Abstract
The number of patients surviving with congenital heart disease (CHD) has soared over the last three decades. Adults constitute the fastest growing segment of the CHD population, now outnumbering children. Research to date on the heart-brain intersection in this population has largely been focused on neurodevelopmental outcomes in childhood and adolescence. Mutations in genes that are highly expressed in heart and brain may cause cerebral dysgenesis. Together with altered cerebral perfusion in utero, these factors are associated with abnormalities of brain structure and brain immaturity in a significant portion of neonates with critical CHD even before they undergo cardiac surgery. In infancy and childhood, the brain may be affected by risk factors related to heart disease itself or to its interventional treatments. As children with CHD become adults, they increasingly develop heart failure, atrial fibrillation, hypertension, diabetes and coronary disease. These acquired cardiovascular comorbidities can be expected to have effects similar to those in the general population on cerebral blood flow, brain volumes, and dementia. In both children and adults, cardiovascular disease may have adverse effects on achievement, executive function, memory, language, social interactions, and quality of life. In summary, against the backdrop of shifting demographics, risk factors for brain injury in the CHD population are cumulative and synergistic. As neurodevelopmental sequelae in children with CHD evolve to cognitive decline or dementia during adulthood, a growing population of CHD can be expected to require support services. We highlight evidence gaps and future research directions.
Keywords: congenital heart disease, neurovascular, neurocognitive
Journal Subject Terms: Cardiovascular Disease, Congenital Heart Disease, Vascular Disease
Introduction
With advances in diagnostic technologies, surgical management, and postoperative care, more than 90% of children with critical congenital heart disease (CHD) are expected to survive to adulthood in the current era. Congenital heart disease is now considered a lifelong condition. Figure 1 illustrates the prevalence of CHD in infants, children, adults and in geriatric subjects in the same population using the Quebec Congenital Heart Disease Database.1–3 From 2000 to 2010, the prevalence of CHD rose by 11% in children compared to 57% in adults. Thus by 2010, two-thirds of the CHD population with severe and other forms of CHD were adults. We estimated that by 2010, 2.4 million people were living with CHD in the US: 1.4 million adults and 1 million children.
Figure 1.
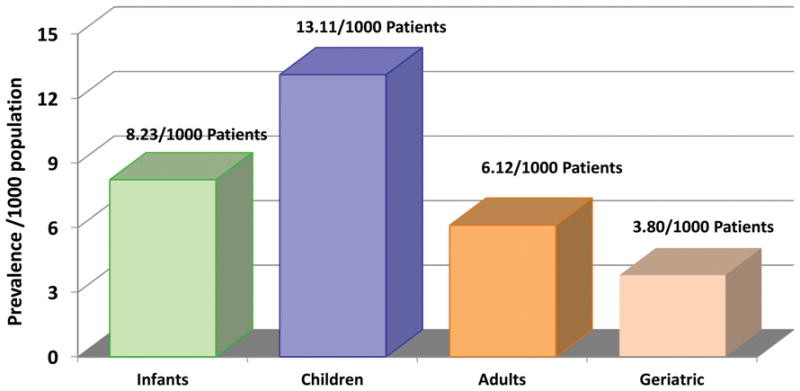
Prevalence of congenital heart disease across the lifespan.3 Reprinted with permission from Mazor Dray E, Marelli AJ. Adult Congenital Heart Disease: Scope of the Problem. Cardiol Clin. 2015;33(4):503–512.
In children with CHD, this notable success has exposed a heightened risk of brain injury and developmental disorders or disabilities.4 Children with complex CHD have been recognized to have a distinct neurobehavioral signature, including mild reduction in cognitive performance, difficulties with social interaction, worse pragmatic language, inattention, impulsive behaviour, and impaired executive function.4 Despite a rich literature on neurodevelopment in children with CHD, few studies have addressed the translation of these findings to neurocognitive function in the adult congenital heart disease population. Evidence in the general adult population suggests that chronic cardiovascular disease (CVD) leads to cerebrovascular injury and dementia.5–7 The aging of the CHD population provides the impetus for this review on the continuum of neurocognitive injury across the lifespan.
A paradigm shift is needed to account for these emerging observations. In Figure 2, we propose a model that sequentially links CHD-related cardiovascular disease across the lifespan to abnormalities in the perinatal, childhood and adult brain. As life expectancy continues to increase, the window of opportunity for repeated injury continues to lengthen. Thus in a lifespan model, neurodevelopmental sequelae in children with CHD evolve during adulthood to cognitive decline or dementia. This review will synthesize and integrate data on the brain structure and function, including neurocognitive and psychosocial sequelae, in congenital heart patients from fetal life to adulthood.
Figure 2.
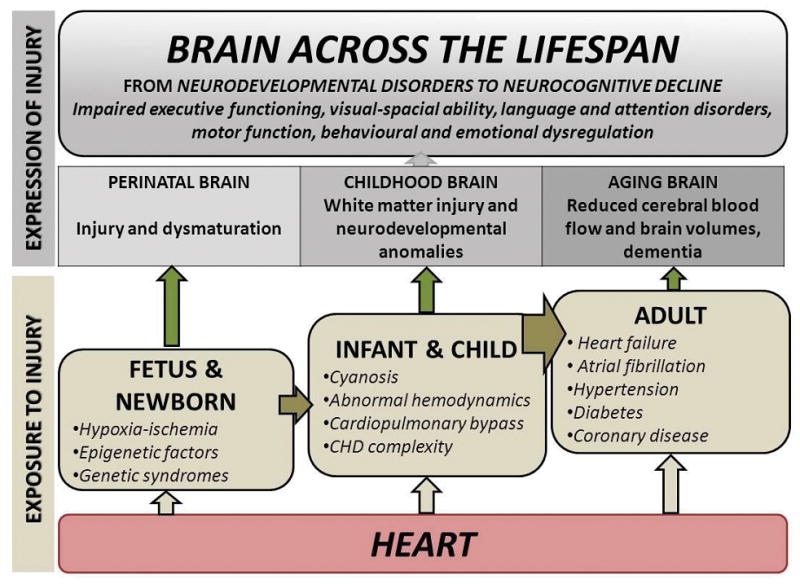
Neurocognitive impairment across the lifespan.
Risk Factors for Adverse Neurologic and Developmental Outcomes in Children
Risk factors for adverse neurocognitive outcomes are multifactorial, interrelated, cumulative, and likely synergistic over time. Neurodevelopmental (ND) and behavioural morbidity in children can derive from genetic & epigenetic factors, patient factors other than genetic disorders (e.g., low birth weight or gestational age), aberrant fetal cerebral perfusion and oxygenation, sequelae of heart disease itself (severe cyanosis, cardiac arrests), and its medical and surgical management (e.g., perioperative hypoxic ischemic injury, stroke, factors associated with prolonged postoperative course). Twenty years ago, the impact of circulatory arrest in infants with CHD undergoing cardiac surgery ushered in an era of clinical trials to assess the effects of intraoperative conduct on neurologic and developmental outcomes (Figure 3).8 Recent data suggest that patient and preoperative risk factors, such as low birth weight or gestational age and social class, as well as cumulative postoperative morbidity, contribute a greater proportion of variability in outcomes after infant heart surgery than intraoperative support techniques.9
Figure 3.
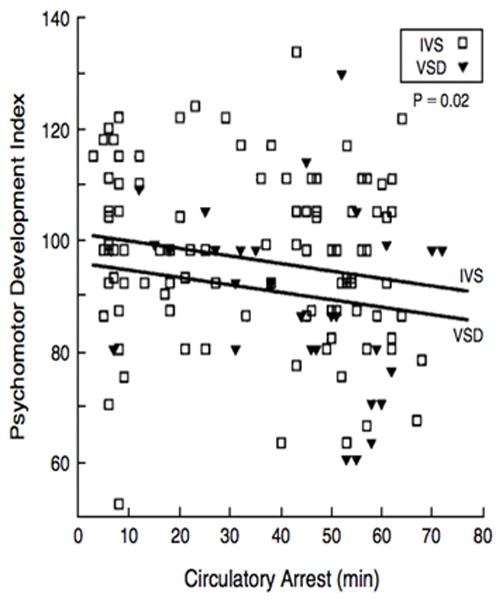
Illustration of the inverse relation between circulatory arrest and psychomotor development index in infants at one-year post the neonatal arterial switch operation for complete transposition of the great arteries.8 Reprinted with permission from Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, Barnes PD, Holmes GL, Hickey PR, Strand RD, Walsh AZ, Helmers SL, Constantinou JE, Carrazana EJ, Mayer JE, Hanley FL, Castaneda AR, Ware JH, Newburger JW. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. The New England Journal of Medicine. 1995;332:549–555.
Genetic disorders are present in about one-third of individuals with congenital heart disease. These include chromosomal disorders (e.g., Trisomy 21), microdeletions (e.g., 22q11 microdeletion), or mutations (e.g., Noonan syndrome). However, known risk factors only explain approximately 30% of observed variation in ND outcome after cardiac surgery in infancy,10–12 suggesting that as yet unknown genetic and epigenetic factors may play an important role. Microdeletions causing CHDs may be associated with specific patterns of neurodevelopmental morbidity. For example, adults with 22q11 deletion have specific deficits in visual-spatial skills, executive functions (problem solving, organization, planning), abstract social thinking, and attentiveness.13 Pathogenic copy number variants, i.e., regions of DNA gains or losses, were reported to be present in 13.9% of infants with single ventricle heart disease, compared to 4.4% of controls, and were associated with inferior neurocognitive and somatic growth.14 Epigenetic factors, the study of protein changes that affect gene regulation without altering core DNA sequence, may also have a role in determining neurocognitive outcome. 15, 16 Finally, genetic polymorphisms affecting host susceptibility and resiliency, such as the apolipoprotein E genotype, may affect the response of the brain to stresses associated with CHD, including cardiopulmonary bypass and perioperative events.17 With a burgeoning population of CHD survivors reaching reproductive age, research on the genetic underpinnings of neurodevelopemental disorders assumes increasing importance.
The Fetal and Perinatal Brain: Injury and Dysmaturation
In response to hypoxia-ischemia, the developing brain acquires characteristic patterns of injury that reflect the “selective vulnerability” of specific cell populations that are maturing at the time of the injury.18 For example, during the early third trimester of gestation, early lineage oligodendrocytes predominate in the brain’s white matter and are vulnerable to insults to which mature myelinating oligodendrocytes are resilient. Given this, neonates born preterm most commonly acquire white matter injury, while term neonates respond to hypoxia-ischemia with a preponderance of injury to grey matter or neuronal structures.18
Recent experimental and clinical data in preterm neonates indicate that the primary issue in white matter injury is cellular maturation arrest rather than cell loss. As such, the primary mechanism of myelination failure in the preterm neonate is a failure of oligodendrocyte progenitor cells to differentiate into myelin forming oligodendrocytes (i.e. dysmaturation) rather than a predominance of necrotic lesions.19, 20 In preterm neonates, white matter dysmaturation is related to modifiable aspects of clinical illness including postnatal infections and procedural pain.21, 22 Of particular clinical relevance are the observations that brain dysmaturation is an important antecedent of adverse developmental outcomes.20, 22, 23
Contrary to what is expected in term neonates, white matter injury is the characteristic pattern in neonates with CHD, and resembles that characteristic of preterm newborns.22, 24, 25 Other focal injuries such as stroke and microhemorrhage are also prevalent. Stroke is also increasingly recognized before and after surgery for CHD, and related in some studies to therapeutic catheterization procedures or regional cerebral perfusion bypass strategies.24 Subtle hemorrhagic brain injury is also commonly seen on MRI after open heart surgery in infancy; these microhemorrhages have been associated with longer total support time and greater number of cardiac catheterizations, and also with worse developmental outcome.11, 26 Importantly, the range of neurodevelopmental sequelae in children with CHD are not fully explained by these focal brain injuries identified before and after cardiac surgery.24 Therefore, the patterns of brain injury seen on diagnostic imaging of neonates with CHD are only the “tip of the iceberg”.
As in the preterm neonate, brain vulnerability in newborns with CHD is primarily a problem of dysmaturation. Converging evidence supports that newborns with CHD have diffuse impairments in the progression and rate of early brain maturation.24, 27–30 Compared to normal term newborns, those with CHD have an immature pattern of brain microstructure and metabolism,27 less mature morphologic scoring,28 and smaller brain volumes.30 These findings likely explain why they are more vulnerable to white matter injury than the conventional “term” patterns of injury. Impaired brain maturation is also an important substrate for postnatal injury, and those with pre-operative brain injury have less robust brain maturation from the pre- to the post-operative scan.24, 31, 32 Consistent with observations in the preterm neonate, neurodevelopmental outcomes at 2 years of age in children born with severe CHD were more strongly related to brain maturity than to brain injury.33 As in the preterm neonate, these data highlight the potential for promoting optimal brain development to improve functional outcomes in neonates with CHD.
Brain maturation slows in fetuses with CHD during the 3rd trimester of pregnancy, when the main brain development events include axon path finding, synapse formation, and refining cortical networks.25, 30 Over this period of dramatic increases in neuronal connectivity and activity, blood flow to the fetal brain is approximately one quarter of the combined ventricular output,34 and fetal cerebral oxygen consumption is almost half of all fetal oxygen consumption.34, 35 In fetuses with CHD studied with phase contrast MRI and T2 mapping based MR oximetry, disrupted streaming of oxygenated blood from the placenta to the brain, and reduced umbilical blood flow and placental oxygen exchange are observed.36 Compared with normal controls, those with some forms of critical CHD have a 10% reduction in the oxygen saturation of blood in the ascending aorta; without a compensatory increase in cerebral blood flow or oxygen extraction, there is an associated one standard deviation reduction in fetal brain volume.36 These in utero cardiovascular disturbances are especially relevant to the issues of brain dysmaturation outlined above given recent observations linking oligodendrocyte precursor cells to postnatal white matter angiogenesis and the early events of myelination through hypoxia-inducible factor.37 Why the fetus with CHD does not compensate for in utero hypoxia with “brain sparing” physiology remains a critical question for future research.
Brain maturation, including the refinement of brain networks and myelination, continues through childhood, providing a significant window for recovery and highlighting the need for a life-span approach to optimizing the outcome trajectory for patients with CHD.38 Fetal interventions to optimize cerebral oxygen delivery at the earliest phase of the lifespan are now on the horizon with the ultimate goal of preventing the onset of brain dysmaturation. As in the preterm neonate, abnormalities in brain maturation evident as early as the third trimester persist through childhood in those with CHD and predict adverse functional outcomes.29, 39, 40 Thus, a lifespan approach to improving neurocognitive outcomes in children with CHD must begin in utero.
The Brain in Children and Adolescents (Figures 3 and 4)
Figure 4.
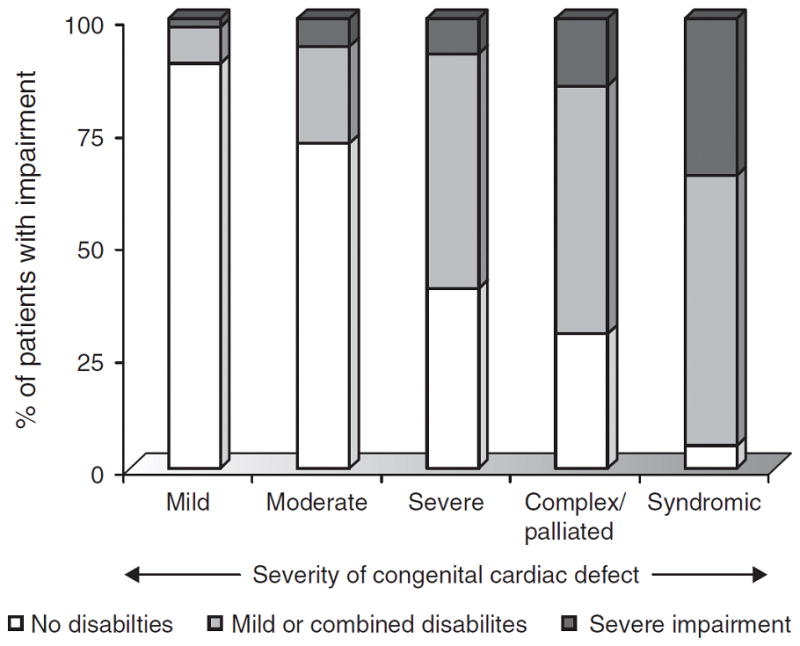
Gradient between congenital heart disease severity and prevalence of neurodevelopmental impairment.4 Reprinted with permission from Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young 2006; 16 (Suppl 1): 92–104.
By school age, children with critical CHD, on average, have lower scores on tests of intelligence and achievement, worse fine motor and gross motor function, and higher likelihood of learning disabilities, use of special services, and abnormalities of speech, language, and behaviour.4, 10 Adverse neurodevelopmental and behavioural outcomes are more likely after repair of complex cardiac lesions, with the greatest neurodevelopmental morbidity in children with single ventricle heart disease, such as hypoplastic left heart syndrome.41 As these children reach adulthood, ND disabilities can limit educational achievements, employability, insurability, and quality of life.42
Brain imaging studies using magnetic resonance imaging (MRI) in school age children and adolescents with CHD suggest that altered white matter microstructure, and resulting differences in network processes (i.e., the connectome), may underlie their cognitive impairment. 32 In addition to diffuse abnormalities (e.g., white matter abnormalities, abnormal T2 hyperintensities, ventriculomegaly), focal or multi-focal abnormalities can occur secondary to thromboembolic events.10 The highest incidence of focal infarction occurs in individuals with cyanotic congenital heart disease; indeed, strokes can be detected by brain MRI in nearly half of cyanotic adults.43 Taken together, brain MRI studies in children and adolescents who underwent infant heart surgery highlight the challenges of discriminating neurocognitive deficits that result from genetic abnormalities, deficient cerebral substrate delivery in fetal life, and postnatal injury.
Neurodevelopmental disabilities can adversely affect learning and the attainment of academic, social, and vocational skills, with greater need for remedial services, including tutoring and special education, as well as physical, occupational, and speech therapy.4 In the CHD population without genetic syndromes or catastrophic events, intelligence quotient is relatively well preserved but specific areas of weakness include motor skills, higher order language (the ability to make up a coherent story when presented with pictures), visual spatial skills, and vigilance and sustained attention. Impairment in visual-spatial ability and planning are well illustrated by the difficulties that school age children and adolescents have shown in completing the Rey Osterreith complex figure, a complicated line drawing which a child is asked to copy (recognition) and then draw from memory (recall).44 This task, which involves perception of shapes within shapes, requires integration of many different areas of the brain. In a population of complex CHD, attention deficit and hyperactivity disorder was reported to be 3–4 times higher than in the general population.45 Indeed, the “lifetime prevalence” of ADHD in adolescents with d-TGA was 19%.10 Impairments in executive functions are a typical feature of the neurobehavioral signature of CHD patients and are associated with behavioural dysregulation and attention problems, lower working memory, and problems with organization and planning abilities. 46, 47 Taken together, these findings explain the high rate of remedial services needed in the critical CHD population. Nearly half of 5 –10 year old children in a mixed population of critical CHD were receiving remedial services.45 By adolescence, 65% of patients with simple d-transposition of the great arteries (d-TGA)10 and 82% with tetralogy of Fallot11 had received remedial academic or behavioural services, such as tutoring, early intervention, occupational therapy, or special education.
In the social and emotional sphere, children and adolescents with CHD often have deficits in social cognition, i.e., the ability to process social information, especially its encoding, storage, retrieval, and interpretation of social situations and relationships.44, 48 Decreased social cognition results in difficulty in “reading” other people, inferring their internal states, and interpreting their actions appropriately. Studies on psychiatric disorders in youth with CHD are limited. In structured psychiatric interviews, adolescents with d-TGA, compared to an optimal control group without known risk factors for brain disorders, had worse clinician-rated global psychosocial functioning, as well as more self-, parent- and clinician-rated symptoms of depression, anxiety, and disruptive behaviours.49 Worse global psychosocial function was associated with lower cognitive function and greater parent stress.
Executive functions include higher-order neurocognitive abilities that facilitate the coordination and organization of actions towards a goal, allowing the individual to adapt to new or complex situations.50 Mediated by the maturation of prefrontal structures, as well as fronto-parietal and sub-cortical networks,51, 52 executive functions depend upon the integrity of white matter pathways that may be disrupted by dysmaturation or postnatal hemodynamic stress. Executive dysfunction in adolescents with critical CHD is associated with worse psychosocial health status and quality of life, 53 suggesting that executive impairments are associated with reduced functioning in everyday life. It is likely that executive dysfunction impairs the ability of patients to adhere to medical recommendations and follow-up.54
Health-related quality of life (HRQOL) is a multidimensional concept which is all the more complex in pediatric populations where patient, family and sibling experience are interrelated making measurement challenging. HRQOL contains elements of both psychosocial and physical health status. Using the Pediatric Cardiac Quality of Life Inventory Testing Study and its corollary studies HRQOL in children, adolescents and their parent-proxy reporters was assessed.55, 56 In 1,605 patient-parent pairs in the US55 and 771 patient-parent pairs in the United Kingdom,56 findings related to impaired HRQOL were consistent. Lower patient- and parent-reported HRQOL scores resulted in greater health care utilization, including a greater number of cardiac surgeries, hospital admissions, and provider visits over a 12-month period. Lower HRQOL scores were also associated with decreased competency (Achenbach Youth Self-Report and Child Behaviour Checklist Total Competency score); impaired self-perception (Global Self-Worth and Self Perception Profile for Children and Adolescents); and increased behavioural and emotional problems (Achenbach Internalizing Problems Summary Scale score and DSM-IV Oriented Scale scores for affective, anxiety, somatic and attention deficit disorders).55 In these populations of patients with pediatric heart disease, the majority of whom had CHD, HRQOL scores remained abnormal across all age categories and respondent types.
Compliance with surveillance recommendations can be impacted by disability in neurodevelopment, in social and emotional domains, in executive function and HRQOL. Patients with CHD are lost to follow-up during the transition to adult healthcare,57 mediated at least in part by diminished working memory and self-organization. Resulting lapses in care affect health care utilization with resulting economic impact. Two thirds of CHD patients fall out of care by the time they reach age 18 with more than half falling out of care during transition years.58, 59 Whereas between the ages of 12 and 30 most emergency room visit rates decrease, CHD patients have higher admission rates via the emergency room.60 Moreover, lapses in care are associated with inappropriate medication regimens and higher rates of urgent cardiac interventions.61 In a cross-sectional multicenter study that examined reasons for lapses in care in over 900 young adult CHD patients found that powerful predictors of care gaps included misinformation related to the perception of the need for care.62 This is likely multifactorial related both to the patient’s capacity and willingness to receive information and health care providers’ ability to deliver it. Improved compliance with follow-up requires improved processes of care delivery particularly during transition of care where gaps are common.58, 59, 63 There is a growing literature on the benefits of health information technology platforms to improve access to multiple elements of care both in the elderly with chronic disease and in youth with life-long conditions.64 A systematic review of 17 internet-delivered health behaviour change interventions for adolescents or young adults revealed the importance of tailored communication including reminders and incentives. 65 These findings underscore the need for targeted interventions leveraging on-line tools to improve care pathways. These strategies seem particularly important in the CHD population, with its high prevalence of executive dysfunction.
Manifestations of Neurodevelopmental Abnormalities in Adults with CHD
Adults with CHD have an increased risk of anxiety, depression, pragmatic language impairment and social cognition issues, worse educational attainment, and underemployment, and delayed progression into independent adulthood.66–69 The prevalence of comorbid psychiatric disorders is 3 to 4 times higher among adults with neurocognitive impairment than in the general population.66, 67 These may result in parental overprotection and impact self-management. 66, 67, 70 Attention deficit, executive dysfunction, mood, language and social cognition issues may manifest themselves as inappropriate behaviour and may limit the ability to form healthy family, work/peer, and romantic relationships. The overall QOL for adults with CHD is reduced compared with the general population.71
There are few quantitative analyses measuring the impact of pediatric and adolescent ND and psychosocial impairments on adults with CHD. In non-CHD populations who have been tracked from childhood through adolescence to adulthood, some relevant data exist on patients with childhood attention deficit disorder (ADHD) and hyperactivity disorder, a common disorder in CHD patients. Adults with ADHD, compared to non-ADHD subjects from the same birth cohort, were found to have higher standardized mortality rate ratios.72 A longitudinal study comprised of 551 subjects with ADHD followed for up to 35 years were found to be at higher risk for impaired mental health, work performance and financial stress scores compared to demographic controls.73 These observations provide the rationale for the growing recognition that wide-ranging neurodevelopmental and psychosocial dysfunction is expected to have significant implications for life success and societal costs as the CHD population continues to grow. In the Dutch CONCOR Study, with almost 1,500 patients at an average age of 39 years, only about two-thirds of patients were employed for more than 12 hours a week. Compared to controls, patients under 40 years of age had significantly lower education, more unemployment and fewer relationships.74
The Aging Brain: Adding Insult to Injury (Figures 5 and 6)
Figure 5.
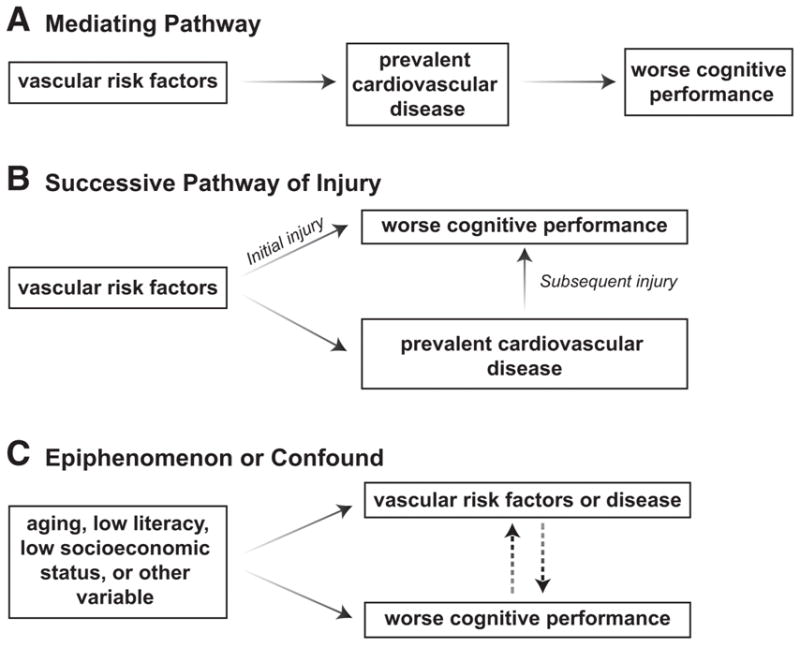
Vascular risk factors and midlife cognition.6 Reprinted with permission from Jefferson AL. Vascular risk factors and midlife cognition: Rethinking the exposure window. Circulation. 2014;129:1548–1550
Figure 6.
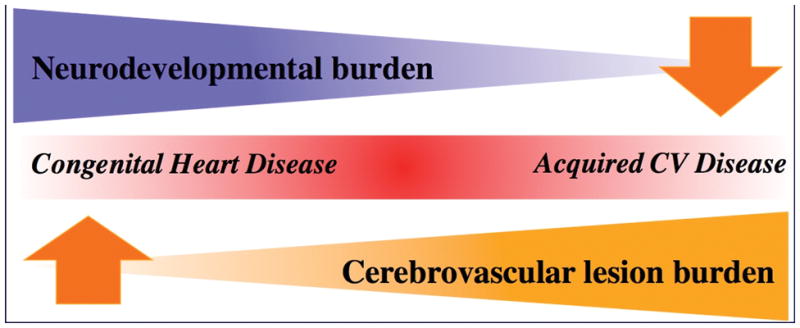
Unifying hypothesis for the continuum of neurocognitive disease in the lifespan of congenital heart disease patients.105 Inspired from Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: How to move forward? Neurology. 2009;72:368–374.
The dynamic nature of cardiovascular morbidity in CHD underscores the challenge of investigating the intersection between heart and brain health in this population. We put forth the concept that impaired neurocognitive development becomes neurocognitive decline in adults with CHD. There is limited research on the long-term impact of CHD on cognitive functioning, structural or functional imaging outcomes, or dementia risk across the lifespan. In the absence of such evidence, we theorize potential connections between CHD and abnormal brain aging based on associations reported in the general population. As described above, brain lesions in infants with CHD are dynamic and associated with widespread impairments in brain maturation that persist, at least, through adolescence. Importantly, the cellular pathways most vulnerable to dysmaturation, are the same pathways that will maintain the brain’s vulnerability to insult including hypoxia-ischemia and inflammation.20 This negative synergy is evident as early as the neonatal period where infants with greater “dysmaturation” are most vulnerable to acquiring brain injuries such as white matter injury. 24 This negative synergy has been conceptualized as “tertiary brain damage”, in that these brain changes predispose a patient to further injury, or prevent brain repair after an initial insult.75 Inflammation and epigenetic modifications may be key mediators of tertiary brain damage.75 Our hypothesis is congruent with increasing evidence in the general population that brain injury from cardiovascular disease precedes clinically evident cognitive decline and increases the risk of Alzheimer’s dementia in the elderly as we review below. Individuals with CHD lesions are expected to develop adult causes of neurocognitive decline earlier in life than the general population because the brain is “primed” for vulnerability to early onset of adult cardiovascular risk factors for brain injury that accompany common forms of critical CHD. In sub-groups of CHD populations, early onset of adult cardiovascular risk factors for brain injury include intraatrial reentrant tachycardia and atrial fibrillation (e.g., in atrial switch procedures or Fontan operations), hypertension (e.g., in coarctation of the aorta), disordered glucose metabolism (e.g., in Fontan procedures and in obesity in adult CHD patients), and coronary artery disease (e.g., in arterial switch operations, Ross procedures and congenital coronary anomalies). This earlier acquisition of brain injury related to adult onset cardiovascular risk factors will be additive with neurocognitive sequelae of brain dysmaturation and acquired injury through childhood and adulthood. We have recently shown the additive burden of heart failure in CHD patients as powerful predictor of stroke.76 Thus, multiple common forms of prevalent cardiovascular disease known to affect older adults, including heart failure with low cardiac output, atrial fibrillation, cardiac arrest, coronary disease and acquired comorbidities in CHD, have been related to abnormal cognitive function and clinical dementia in elders without CHD are reviewed below.
Heart failure and low cardiac output have been increasingly recognized as a complication of CHD.77 The chronic mismatch between ventricular preload and afterload resulting from abnormalities of cardiac anatomy in CHD patients result in sub-clinical heart failure.78 Progressive heart failure accounts for approximately one third of deaths in CHD patients.79 In non-CHD older adults, heart failure has been linked to impairment in global cognition, language, attention, executive functioning, and episodic memory,80 as well as structural imaging changes seen with magnetic resonance imaging.81 Epidemiological data suggest that heart failure is associated with an increased risk of dementia of the Alzheimer’s type over a 9-year follow-up period.82 The well-established association between heart failure and corresponding reductions in cerebral blood flow83 is likely due to poor left ventricular ejection fraction and reduced cardiac output.84 In older patients lower cardiac output was associated with worse executive functioning skills85 and increased white matter hyperintensities seen on brain magnetic resonance imaging.86 Epidemiological data from the Framingham Heart Study showed that, among more than 1000 adults age 40–89 years, lower ejection fraction was associated with worse visuospatial episodic memory and object recognition performances, even when excluding participants with prevalent cardiovascular disease.87 Data from the same epidemiological cohort suggested that, among 1504 adults age 34–84 years, lower cardiac output was associated with smaller total brain volume, even after adjustment for vascular risk factors.5 More recently, a correlation between reduced cardiac output and smaller brain volume has been independently replicated in an Icelandic cohort of older adults.88 Moreover, among adults age 60 and older in the Framingham Offspring Study, lower cardiac output was associated with a two-fold increase in dementia over a median follow-up period of nearly 8 years.7 Similar associations have been reported in an independent cohort of older adults in whom lower cardiac output was associated not only with a greater risk of dementia but also with its precursor, mild cognitive impairment.88
Atrial fibrillation is perhaps one of the best examples of the abnormal offsetting of biological and pathological cardiac age in CHD populations. The lifetime risk of atrial fibrillation in a 20 year old with CHD is equivalent to that of a 55 year old without CHD, with more than 50% of patients with severe CHD developing atrial arrhythmias by age 65 years.89 The presence of atrial fibrillation is associated with abnormal brain aging. Data from the population-based Rotterdam Study of adults aged 55 to 106 years suggest that atrial fibrillation confers an increased risk for cognitive impairment and incident dementia (OR=2.3) compared to peers without atrial fibrillation.90 Meta-analyses of data comprising more than 46,000 participants suggest that individuals with atrial fibrillation have a two-fold higher risk of dementia that further increases in the presence of recent stroke.91
Traditional vascular risk factors including high blood pressure, diabetes and coronary disease further exacerbate the growing burden of systemic complications as individuals with CHD age. In a population-based ACHD cohort consisting of 3,239 patients surviving to age 65 and followed for up to 15 years, predictors of mortality included dementia, diabetes and myocardial infarction.2 Although risk factors are in part driven by age, and hypertension may occur in the context of specific CHD disorders such as coarctation of the aorta,92 atherosclerotic disease is observed in younger cohorts with CHD. Billet et al.93 found that adults with ACHD were more likely to have hypertension, stroke, and chronic kidney disease (a coronary artery disease risk equivalent) than age-matched controls without ACHD. Moons et al. reported that at least 80% of adults with ACHD had at least one coronary artery disease risk factor, with the rates of hypertension and obesity being higher than in the general population.70 The mean age in both of these studies was only 26 to 28 years, suggesting that ACHD patients may not only harbour risk factors for many years but may also have a higher risk burden over their lifetime.
Hypertension and diabetes have both been linked to abnormal brain health. Hypertension alters the cerebral vasculature, predisposing to infarction of both gray and white matter.94 Even in the absence of hypertension, subclinical elevations in blood pressure are related to worse cognitive function. A recent meta-analysis of up to 4000 participants free of clinical dementia or stroke yielded evidence that elevated systolic blood pressure was associated with impairment of global cognition and episodic memory even after adjustment for vascular comorbidities.95 Diabetes has been linked to an increased risk of clinical dementia possibly mediated by impaired insulin signalling in the brain and inflammatory or oxidative processes.96, 97.
Coronary disease may occur in the context of specific CHD lesions. Of 400 patients who had undergone the arterial switch operation for d-transposition of the great arteries and were followed for an average of 25 years from birth, 5% had obstructive coronary disease.98 In patients with coarctation of the aorta, coronary disease was found to be independently associated with poor risk factor control.92 Coronary artery disease, especially atherosclerosis, has been linked to abnormal brain aging in multiple studies. In men participating in the Honolulu-Asia Aging Study, coronary heart disease was associated with an increased risk for vascular dementia (OR=2.5).99 Cross-sectional population-based data from more than 2000 Rotterdam Study participants suggested that atherosclerosis was associated with dementia (OR=1.3 to 1.9), including both Alzheimer’s disease (OR=1.3 to 1.8) and vascular dementia (OR=1.9 to 3.2).100 Longitudinal follow-up over 9 years for this population-based cohort suggested an increase in the risk of dementia.101 Associations between vascular risk factors and compromises in cognitive health appear to begin as early as mid-life or earlier.102 Blood pressure elevations appear to exert effects on late-life cognitive health and dementia risk beginning as early as mid-life.103 Mid-life diabetes (vs. no diabetes) is associated with greater cognitive decline over a 20 year follow-up period, and individuals with poorly controlled diabetes have worse cognitive decline than individuals with well-controlled diabetes.104 Recent data suggest the exposure window may be even earlier than mid-life. Longitudinal exposure to higher systolic and diastolic blood pressures and higher fasting blood glucose starting in early-life corresponded to worse episodic memory, information processing speed, and executive function performances approximately 25 years later in mid-life.102 In light of post-mortem evidence that the pathogenesis of Alzheimer’s disease begins decades before clinical symptoms manifest in late-life, it is plausible that vascular risk exposure may affect brain health much earlier than previously appreciated.6
Competing Gradients of Neurological Injury (Figure 6)
We began with Figure 1 to demonstrate how cumulative cardiovascular injury in CHD impacts the brain sequentially across the age continuum. In Figure 6, we illustrate two gradients of neurovascular injury.105 As the cardiovascular disease burden shifts from factors associated with CHD to acquired cardiovascular disease, the decreasing gradient of neurodevelopmental abnormalities is replaced by an increasing gradient of neurovascular disease. This figure highlights the concept that there comes a point when the brain is no longer developing and thus the gradient of abnormal neurocognitive development wanes to be replaced by the increasing burden of neurological injury or even decline. Together these gradients magnify the potential for expression of neurological injury in the CHD patient across the lifespan. Thus, abnormal neurodevelopment in infants, children, and adolescents may become neurocognitive decline as patients with CHD age. We have already shown that young patients with CHD have aging hearts.89 The question now is do these same patients have brains that show evidence of premature aging.
Conclusions
Dramatic advances have occurred in the survival of patients with CHD. At the present time, 9 in 10 children survive until adulthood, a minimum of 1 in 150 Americans has a congenital heart defect, and today there are more adults than children with CHD. In this first review of the relationship between heart health and brain health in CHD patients across the lifespan, we illustrate the link between cardiovascular and neurovascular diseases as patients age. In the absence of data on the impact of cardiovascular disease on neurovascular disease in CHD populations, we show the importance of acquired cardiovascular complications in CHD patients on cerebral blood flow, brain volumes and dementia. We put forth the idea that impaired neurocognitive development becomes neurocognitive decline in adults with CHD. We provide two conceptual models: the first illustrating the cumulative burden of cardiovascular injury expected to impact the brain in CHD populations and the second illustrating how neurovascular disease is expected to replace neurodevelopmental disabilities as patients age and CHD persists across the lifespan. This review has for the first time synthesized and integrated existing data on the brain structure and function, including neurocognitive and psychosocial sequelae, in CHD patients from fetal life to adulthood.
Future directions
This synthesis of available evidence has highlighted substantial knowledge gaps. Table 1 summarizes translational research objectives that are likely to result in patient and family- centered outcomes. In lifelong conditions such as CHD, the ‘nature’ vs ‘nurture’ dialogue is becoming increasingly important. It is believed that a ‘nurturing’ model of resilience and mitigation for neurodevelopmental and neurovascular risk factors is becoming increasingly important to optimize cognitive health and psychosocial functioning. Research using longitudinal cohorts is needed to measure serial outcomes from fetal life and early infancy to adulthood. In young patients who are stable, cardiac dysfunction even when clinically silent should be minimized. In older CHD adults, we need to understand if acquired cardiovascular risk factors are effect modifiers of brain injury. Although we have proposed a link between neurodevelopmental disabilities in children and neurocognitive decline in adults, the differences need to be characterized, explored and leveraged for meaningful intervention and prevention. Avoidable pathways of injury and modifiable risk factors across the lifespan need to be identified. Successfully identifying these pathways will require consideration of the wide range of hemodynamic disturbances in various congenital lesions and their differing risks for brain damage, from stroke or white matter injury.27, 106 A better understanding of the lesion-specific alterations in fetal hemodynamics, and their impact on the brain, should open the window to new opportunities to promote optimal brain development from the earliest days.36 Moreover, a life-span perspective on neurocognitive function in patients with CHD is warranted to plan resource allocation and document the potential benefits of early intervention and prevention. Children are the adults of our future. Reducing the cumulative burden of brain injury and cognitive morbidity should be a priority for clinical care and research in the aging CHD population.
Table 1.
Future directions for translational research resulting in improved patient and family centered outcomes.
| Understanding of the relative importance of the risk factors for neurocognitive disability in CHD |
|
| Interventions to promote brain health: |
|
| Improvement of patient-centered and societal outcomes by: |
|
CHD= congenital heart disease
Supplementary Material
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime Prevalence of Congenital Heart Disease in the General Population from 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 2.Afilalo J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Marelli AJ. Geriatric congenital heart disease burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509–1515. doi: 10.1016/j.jacc.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 3.Mazor Dray E, Marelli AJ. Adult Congenital Heart Disease: Scope of the Problem. Cardiol Clin. 2015;33:503–512. doi: 10.1016/j.ccl.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16(Suppl 1):92–104. doi: 10.1017/S1047951105002398. [DOI] [PubMed] [Google Scholar]
- 5.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jefferson AL. Vascular risk factors and midlife cognition: rethinking the exposure window. Circulation. 2014;129:1548–1550. doi: 10.1161/CIRCULATIONAHA.114.008906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jefferson AL, Beiser AS, Himali JJ, Seshadri S, O’Donnell CJ, Manning WJ, Wolf PA, Au R, Benjamin EJ. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation. 2015;131:1333–1339. doi: 10.1161/CIRCULATIONAHA.114.012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, Barnes PD, Holmes GL, Hickey PR, Strand RD, Walsh AZ, Helmers SL, Constantinou JE, Carrazana EJ, Mayer JE, Hanley FL, Castaneda AR, Ware JH, Newburger JW. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 9.Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, Pike N, Sood E, Mahle WT, Cooper DS, Dunbar-Masterson C, Krawczeski CD, Lewis A, Menon SC, Pemberton VL, Ravishankar C, Atz TW, Ohye RG, Gaynor JW. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, Rappaport LA, Wernovsky G, Jonas RA, Newburger JW. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellinger DC, Rivkin MJ, DeMaso D, Robertson RL, Stopp C, Dunbar-Masterson C, Wypij D, Newburger JW. Adolescents with tetralogy of Fallot: neuropsychological assessment and structural brain imaging. Cardiol Young. 2015;25:338–347. doi: 10.1017/S1047951114000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, Ghanayem NS, Goldberg CS, Hovels-Gurich H, Ichida F, Jacobs JP, Justo R, Latal B, Li JS, Mahle WT, McQuillen PS, Menon SC, Pemberton VL, Pike NA, Pizarro C, Shekerdemian LS, Synnes A, Williams I, Bellinger DC, Newburger JW International Cardiac Collaborative on Neurodevelopment I. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135:816–825. doi: 10.1542/peds.2014-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swillen A, Vogels A, Devriendt K, Fryns JP. Chromosome 22q11 deletion syndrome: update and review of the clinical features, cognitive-behavioral spectrum, and psychiatric complications. Am J Med Genet. 2000;97:128–135. doi: 10.1002/1096-8628(200022)97:2<128::aid-ajmg4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Carey AS, Liang L, Edwards J, Brandt T, Mei H, Sharp AJ, Hsu DT, Newburger JW, Ohye RG, Chung WK, Russell MW, Rosenfeld JA, Shaffer LG, Parides MK, Edelmann L, Gelb BD. Effect of copy number variants on outcomes for infants with single ventricle heart defects. Circ Cardiovasc Genet. 2013;6:444–451. doi: 10.1161/CIRCGENETICS.113.000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, Romano-Adesman A, Bjornson RD, Breitbart RE, Brown KK, Carriero NJ, Cheung YH, Deanfield J, DePalma S, Fakhro KA, Glessner J, Hakonarson H, Italia MJ, Kaltman JR, Kaski J, Kim R, Kline JK, Lee T, Leipzig J, Lopez A, Mane SM, Mitchell LE, Newburger JW, Parfenov M, Pe’er I, Porter G, Roberts AE, Sachidanandam R, Sanders SJ, Seiden HS, State MW, Subramanian S, Tikhonova IR, Wang W, Warburton D, White PS, Williams IA, Zhao H, Seidman JG, Brueckner M, Chung WK, Gelb BD, Goldmuntz E, Seidman CE, Lifton RP. De novo mutations in histone-modifying genes in congenital heart disease. Nature. 2013;498:220–223. doi: 10.1038/nature12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. Int Rev Neurobiol. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- 17.Gaynor JW, Kim DS, Arrington CB, Atz AM, Bellinger DC, Burt AA, Ghanayem NS, Jacobs JP, Lee TM, Lewis AB, Mahle WT, Marino BS, Miller SG, Newburger JW, Pizarro C, Ravishankar C, Santani AB, Wilder NS, Jarvik GP, Mital S, Russell MW. Validation of association of the apolipoprotein E epsilon2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants. J Thorac Cardiovasc Surg. 2014;148:2560–2566. doi: 10.1016/j.jtcvs.2014.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Back SA, Miller SP. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Ann Neurol. 2014;75:469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, Miller SP. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71:385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013;81:2082–2089. doi: 10.1212/01.wnl.0000437298.43688.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ball G, Pazderova L, Chew A, Tusor N, Merchant N, Arichi T, Allsop JM, Cowan FM, Edwards AD, Counsell SJ. Thalamocortical Connectivity Predicts Cognition in Children Born Preterm. Cereb Cortex. 2015;25:4310–4318. doi: 10.1093/cercor/bhu331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimitropoulos A, McQuillen PS, Sethi V, Moosa A, Chau V, Xu D, Brant R, Azakie A, Campbell A, Barkovich AJ, Poskitt KJ, Miller SP. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–248. doi: 10.1212/WNL.0b013e31829bfdcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McQuillen PS, Goff DA, Licht DJ. Effects of congenital heart disease on brain development. Prog Pediatr Cardiol. 2010;29:79–85. doi: 10.1016/j.ppedcard.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soul JS, Robertson RL, Wypij D, Bellinger DC, Visconti KJ, du Plessis AJ, Kussman BD, Scoppettuolo LA, Pigula F, Jonas RA, Newburger JW. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J Thorac Cardiovasc Surg. 2009;138:374–381. doi: 10.1016/j.jtcvs.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 28.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Zimmerman RA, Spray TL, Gaynor JW, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. discussion 536–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Rhein M, Buchmann A, Hagmann C, Huber R, Klaver P, Knirsch W, Latal B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137(Pt 1):268–276. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- 30.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr, Guizard N, McGrath E, Geva J, Annese D, Dunbar-Masterson C, Trainor B, Laussen PC, du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, Heinle JS, Graves DE, Fraser CD., Jr Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rollins CK, Watson CG, Asaro LA, Wypij D, Vajapeyam S, Bellinger DC, DeMaso DR, Robertson RL, Jr, Newburger JW, Rivkin MJ. White matter microstructure and cognition in adolescents with congenital heart disease. J Pediatr. 2014;165:936–944. e931–932. doi: 10.1016/j.jpeds.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, Finucane K, Brizard C, Dance B, Shekerdemian LS. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph A, editor. Congenital Diseases of the Heart: Clinical-Physiological Considerations. 3. Wiley-Blackwell; 2009. [Google Scholar]
- 35.Sun L, Al-Rujaib M, Jaeggi E, Kingdom J, Windrim R, Sled JG, Macgowan C, Seed M. Preliminary hemodynamic reference ranges for the normal late gestation human fetus by phase contrast MRI and T2 mapping. Ultrasound Obstet Gynecol. 2014;44(S1):132. [Google Scholar]
- 36.Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, Grosse-Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, Hickey E, Miller S, Seed M. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E, Rowitch DH. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158:383–396. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Ment LR, Kesler S, Vohr B, Katz KH, Baumgartner H, Schneider KC, Delancy S, Silbereis J, Duncan CC, Constable RT, Makuch RW, Reiss AL. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503–511. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalpakidou AK, Allin MP, Walshe M, Giampietro V, Nam KW, McGuire P, Rifkin L, Murray RM, Nosarti C. Neonatal brain injury and neuroanatomy of memory processing following very preterm birth in adulthood: an fMRI study. PLoS One. 2012;7:e34858. doi: 10.1371/journal.pone.0034858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wernovsky G. Outcomes regarding the central nervous system in children with complex congenital cardiac malformations. Cardiol Young. 2005;15(Suppl 1):132–133. doi: 10.1017/s1047951105001162. [DOI] [PubMed] [Google Scholar]
- 42.Bellinger DC, Newburger JW, Wypij D, Kuban KC, duPlesssis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen AS, Idorn L, Thomsen C, von der Recke P, Mortensen J, Sorensen KE, Thilen U, Nagy E, Kofoed KF, Ostrowski SR, Sondergaard L. Prevalence of cerebral and pulmonary thrombosis in patients with cyanotic congenital heart disease. Heart. 2015;101:1540–1546. doi: 10.1136/heartjnl-2015-307657. [DOI] [PubMed] [Google Scholar]
- 44.Bellinger DC. Are children with congenital cardiac malformations at increased risk of deficits in social cognition? Cardiol Young. 2008;18:3–9. doi: 10.1017/S104795110700176X. [DOI] [PubMed] [Google Scholar]
- 45.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–e767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 46.Calderon J, Bellinger DC. Executive function deficits in congenital heart disease: why is intervention important? Cardiol Young. 2015;25:1238–1246. doi: 10.1017/S1047951115001134. [DOI] [PubMed] [Google Scholar]
- 47.Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. J Int Neuropsychol Soc. 2015;21:34–49. doi: 10.1017/S1355617714001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellinger DC. Theory of mind deficits in children with congenital heart disease. Dev Med Child Neurol. 2010;52:1079–1080. doi: 10.1111/j.1469-8749.2010.03734.x. [DOI] [PubMed] [Google Scholar]
- 49.DeMaso DR, Labella M, Taylor GA, Forbes PW, Stopp C, Bellinger DC, Rivkin MJ, Wypij D, Newburger JW. Psychiatric disorders and function in adolescents with d-transposition of the great arteries. J Pediatr. 2014;165:760–766. doi: 10.1016/j.jpeds.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diamond A. Executive functions. Annual review of psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 52.Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral cortex (New York, NY: 1991) 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 53.Neal AE, Stopp C, Wypij D, Bellinger DC, Dunbar-Masterson C, DeMaso DR, Newburger JW. Predictors of health-related quality of life in adolescents with tetralogy of Fallot. The Journal of pediatrics. 2015;166:132–138. doi: 10.1016/j.jpeds.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brock LL, Brock CD, Thiedke CC. Executive function and medical non-adherence: a different perspective. International journal of psychiatry in medicine. 2011;42:105–115. doi: 10.2190/PM.42.2.a. [DOI] [PubMed] [Google Scholar]
- 55.Marino BS, Tomlinson RS, Wernovsky G, Drotar D, Newburger JW, Mahony L, Mussatto K, Tong E, Cohen M, Andersen C, Shera D, Khoury PR, Wray J, Gaynor JW, Helfaer MA, Kazak AE, Shea JA Pediatric Cardiac Quality of Life Inventory Testing Study C. Validation of the pediatric cardiac quality of life inventory. Pediatrics. 2010;126:498–508. doi: 10.1542/peds.2009-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wray J, Franklin R, Brown K, Cassedy A, Marino BS. Testing the pediatric cardiac quality of life inventory in the United kingdom. Acta Paediatr. 2013;102:e68–73. doi: 10.1111/apa.12074. [DOI] [PubMed] [Google Scholar]
- 57.Iversen K, Vejlstrup NG, Sondergaard L, Nielsen OW. Screening of adults with congenital cardiac disease lost for follow-up. Cardiology in the young. 2007;17:601–608. doi: 10.1017/S1047951107001436. [DOI] [PubMed] [Google Scholar]
- 58.Mackie AS, Ionescu-Ittu R, Therrien J, Pilote L, Abrahamowicz M, Marelli AJ. Children and adults with congenital heart disease lost to follow-up: who and when? Circulation. 2009;120:302–309. doi: 10.1161/CIRCULATIONAHA.108.839464. [DOI] [PubMed] [Google Scholar]
- 59.Reid GJ, Irvine MJ, McCrindle BW, Sananes R, Ritvo PG, Siu SC, Webb GD. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(3 Pt 1):e197–205. doi: 10.1542/peds.113.3.e197. [DOI] [PubMed] [Google Scholar]
- 60.Gurvitz MZ, Inkelas M, Lee M, Stout K, Escarce J, Chang RK. Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood. J Am Coll Cardiol. 2007;49:875–882. doi: 10.1016/j.jacc.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 61.Yeung E, Kay J, Roosevelt GE, Brandon M, Yetman AT. Lapse of care as a predictor for morbidity in adults with congenital heart disease. International journal of cardiology. 2008;125:62–65. doi: 10.1016/j.ijcard.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 62.Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, Ting J, Kuehl K, Earing M, Webb G, Houser L, Opotowsky A, Harmon A, Graham D, Khairy P, Gianola A, Verstappen A, Landzberg M Alliance for Adult Research in Congenital C, Adult Congenital Heart A. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial) J Am Coll Cardiol. 2013;61:2180–2184. doi: 10.1016/j.jacc.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sable C, Foster E, Uzark K, Bjornsen K, Canobbio MM, Connolly HM, Graham TP, Gurvitz MZ, Kovacs A, Meadows AK, Reid GJ, Reiss JG, Rosenbaum KN, Sagerman PJ, Saidi A, Schonberg R, Shah S, Tong E, Williams RG American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young CoCNCoCC, Council on Peripheral Vascular D. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454–1485. doi: 10.1161/CIR.0b013e3182107c56. [DOI] [PubMed] [Google Scholar]
- 64.Rozenblum R, Miller P, Pearson D, Marelli A. Information technology for patient empowerment in healthcare. Berlin, Germany: Walter de Gruyter Inc; 2015. Patient-centered healthcare, patient engagement and health information technology: the perfect storm; pp. 3–22. [Google Scholar]
- 65.Crutzen R, de Nooijer J, Brouwer W, Oenema A, Brug J, de Vries NK. Strategies to facilitate exposure to internet-delivered health behavior change interventions aimed at adolescents or young adults: a systematic review. Health education & behavior : the official publication of the Society for Public Health Education. 2011;38:49–62. doi: 10.1177/1090198110372878. [DOI] [PubMed] [Google Scholar]
- 66.Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, Ong L, Colman J, Oechslin E, Nolan RP. Depression and anxiety in adult congenital heart disease: predictors and prevalence. Int J Cardiol. 2009;137:158–164. doi: 10.1016/j.ijcard.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 67.Kovacs AH, Sears SF, Saidi AS. Biopsychosocial experiences of adults with congenital heart disease: review of the literature. Am Heart J. 2005;150:193–201. doi: 10.1016/j.ahj.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 68.van Rijen EH, Utens EM, Roos-Hesselink JW, Meijboom FJ, van Domburg RT, Roelandt JR, Bogers AJ, Verhulst FC. Psychosocial functioning of the adult with congenital heart disease: a 20–33 years follow-up. Eur Heart J. 2003;24:673–683. doi: 10.1016/s0195-668x(02)00749-2. [DOI] [PubMed] [Google Scholar]
- 69.Kamphuis M, Vogels T, Ottenkamp J, Van Der Wall EE, Verloove-Vanhorick SP, Vliegen HW. Employment in adults with congenital heart disease. Arch Pediatr Adolesc Med. 2002;156:1143–1148. doi: 10.1001/archpedi.156.11.1143. [DOI] [PubMed] [Google Scholar]
- 70.Moons P, De Bleser L, Budts W, Sluysmans T, De Wolf D, Massin M, Gewillig M, Pasquet A, Suys B, Vliers A. Health status, functional abilities, and quality of life after the Mustard or Senning operation. Ann Thorac Surg. 2004;77:1359–1365. doi: 10.1016/j.athoracsur.2003.09.073. discussion 1365. [DOI] [PubMed] [Google Scholar]
- 71.Kamphuis M, Ottenkamp J, Vliegen HW, Vogels T, Zwinderman KH, Kamphuis RP, Verloove-Vanhorick SP. Health related quality of life and health status in adult survivors with previously operated complex congenital heart disease. Heart. 2002;87:356–362. doi: 10.1136/heart.87.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barbaresi WJ, Colligan RC, Weaver AL, Voigt RG, Killian JM, Katusic SK. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131:637–644. doi: 10.1542/peds.2012-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brook JS, Brook DW, Zhang C, Seltzer N, Finch SJ. Adolescent ADHD and adult physical and mental health, work performance, and financial stress. Pediatrics. 2013;131:5–13. doi: 10.1542/peds.2012-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zomer AC, Vaartjes I, Uiterwaal CS, van der Velde ET, Sieswerda GJ, Wajon EM, Plomp K, van Bergen PF, Verheugt CL, Krivka E, de Vries CJ, Lok DJ, Grobbee DE, Mulder BJ. Social burden and lifestyle in adults with congenital heart disease. Am J Cardiol. 2012;109:1657–1663. doi: 10.1016/j.amjcard.2012.01.397. [DOI] [PubMed] [Google Scholar]
- 75.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? The Lancet. Neurology. 2012;11:556–566. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 76.Lanz J, Brophy JM, Therrien J, Kaouache M, Guo L, Marelli AJ. Stroke in Adults With Congenital Heart Disease: Incidence, Cumulative Risk, and Predictors. Circulation. 2015;132:2385–2394. doi: 10.1161/CIRCULATIONAHA.115.011241. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez FH, 3rd, Marelli AJ. The epidemiology of heart failure in adults with congenital heart disease. Heart Fail Clin. 2014;10:1–7. doi: 10.1016/j.hfc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Roche SL, Redington AN. The failing right ventricle in congenital heart disease. Can J Cardiol. 2013;29:768–778. doi: 10.1016/j.cjca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 79.Nieminen HP, Jokinen EV, Sairanen HI. Causes of late deaths after pediatric cardiac surgery: a population-based study. J Am Coll Cardiol. 2007;50:1263–1271. doi: 10.1016/j.jacc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 80.Trojano L, Antonelli Incalzi R, Acanfora D, Picone C, Mecocci P, Rengo F. Cognitive impairment: a key feature of congestive heart failure in the elderly. J Neurol. 2003;250:1456–1463. doi: 10.1007/s00415-003-0249-3. [DOI] [PubMed] [Google Scholar]
- 81.Vogels RL, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail. 2007;9:1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 83.Jefferson AL. Cardiac output as a potential risk factor for abnormal brain aging. J Alzheimers Dis. 2010;20:813–821. doi: 10.3233/JAD-2010-100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Putzke JD, Williams MA, Rayburn BK, Kirklin JK, Boll TJ. The relationship between cardiac function and neuropsychological status among heart transplant candidates. J Card Fail. 1998;4:295–303. doi: 10.1016/s1071-9164(98)90235-4. [DOI] [PubMed] [Google Scholar]
- 85.Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, Cohen RA. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc. 2007;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O’Donnell CJ, Wolf PA, Manning WJ, Beiser AS, Benjamin EJ. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study) Am J Cardiol. 2011;108:1346–1351. doi: 10.1016/j.amjcard.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sabayan B, van Buchem MA, Sigurdsson S, Zhang Q, Harris TB, Gudnason V, Arai AE, Launer LJ. Cardiac hemodynamics are linked with structural and functional features of brain aging: the age, gene/environment susceptibility (AGES)-Reykjavik Study. J Am Heart Assoc. 2015;4:001294. doi: 10.1161/JAHA.114.001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 90.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 91.Kwok CS, Loke YK, Hale R, Potter JF, Myint PK. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology. 2011;76:914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 92.Roifman I, Therrien J, Ionescu-Ittu R, Pilote L, Guo L, Kotowycz MA, Martucci G, Marelli AJ. Coarctation of the aorta and coronary artery disease: fact or fiction? Circulation. 2012;126:16–21. doi: 10.1161/CIRCULATIONAHA.111.088294. [DOI] [PubMed] [Google Scholar]
- 93.Billett J, Cowie MR, Gatzoulis MA, Vonder Muhll IF, Majeed A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008;94:1194–1199. doi: 10.1136/hrt.2007.122671. [DOI] [PubMed] [Google Scholar]
- 94.Moss MB, Jonak E. Cerebrovascular disease and dementia: a primate model of hypertension and cognition. Alzheimers Dement. 2007;3(2 Suppl):002. doi: 10.1016/j.jalz.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 95.Gifford KA, Badaracco M, Liu D, Tripodis Y, Gentile A, Lu Z, Palmisano J, Jefferson AL. Blood pressure and cognition among older adults: a meta-analysis. Arch Clin Neuropsychol. 2013;28:649–664. doi: 10.1093/arclin/act046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;5:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 98.Khairy P, Clair M, Fernandes SM, Blume ED, Powell AJ, Newburger JW, Landzberg MJ, Mayer JE., Jr Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation. 2013;127:331–339. doi: 10.1161/CIRCULATIONAHA.112.135046. [DOI] [PubMed] [Google Scholar]
- 99.Ross GW, Petrovitch H, White LR, Masaki KH, Li CY, Curb JD, Yano K, Rodriguez BL, Foley DJ, Blanchette PL, Havlik R. Characterization of risk factors for vascular dementia: the Honolulu-Asia Aging Study. Neurology. 1999;53:337–343. doi: 10.1212/wnl.53.2.337. [DOI] [PubMed] [Google Scholar]
- 100.Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 101.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 102.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 104.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, Selvin E. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, Azakie A, Karl T, Miller SP. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38(2 Suppl):736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


