Abstract
Background
Injectable naltrexone for alcohol use disorders (AUDs) has been efficacious in several studies. It has not been (1) compared head-to-head with oral naltrexone, or (2) examined in the hospital setting as an intervention that might facilitate treatment attendance after hospital discharge.
Methods
Fifty-four hospitalized veterans identified as having DSM-IV alcohol dependence were randomized to receive: (1) a 50 mg oral naltrexone plus a 30-day prescription, or (2) a 380 mg intramuscular naltrexone injection prior to discharge. Of 113 veteran inpatients deemed eligible based on screening criteria, 54 met final eligibility criteria and were enrolled and randomized. Baseline data included demographics, alcohol consumption and co-morbidity. Measures of treatment initiation and engagement and alcohol consumption were reassessed at 14-day and 45-day follow ups.
Results
Thirty-five participants (64.8%) completed the entire study protocol (received a study medication and completed 14- and 45-day follow ups). Among those who received a study medication (n=45), 77.8% completed all follow-up interviews. This pilot study was not designed to have sufficient statistical power for hypothesis testing, and thus, as expected, there were no significant differences between groups in medication adherence (self-report of > 80% of daily doses taken in oral group; receipt of second injection in the injection group), treatment engagement (at least treatment 3 visits in the 30 days post-discharge, and 2 or more visits per month in each of the 3 months following discharge) or alcohol consumption at 14 or at 45 days (p>0.05). The median number of drinks among the entire cohort in the two weeks prior to hospitalization (128 drinks) was significantly higher than at Day 14 (0 drinks, p<0.001) or Day 45 (0 drinks, p<0.001). Rates of medication adherence were 62% in the oral group and 61% in the injection group
Conclusions
Results indicate feasibility for larger, more definitive study. Both groups had significant reductions in alcohol consumption over time and high treatment engagement rates. Both oral and injectable formulations are feasible to initiate prior to discharge for hospital inpatients identified as having an AUD.
Keywords: alcohol dependence, alcohol use disorder, naltrexone, hospital, treatment engagement
Introduction
Alcohol use disorder (AUD) is a highly prevalent and crucial health issue in the U.S. In 2013, 14.7 million people met criteria for alcohol dependence or abuse, but only 10% received needed treatment (Substance Abuse and Mental Health Services Administration 2014). Rates of alcohol use disorders (AUDs) among veterans (particularly among combat-exposed veterans) exceeded those in the general population, and fewer than 4% of veterans receiving care in the Department of Veterans Healthcare System with high alcohol consumption initiate specialized addiction treatments (Glass et al. 2010). Factors more common among veterans, such as traumatic brain injury and post-traumatic stress disorder, may interfere with their participation in treatment (Bernhardt 2009). Further, alcohol and other substance use disorders have been found to be the most stigmatized of the psychiatric conditions, and stigma has been associated with poorer adherence to pharmacological treatment (DiIorio et al. 2003; Livingston and Boyd 2010).
High levels of alcohol consumption and AUD cause myriad disorders involving the cardiovascular, gastrointestinal, hepatic, neurologic, renal, and endocrine systems (Rehm et al. 2010; Ries et al. 2014). Patients with AUDs, including veterans, are therefore more frequently hospitalized than the general population. The prevalence of AUDs among hospitalized patients has been estimated to be 4 to 5 times that of the general adult, non-hospitalized population (Bostwick and Seaman 2004; Roche et al., 2006).
Even when an AUD precipitates or complicates a hospitalization, recommended treatment is rarely accessed post-discharge. While brief advice to cut down on alcohol use has been successful in facilitating behavior change in outpatient settings, such strategies have been ineffective in the inpatient setting, likely due to the increased severity of AUDs among hospital inpatients vs. the general population (Saitz et al., 2007; Saitz, 2010). The transition from hospital or other institutional settings to community is a critical phase for individuals with AUDs, and methods to foster treatment initiation before discharge and engagement after discharge are needed. In VA health care facilities in 2013, rates of follow-up addiction treatment within 60 days of receiving detoxification for alcohol or opioids in inpatient or outpatient settings were 50% nationally (range 13–77%) (Timko et al. 2016).
Pharmacotherapy to curb alcohol use is one intervention, which might be initiated prior to a hospital discharge to facilitate reduction in alcohol consumption and, subsequently, improve treatment engagement. The FDA-approved pharmacotherapy naltrexone is available in two forms (oral and injectable formulations) to reduce craving for alcohol and assist patients in their recovery from AUDs. Non-adherence to daily at-home dosing has been a barrier to sustained recovery when oral preparations are used (Krystal et al., 2001; Pettinati et al., 2006; Volpicelli et al., 1997). Long-acting, monthly injectable naltrexone may overcome the challenge of adhering to a daily oral medication. If medication adherence is major barrier to reducing alcohol use after hospital discharge, reducing long-acting naltrexone injection prior to discharge from a controlled environment (i.e. hospital or residential facility) might more effectively reduce alcohol use after discharge, and facilitate meaningful participation in behavioral treatment. Injectable naltrexone in addition to behavioral treatment has been efficacious in several studies (Ciraulo et al., 2008; Comer et al., 2006; Garbutt et al., 2005). However, it has yet to be examined (1) in head-to-head comparison with daily oral naltrexone, or (2) in the inpatient hospital setting as an intervention that might facilitate behavioral treatment attendance after hospital discharge. To begin to address this knowledge gap, this study sought to pilot methodology which could evaluate the effectiveness of oral naltrexone (taken orally once daily at home) vs. long-acting injectable naltrexone (30-day duration of action) initiated prior to discharge from an inpatient hospitalization.
As a pilot proof-of-concept study, the work primarily sought to determine the feasibility of subject recruitment and of protocol completion by a substantial majority (> 70%) of enrolled participants. Protocol completion was defined as participants completing all 3 data collection visits: baseline, day 14, and day 45. We also sought to calculate variance estimates to use in sample size estimation for future clinical study.
Materials and Methods
The Health Sciences Institutional Review Board (IRB) of the University of Wisconsin and the Research and Development Committee of the William S. Middleton Memorial Veterans Hospital approved study methods. Methodology is in accordance with the Helsinki Declaration as revised in 2004.
Study design and participants
Veterans hospitalized for an acute medical or psychiatric (i.e. not necessarily for purposes of medically managed detoxification) illness at the William S. Middleton Memorial Hospital and for whom alcohol withdrawal symptom monitoring was ordered by the admitting physician were recruited. To identify potentially eligible hospitalized veterans, study team members with appropriate clinical privileges and access (Drs. Brown and Busch) received electronic notifications via the VA electronic health record (EHR) when symptom monitoring for alcohol withdrawal was ordered at the time of hospital admission. This identification was followed by a chart review to screen patients for basic eligibility criteria. A study coordinator approached patients who were at least 18 years old who had a clinically established diagnosis of alcohol dependence based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (APA 2000). The diagnosis was established based on clinical interview by an addiction counselor or addiction medicine physician, a process which is standard policy for any veteran for whom alcohol withdrawal monitoring is ordered at the study hospital and is an interaction documented in the electronic health record. Individuals were excluded if they had past-year opioid dependence, daily use of opioid analgesics, acute hepatitis or liver failure, suicidality, or if they were unable to provide written informed consent. Women were excluded if they were pregnant or currently breastfeeding. Before randomization, patients received a brief questionnaire, a liver function test, and a urine pregnancy test to verify eligibility. Pregnancy tests were repeated one month into the study. Baseline measurements were collected in the hospital prior to discharge. Telephone follow-up interviews occurred 14 and 45 days post-discharge. Medical records review was undertaken to ascertain attendance to treatment visits over the 90 days subsequent to the index hospitalization. Participants were compensated $50 for completing the baseline interview and $50 for the day 45 interview for a maximum total compensation of $100 per participant. Study procedures were recorded in an electronic subject tracking database maintained in REDCap, a secure, web-based application designed for research (Harris et al. 2009).
Interventions
The study coordinator, in the process of informed consent and enrollment, discussed the potential risks and benefits of naltrexone. Pertinent material to that end was included in the IRB-approved study consent document to be reviewed with potential participants. This risk-benefit material was guided by package inserts and the Micromedex Drug Reference (Micromedex). Eligible and consenting veterans were randomized 1:1 to receive either: (1) a 50 mg oral naltrexone plus a 30-day prescription, or (2) a 380 mg intramuscular naltrexone injection prior to discharge. Participants randomized to the injectable naltrexone group were administered a single 380 mg intramuscular injection of naltrexone prior to discharge, which had a 30-day duration of action. Participants were scheduled for a return MD clinic visit one month later to receive a second injection. Those residing outside of the hospital’s metropolitan area who received their primary care at a community-based VA clinic had the option to receive their second injection at that clinic. Individuals randomized to the oral naltrexone group received an initial 50 mg oral dose of naltrexone prior to hospital discharge plus a 30-day prescription for oral naltrexone, as well as a 15-day refill to be used after day 30. Refills in the oral condition could be received by mail or picked up in person at their preferred VA pharmacy.
Standard hospital-based care for all hospitalized veterans monitored for withdrawal included assessment and motivational enhancement by an addiction counselor to encourage engagement in addiction treatment. National VA performance measures monitor outpatient addiction treatment follow-up within 2 weeks of discharge for all patients diagnosed with AUD.
Randomization
The 1:1 randomization sequence was computer-generated. Sequentially numbered opaque envelopes were used to conceal randomization, which were opened by the interviewer following consent and the baseline interview. The randomization slip and consent form were immediately delivered to the pharmacy, which entered an order for the appropriate medication into the EHR for the PI to sign.
Outcomes and measurements
As a pilot study, the primary goal was to demonstrate a feasible study design. We recorded sufficient information to estimate rates of recruitment, randomization, intervention delivery, medication adherence, adverse events, and interview completion. We calculated enrollment per month (we sought to enroll approximately 6 participants per month in order to complete the study within 12 months) and rate of protocol completion. Protocol completion was defined as receiving the appropriate study medication after randomization and participating in all three phases of data collection (baseline, day 14, and day 45). Because this was a pilot study, we did not continue to interview patients who did not receive a study medication. Thus, we calculated protocol completion in two ways: 1) among all patients who were randomized; and 2) among those who were randomized and received a study medication (and thus, were contacted by the study team to complete the day 14 and day 45 interview). We also sought to calculate variance estimates to use in sample size estimation for the proposed primary and secondary outcome measures of a future clinical trial.
To assess medication adherence, participants assigned to the oral medication group were asked to self-report the number of missed doses over the previous 14 days at each follow-up. Adherence was operationalized as taking ≥ 80% of daily oral naltrexone doses. In the injectable naltrexone condition, medication adherence was defined as receiving the second naltrexone injection. We asked veterans how the medication was affecting them using open-ended questions. We monitored and recorded adverse study events in a web-based application managed by the University of Wisconsin’s Health Sciences IRB.
The primary outcome planned for the future clinical trial was the receipt of post-discharge addiction treatment from substance use disorder specialists. For analytic purposes, treatment initiation was defined as attendance to at least 1 specialist treatment visit in the 2 weeks after hospital discharge. Treatment engagement was examined in 2 ways: at least 3 visits in the 30 days post-discharge, and 2 or more visits per month in each of the 3 months following discharge. Treatment was ascertained via a chart review of medical records beginning on the date each individual participant was discharged from the inpatient hospital setting and ending 90 days thereafter. Note titles and visit locations were abstracted from the EHR and used to identify visits associated with treatment from substance use disorder specialists. The research team, which included a staff physician and psychologist at the study site, consulted with a clinical applications coordinator to determine appropriate data elements for the chart review. The chart review captured visits occurring at any VA medical center or community-based VA clinic.
Secondary outcomes included post-discharge alcohol consumption and hospital readmissions. Alcohol consumption for the prior 14-day period was queried at each interview via the Timeline Follow-back Method (TLFB) (Sobell and Sobell, 1992). We calculated the presence of heavy drinking days (yes/no; > 4 standard drinks on a day in the last 14), number of heavy drinking days (range 0–14), and the total number of drinks in the past 14 days. At baseline, veterans were asked to complete this interview in reference to the 14 days prior to their hospitalization. The chart review captured hospital admissions occurring at any VA hospital locations in psychiatric, general medical, surgical, or intensive care wards in the 90 days after the index hospitalization.
Sociodemographic characteristics were obtained using the Addiction Severity Index-Lite (Cacciola et al., 2007; McLellan et al., 1992). To evaluate the feasibility of data collection for a future trial, in which co-morbid mental health conditions would be used as statistical controls and potential moderating variables, we administered the Generalized Anxiety Disorder Screener (GAD-7) (Löwe et al., 2008) to measure anxiety severity, the Patient Health Questionnaire (PHQ-9) (Manea et al., 2015) to measure depression, and the Mini International Neuropsychiatric Instrument (MINI) (Sheehan et al., 1998) to assess for DSM-IV post-traumatic stress disorder.
Sample size estimation
As above, we sought to obtain a sample of approximately 50 subjects based on available funding and time available for recruitment, expecting an accrual rate of approximately 6 subjects per month. Approximately 25 subjects per group would allow for the calculation of 95% confidence intervals (CI) around the percentage of patients obtaining treatment in each group with an interval width of 40% if treatment utilization was 50% and down to an interval width of 27% if treatment utilization was 90%. In addition, 25 subjects per group would allow us to calculate 95% CIs around means of our secondary outcomes with accuracy of +/− 41.3% of the standard deviation (SD). For example, if the SD = 2, our 95% CI width would be +/− 0.826. As per published recommendations for pilot studies, we did not seek to obtain adequate statistical power to evaluate between-group differences in the study outcomes (Eldridge et al., 2016).
Statistical Analyses
Subject characteristics, follow-up rates, medication adherence, and engagement rates were compared between treatment groups with t-test, Wilcoxon rank sum tests, and Fisher’s exact tests. Total number of drinks and total number heavy drinking days over time were summarized with median (range) at each time point. Comparison of the change in outcome variables from baseline regardless of treatment group was done with paired t-tests. Analysis was based upon modified intention-to-treat principle, wherein veterans not receiving any intervention were not analyzed, because we did not proactively contact these patients for follow-up assessments (Cook and DeMets, 2007). One veteran who was randomized to injectable naltrexone received oral and was analyzed as randomized.
Results
Participant flow
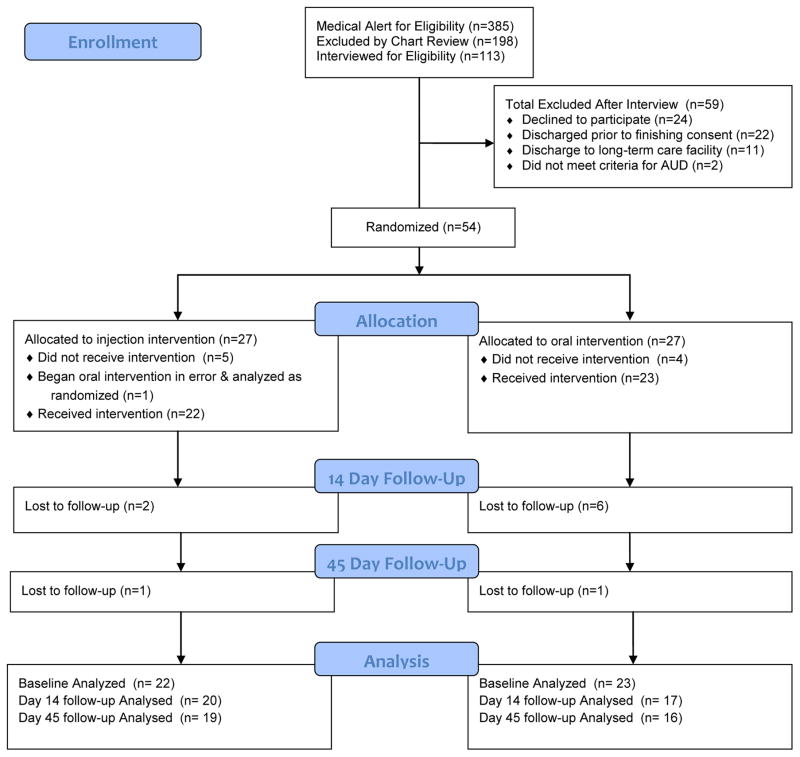
Recruitment and loss to follow up are depicted in the CONSORT diagram in Figure 1. During the study period, there were a total of 385 electronic medical record alerts of hospital admissions who were to be observed for the possible development of alcohol withdrawal symptoms. Of these, 74 veterans were not screened due to discharge prior to being approached or lack of available research staff, and 198 patients were excluded via chart review for applicable exclusions. One hundred thirteen veterans were contacted and interviewed for eligibility. Exclusionary criteria included: plan for discharge to a long-term controlled environment (e.g. skilled nursing facility), current opioid use or use disorder, psychosis, suicidal or homicidal ideation, medical contraindications, already on naltrexone, geographically distant residence, unable to complete consent, or if the patient did not meet alcohol dependence criteria. The most prevalent reasons for participant exclusions included 32% of patients having current medical contraindications (e.g. hepatitis, pancreatitis) and 29% of patients with current opioid use or physical dependence. A total of 59 eligible patients were excluded for the following reasons: twenty-six declined to participate, 20 were discharged prior to finishing consent, 11 were excluded due to plans to discharge to a controlled facility, and 2 did not meet criteria for alcohol dependence based upon baseline clinical interview by an addiction medicine physician or addiction counselor.
Figure 1.
Study recruitment/retention CONSORT diagram
Of the 54 participants enrolled in the study, 27 were randomized to the injectable naltrexone group and 27 were randomized to the oral naltrexone group. The specific hospital wards from which veterans were recruited appear in Table 1. Assignment to study arm did not significantly differ based upon hospital ward location. Of the veterans recruited, 13 (n=7 in injection group, n=6 in oral group) transitioned from the hospital to a brief (7–21 days) stay in a residential treatment unit. Since this constituted a controlled environment, where risk for ongoing alcohol consumption would be negligible, medication administration occurred immediately prior to discharge from this environment, and follow-up measures were timed for 14 and 45 days after discharge from this environment. Twenty-one of 27 participants who were randomized to receive the injection received the intervention; one received oral and was analyzed as randomized (n = 22). Of the 27 randomized to the oral intervention group, 23 received an oral dose at baseline. Follow-up rates for the sample receiving medication (n = 45) were 82% at day 14 and 77% at day 45, with no significant difference in retention between study groups.
Table 1.
Baseline sample characteristics.
| Injection (n=22) | Oral (n=23) | P-value | |
|---|---|---|---|
| Sex, male | 21 (95.5%) | 22 (95.7%) | 1 |
| Age, mean (SD) | 48.3 (12.9) | 50.9 (14.5) | 0.529 |
| Race, white | 20 (90.9%) | 20 (87.0%) | 1 |
| Education, HS grad | 20 (90.9%) | 22 (95.7%) | 1 |
| PHQ-9 score mean (SD) | 13.9 (6.9) | 13.5 (6.9) | 0.834 |
| GAD-7 score mean (SD) | 12.6 (6.7) | 11.4 (6.6) | 0.563 |
| # drinks in last 14 days (range) | 115 (0 – 308) | 137 (0 – 364) | 0.972 |
| Hospital location | 0.676 | ||
| Psychiatry | 11 (50%) | 15 (65.2%) | |
| Medical | 3 (13%) | 2 (8.6%) | |
| Surgical | 1 (4.5%) | 0 (0%) | |
| Residential unit* | 7 (30.4%) | 6 (26.1%) |
13 participants (6 in injection group, 7 in oral group) transitioned from hospital to a residential unit (7–21 day stay). Medication administration occurred at the time of discharge from the residential unit for these participants and 14- and 45-day follow-up assessments were timed based on discharge from the residential unit.
Sample Characteristics
Table 1 contains baseline characteristics for participants in each study group. Mean ages were 51 (SD=15) and 48 (SD=13) for oral and injection groups respectively. Eighty-nine percent of the sample was Caucasian, 96% were male, 93% had at least completed high school, and 87% had an annual income of 50K or less. There were no significant differences between study groups in alcohol consumption, PHQ-9 scores, or GAD-7 scores. Baseline characteristics for the 9 veterans (5 in injection arm, 4 in oral arm) who consented but did not receive study medication were examined and found not to differ significantly by medication assignment, from the overall sample, or from participants who did not complete study follow up.
Outcomes and Estimation of Feasibility
Rates of protocol completion are as follows. Among those who were randomized (n=54), 64.8% (n=35) received a study medication and completed all follow-ups. Among those who received a study medication and were proactively followed (n=45), 77% completed all follow-ups. The accrual time frame for recruitment (n = 54) was from September 5, 2013 to April 20, 2015, for a total accrual time of 19 months, and an overall accrual rate of 2.8 subjects per month on average. Barriers to achieving the goal of 6 participants per month overall included primarily the following: (1) a higher-than-expected rate of ongoing opioid use in the population, which was an exclusion criteria, and (2) transitions in study team membership and need for IRB protocol revision, which led to a 3-month period (July-October 2014) during which we could not enroll patients. The rate of recruitment improved significantly when a researcher with privileges to screen using the electronic medical record and who had a clinical role with the Alcohol and Drug Treatment program (Dr. Busch) took over study eligibility determination, consent and enrollment tasks; recruitment rate improved from 1.9 subjects to 4.4 subjects per month.
Post-discharge specialty addictions treatment engagement and retention are described in Table 2. Rates of initial engagement (at least one visit within 14 days after hospital discharge) were high (86.4% for injection group, 78.3% for oral group) and did not differ significantly between treatment groups. The proportion receiving at least 3 visits in the first month was similar across groups (68.2% for injection group, 65.2% for oral group). The proportion retained in treatment over 3 months (2 visits per month) was 40.9% for injection group and 21.7% for oral group. This difference did not attain statistical significance. The absolute number of visits attended over 90 days also did not differ significantly by treatment group (p = 0.099).
Table 2.
Treatment attendance in injection vs oral group reported as N (%), median (interquartile range), or mean (standard deviation)
| Specialty treatment attendance post-hospitalization | Injection (n = 22) | Oral(n = 23) | Odds Ratio (95%CI) | P-value |
|---|---|---|---|---|
| Days 0 – 14* | ||||
| ≥ 1 visit | 19 (86.4%) | 18 (78.3%) | 1.71 (0.35, 9.98) | 0.699 |
| Total number: median (IQR) | 2.5 (2.0 – 4.0) | 2.0 (1.0 – 3.5) | --- | 0.503 |
| Total number: mean (SD) | 4.7 (9.6) | 3.3 (4.5) | ||
| Days 0 – 30* | ||||
| ≥ 3 visits | 15 (68.2%) | 15 (65.2%) | 1.14 (0.32, 4.12) | 1 |
| Total number: median (IQR) | 4.5 (2.0 – 7.0) | 3.0 (2.0 – 6.5) | --- | 0.444 |
| Total number: mean (SD) | 9.0 (18.5) | 6.7 (9.3) | ||
| Days 0 – 90* | ||||
| ≥ 2 in each month | 9 (40.9%) | 5 (21.7%) | 2.42 (0.66, 9.80) | 0.208 |
| Total number: median (IQR) | 8.5 (6.0 – 19.0) | 5.0 (2.5 – 16.0) | --- | 0.099 |
| Total number: mean (SD) | 22.5 (48.1) | 14.2 (23.3) |
The median number of drinks in the last 14 days (128) prior to hospitalization among the entire cohort was significantly higher than day 14 (0, p<0.001) and day 45 (0, p<0.001). The median number of heavy drinking days in last 14 days prior to baseline among entire cohort (11) was significantly higher than day 14 (0, p<0.001) and day 45 (0, p<0.001). There were no differences at any time point between treatment groups in either outcome (p > 0.05), and alcohol consumption descriptors did not differ between groups at any time point. Group comparisons on consumption at each time point are shown in Table 3. Percentages of participants who had ≥1 hospital readmission by day 45 were similar between treatment groups (p>0.05), and similar among medication adherent (62% of injection group and 61% of oral group met study definition of adherence, p > 0.05) verses non-adherent groups, indicating no significant differences in either outcome. Twenty-six percent of the total sample (n=9) had ≥1 hospital readmission with 17% (n=3) of participants readmitted within the medication adherent group and 43% (n=6) non-adherent participants readmitted by day 45 (p>0.05).
Table 3.
Drinking amount and heavy drinking days in previous 14 days in injection vs oral group reported as median (interquartile range) and mean (standard deviation)
| Baseline | Day 14 | Day 45 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Injection (n=22) | Oral (n=23) | P-value | Injection (n=20) | Oral (n=17) | P-value | Injection (n=19) | Oral (n=16) | P-value | |
| Total Drinks | 114.5 (0 – 308) 140.2 (95.6) |
136.5 (0 – 364) 131.8 (86.0) |
0.972 | 0 (0 – 270) 28.8 (69.9) |
0 (0 – 66) 5.2 (16.3) |
0.398 | 0 (0 – 240) 19.3 (57.6) |
1 (0 – 355) 26.5 (87.9) |
0.324 |
| No drinks last 14 days – yes | 3 (13.6%) | 2 (9.1%) | 1 | 9 (56.2%) | 11 (68.8%) | 0.716 | 13 (72.2%) | 8 (50.0%) | 0.291 |
| Heavy Drinking Days | 10 (0 – 14) 8.9 (4.7) |
12 (0 – 14) 10.4 (4.7) |
0.176 | 0 (0 – 14) 2.2 (4.8) |
0 (0 – 11) 0.7 (2.8) |
0.161 | 0 (0 – 14) 1.2 (3.4) |
0 (0 – 14) 1.3 (3.6) |
0.889 |
| No heavy drinking days – yes | 3 (13.6%) | 3 (13.6%) | 1 | 12 (75.0%) | 15 (93.8%) | 0.333 | 14 (77.8%) | 12 (75.0%) | 1 |
Effect sizes for treatment engagement and drinking outcomes were also calculated. The effect was considered small (Cohen’s D 0.25; 95% CI for Cohen’s D ranging from 0 – 0.80) and did not differ between treatment arms as expected for this small pilot trial. However, the data on both treatment engagement and drinking outcomes was highly right skewed (hence, the use of median as the preferred measure of central tendency for study outcomes), calling into question the precision of this estimate.
Discussion
The results of the current work indicate potential feasibility for initiating a larger randomized clinical trial of alcohol pharmacotherapy for veterans with alcohol use disorder during acute hospitalization. The recruitment rate did not reach the expected goal of 6 patients per month overall. This was primarily driven by high rates of regular opioid use in the target population and by transitions in research team roles. Future larger study would likely require: (1) either a longer period of recruitment and expansion of recruitment sites, (2) plans at the outset for the research team member(s) engaged in recruitment and enrollment to have specific expertise in the assessment of substance use disorder and protocols and practices in local treatment. In addition, it may be necessary to include all patients with alcohol withdrawal risk, regardless of whether or not an addiction counselor assessed patients as having alcohol dependence, and instead relying on a research assessment to identify alcohol use disorder. It is possible that research assessments would be more sensitive in identifying latent disorder in the population. Protocol completion rates among those randomized were modest at 45 days (64.8%), which approached our goal of 70%. Conducting the same level of proactive outreach with all participants even when they do not receive a study medication, would likely allow us to meet this goal (approximately 77% of those who received a study medication, and thus were proactively followed, completed all follow-up interviews).
Given prior literature indicating that many inpatients with AUDs forgo follow-up treatment (Blondell et al., 2011; Saitz et al., 2007), we were surprised that the vast majority of veterans in both study groups saw an addiction treatment specialist in the last 14 days after hospital discharge. In light of these findings, and because inpatients in need of alcohol detoxification are likely to have extensive treatment needs, our findings indicate that the most optimal measure of follow-up care would be a measure based on continued engagement in substance use disorder treatment, rather than the initiation of any treatment. For instance, we found that 40.9% of patients receiving injectable naltrexone versus 21.7% of patients receiving oral naltrexone attended at least two specialty addiction treatment visits per month in the following three months. Keeping patients engaged in treatment is desirable (McKay, 2005; McLellan et al., 2000) and is known to improve addiction-related outcomes (Blodgett et al., 2014; McKay, 2009)
Because the current feasibility study did not include an inactive control group, we are unable to determine the extent these treatment initiation rates could be attributed to receiving oral or injectable naltrexone. While a goal of close to 100% follow-up of inpatients within 14 days is desirable, previous trials have found relatively modest rates of addiction treatment services when patients were identified opportunistically through alcohol screening (Saitz et al., 2007) or through a need for detoxification (Blondell et al., 2011). Several contextual factors may have contributed to the higher rates in the current study. In 2008, the Department of Veterans Affairs instituted a performance measure monitoring 14-day outpatient follow-up of inpatients with AUDs (Uniform Mental Health Services in VA Medical Centers and Clinics Handbook).
In regards to the primary outcomes of study feasibility, we were able to recruit, retain, and interview subjects, but there were also issues that could complicate this intervention and should be considered in future research and clinical practice. Our finding that many veterans with alcohol dependence were on chronic opioids was surprising and important, because (1) use of naltrexone will be limited to a smaller segment of the population, and (2) modifications to pain management plans are likely warranted in individuals with at-risk and problem drinking due to complications such as opioid overdose, serious GI events, and hepatic injury.
Of additional potential interest was the common occurrence of regular opioid pain medication use among veterans screened for participation. Chronic heavy alcohol consumption is an independent risk factor for mortality due to toxicity from acetaminophen, a commonly used over-the-counter analgesic (Schmidt et al., 2002). Even therapeutic doses of acetaminophen have been associated with hepatic injury and mortality among those with problem drinking (Dart et al., 2010; Kuffner et al. 2007). Also commonly used in the setting of chronic pain, non-steroidal anti-inflammatory agents (NSAIDs) present significant risk in the setting of heavy alcohol consumption, with an AUD history conferring an odds ratio 10.2 for severe GI events (Neutel and Appel, 2000). Further, alcohol is involved in a significant proportion (19%) of opioid-related emergency department visits and hospitalizations (Jones et al., 2014). Pain management considerations may require significant attention among individuals with AUD.
Consideration should be given to alternative pharmacotherapies for chronic pain and to treatments that reduce both chronic pain and alcohol consumption. Targeted psychological pain interventions have been effective in samples of chronic pain patients in treatment for use disorders, and may represent an important consideration for pain management in this population (Ilgen et al., 2016). Additionally, pharmacotherapies effective for painful conditions (e.g. gabapentin, pregabalin) may also assist in reductions of alcohol consumption (Martinotti et al., 2013; Myrick et al., 2009).
As expected, drinking decreased in both groups overall, which is in line with prior evidence indicating naltrexone’s effectiveness in reducing heavy drinking in outpatient settings (Jonas et al., 2014). In this particular study, how much of the reduction in alcohol consumption was due to the medication or natural process of recovery after leaving the hospital is uncertain due to the lack of a control group.
With slight modifications to the current study methods as mentioned above, a future study of similar design could test the following hypotheses: (1) that hospital-administered, long-acting injectable naltrexone reduces alcohol use more than daily oral naltrexone following hospitalization, (2) that this reduced alcohol consumption is associated with greater engagement in substance abuse behavioral treatment, (3) that injectable naltrexone and improved adherence to oral naltrexone is associated with improved likelihood of attending initial visits for substance abuse behavioral treatment when compared to patients non-adherent to recommended naltrexone dosing, and (4) that assignment to injectable naltrexone and improved adherence to oral naltrexone are associated with reduced alcohol consumption following hospital discharge when compared with non-adherent subjects receiving oral naltrexone.
Limitations
As a pilot study, the small sample size was useful to gain information about feasibility, However, as appropriate for a pilot study, we did not seek to gain sufficient statistical power to detect significant differences in outcomes between study groups (Eldridge et al., 2016). Also, as previously mentioned, the lack of an inactive control group limits ability to attribute improvements over time to medication conditions. The homogeneity of the sample (predominantly Caucasian male veterans) limits the potential generalizability of findings.
Injectable naltrexone is currently viewed (and frequently only covered by third-party payers) as an outpatient medication. Study results, however, indicate that the medication may be feasibly delivered during a “teachable moment,” such as a hospitalization for (or hospitalization complicated by) alcohol-related issues or a residential treatment stay. If future studies demonstrate the efficacy of naltrexone in hospitalized patients, policies allowing for the administration of injectable naltrexone prior to discharge from institutional settings may foster improvements not only in patient-oriented outcomes (i.e. improved treatment uptake and reduced alcohol consumption after hospital discharge), but may also drive cost savings via reductions in repeat emergency room utilization and hospitalization due to alcohol intoxication or other alcohol-related issues. Consideration of the presence of AUD and management modifications are likely significant in the care of individuals with chronic painful conditions.
Acknowledgments
Funding: University of Wisconsin Clinical and Translational Sciences Award (NIH-NCATS 4 UL1 TR000427 10). The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This material is also the result of work supported with resources and the use of facilities at the William S. Middleton Memorial Veterans Hospital. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government. The authors have no financial conflict of interest to declare.
Footnotes
ClinicalTrials.gov Identifier: NCT01856712
References
- APA. Diagnostic and Statistical Manual of Mental Disorders (4th Edition, Text Revised) Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- Bernhardt A. Rising to the Challenge of Treating OEF/OIF Veterans with Co-occurring PTSD and Substance Abuse. Smith College Studies in Social Work. 2009;79(3–4):344–67. [Google Scholar]
- Blodgett JC, Maisel NC, Fuh IL, Wilbourne PL, Finney JW. How Effective Is Continuing Care for Substance Use Disorders? A Meta-Analytic Review. Journal of Substance Abuse Treatment. 2014;46:87–97. doi: 10.1016/j.jsat.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondell RD, Frydrych LM, Jaanimägi U, Ashrafioun L, Homish GG, Foschio EM, Bashaw HL. A Randomized Trial of Two Behavioral Interventions to Improve Outcomes Following Inpatient Detoxification for Alcohol Dependence. Journal of Addictive Diseases. 2011;30:136–48. doi: 10.1080/10550887.2011.554777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick JM, Seaman JS. Hospitalized Patients and Alcohol: Who Is Being Missed? General Hospital Psychiatry. 2004;26:59–62. doi: 10.1016/j.genhosppsych.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, RH Pain and Use of Alcohol to Manage Pain: Prevalence and 3-Year Outcomes among Older Problem and Non-Problem Drinkers. Addiction. 2005;100:777–86. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. Initial Evidence for the Reliability and Validity of a ‘Lite’ Version of the Addiction Severity Index. Drug and Alcohol Dependence. 2007;87:297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Dong Q, Silverman BL, Gastfriend DR, Pettinati HM. Early Treatment Response in Alcohol Dependence With Extended-Release Naltrexone. The Journal of Clinical Psychiatry. 2008;69:190–95. doi: 10.4088/jcp.v69n0204. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O’Brien C. Injectable, Sustained-Release Naltrexone for the Treatment of Opioid Dependence: A Randomized, Placebo-Controlled Trial. Archives of General Psychiatry. 2006;63:210–18. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RC, Green JL, Kuffner EK, Heard K, Sproule B, Brands B. The Effects of Paracetamol (Acetaminophen) on Hepatic Tests in Patients Who Chronically Abuse Alcohol - a Randomized Study. Alimentary Pharmacology & Therapeutics. 2010;32:478–86. doi: 10.1111/j.1365-2036.2010.04364.x. [DOI] [PubMed] [Google Scholar]
- DiIorio C, Shafer PO, Letz R, Henry T, Schomer DL, Yeager K. The Association of Stigma with Self-Management and Perceptions of Health Care among Adults with Epilepsy. Epilepsy & Behavior. 2003;4:259–67. doi: 10.1016/s1525-5050(03)00103-3. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW. Efficacy and Tolerability of Long-Acting Injectable Naltrexone for Alcohol Dependence: A Randomized Controlled Trial. JAMA. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Glass JE, Perron BE, Ilgen MA, Chermack ST, Ratliff S, Zivin K. Prevalence and Correlates of Specialty Substance Use Disorder Treatment for Department of Veterans Affairs Healthcare System Patients with High Alcohol Consumption. Drug & Alcohol Dependence. 2010;112:150–55. doi: 10.1016/j.drugalcdep.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. Journal of Biomedical Informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann NG, Olofsson O, Salen B, Wickstrom L. Prevalence of Abuse and Dependency in Chronic Pain Patients. The International Journal of the Addictions. 1995;30:919–27. doi: 10.3109/10826089509055820. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Bohnert A, Chermack S, Conran C, Jannausch M, Trafton J, Blow FC. A Randomized Trial of a Pain Management Intervention for Adults Receiving Substance Use Disorder Treatment. Addiction. 2016;111:1385–93. doi: 10.1111/add.13349. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC. Pharmacotherapy for Adults with Alcohol Use Disorders in Outpatient Settings: A Systematic Review and Meta-Analysis. JAMA. 2014;311:1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Jones CM, Paulozzi LJ, Mack KA. Alcohol Involvement in Opioid Pain Reliever and Benzodiazepine Drug Abuse-Related Emergency Department Visits and Drug-Related Deaths - United States, 2010. MMWR. Morbidity and Mortality Weekly Report. 2014;63:881–85. [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the Treatment of Alcohol Dependence. New England Journal of Medicine. 2001;345:1734–39. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Kuffner EK, Green JL, Bogdan GM, Palmer RB, Heard K, Slattery JT, Dart RC. The Effect of Acetaminophen (Four Grams a Day for Three Consecutive Days) on Hepatic Tests in Alcoholic Patients--a Multicenter Randomized Study. BMC Medicine. 2007;5:13. doi: 10.1186/1741-7015-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larance B, Campbell G, Peacock A, Nielsen S, Bruno R, Hall W, Lintzeris N, Cohen M, Degenhardt L. Pain, Alcohol Use Disorders and Risky Patterns of Drinking among People with Chronic Non-Cancer Pain Receiving Long-Term Opioid Therapy. Drug and Alcohol Dependence. 2016;162:79–87. doi: 10.1016/j.drugalcdep.2016.02.048. [DOI] [PubMed] [Google Scholar]
- Livingston JD, Boyd JE. Correlates and Consequences of Internalized Stigma for People Living with Mental Illness: A Systematic Review and Meta-Analysis. Social Science & Medicine. 2010;71:2150–61. doi: 10.1016/j.socscimed.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Löwe B, Decker O, Müller S, Brahler E, Schellberg D, Herzog W, Herzberg PY. Validation and Standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the General Population. Medical Care. 2008;46:266–74. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- Manea L, Gilbody S, McMillan D. A Diagnostic Meta-Analysis of the Patient Health Questionnaire-9 (PHQ-9) Algorithm Scoring Method as a Screen for Depression. General Hospital Psychiatry. 2015;37:67–75. doi: 10.1016/j.genhosppsych.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Lupi M, Sarchione F, Santacroce R, Salone A, De Berardis D, Serroni N, Cavuto M, Signorelli M, Aguglia E, Valchera A, Iasevoli F, Di Giannantonio M. The Potential of Pregabalin in Neurology, Psychiatry and Addiction: A Qualitative Overview. Current Pharmaceutical Design. 2013;19:6367–74. doi: 10.2174/13816128113199990425. [DOI] [PubMed] [Google Scholar]
- McKay JR. Is There a Case for Extended Interventions for Alcohol and Drug Use Disorders? Addiction. 2005;100:1594–1610. doi: 10.1111/j.1360-0443.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- McKay JR. Continuing Care Research: What We’ve Learned and Where We’re Going. Journal of Substance Abuse Treatment. 2009;36:131–45. doi: 10.1016/j.jsat.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug Dependence, a Chronic Medical Illness: Implications for Treatment, Insurance, and Outcomes Evaluation. JAMA. 2000;284:1689–95. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Micromedex. [Accessed 4/14/17]; http://www.micromedexsolutions.com/micromedex2/librarian/PFDefaultActionId/evidencexpert.DoIntegratedSearch#.
- Murphy L, Laura, Ng K, Su V, Woodworth-Giroux S, Levy TS, Sproule BA, Furlan AD. Approach to the Pharmacological Management of Chronic Pain in Patients with an Alcohol Use Disorder. Journal of Pain Research. 2015;8:851–57. doi: 10.2147/JPR.S88900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Randall PK, Boyle E, Anton RF, Becker HC, Randall CL. A Double-Blind Trial of Gabapentin versus Lorazepam in the Treatment of Alcohol Withdrawal. Alcoholism, Clinical and Experimental Research. 2009;33:1582–88. doi: 10.1111/j.1530-0277.2009.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutel CI, Appel WC. The Effect of Alcohol Abuse on the Risk of NSAID-Related Gastrointestinal Events. Annals of Epidemiology. 2000;10:246–50. doi: 10.1016/s1047-2797(00)00040-5. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The Status of Naltrexone in the Treatment of Alcohol Dependence: Specific Effects on Heavy Drinking. Journal of Clinical Psychopharmacology. 2006;26:610–25. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges G, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, Taylor B. The Relation between Different Dimensions of Alcohol Consumption and Burden of Disease: An Overview. Addiction. 2010;105:817–43. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries R, Fiellin D, Miller S, Saitz R. ASAM Principles of Addiction Medicine. 5. Lippincott Williams & Wilkins; Philadelphia, PA: 2014. [Google Scholar]
- Roche AM, Freeman T, Skinner N. From Data to Evidence, to Action: Findings from a Systematic Review of Hospital Screening Studies for High Risk Alcohol Consumption. Drug and Alcohol Dependence. 2006;83:1–14. doi: 10.1016/j.drugalcdep.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Saitz R. Alcohol Screening and Brief Intervention in Primary Care: Absence of Evidence for Efficacy in People with Dependence or Very Heavy Drinking. Drug and Alcohol Review. 2010;29:631–40. doi: 10.1111/j.1465-3362.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Palfai TP, Cheng DM, Horton NJ, Freedner N, Dukes K, Kraemer KL, Roberts MS, Guerriero RT, Samet JH. Brief Intervention for Medical Inpatients with Unhealthy Alcohol Use: A Randomized, Controlled Trial. Annals of Internal Medicine. 2007;146:167–76. doi: 10.7326/0003-4819-146-3-200702060-00005. [DOI] [PubMed] [Google Scholar]
- Schmidt LE, Dalhoff K, Poulsen HE. Acute versus Chronic Alcohol Consumption in Acetaminophen-Induced Hepatotoxicity. Hepatology (Baltimore, Md) 2002;35:876–82. doi: 10.1053/jhep.2002.32148. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett K, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Shemory ST, Pfefferle KJ, Gradisar IM. Modifiable Risk Factors in Patients With Low Back Pain. Orthopedics. 2016;39:e413–416. doi: 10.3928/01477447-20160404-02. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back. In: Litten Raye Z, Allen John P., editors. Measuring Alcohol Consumption. Humana Press; 1992. pp. 41–72. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Mental Health Findings. US Department of Health and Human Services; 2014. (SMA) 14-4887. NSDUH Series H-49. http://www.samhsa.gov/data/sites/default/files/NSDUHmhfr2013/NSDUHmhfr2013.pdf. [PubMed] [Google Scholar]
- Timko C, Gupta S, Schultz N, Harris AHS. Veterans’ Service Utilization Patterns After Alcohol and Opioid Detoxification in VHA Care. Psychiatric Services. 2016;67:460–64. doi: 10.1176/appi.ps.201400579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and Alcohol Dependence: Role of Subject Compliance. Archives of General Psychiatry. 1997;54:737–42. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]