. Determinants included the A) AVF factor score at baseline, B) number of APOE ε4 alleles and C) ‘neurodegenerative factor’ factor (NDF) score at baseline. Pearson’s correlation coefficients (r) and associated p-values are shown. The ANOVA p-value is also shown for the relationship with the number of APOE ε4 alleles. Individuals with MCI who converted to AD during the two-year follow-up are shown in gray while those who did not convert are shown in white. Difference defined as value at follow-up minus value at baseline. Covariates were not included to show the uncorrected data in addition to the models accounting for covariates.
4 Discussion
The current work demonstrates that two statistically distinct classes of degenerative change indexed by structural MRI are important independent predictors of longitudinal cognitive decline in individuals with mild cognitive impairment (MCI). To demonstrate this, we first replicated the factor analysis we recently published in a distinct dataset (Coutu et al., 2016), showing two distinct classes of degenerative changes both involving hippocampal changes: one class interpreted as representing ‘age- and vascular-related’ processes involving white matter microstructure, white matter lesions and ventricular changes, and one class representing ‘neurodegenerative’ cortical changes. The current work supported part of this previous interpretation by showing that only the ‘neurodegenerative’ factor correlated specifically with both CSF t-tau and p-tau181, representative markers of neuronal injury (Jack Jr, 2012; Jack Jr et al., 2010), though both classes were related independently to CSF Aβ1–42. Progression of the ‘age- and vascular-related’ factor and of the ‘neurodegenerative’ factor were independently related to longitudinal cognitive decline as measured with the Mini-Mental State Examination (MMSE) and other clinical scales after the course of two years. However, the ‘age- and vascular-related’ factor was associated with a greater attributable cognitive loss than the ‘neurodegenerative’ factor in individuals who converted to AD. This suggests that preventing decline in white matter, ventricular and vasculodegenerative processes may slow cognitive decline to a degree that is at least equivalent to treating the neurodegenerative aspect of the disease, even in individuals known to have little to no obvious vascular comorbidity. Future studies will investigate the potential delay in time-to-onset of dementia that could be possible by treating these processes as well as the determinants of progression in both factors to help devise a therapeutic strategy.
While longitudinal progression of the ‘age- and vascular-related’ factor score was related to a decline in MMSE, the factor score at baseline did not predict greater cognitive decline. This indicates that a longitudinal change in these factors impacts cognition, such as sporadic or continuous vascular deficits leading to increased white matter lesion volume and more generally white matter damage, whereas the baseline level is not as important in determining future cognitive decline. In contrast, both the ‘neurodegenerative’ factor at baseline and its longitudinal progression predicted greater cognitive decline, suggesting accelerating decline. In addition, decline in MMSE was further predicted by a lower MMSE at baseline, and this demonstrated the importance of including this variable in the model, as individuals who are closer to conversion to AD tend to decline faster, mitigating the limitation of our linear model. Indeed, we did not find the same predictor effect of both ‘neurodegenerative’ factor and cognition at baseline on change in ADAS-Cog and CDR-SB, which suggests those clinical scales are more accurately descriptive of a linear change in cognitive impairment than MMSE.
The progression of the ‘age- and vascular-related’ factor was predicted by having two APOE ε4 alleles and by a lower factor score at baseline. Of note, converters deviated from the latter relationship and had disproportionately greater progression of the ‘age- and vascular-related’ factor than expected from their baseline factor score, suggesting they may have been subjected to greater white matter damage and vascular burden than is normally seen with age. These associations are consistent with the notion that risk for future vascular insults is partly determined by a history of stroke and cardiovascular disease (Burn et al., 1994; Wolf et al., 1991) and that individuals with greater white matter lesion volume show a more rapid lesion progression over time compared to individuals with lower baseline volumes (Burton et al., 2006; Gouw et al., 2008). The presence of APOE ε4 alleles has also been linked to recurrence of ischemic cerebrovascular disease, which supports our interpretation (Kim et al., 2003). Furthermore, prior studies observed that APOE may modulate the effects of vascular conditions on white matter lesions (de Leeuw et al., 2004), especially in carriers of two APOE ε4 alleles (Godin et al., 2009), but also modulate effects on cognitive decline and dementia trajectories in middle-aged and older cohorts (Carmelli et al., 1998; Haan et al., 1999; Hofman et al., 1997). These findings support the notion that APOE may enhance risk for AD through yet unclear cerebrovascular mechanisms (Yip et al., 2005). Future work will aim to further include and understand the contribution of other markers of small-vessel disease, such as cerebral microbleeds. However, such vascular insults are generally known to be highly correlated with white matter lesion burden, even in AD (Pettersen et al., 2008).
It was posited in our previous study (Coutu et al., 2016) that the factor analysis may have partitioned the contribution of two distinct pathologies affecting the hippocampus. The current study shows further credence to this theory, as the changes over time in each factor score were uncorrelated, despite being both related to the change in hippocampal volume. While the absence of correlation between factors in the cross-sectional datasets is a direct result of the factor analysis, it was not necessarily expected that the progression of factors over time would be independent. This suggests the presence of two statistically distinct processes that independently affect both the hippocampal volume and MMSE. Evidence for these two distinct processes exists in the literature. On one hand, hippocampal volume as a marker of AD neurodegenerative pathology is well-established (Atiya et al., 2003), and this is accounted for by the ‘neurodegenerative’ factor with its correlation to CSF levels of t-tau and p-tau181 representative of neuronal loss and injury (Jack Jr, 2012; Jack Jr et al., 2010). On the other hand, it is also known that hippocampal volume is reduced in vascular dementia to a similar extent as in AD (Du et al., 2002; Fein et al., 2000; Laakso et al., 1996) and that untreated hypertension may lead to a greater reduction in hippocampal volume in non-demented older adults (den Heijer et al., 2005), and this independent effect is well-represented by the ‘age- and vascular-related’ factor strongly associated with white matter damage and lesion burden. These notions suggest that the combination of those two processes might lead to a faster clinical manifestation of the disease, which may or may not be made evident through the observation of white matter damage or other imaging markers. A recent study also showed that both white matter lesions and amyloid burden independently and additively contribute to longitudinal cognitive decline in older adults (Vemuri et al., 2015). However, in this study, both classes of degenerative change were independently related to levels of CSF Aβ1–42. While this remains to be further investigated, our results are not necessarily at odds with this previous study, as we found that there is part of the variance of CSF Aβ1–42 that is associated with the ‘neurodegenerative’ factor independently from the ‘age- and vascular-related’ factor associated with white matter lesions. Furthermore, ischemia has previously been showed to promote Aβ1–42 accumulation through both increased production and reduced clearance (Iadecola, 2010), which may explain the relationship between the ‘age- and vascular-related’ factor and CSF Aβ1–42. The correlational analyses alone cannot provide conclusive mechanistic insight and more work is necessary to determine the pathologic bases of the imaging factors described. Regardless, the recognition and demonstration of a disease pathway involving white matter and vascular pathology that is distinct from neurodegenerative AD pathology but affect critically-involved structures in AD such as the hippocampus would help further the current efforts to prevent and treat AD. Indeed, comprehensive treatment of vascular risk factors reduced the risk of developing AD in an MCI population, compared to treatment of only some vascular risk factors (Li et al., 2011), and led to slower progression of white matter lesions in individuals with AD (Richard et al., 2010). Therefore, while the ‘age- and vascular-related’ process seems to be unrelated to neurodegenerative changes such as cortical atrophy and increased CSF t-tau and p-tau181, it remains clinically important as it may lead to further progression towards dementia as assessed with clinical outcomes. Further research also remains to be done to investigate synergistic effects of both cerebrovascular disease and AD neurodegenerative processes, which are observed together in about 30 to 45% of older adults with dementia and especially in the oldest old (Jellinger and Attems, 2010; Kalaria and Ballard, 1999; Kawas et al., 2015), though to a lesser extent in the sample studied here due to exclusion of vascular comorbidities in ADNI. The potential for synergy of these common co-existing pathologies has previously been detailed (Attems and Jellinger, 2014; Iadecola, 2010), and there is previous recognition that vascular risk factors are associated with faster cognitive decline in incident AD (Helzner et al., 2009). The framework built in this study differs from previous studies as it does not classify individuals as either suffering from age- and vascular-associated processes or neurodegenerative processes, but instead provides the basis to rate each process independently on a continuum. Such a framework is expected to be useful to evaluate synergistic effects in patient populations suffering from multiple pathologies.
The current work has limitations. First, the white matter lesions were segmented from T1- weighted images instead of T2-weighted images with fluid-attenuated inversion-recovery (FLAIR). Despite the high correlation and concordance of those two measures, it was found that the factor loading of white matter lesions in the ‘age- and vascular-related’ factor was lower when using FLAIR-based estimates. This suggests potentially greater sensitivity of T1-based white matter changes to this process, and differences in the two methods should be investigated in future studies. It may also suggest that the ‘age- and vascular-related’ factor may be more strongly related to white matter atrophy and ventricular expansion than vascular processes. However, it is important to note that the ADNI has focused on recruiting highly selected cases of AD and potentially preclinical AD, excluding individuals with vascular comorbidities. Indeed, it has recently been shown that participants in the ADNI have lower white matter lesion burden than other similar studies (Ramirez et al., 2015). Despite this, we still found a distinct process related to white matter lesions replicated in two separate ADNI datasets and this process had an impact on cognitive decline greater than the neurodegenerative aspect of the disease. Replication in a more ecological sample with greater vascular co-morbidities on par to what is observed in general population would be beneficial. For instance, greater co-existence and overlap of longitudinal trajectories of both classes of degenerative changes may be observed in such as sample. Another limitation is that the measurement properties may influence the factor scores (e.g. volume measurements may cluster together), which would change the interpretation. However, hippocampal volume was important in both factors demonstrating that it is possible to have different types of measurements within one factor. The factor analysis also did not account for potential covariation found naturally in younger adults between the five imaging measures investigated and accounting for this premorbid state is a goal of future study. Finally, while a greater cognitive loss was attributed to the progression of the ‘age- and vascular-related’ factor than to the progression of the ‘neurodegenerative’ factor, it is possible that this is only true in the MCI state that is bordering on conversion. Furthermore, the general linear model used here to make that assessment has several assumptions, one of which being the assumption of a linear decline, which is also a limitation in representing actual clinical progression. Future work will focus on using a larger dataset from ADNI with longer longitudinal follow-up and a greater number of converters to allow more in-depth analysis using proportional hazards models. Despite these limitations, the current study shows the importance of considering vascular and white matter pathologies in understanding cognitive decline in individuals on the path towards a clinical diagnosis of AD.
Supplementary Material
Two distinct factors derived from structural MRI were linked to progression in MCI
A factor interpreted as ‘neurodegenerative’ related to AD-specific cortical atrophy
The other ‘age- and vascular-related’ factor related to white matter lesions
This other factor more strongly related to cognitive decline over two years in MCI
This was true despite selection against vascular comorbidities in the ADNI cohort
Acknowledgments
Funding: This study was supported by the National Institutes of Health grants R01NR010827, P41RR14075, S10RR021110, S10RR023401, S10RR019307, S10RR019254 and S10RR023043.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (NIH Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
AD: Alzheimer’s disease; MCI: mild cognitive impairment; MMSE: Mini-Mental State Examination; ADAS-Cog:Alzheimer’s Disease Assessment Scale – Cognitive; CDR-SB: Clinical Dementia Ration – Sum of Boxes; AVF: ‘age-and vascular-related’ factor; NDF: ‘neurodegenerative’ factor; ADNI: Alzheimer’s Disease Neuroimaging Initiative
Disclosure: The authors have no actual or potential conflicts of interest relevant to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atiya M, Hyman BT, Albert MS, Killiany R. Structural magnetic resonance imaging in established and prodromal Alzheimer disease: a review. Alzheimer Dis Assoc Disord. 2003;17:177–95. doi: 10.1097/00002093-200307000-00010. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease – lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–55. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire Community Stroke Project Stroke. 1994;25:333–337. doi: 10.1161/01.STR.25.2.333. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O’Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am J Geriatr Psychiatry. 2006;14:842–849. doi: 10.1097/01.JGP.0000236596.56982.1c. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Miller B, Wolf PA, Jarvik GP, Schellenberg GD. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology. 1998;50:1580–1585. doi: 10.1212/WNL.50.6.1580. [DOI] [PubMed] [Google Scholar]
- Coutu JP, Goldblatt A, Rosas HD, Salat DH. White Matter Changes are Associated with Ventricular Expansion in Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. J Alzheimers Dis. 2016;49:329–342. doi: 10.3233/JAD-150306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FEE, Richard F, de Groot JC, van Duijn CM, Hofman A, Van Gijn J, Breteler MBMB. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke. 2004;35:1057–60. doi: 10.1161/01.STR.0000125859.71051.83. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, Koudstaal PJ, Breteler MMB. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64:263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling Ra, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling Ra, Killiany RJ, Albert MS, Hyman BT, Blacker D, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Laakso MP, Zhu XP, Jagust WJ, Yaffe K, Kramer JH, Miller BL, Reed BR, Norman D, Chui HC, Weiner MW. Effects of subcortical ischemic vascular dementia and AD on entorhinal cortex and hippocampus. Neurology. 2002;58:1635–1641. doi: 10.1212/WNL.58.11.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/WNL.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Godin O, Tzourio C, Maillard P, Alpérovitch A, Mazoyer B, Dufouil C. Apolipoprotein E genotype is related to progression of white matter lesion load. Stroke. 2009;40:3186–90. doi: 10.1161/STROKEAHA.109.555839. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Coresh J, Catellier DJ, Sharrett aR, Rose KM, Coker LH, Shibata DK, Knopman DS, Jack CR, Jr, Mosley TH. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis risk in communities (ARIC) study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, Van Der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJG. Heterogeneity of small vessel disease3: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Van Der Flier WM, Fazekas F, Van Straaten ECW, Pantoni L, Poggesi A, Inzitari D, Erkinjuntti T, Wahlund LO, Waldemar G, Schmidt R, Scheltens P, Barkhof F. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: The leukoaraiosis and disability study. Stroke. 2008;39:1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio Ta, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–6. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–8. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman a, Ott a, Breteler MM, Bots ML, Slooter aJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–4. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR., Jr Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012;263:344–61. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, Whitwell LJ, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DLG, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ, Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–61. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119:421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13:S115–23. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old. Neurology. 2015;85:535–542. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Han SR, Chung SW, Kim BS, Lee KS, Kim YI, Yang DW, Kim KS, Kim JW. The apolipoprotein E epsilon4 haplotype is an important predictor for recurrence in ischemic cerebrovascular disease. J Neurol Sci. 2003;206:31–7. doi: 10.1016/s0022-510x(02)00361-1. [DOI] [PubMed] [Google Scholar]
- Laakso M, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, Hanninen T, Vainio P, Soininen H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia. Neurology. 1996;46:678–81. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Zhang M, Xu Z, Gao C, Fang C, Yan J, Zhou H. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–91. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, Sano M, Bieliauskas L, Geldmacher D, Clark C, Thal LJ. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study Alzheimer Dis Assoc Disord. 1997 9236948. [PubMed] [Google Scholar]
- Morris The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Pantoni L. Cerebral small vessel disease3: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- Pettersen JA, Sathiyamoorthy G, Gao FQ, Szilagyi G, Nadkarni NK, St George-Hyslop P, Rogaeva E, Black SE. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–795. doi: 10.1001/archneur.65.6.790. [DOI] [PubMed] [Google Scholar]
- Ramirez J, McNeely AA, Scott CJM, Masellis M, Black SE. White matter hyperintensity burden in elderly cohort studies. The Sunnybrook Dementia Study, Alzheimer’s Disease Neuroimaging Initiative, and Three-City Study. Alzheimer’s Dement. 2015;12:203–210. doi: 10.1016/j.jalz.2015.06.1886. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–18. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E, Gouw AA, Scheltens P, van Gool WA. Vascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the evaluation of vascular care in Alzheimer’s disease (EVA) study. Stroke. 2010;41:554–6. doi: 10.1161/STROKEAHA.109.571281. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Terry RD, Fuld Pa, Katzman R, Peck a. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Rostrup E, Gouw AA, Vrenken H, van Straaten ECW, Ropele S, Pantoni L, Inzitari D, Barkhof F, Waldemar G. The spatial distribution of age-related white matter changes as a function of vascular risk factors--results from the LADIS study. Neuroimage. 2012;60:1597–607. doi: 10.1016/j.neuroimage.2012.01.106. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, Raman MR, Machulda MM, Mielke MM, Lowe VJ, Senjem ML, Gunter JL, Rocca WA, Roberts RO, Petersen RC, Jack CR. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015;138:761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Van Oostenbrugge R, Pantoni L, Speck O, Stephan BCM, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.STR.22.3.312. [DOI] [PubMed] [Google Scholar]
- Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–65. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.