Abstract
Background
Current guidelines recommend at least 24-hour Holter monitoring at 6-month intervals to evaluate the recurrence of atrial fibrillation (AF) following surgical ablation. In this prospective multicenter study, conventional intermittent methods of AF monitoring were compared to continuous monitoring using an implantable loop recorder (ILR).
Methods
From 8/2011 to 1/2014, 47 patients receiving surgical treatment for AF at two institutions had an ILR placed at the time of surgery. Each atrial tachyarrhythmia (ATA) of two or more minutes was saved. Patients transmitted ILR recordings bimonthly or following any symptomatic event. Up to 27 minutes of data was stored before files were overwritten. Patients also received ECG and 24-hour Holter monitoring at 3, 6, and 12-months. ILR compliance was defined as any transmission between 0–3 months, 3–6 months, or 6–12 months. Freedom from ATAs were calculated and compared.
Results
ILR compliance at 12-months was 93% compared to ECG and Holter compliance of 85% and 76% respectively. ILR devices reported a total of 20,878 ATAs. Of these, 11% of episodes were available for review and 46% were confirmed as AF. Freedoms from ATAs were no different between continuous and intermittent monitoring at one year. Symptomatic events accounted for 187 episodes. However, only 10% were confirmed as AF.
Conclusions
ILR was equivalent at detecting ATAs compared to Holter or ECG. However, the high rate of false positives and limited number of events available for review present barriers to broad implementation of this form of monitoring. Very few symptomatic events were AF upon review.
Keywords: Ablation, Atrial Fibrillation, Devices, Practice Guidelines
Atrial fibrillation (AF) is the most common of all cardiac arrhythmias and accounts for nearly one-third of all hospital admissions due to heart rhythm irregularities. [1] A recent study predicted that the number of Americans diagnosed with AF will grow to over 10 million by the year 2050. [2] AF surgery is still underutilized and only 40% of patients with a history of AF referred for concomitant cardiac surgery currently receive an ablation procedure. A more accurate means of follow-up would allow for better postoperative management strategy, especially regarding decisions to continue anti-arrhythmic and anticoagulant medication. [3]
While surgical treatment for AF has been performed for almost 30 years, most of the historical series reported only the recurrence of symptomatic AF or used only intermittent electrocardiogram (ECG) follow-up. While it has been demonstrated that episodes of early postoperative atrial arrhythmias usually resolve within the first month following the Cox-Maze procedure, some do persist. [4] Several studies have demonstrated that complaints of palpitations often result from atrial or ventricular premature beats and are not an accurate predictor of recurrent AF. [5, 6]
Traditional methods of patient follow-up after treatment for AF have relied on intermittent monitoring. Early studies depended on symptomatic patients’ ability to accurately report their rhythm and incorporated only ECG follow-up. [6–9] Therefore, in 2007, an expert panel of electrophysiologists, cardiologists, and cardiac surgeons formed the Heart Rhythm Society Task Force and developed recommendations for catheter and surgical ablation of AF, which included guidelines for post-treatment follow-up and rhythm monitoring. [10]
The consensus statement of this expert panel set standards for reporting outcomes to include a minimal assessment of symptomatic AF and search for asymptomatic AF with prolonged cardiac rhythm monitoring at specified intervals. The basis of follow-up includes a 3-month visit followed by visits every 6-months for two years. Patients should receive ECG monitoring at each visit and 24-hour Holter monitoring is recommended for patients with persistent or longstanding persistent AF every 6 months. An episode of atrial tachyarrhythmias (ATA) thirty seconds or more in duration is considered a recurrence.
The optimal duration of prolonged monitoring is controversial. A number of investigators have concluded that continuous monitoring is the best follow-up strategy and is more sensitive at detecting recurrent AF episodes. [11–13] However, there have been few studies in surgical patients, particularly after a Cox-Maze (CM) procedure [14, 15] Since the CM has a high reported success rate, the utility of more prolonged monitoring periods may not be as helpful as when it is employed after less effective interventions.
A continuously recording implantable loop recorder (ILR) is theoretically the best follow-up and should be the most accurate way to define both symptomatic and asymptomatic episodes. The Reveal XT 9529 (Medtronic, Inc., Minneapolis, MN) is a small, leadless FDA-approved ILR which continuously monitors a patient’s cardiac rhythm (Figure 1). Recording can be triggered by patient activation as well as automatically. Data collection can be transmitted to health care providers via interrogation at clinic visits as well as electronically via the Medtronic CareLink® system.
Figure 1.
Reveal XT implantable loop recording device
The goal of this prospective observational study was to compare traditional means of arrhythmia assessment (ECG and Holter monitoring) to an ILR device.
PATIENTS and METHODS
This study was approved by the Washington University School of Medicine Institutional Review Board. Written informed consent and permission for release of information was obtained from each patient prior to enrollment. All data were entered prospectively into a custom longitudinal database.
Patient Selection
A total of 47 consecutive patients who received an ILR following a surgical ablation procedure were followed between August 2011 and January 2014. Inclusion criteria included patients over the age of 18 with AF who were scheduled to receive an elective surgical ablation procedure which included a Cox-Maze IV (CMIV) lesion set or pulmonary vein isolation (PVI). Exclusion criteria included patients with a preoperative permanent pacemaker, a projected lifespan of ≤ 6 months, or patients requiring emergent cardiac surgery.
Surgical Procedure
An ILR was placed subcutaneously in the left chest wall following a surgical ablation procedure which included a either a standalone or concomitant RF CMIV ablation, left or right atrial ablation, or pulmonary vein isolation. All surgical ablations and device implantations were performed by one of three experienced AF surgeons at a tertiary university hospital.
Patients were discharged on class I or III antiarrhythmic drugs and warfarin, unless contraindicated; antiarrhythmic agents were discontinued 2 months postoperatively if patients were in normal sinus rhythm. Calcium channel blockers or beta blockers were not considered as antiarrhythmic drugs.
ILR Monitoring and Transmission
The Reveal XT detects ATA episodes based on R wave variability. The R-R interval is measured and the differences are plotted on a Lorenz Plot. Highly irregular R wave intervals seen during AF produces a Lorenz Plot that is very widespread. Using a proprietary algorithm, the device is triggered to record when a widened Lorenz plot is detected.
The ILR stored up to 30 arrhythmia episodes of each type in an episode log. For each ATA episode, the ILR stored an EGM of the first 2 minutes of the episode. Twenty-seven minutes of EGM storage was available for automatically detected episodes. When the available memory was full, the oldest stored EGM recording was overwritten.
When the patient experienced symptoms, the patient prompted the ILR to record a symptomatic episode. Up to 10 patient activated symptom episodes could be stored in the episode log. Twenty-two and a half minutes of EGM storage were available for the 3 most recent symptom episodes in the episode log. Each symptom episode consisted of 6.5 minutes of ECG before activation and 1 minute after activation.
Transmissions from the patient’s home were performed via the Medtronic Carelink® remote monitoring system which uploads data through the patient’s telephone line. Data were transmitted bimonthly to the operative surgeon’s institution.
Adjudication Process
Each episode of AF was recorded either automatically by the ILR or as a symptom-triggered episode by the patient. Each available episode was reviewed by study personnel trained to read Reveal XT electrograms. Any episode with an undetermined rhythm was further reviewed by a board certified electrophysiologist.
Follow up
Patients were prospectively followed at 3, 6, 12 months postoperatively. ILR data was transmitted by the patient from home bimonthly or after 3 patient-activated symptomatic episodes were recorded. At each follow-up, patients had an electrocardiogram (ECG) and a 24-hour Holter monitor. ILR compliance was defined as any transmission between 0–3 months, 3–6 months, or 6–12 months. Episodes triggering ILR recording were adjudicated and either confirmed or rejected as AF. Compliance and freedoms from AF were evaluated at 3, 6, 12-months following a 3-month blanking period as defined by the HRS/EHRA/ACAS consensus statements. [10] Recurrence was defined as any episode of AF, atrial flutter, or atrial tachyarrhythmias that lasted longer than 30 seconds. [10] When detecting recurrences among ILRs, the two transmissions prior to the given time point were used. All episodes of patient- triggered symptomatic events were adjudicated to determine the presence of AF.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or as median with range. Categorical variables were expressed as frequencies and percentages with outcomes compared using the χ2. All data analyses were performed using SYSTAT 13 software (Systat Software, Inc., Chicago, IL).
RESULTS
Demographics
Preoperative demographics can be found in Table 1. Forty percent of patients had a history of paroxysmal AF while the remaining 60% were in either persistent or longstanding persistent AF. The majority of patients received a biatrial Cox-Max IV procedure (42/47, 89%) while 3 patients received a pulmonary vein isolation (3/47, 7%) and 1 patient each received either a left or right atrial radiofrequency ablation set (2/47, 4%). A stand-alone CMIV procedure was performed in 14 patients (30%) while 33 patients (70%) received a concomitant procedure. These procedures included: 21 mitral valve procedures ± coronary artery bypass graft procedures (CABG) ± Tricuspid valve (64%), 6 CABG ± aortic valve procedures (18%), and 6 other procedures (18%).
Table 1.
Preoperative Demographics
| Age (years) | 67 ± 10 |
| Male gender (%) | 27/47 (57%) |
| AF duration (years) | 5.6 ± 8.4 |
| Paroxysmal AF (%) | 18/47 (38%) |
| Persistent AF (%) | 19/47 (40%) |
| Longstanding Persist (%) | 10/47 (21%) |
| NYHA class III or IV (%) | 10/47 (21%) |
| LVEF (%) | 58 ± 7% |
| Failed catheter ablation (%) | 13/47 (28%) |
| LA diameter (cm) | 5 ± 0.9 |
AF = Atrial fibrillation, LVEF = Left ventricular ejection fraction, NYHA = New York Heart Association
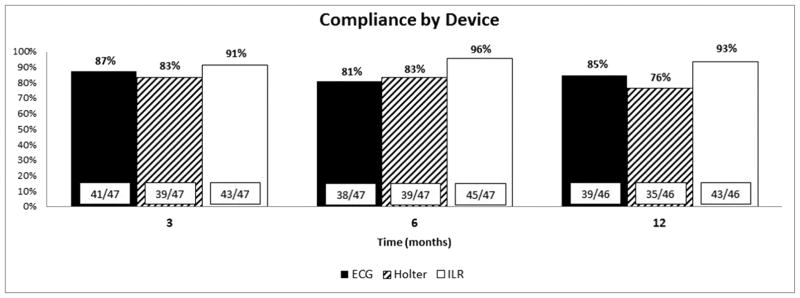
Follow-up Device Compliance
Device compliance was >90% for ILRs and highest at all-time points when compared to ECG and Holter (Figure 2). At 6 months, ILR compliance was 96% compared to ECG and Holter monitoring, which were 81% and 83% respectively (p = 0.073). At one 1 year, ILR compliance was 91% compared to ECG and Holter which were 85% and 76% respectively (p = 0.067).
Figure 2.
Follow-up compliance by arrhythmia monitoring device
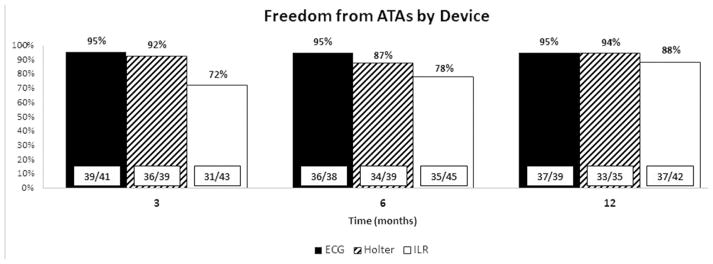
Freedoms from ATAs
ILRs recorded a total of 20,878 ATAs during the course of the study. Given the data storage limitations of the device, only 2,249 (11%) of episodes were available for adjudication to confirm the presence of ATAs. Of those available for review, 1,034 episodes (46%) were confirmed as ATAs. Freedoms from ATAs as detected by arrhythmia monitoring device are shown in Figure 3. Freedom from ATAs at one year was 95% compared to ECG and Holter monitoring, which were 95% and 94% respectively (p = 0.451). While there was a trend toward a difference at 3 and 6 months when comparing ILR to Holter and ECG (p = 0.096 and p = 0.093 respectively), this difference disappeared at 1 year.
Figure 3.
Freedoms from atrial fibrillation as determined by capture method
ATAs not Captured by Holter
There were 34 patients that received both a Holter monitor and transmitted ILR data at 1 year. Of these, only 3 patients (9%) were found to demonstrate ATAs within their last 2 transmissions prior to their 1-year follow up which were not captured by Holter monitoring. Mean ATA burden (% of time in ATA/day) among this subgroup was 0.63% (range 0.48% – 0.85%). The longest recorded ATA episode was 98 minutes of AF.
Symptomatic Triggers
Patients were able to log up to 10 symptomatic episodes and store up to three 7.5 min EGMs for a total of 22.5 min before data were overwritten. Activating a symptomatic episode records an EGM 6.5 minutes prior to activation and 1 minute following activation. Twenty three patients activated 187 symptomatic episodes. Of these, 158 episodes (84%) were available for adjudication. Upon review, 19 episodes (12%) were confirmed as ATAs with the vast majority of false positives showing normal sinus rhythm followed by PACs and PVCs.
COMMENT
Our study showed an increasing trend in compliance when using ILR compared to both ECG and Holter monitoring. However, freedoms from ATAs were not different when comparing these different monitoring modalities. This study is one of the largest in the literature comparing conventional arrhythmia monitoring to ILR in the setting of a surgical ablation. Moreover, the episodes recorded by the ILRs were carefully adjudicated for accuracy.
In a previous study comparing conventional arrhythmia monitoring to ILR following surgical ablation, the investigators showed that ILRs detected more episodes of ATAs when compared to 24-hour Holter monitoring. [14] This study however performed only a left atrial lesion set during concomitant cardiac procedures. A left atrial lesion has been previously shown to be less effective at restoring normal sinus rhythm than a biatrial lesion set. [16] The majority of patients in our study received a CMIV procedure which utilizes bipolar radiofrequency ablation and cryoablation to create a biatrial lesion set. It is possible that at one year, the ILR was unable to detect a difference when compared to ECG or Holter monitoring because the recurrence rate has been shown to be low after this procedure, particularly compared to a left atrial lesion set alone. [17, 18] For either ablation procedures with either a very low or high success rate, the utility of ILRs and continuous monitoring may be less apparent.
Based on our findings, the primary benefit of ILR use was its ability to increase patient compliance. Between 6–12 months, 93% of patients submitted at least one transmission of ILR data. In our experience, implantable devices are particularly useful in obtaining follow-up from patients who are unlikely to return for follow-up visits due to either the distance of their home from the implanting center, socioeconomic factors, or lack of motivation.
This study identified two major limitations of the ILR device. These included 1) a limited storage capacity, and 2) high rate of false positives. The version of ILR used in this study was only capable of storing 49.5 minutes of EGMs: 3 symptomatic EGMs of 7.5 minutes each and 27 minutes of EGMs using automatically detected ATAs. Additional events overwrote the oldest previously recorded EGM. Even with patients uploading data bimonthly, only 11% of the overall data were available for adjudication according to recorded patient’s logs. Furthermore logs were overwritten if there were more than 30 episodes per type of arrhythmia (AF, AT, etc) or over 10 symptomatic episodes. Given this, the device only transmitted a fraction of potential ATAs. Only 11% of the EGMs were available for adjudication. Data retrieval could theoretically be increased by increasing data transmission frequency however this is likely to decrease patient compliance, especially at the later time points.
Results from this study also revealed a high degree of false positives reported by the ILR. In this study, of the 11% of EGMs available for adjudication, 64% were found to represent false positives. This is in contrast to previously reported data showing a false positive rate ranging between 18–61%. [11, 19–21] In both studies, a trained examiner adjudicated documented episodes of ATAs so it is unclear at this time why our study showed a higher incidence. It may have been due to a different patient population or a more intensive review of the EGMs both by a trained examiner and a board certified electrophysiologist. Newer devices have attempted to address the issue of high false positives with changes to both the device hardware and algorithm [22]
Because of the need for frequent uploads and the amount of false positives, the amount of data requiring review is substantial. We estimate it took approximately 2 minutes to adjudicate each EGM. In this study with 47 patients, 2,249 EGMs were available for review using bimonthly transmission. Therefore we calculate the time required to review all EGMs in this study was 75 hours. Such a time commitment requires a dedicated support staff well trained in EGM interpretation. This level of support specialization is likely beyond the scope of most general cardiothoracic surgery programs.
Notably, when evaluating symptomatic events, most events available for review were not found to be ATAs. This is an important finding of this study and would suggest that symptomatic recurrence rates would overestimate the real failure rate.
Limitations
This study has several limitations. This is a relatively small study and thus subject to selection bias and type 2 statistical errors. A larger multicenter study would be needed to fully define the utility of ILRs. Moreover in our study, the majority of patients underwent a Cox-Maze IV procedure and our ATA recurrence rate was low, less than 10%. It would be possible that a larger patient cohort with a higher failure rate would demonstrate a clinical benefit of using ILRs.
It is acknowledged that more frequent downloads of the ILRs would have yielded more EGMs available for adjudication. However, we were able to adjudicate over 2,200 EGMs and it is unlikely that having more available for analysis would have changed the high false positive rate that we observed with ILRs.
Conclusion
ILRs had similar rates of detecting AF when compared to Holter monitoring or ECG. However, the high rate of false positives and limited number of events available for review present barriers to broad implementation for this form of monitoring. Of interest, very few symptomatic events were actually AF upon review suggesting the unreliability of symptoms in defining recurrent AF.
Acknowledgments
Supported by National Institute of Health grants R01 HL032257, T32 HL007776 and Medtronic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fuster V, Ryden LE, Cannom DS, et al. Acc/aha/esc 2006 guidelines for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on practice guidelines and the european society of cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): Developed in collaboration with the european heart rhythm association and the heart rhythm society. Circulation. 2006;114(7):e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (atria) study. Jama. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 4.Ishii Y, Gleva MJ, Gamache MC, et al. Atrial tachyarrhythmias after the maze procedure: Incidence and prognosis. Circulation. 2004;110(11 Suppl 1):II164–168. doi: 10.1161/01.CIR.0000138400.44799.65. [DOI] [PubMed] [Google Scholar]
- 5.Vasamreddy CR, Dalal D, Dong J, et al. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. Journal of cardiovascular electrophysiology. 2006;17(2):134–139. doi: 10.1111/j.1540-8167.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 6.Klemm HU, Ventura R, Rostock T, et al. Correlation of symptoms to ecg diagnosis following atrial fibrillation ablation. Journal of cardiovascular electrophysiology. 2006;17(2):146–150. doi: 10.1111/j.1540-8167.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 7.Damiano RJ, Jr, Gaynor SL, Bailey M, et al. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the cox maze procedure. The Journal of thoracic and cardiovascular surgery. 2003;126(6):2016–2021. doi: 10.1016/j.jtcvs.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Mehall JR, Kohut RM, Jr, Schneeberger EW, Merrill WH, Wolf RK. Absence of correlation between symptoms and rhythm in “symptomatic” atrial fibrillation. The Annals of thoracic surgery. 2007;83(6):2118–2121. doi: 10.1016/j.athoracsur.2007.02.084. [DOI] [PubMed] [Google Scholar]
- 9.Gillinov AM, Bhavani S, Blackstone EH, et al. Surgery for permanent atrial fibrillation: Impact of patient factors and lesion set. The Annals of thoracic surgery. 2006;82(2):502–513. doi: 10.1016/j.athoracsur.2006.02.030. discussion 513–504. [DOI] [PubMed] [Google Scholar]
- 10.Calkins H, Kuck KH, Cappato R, et al. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the european heart rhythm association (ehra), a registered branch of the european society of cardiology (esc) and the european cardiac arrhythmia society (ecas); and in collaboration with the american college of cardiology (acc), american heart association (aha), the asia pacific heart rhythm society (aphrs), and the society of thoracic surgeons (sts). Endorsed by the governing bodies of the american college of cardiology foundation, the american heart association, the european cardiac arrhythmia society, the european heart rhythm association, the society of thoracic surgeons, the asia pacific heart rhythm society, and the heart rhythm society. Heart rhythm: the official journal of the Heart Rhythm Society. 2012;9(4):632–696. e621. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Hindricks G, Pokushalov E, Urban L, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: Results of the xpect trial. Circulation Arrhythmia and electrophysiology. 2010;3(2):141–147. doi: 10.1161/CIRCEP.109.877852. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart rhythm: the official journal of the Heart Rhythm Society. 2006;3(12):1445–1452. doi: 10.1016/j.hrthm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Charitos EI, Stierle U, Ziegler PD, et al. A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: Insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation. 2012;126(7):806–814. doi: 10.1161/CIRCULATIONAHA.112.098079. [DOI] [PubMed] [Google Scholar]
- 14.Hanke T, Charitos EI, Stierle U, et al. Twenty-four-hour holter monitor follow-up does not provide accurate heart rhythm status after surgical atrial fibrillation ablation therapy: Up to 12 months experience with a novel permanently implantable heart rhythm monitor device. Circulation. 2009;120(11 Suppl):S177–184. doi: 10.1161/CIRCULATIONAHA.108.838474. [DOI] [PubMed] [Google Scholar]
- 15.Ip JH, Viqar-Syed M, Grimes D, et al. Surveillance of af recurrence post-surgical af ablation using implantable cardiac monitor. Journal of interventional cardiac electrophysiology: an international journal of arrhythmias and pacing. 2012;33(1):77–83. doi: 10.1007/s10840-011-9600-2. [DOI] [PubMed] [Google Scholar]
- 16.Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: A meta-analysis. The Journal of thoracic and cardiovascular surgery. 2006;131(5):1029–1035. doi: 10.1016/j.jtcvs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Saint LL, Bailey MS, Prasad S, et al. Cox-maze iv results for patients with lone atrial fibrillation versus concomitant mitral disease. The Annals of thoracic surgery. 2012;93(3):789–794. doi: 10.1016/j.athoracsur.2011.12.028. discussion 794–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weimar T, Schena S, Bailey MS, et al. The cox-maze procedure for lone atrial fibrillation: A single-center experience over 2 decades. Circulation Arrhythmia and electrophysiology. 2012;5(1):8–14. doi: 10.1161/CIRCEP.111.963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Castellano N, Fernandez-Cavazos R, Moreno J, et al. The cor trial: A randomized study with continuous rhythm monitoring to compare the efficacy of cryoenergy and radiofrequency for pulmonary vein isolation. Heart rhythm: the official journal of the Heart Rhythm Society. 2014;11(1):8–14. doi: 10.1016/j.hrthm.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Verma A, Champagne J, Sapp J, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Internal Medicine. 2013;173(2):149–56. doi: 10.1001/jamainternmed.2013.1561. [DOI] [PubMed] [Google Scholar]
- 21.Kapa S, Epstein AE, Callans DJ, et al. Assessing arrhythmia burden after catheter ablation of atrial fibrillation using an implantable loop recorder: the ABACUS study. Journal of Cardiovascular Electrophysiology. 2013;24(8):875–81. doi: 10.1111/jce.12141. [DOI] [PubMed] [Google Scholar]
- 22.Pürerfellner H, Sanders P, Pokushalov E, et al. Miniaturized Reveal LINQTM Insertable Cardiac Monitoring (ICM) System: First in Man Experience. Heart Rhythm. 2015;(6):1113–9. doi: 10.1016/j.hrthm.2015.02.030. [DOI] [PubMed] [Google Scholar]